Abstract
A 32-year-old pregnant woman from southeastern Connecticut presents to her physician in July at 26 weeks’ gestation because of a skin lesion. She reports she has had fatigue, arthralgia, and headache for 2 days and a rash in her left axilla for 1 day. She lives in a wooded area and works in her garden frequently. Six weeks earlier, she had removed a small tick that was attached behind her right knee. On physical examination, she is afebrile. She has an erythematous, oval macular lesion, 7 to 8 cm in diameter, in her left axilla, with enhanced central erythema; no other abnormalities are noted. How should her case be managed?
THE CLINICAL PROBLEM
Lyme disease, a zoonosis, is transmitted by certain ixodid ticks and is the most common reportable vectorborne disease in the United States, where it is caused only by the spirochete Borrelia burgdorferi sensu stricto (hereafter termed B. burgdorferi).1–3 In Europe and in Asia, B. afzelii, B. garinii, and other related species, in addition to B. burgdorferi, cause Lyme disease.2 The most common sign of Lyme disease is erythema migrans (Fig. 1A).1–3 Erythema migrans usually begins as a small erythematous papule or macule that appears at the site of the tick bite 1 to 2 weeks later (range, 3 to 32 days) and subsequently enlarges.4–7 The lesion may have centrally located vesicles or necrotic areas (Fig. 1B). Erythema migrans may be asymptomatic, mildly pruritic, or, in rare cases, painful; if untreated, lesions may become 61 cm (2 ft) in diameter or larger and may last for 3 to 4 weeks before resolving.4–7 Erythema migrans lesions may occur anywhere on the body surface, although common sites are the groin, axilla, waist, back, legs, and, in children, the head and neck. Although reputed to have a bull’s-eye appearance, approximately two thirds of single erythema migrans lesions either are uniformly erythematous or have enhanced central erythema without clearing around it.4–7 In some patients, erythema migrans is asymptomatic, but many patients have nonspecific symptoms, including fatigue, headache, arthralgia, myalgia, and, less often, fever.4–7 An erythema migrans–like skin lesion can also be a sign of southern tick-associated rash illness, which is associated with the bite of the Lone Star tick, Amblyomma americanum.8
Figure 1. Types of Erythema Migrans.
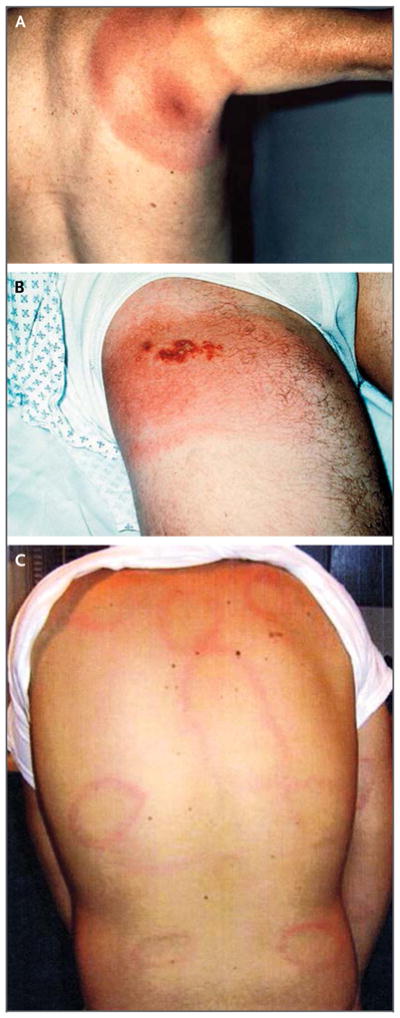
Panel A shows a single erythema migrans lesion, Panel B shows vesicular erythema migrans, and Panel C shows multiple erythema migrans lesions.
Most patients with erythema migrans (about 80%) have a single lesion, but the bacteria can disseminate hematogenously to other sites in the skin and to extracutaneous sites. The most common sign of early disseminated infection is multiple, often smaller erythema migrans lesions (Fig. 1C).7,9 The likelihood of hematogenous dissemination may be related to genotypic characteristics of the infecting strain of B. burgdorferi.10,11 Extracutaneous signs of disseminated Lyme disease that may occur, with or without erythema migrans, include neurologic conditions, such as cranial-nerve (particularly facial-nerve) palsy and meningitis that mimics aseptic meningitis, as well as carditis, which is most commonly manifested as heart block.1,2,9 Arthritis (most often affecting the knee) is a late sign of disseminated Lyme disease, occurring weeks to months after initial infection; it occurs in less than 10% of all cases, because most patients are treated and cured at an earlier stage of the illness.1,2,7,9
The incidence of Lyme disease in the United States has been increasing since national surveillance with the use of a standardized case definition was instituted in 1991.1,3 Although both under-reporting and overreporting occur in this passive surveillance system, the number of cases reported annually has risen from about 10,000 in 1992 to 25,000 to 30,000 currently.3 The actual number of annual cases may be as high as 300,000.12 The great majority of cases occur in New England and the mid-Atlantic states, with additional foci in northern midwestern states (Wisconsin and Minnesota).3 Less frequently, Lyme disease occurs in the Pacific coastal regions of Oregon and northern California.3 Although the geographic range of Lyme disease remains limited, it has been expanding. The incidence of Lyme disease is highest among children 5 to 14 years of age and middle-aged adults (40 to 50 years of age), and it is slightly more common among males than among females.3
The major natural reservoirs for B. burgdorferi are mice, chipmunks, and other small mammals, as well as birds.13,14 Deer are not competent hosts for B. burgdorferi but are important in sustaining the life cycle of the vector ticks. In the United States, Lyme disease is transmitted only by Ixodes scapularis ticks (deer ticks) in the eastern and northern midwestern states and by I. pacificus ticks in the western United States. These ticks feed once during each of the three stages of their life cycle (larva, nymph, and adult) (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). They acquire B. burgdorferi by feeding on an infected animal and may transmit the infection to a human during a subsequent blood meal.13,14 Transmission is most likely during the nymphal stage, since nymphs are abundant in the spring and early summer and are small and difficult to detect.15 Correspondingly, the peak incidence of erythema migrans is during the spring and summer months.3 Risk factors for Lyme disease include occupational and recreational exposure to fields and to woods in endemic areas, as well as outdoor activities such as gardening on residential properties near woodlands.14,16 Ixodid ticks are also vectors for a number of other infectious agents that may produce coinfections with B. burgdorferi, with clinical manifestations that range from asymptomatic to severe and life-threatening (Table 1).1 Although there has been concern about in utero transmission of B. burgdorferi, studies have shown no evidence of congenital Lyme disease.19,20
Table 1.
Potential Coinfections.*
| Infectious Agent† | Characteristics |
|---|---|
| Babesia microti1 | An intraerythrocytic parasite that can cause fever and anemia; usually cleared spontaneously by immunocompetent persons; may cause life-threatening illness in persons who are elderly or immunocompromised |
| Anaplasma phagocytophilum1 | An intracellular bacterium that may cause severe acute illness, with fever, leukopenia, and thrombocytopenia |
| Deer tick virus17 (a type of Powassan virus) | Can cause a serious, sometimes fatal encephalitis |
| Borrelia miyamotoi18 | Member of the relapsing-fever group of borrelia‡ |
| Ehrlichia species Wisconsin | Intracellular bacterium‡ |
Coinfections should be considered when patients with Lyme disease have severe or prolonged manifestations of infection or have anemia, leukopenia, thrombocytopenia, or unusually high or persistent fever.
Like infection with B. burgdorferi, infections with these organisms are transmitted by ixodes ticks.
There are few reports of humans infected with either B. miyamotoi or ehrlichia species Wisconsin, so the frequency and full spectrum of their manifestations remain to be determined.
STRATEGIES AND EVIDENCE
DIAGNOSIS
The diagnosis of erythema migrans is based on the clinical history, the distinctive character of the condition, and a history of potential exposure to ticks in an area where Lyme disease is endemic. The differential diagnosis is shown in Table 2.
Table 2.
Differential Diagnosis of Erythema Migrans.
| Condition | Characteristics |
|---|---|
| Single erythema migrans lesion | Erythematous macule or papule at site of tick bite (although the tick is often not seen); enlarges relatively rapidly to 5–30 cm or more in diameter; typically flat and annular; usually uniformly erythematous or with heightened central erythema; may have central clearing; without treatment, persists for average of 3–4 wk* |
| Nummular eczema | Lesion usually smaller and less erythematous than erythema migrans lesion; does not enlarge rapidly; pruritic; well demarcated; skin may be thickened or weepy |
| Tinea (ringworm) | Rash with raised margins and scale on the edges; central clearing is typical; pruritic |
| Granuloma annulare | Small (2–5 cm in diameter), circular rash with erythematous papules and clear center; develops over weeks; often on dorsum of extremities |
| Cellulitis | Area of inflammation often at site of trauma to skin; warm; enlarges rapidly; rarely circular; may be tender and associated with fever |
| Insect bite | Often raised papule with central punctum; pruritic; usually smaller than erythema migrans lesion; rarely continues to enlarge |
| Spider bite | Necrotic lesion with central eschar; often very painful |
| Hypersensitivity to tick bite | Small lesion, does not expand as erythema migrans does; present at time tick bite is recognized or soon after; uniformly erythematous; often pruritic |
| Multiple erythema migrans lesions | Multiple ringlike lesions; typically do not enlarge rapidly; a larger, primary lesion may be present; often associated with systemic symptoms |
| Erythema multiforme | Multiple lesions, often quite small; mucosa, palms, and soles may be involved; cause may be apparent (e.g., drug or infection) |
| Urticaria | Pruritic, raised lesions; may appear and disappear rapidly |
A similar lesion is found in southern tick-associated rash illness (STARI), which occurs primarily in southeastern and south central states. STARI does not have extracutaneous manifestations. The cause of STARI is unclear; no diagnostic test is available.8
Serologic tests for the diagnosis of B. burgdorferi infection are generally of little use in patients with erythema migrans.21–23 Two-tier serologic testing for antibodies to B. burgdorferi is recommended (a quantitative test, usually an enzyme-linked immunosorbent assay [ELISA] of the concentration of antibodies to B. burgdorferi and, if results are positive or equivocal, a Western blot)1; however, it has poor sensitivity in patients with erythema migrans during the acute phase (positive results in only 25 to 40% of patients without evidence of dissemination).21–23 The proportion of patients who test positive during the acute phase is higher among those with disseminated disease, but false negative results remain common (occurring in as many as 50% of cases).21–23 Even in the convalescent phase after antimicrobial treatment, a substantial proportion of patients with erythema migrans (half of those without dissemination and a quarter of those with dissemination) do not have a positive test result21–23; presumably, elimination of the organism dampens the antibody response. ELISA for antibodies against the C6 peptide of the variable major protein–like sequence expressed lipoprotein (C6VlsE) as a single test for Lyme disease at any stage has sensitivity and specificity similar to or better than those of conventional ELISA, but its specificity is inferior to that of the two-tier test.24 The sensitivity of two-tier testing is much better in patients either with early disseminated neurologic or cardiac Lyme disease (80 to 100%) or with late manifestations of Lyme disease such as arthritis (nearly 100%).21–23 Other testing strategies, such as the use of a C6VlsE ELISA as a second-tier test with conventional ELISA, have been suggested but still have suboptimal sensitivity for the detection of early Lyme disease.25 Although tests for antibodies have good sensitivity and specificity in patients who have had untreated infection for a month or longer, these tests should not be used for screening persons with a low probability of infection, such as those with only nonspecific symptoms such as fatigue or pain, because the positive predictive value in such patients is poor.1
As with most infections, after antibodies develop in Lyme disease, they may persist for many years, and the presence of these antibodies (both IgM and IgG) is an indication of previous exposure to the organism, not necessarily of active infection.26,27 Results of tests to directly detect bacteria in patients with erythema migrans, such as culture of either blood or biopsy samples from the lesion, sometimes combined with polymerase-chain-reaction assays, are generally not available for weeks; such tests are therefore not useful in practice.28
TREATMENT
Randomized trials have assessed several different antimicrobial agents for the treatment of erythema migrans. The currently recommended treatment regimens are summarized in Table 3. In these trials, rates of cure (defined as complete resolution of signs and symptoms shortly after the completion of treatment) have been about 90% with doxycycline, amoxicillin, or cefuroxime axetil.1 With rare exceptions, patients who were not cured continued to have only nonspecific symptoms, such as fatigue or arthralgia. If a patient has a contraindication to all those drugs, macrolide antibiotics (e.g., azithromycin, clarithromycin, or erythromycin) are an option, but they are somewhat less effective, with cure rates of about 80%.1 Most of the trials involved treatment for either 14 or 21 days. In one double-blind, randomized, controlled trial, 180 patients with erythema migrans received doxycycline for either 10 days or 20 days or received one dose of ceftriaxone followed by 10 days of doxycycline.30 There were no significant differences in outcomes (clinical cure and results of neurocognitive tests) among the three groups at any of the follow-up evaluations (at 20 days and 3, 12, and 30 months). Another randomized, controlled trial, in which 140 patients with early disseminated Lyme disease (almost all of whom had multiple erythema migrans lesions) were treated either for 14 days with ceftriaxone or for 21 days with doxycycline, also showed no significant between-group differences in cure rates.31 Resistance of B. burgdorferi to recommended antimicrobial agents has not been reported. First-generation cephalosporins, such as cephalexin, are not effective in treating Lyme disease.32
Table 3.
Treatment of Lyme Disease.
| Condition and Recommended Drug | Dose* | Duration† | Comments‡ |
|---|---|---|---|
| days | |||
| Erythema migrans | |||
|
| |||
| Doxycycline (for patients ≥8 yr of age) | 200 mg/day (pediatric dose, 4 mg/kg/day) orally, divided into two doses per day | 14 (range, 10–21) | Do not use to treat children <8 yr of age or women who are pregnant or lactating; warn patient about exposure to sun, since photosensitivity rash occurs in 20–30% of patients; drug has good penetration into the central nervous system; patient should take drug with fluids to minimize nausea and gastrointestinal irritation; also effective against granulocytic anaplasmosis but not against babesiosis |
|
| |||
| Amoxicillin | 1500 mg/day (pediatric dose, 50 mg/kg/day) orally, divided into three doses per day | 14 (range, 14–21) | This agent is not effective against granulocytic anaplasmosis or babesiosis |
|
| |||
| Cefuroxime axetil | 1000 mg/day (pediatric dose, 30 mg/kg/day) orally, divided into two doses per day | 14 (range, 14–21) | This agent is not effective against granulocytic anaplasmosis or babesiosis |
|
| |||
| Meningitis§ | |||
|
| |||
| Ceftriaxone | 2 g/day (pediatric dose, 50–75 mg/kg/day) intravenously once per day | 14 (range, 10–28) | Treatment has risks associated with indwelling catheters, including infection, and can cause pseudolithiasis in the gallbladder |
|
| |||
| Cefotaxime | 6 g/day (pediatric dose, 150– 200 mg/kg/day) intravenously, divided into doses administered every 8 hr | 14 (range, 10–28) | Treatment has risks associated with indwelling catheters, including infection |
|
| |||
| Cranial-nerve palsy without clinical evidence of meningitis¶ | |||
|
| |||
| Doxycycline (for patients ≥8 yr of age) | 200 mg/day (pediatric dose, 4 mg/kg/day) orally, divided into two doses per day | 14 (range, 14–21) | |
| Amoxicillin | 1500 mg/day (pediatric dose, 50 mg/kg/day) orally, divided into three doses per day | 14 (range, 14–21) | See comments for drugs used to treat erythema migrans; there is not good evidence that treatment changes the outcome of facial palsy, but it does prevent additional sequelae of infection |
| Cefuroxime axetil | 1000 mg/day (pediatric dose, 30 mg/kg/day) orally, divided into two doses per day | 14 (range, 14–21) | |
|
| |||
| Carditis | |||
|
| |||
| Same oral agents as for erythema migrans; same parenteral agents as for meningitis | Same doses as for oral and parenteral agents used to treat erythema migrans | 14 (range, 14–21) | Patients who are symptomatic should be hospitalized, monitored, and treated initially with a parenteral agent such as ceftriaxone; some patients with advanced heart block require a temporary pacemaker; after advanced block resolves, treatment may be completed with an oral agent |
|
| |||
| Arthritis | |||
|
| |||
| Same oral agents as for erythema migrans; same parenteral agents as for meningitis | Same doses as for oral and parenteral agents used to treat erythema migrans | 28 | Nonsteroidal antiinflammatory agents are often helpful as adjunctive treatment; for patients in whom arthritis persists or recurs, most experts recommend a second 28-day course of oral treatment; 14–28 days of parenteral treatment is an alternative |
For each drug, the maximum pediatric dose is the adult dose.
Recommendations are from the Infectious Diseases Society of America.
A reaction similar to the Jarisch–Herxheimer reaction may occur in the first 24 hours after treatment is begun.
There is evidence from Europe that treatment of meningitis with doxycycline administered orally is as good as parenteral treatment, although the species of borrelia that cause Lyme meningitis in Europe may be different from that in the United States.29
Doxycycline is preferable because of its good penetration into the central nervous system.
About 15% of patients have a reaction similar to the Jarisch–Herxheimer reaction (increased temperature, myalgia, and arthralgia) within 24 hours after treatment is begun with any recommended antimicrobial agent, as a result of an increase in circulating toxins associated with lysis of spirochetes. 1 The reaction resolves without serious consequences within 24 to 48 hours. Nonsteroidal antiinflammatory drugs may alleviate the symptoms of this reaction.
PREVENTION
Although avoidance of tick-infested areas is the best way to prevent Lyme disease, occupational and recreational activities and the proximity of residential areas to woodlands often make such directives impractical. Application of insect repellents that contain N,N-diethyl-meta-toluamide (DEET) in concentrations of at least 20% or covering skin with long pants and shirts is effective in preventing tick bites. Bathing within 2 hours after exposure may also be effective, because ticks take longer than 2 hours to fully attach. Regularly checking clothes and the entire body surface for ticks and removing them is recommended.33 Reducing the number of ticks on properties by spraying acaricides, using tubes that contain cotton balls infused with the insecticide permethrin, and removing leaf litter may help reduce the risk.33 A vaccine to prevent Lyme disease in humans was withdrawn from the market because of poor sales and is not currently available,33 although new vaccines are being developed.34
B. burgdorferi bacteria live in the midgut of ticks. As the tick becomes engorged with blood during feeding, bacteria replicate and migrate to the tick’s salivary glands, from which the organism can be injected into the host. Studies of the transmission of B. burgdorferi to humans are consistent with studies in animals indicating that transmission from infected nymphal ticks generally occurs only after 36 to 48 hours of attachment, and transmission from adult ticks occurs after an even longer period (≥48 hours).15,35,36 In a study comparing doxycycline with placebo in participants who had been bitten by nymphal ticks for which the duration of feeding could be assessed, the risk of erythema migrans in the placebo group was 25% (3 of 12 bites) if the tick had fed for 72 or more hours and 0% (0 of 48 bites) if the tick had fed for less than 72 hours.15 Nearly 75% of deer ticks removed from humans have fed for less than 48 hours.37 Consequently, even in areas where Lyme disease is highly endemic, the risk of disease transmission from a recognized bite is low (1 to 3%).38 The risk of transmission from an unrecognized bite may be greater, since an undetected tick is more likely to feed to repletion.37 Because additional bites are common (and may be unrecognized), a recognized tick bite is an indication that the person is at risk for tickborne illnesses.15 Routine serologic testing of persons bitten by a deer tick is not useful.15,21
In a randomized, controlled trial involving persons 12 years of age or older, a single 200-mg dose of doxycycline administered within 72 hours after removal of a deer tick was 87% effective (95% confidence interval, 25 to 98) in preventing Lyme disease.15 A meta-analysis indicated that 50 people bitten by a deer tick would need to be treated to prevent one case of erythema migrans, so antimicrobial prophylaxis is not recommended routinely, although it may be indicated for persons who remove an engorged nymphal deer tick.15,38,39 Anxious patients may be reassured that the risk of Lyme disease is very low and that, if it develops, treatment is very effective. Although there are highly sensitive molecular tests for the presence of B. burgdorferi in ticks, the value of these tests for predicting the risk of Lyme disease in a human bitten by a tick that is positive for B. burgdorferi is unknown, and there is no evidence that such testing is useful.
AREAS OF UNCERTAINTY
In a minority of patients who receive recommended treatment for Lyme disease, objective signs resolve, but subjective symptoms such as fatigue, arthralgia, and myalgia persist for weeks, months, or longer. These are classified as post–Lyme disease symptoms if they persist for less than 6 months and as post–Lyme disease syndrome if they are disabling and persist for 6 months or longer.40 The cause and frequency of this problem are unclear.40,41 Such nonspecific symptoms are common in the general population without Lyme disease. The positive predictive value of serologic tests for Lyme disease in patients with only nonspecific symptoms is poor,42 so misdiagnosis based on false positive serologic test results is common.1,40–42 Moreover, extensive publicity as well as misinformation on the Internet about “chronic” Lyme disease, a condition for which there is no clear definition or scientific evidence of its existence, may increase anxiety on the part of patients about the consequences of the illness and may confound assessments of treatment outcomes.41,43 In patients with two or more episodes of erythema migrans, often occurring years apart, it has been shown that the episodes were caused by different strains of bacteria, indicating reinfection rather persistence of infection with the original organism.44,45 Several carefully conducted, placebo-controlled, randomized trials of prolonged antimicrobial treatment in patients with persistent subjective symptoms after treatment for Lyme disease have shown a minimal benefit or none and a substantial risk of adverse effects.1,40,41,46,47 Consequently, prolonged antimicrobial treatment for subjective symptoms is not recommended in patients whose objective signs of Lyme disease have resolved in response to conventional therapy. Consideration of other causes of persistent symptoms is warranted. 40,41,48 In most of these patients, nonspecific symptoms resolve over time without additional antimicrobial treatment.40,41
GUIDELINES
The Infectious Diseases Society of America has published guidelines for the management of Lyme disease1 that are similar to those of other professional groups, including the American Academy of Neurology and professional societies in European countries.49,50 The recommendations in this article are consistent with these guidelines (Table 3).
CONCLUSIONS AND RECOMMENDATIONS
The patient described in the vignette has a skin lesion that is consistent with erythema migrans, 6 weeks after having removed a tick from another location on her body. I would recommend that this patient take 500 mg of amoxicillin three times a day for 14 days, because doxycycline is contraindicated in pregnant women. I would reassure her that the outcomes of treatment are excellent and that congenital Lyme disease has never been documented. I would advise her to beware of misinformation on the Internet. Even if the tick removed 6 weeks earlier had been an engorged nymphal deer tick, chemoprophylaxis would not have been indicated, because only doxycycline has been shown to be effective as chemoprophylaxis. Because the previously recognized tick was removed more than 1 month earlier and the site of the tick bite differed from that of the current erythema migrans lesion, this illness is probably unrelated to the tick bite, and chemoprophylaxis administered at that time would not have prevented it.
Supplementary Material
KEY CLINICAL POINTS.
LYME DISEASE
Erythema migrans lesions often do not have central clearing; the majority either are uniformly erythematous or have enhanced central erythema.
Antibody testing of patients with erythema migrans is not indicated routinely because of poor sensitivity in detecting early infection.
Treatment with doxycycline, amoxicillin, or cefuroxime is safe and highly efficacious for early Lyme disease.
A single 200-mg dose of doxycycline reduces the risk of Lyme disease in persons bitten by Ixodes scapularis ticks; however, it is not indicated routinely (given the low risk of transmission from a tick bite even in areas where the disease is endemic) and is contraindicated for pregnant women and for children younger than 8 years of age.
There is no evidence that patients treated for Lyme disease who have persistent, nonspecific symptoms (e.g., arthralgia and fatigue) have persistent infection; the risks of prolonged treatment with antimicrobial agents far outweigh the benefits, if any.
Footnotes
Dr. Shapiro reports providing medical-record review for law firms for malpractice cases regarding Lyme disease. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the author are available with the full text of this article at NEJM.org.
References
- 1.Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–134. doi: 10.1086/508667. Erratum, Clin Infect Dis 2007;45:941. [DOI] [PubMed] [Google Scholar]
- 2.Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379:461–73. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Lyme disease data. ( http://www.cdc.gov/lyme/stats)
- 4.Tibbles CD, Edlow JA. Does this patient have erythema migrans? JAMA. 2007;297:2617–27. doi: 10.1001/jama.297.23.2617. [DOI] [PubMed] [Google Scholar]
- 5.Steere AC, Sikand VK. The presenting manifestations of Lyme disease and the outcomes of treatment. N Engl J Med. 2003;348:2472–4. doi: 10.1056/NEJM200306123482423. [DOI] [PubMed] [Google Scholar]
- 6.Nadelman RB, Nowakowski J, Forseter G, et al. The clinical spectrum of early Lyme borreliosis in patients with culture-confirmed erythema migrans. Am J Med. 1996;100:502–8. doi: 10.1016/s0002-9343(95)99915-9. [DOI] [PubMed] [Google Scholar]
- 7.Smith RP, Schoen RT, Rahn DW, et al. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann Intern Med. 2002;136:421–8. doi: 10.7326/0003-4819-136-6-200203190-00005. [DOI] [PubMed] [Google Scholar]
- 8.Wormser GP, Masters E, Nowakowski J, et al. Prospective clinical evaluation of patients from Missouri and New York with erythema migrans-like skin lesions. Clin Infect Dis. 2005;41:958–65. doi: 10.1086/432935. [DOI] [PubMed] [Google Scholar]
- 9.Wormser GP, McKenna D, Carlin J, et al. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med. 2005;142:751–5. doi: 10.7326/0003-4819-142-9-200505030-00011. [DOI] [PubMed] [Google Scholar]
- 10.Wormser GP, Brisson D, Liveris D, et al. Borrelia burgdorferi genotype predicts the capacity for hematogenous dissemination during early Lyme disease. J Infect Dis. 2008;198:1358–64. doi: 10.1086/592279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanincova K, Mukherjee P, Ogden NH, et al. Multilocus sequence typing of Borrelia burgdorferi suggests existence of lineages with differential pathogenic properties in humans. PLoS One. 2013;8(9):e73066. doi: 10.1371/journal.pone.0073066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC provides estimate of Americans diagnosed with Lyme disease each year. Press release of the Centers for Disease Control and Prevention. 2013 Aug 13; ( http://www.cdc.gov/media/releases/2013/p0819-lyme-disease.html)
- 13.Lane RS, Piesman J, Burgdorfer W. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu Rev Entomol. 1991;36:587–609. doi: 10.1146/annurev.en.36.010191.003103. [DOI] [PubMed] [Google Scholar]
- 14.Walker DH, Barbour AG, Oliver JH, et al. Emerging bacterial zoonotic and vector-borne diseases: ecological and epidemiological factors. JAMA. 1996;275:463–9. [PubMed] [Google Scholar]
- 15.Nadelman RB, Nowakowski J, Fish D, et al. Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345:79–84. doi: 10.1056/NEJM200107123450201. [DOI] [PubMed] [Google Scholar]
- 16.Ley C, Olshen EM, Reingold AL. Case-control study of risk factors for incident Lyme disease in California. Am J Epidemiol. 1995;142(Suppl):S39–S47. doi: 10.1093/aje/142.supplement_9.s39. [DOI] [PubMed] [Google Scholar]
- 17.Ei Khoury MY, Camargo JF, Wormser GP. Changing epidemiology of Powassan encephalitis in North America suggests the emergence of the deer tick virus subtype. Expert Rev Anti Infect Ther. 2013;11:983–5. doi: 10.1586/14787210.2013.837805. [DOI] [PubMed] [Google Scholar]
- 18.Branda JA, Rosenberg ES. Borrelia miyamotoi: a lesson in disease discovery. Ann Intern Med. 2013;159:61–2. doi: 10.7326/0003-4819-159-1-201307020-00009. [DOI] [PubMed] [Google Scholar]
- 19.Gerber MA, Zalneraitis EL. Childhood neurologic disorders and Lyme disease during pregnancy. Pediatr Neurol. 1994;11:41–3. doi: 10.1016/0887-8994(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 20.Strobino BA, Williams CL, Abid S, Chalson R, Spierling P. Lyme disease and pregnancy outcome: a prospective study of two thousand prenatal patients. Am J Obstet Gynecol. 1993;169:367–74. doi: 10.1016/0002-9378(93)90088-z. [DOI] [PubMed] [Google Scholar]
- 21.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of Lyme borreliosis. Clin Microbiol Rev. 2005;18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steere AC, McHugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis. 2008;47:188–95. doi: 10.1086/589242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wormser GP, Nowakowski J, Nadelman RB, Visintainer P, Levin A, Aguero-Rosenfeld ME. Impact of clinical variables on Borrelia burgdorferi-specific antibody seropositivity in acute-phase sera from patients in North America with culture-confirmed early Lyme disease. Clin Vaccine Immunol. 2008;15:1519–22. doi: 10.1128/CVI.00109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wormser GP, Schriefer M, Aguero-Rosenfeld ME, et al. Single-tier testing with the C6 peptide ELISA kit compared with two-tier testing for Lyme disease. Diagn Microbiol Infect Dis. 2013;75:9–15. doi: 10.1016/j.diagmicrobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis. 2011;53:541–7. doi: 10.1093/cid/cir464. [DOI] [PubMed] [Google Scholar]
- 26.Kalish RA, McHugh G, Granquist J, Shea B, Ruthazer R, Steere AC. Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10–20 years after active Lyme disease. Clin Infect Dis. 2001;33:780–5. doi: 10.1086/322669. [DOI] [PubMed] [Google Scholar]
- 27.Hilton E, Tramontano A, DeVoti J, Sood SK. Temporal study of immunoglobin M seroreactivity to Borrelia burgdorferi in patients treated for Lyme borreliosis. J Clin Microbiol. 1997;35:774–6. doi: 10.1128/jcm.35.3.774-776.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liveris D, Schwartz I, McKenna D, et al. Comparison of five diagnostic modalities for direct detection of Borrelia burgdorferi in patients with early Lyme disease. Diagn Microbiol Infect Dis. 2012;73:243–5. doi: 10.1016/j.diagmicrobio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ljøstad U, Skogvoll E, Eikeland R, et al. Oral doxycycline versus intravenous ceftriaxone for European Lyme neuroborreliosis: a multicentre, non-inferiority, double-blind, randomised trial. Lancet Neurol. 2008;7:690–5. doi: 10.1016/S1474-4422(08)70119-4. Erratum, Lancet Neurol 2008;7:675. [DOI] [PubMed] [Google Scholar]
- 30.Wormser GP, Ramanathan R, Nowakowski J, et al. Duration of antibiotic therapy for early Lyme disease: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138:697–704. doi: 10.7326/0003-4819-138-9-200305060-00005. [DOI] [PubMed] [Google Scholar]
- 31.Dattwyler RJ, Luft BJ, Kunkel MJ, et al. Ceftriaxone compared with doxycycline for the treatment of acute disseminated Lyme disease. N Engl J Med. 1997;337:289–94. doi: 10.1056/NEJM199707313370501. [DOI] [PubMed] [Google Scholar]
- 32.Nowakowski J, McKenna D, Nadelman RB, et al. Failure of treatment with cephalexin for Lyme disease. Arch Fam Med. 2000;9:563–7. doi: 10.1001/archfami.9.6.563. [DOI] [PubMed] [Google Scholar]
- 33.Hayes EB, Piesman J. How can we prevent Lyme disease? N Engl J Med. 2003;348:2424–30. doi: 10.1056/NEJMra021397. [DOI] [PubMed] [Google Scholar]
- 34.Wressnigg N, Pöllabauer E-M, Aichinger G, et al. Safety and immunogenicity of a novel multivalent OspA vaccine against Lyme borreliosis in healthy adults: a double-blind, randomised, dose-escalation phase 1/2 trial. Lancet Infect Dis. 2013;13:680–9. doi: 10.1016/S1473-3099(13)70110-5. [DOI] [PubMed] [Google Scholar]
- 35.Piesman J. Dynamics of Borrelia burgdorferi transmission by nymphal Ixodes dammini ticks. J Infect Dis. 1993;167:1082–5. doi: 10.1093/infdis/167.5.1082. [DOI] [PubMed] [Google Scholar]
- 36.Piesman J, Maupin GO, Campos EG, Happ CM. Duration of adult female Ixodes dammini attachment and transmission of Borrelia burgdorferi, with description of a needle aspiration isolation method. J Infect Dis. 1991;163:895–7. doi: 10.1093/infdis/163.4.895. [DOI] [PubMed] [Google Scholar]
- 37.Falco RC, Fish D, Piesman J. Duration of tick bites in a Lyme disease-endemic area. Am J Epidemiol. 1996;143:187–92. doi: 10.1093/oxfordjournals.aje.a008728. [DOI] [PubMed] [Google Scholar]
- 38.Warshafsky S, Lee DH, Francois LK, Nowakowski J, Nadelman RB, Wormser GP. Efficacy of antibiotic prophylaxis for the prevention of Lyme disease: an updated systematic review and meta-analysis. J Antimicrob Chemother. 2010;65:1137–44. doi: 10.1093/jac/dkq097. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro ED. Doxycycline for tick bites — not for everyone. N Engl J Med. 2001;345:133–4. doi: 10.1056/NEJM200107123450209. [DOI] [PubMed] [Google Scholar]
- 40.Feder HM, Jr, Johnson BJB, O’Connell S, et al. A critical appraisal of “chronic Lyme disease. N Engl J Med. 2007;357:1422–30. doi: 10.1056/NEJMra072023. [DOI] [PubMed] [Google Scholar]
- 41.Lantos PM. Chronic Lyme disease: the controversies and the science. Expert Rev Anti Infect Ther. 2011;9:787–97. doi: 10.1586/eri.11.63. [DOI] [PubMed] [Google Scholar]
- 42.Seltzer EG, Shapiro ED. Misdiagnosis of Lyme disease: when not to order serologic tests. Pediatr Infect Dis J. 1996;15:762–3. doi: 10.1097/00006454-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Cooper JD, Feder HM., Jr Inaccurate information about Lyme disease on the Internet. Pediatr Infect Dis J. 2004;23:1105–8. [PubMed] [Google Scholar]
- 44.Nadelman RB, Wormser GP. Reinfection in patients with Lyme disease. Clin Infect Dis. 2007;45:1032–8. doi: 10.1086/521256. [DOI] [PubMed] [Google Scholar]
- 45.Nadelman RB, Hanincová K, Mukherjee P, et al. Differentiation of reinfection from relapse in recurrent Lyme disease. N Engl J Med. 2012;367:1883–90. doi: 10.1056/NEJMoa1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klempner MS, Hu LT, Evans J, et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med. 2001;345:85–92. doi: 10.1056/NEJM200107123450202. [DOI] [PubMed] [Google Scholar]
- 47.Halperin JJ. Prolonged Lyme disease treatment: enough is enough. Neurology. 2008;70:986–7. doi: 10.1212/01.WNL.0000291407.40667.69. [DOI] [PubMed] [Google Scholar]
- 48.Hatcher S, Arroll B. Assessment and management of medically unexplained symptoms. BMJ. 2008;336:1124–8. doi: 10.1136/bmj.39554.592014.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halperin JJ, Shapiro ED, Logigian E, et al. Practice parameter: treatment of nervous system Lyme disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2007;69:91–102. doi: 10.1212/01.wnl.0000265517.66976.28. Erratum, Neurology 2008;70: 1223. [DOI] [PubMed] [Google Scholar]
- 50.Baker PJ. Chronic Lyme disease: in defense of the scientific enterprise. FASEB J. 2010;24:4175–7. doi: 10.1096/fj.10-167247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


