Abstract
Background
Shoulder arthroplasty is increasing in the United States. Reverse shoulder arthroplasty (RSA) has emerged as an alternative treatment for end-stage glenohumeral pathology. Until recently, administrative coding practices have not differentiated RSA from traditional total shoulder arthroplasty (TSA), and thus national procedural volume has been unknown. The purpose of this study was to define the utilization, patient characteristics, indications and complications for RSA, and contrast these to TSA and hemiarthroplasty (HA).
Methods
The 2011 Nationwide Inpatient Sample (HCUP-NIS) dataset was queried using ICD-9-CM codes to identify patients undergoing RSA, TSA, or HA. We used weighted estimates of national procedure volume, per-capita utilization, patient comorbidities, and inpatient complications denned by the Agency for Healthcare Research and Quality (AHRQ) and identified them using standard methods described by Elixhauser. ANOVA statistical analysis was used and significance was denned as p value <0.05.
Results
In 2011, 66,485 patients underwent shoulder arthroplasty; there were 21,692 cases of RSA, 29,359 of TSA, and 15,434 of HA. Utilization of RSA and TSA increased between 2002-2011, and decreased for HA. RSA patients were older (72.7 years vs 67.4 TSA vs 66.8 HA) and more commonly female. Comorbidity burden was highest in patients undergoing HA. Inpatient complications were highest after RSA (p < 0.001). When compared to TSA, RSA was more commonly used in the setting of rotator cuff disease, and posttraumatic sequelae (p<0.001).
Conclusions
Our findings represent the first national estimates of RSA within the United Sates. RSA is a significant contributor to increasing shoulder arthroplasty utilization nationally representing one-third of arthroplasty cases. Conditions traditionally managed with HA in older populations appear to now be more commonly managed with RSA. RSA is performed on older patients with expanded indications.
Introduction
For decades, total shoulder arthroplasty (TSA) has been the gold-standard treatment for end stage arthritis of the glenohumeral joint. In appropriate patients, the efficacy of TSA provides long-term survival and satisfaction rates exceeding 86–95%1,2. However, TSA in patients with concomitant rotator cuff pathology has been associated with early failure due to high rates of glenoid loosening. Thus, these patients were traditionally offered hemiarthroplasty or humeral head resurfacing3. Recently, however, reverse shoulder arthroplasty (RSA) has emerged as an alternative surgical option.
RSA provides a mechanical advantage for shoulder elevation in patients with rotator cuff disease4. Early RSA designs suffered catastrophic failures from glenoid loosening5. Modern designs, however, have shown improved results6, and RSA survival rates have exceeded 85% at 10 years7,8. In November of 2003, the Food and Drug Administration (FDA) approved RSA arthroplasty in the United States. Since that time, RSA has been popularized for addressing a wide variety of shoulder conditions; these include glenohumeral arthritis, rotator cuff arthropathy, failed conventional total shoulder arthroplasty, fracture sequelae, rheumatoid arthritis with irreparable rotator cuff tears, proximal humerus tumors and proximal humerus fractures9-16.
The volume of shoulder arthroplasty has been increasing since the early 1990's9,18-20. From 1990 to 2004 previous studies describe steadily increasing rates of TSA18,19, outpaced only by the sharply increasing rates of hip and knee arthroplasty20. However, since FDA approval, the overall volume of shoulder arthroplasty has accelerated18,20. An aging population, improved implant designs, and broader indications have all been implicated for increasing volume and utilization18. Some postulate that RSA has also been the driver of these volume increases18. Until 2011, TSA and RSA were coded identically in administrative claims databases; both shared the International Classification of Disease, Ninth Revision (ICD-9) code, 81.80. Delineating United States (US) national volume of each has not been previously possible, and only small, single-institution, case series exist11,21.
Given the paucity of epidemiological data, the purpose of this study was to define the national utilization, patient characteristics and indications, and inpatient complications of patients undergoing RSA in the US using the well-established Nationwide Inpatient Sample (NIS) database. A secondary goal was to compare these findings to patients undergoing TSA and hemiarthroplasty.
Patients and Methods
This is a multicenter observational epidemiologic study of prospectively collected data of primary total shoulder arthroplasties conducted in the United States in 2011. This study was exempt from IRB approval.
Data Source
The Nationwide Inpatient Sample (NIS) NIS is the largest national all payer database for inpatient hospital stays22. First published in 1988, it has subsequently been updated annually, up to 2011. As of 2011, the NIS captured data from 46 states, covering 97% of the U.S. population. All non-Federal, short-term, general, and specialty hospitals in the U.S. are eligible for inclusion, including long-term care facilities. Participating hospitals are stratified according to size and geographic location. Within each strata, the NIS approximates a randomly generated 20% sample of all discharges. A multiplier unique to each strata is then applied in order to provide national estimates for a given data point. In 2011, the NIS captured over 8 million discharges, and the multipliers were used to provide weighted averages for an estimated 38.5 million inpatient stays. The estimated data points include over 100 variables, encompassing patient demographic, medical comorbidities, and morbidity outcomes, as well as hospital characteristics and financial information. Notably, the database includes only in-hospital events. Events that occur after a patient's discharge or during subsequent admissions are not linked.
Participants, Sample Size & Interventions
We included all patients from the 2011 NIS database with an ICD-9-CM procedure code (International Classification of Diseases, Ninth Revision, Clinical Modification) for primary shoulder arthroplasty (either HA 81.81; TSA: 81.80; or RSA: 81.88). Patients younger than 40 years or older than 95 years were excluded. Revision shoulder arthroplasty cases were excluded (ICD-9 codes 996.4x, 996.66, and 996.77). Prior to 2011, RSA and TSA shared a common code (81.80), but for the first time in 2011 RSA was given a unique identifier (81.88). Thus, 2011 is the first year in which these procedures could be distinguished. Ultimately, 66,485 procedures were identified. The FDA did not approve RSA for use in the United States until 200318. Thus, in order to provide a comparison with procedure volume and patient characteristics prior to the introduction of RSA, we included a mirrored cohort from the 2002 NIS database, consisting of 24,677 patients, using the same selection criteria. US census data of population estimates by age was used to estimate the number of persons in the US age 40–95 in 2002 and 201123,24.
Indications and Comorbidities
Indications were identified by querying the primary ICD-9-CM code for incidences of osteoarthritis (715.xx), proximal humerus fracture (812.0x), proximal humerus nonunion/malunion (733.8x), aseptic necrosis (733.41), rotator cuff tear arthropathy (716.91), disorders of shoulder bursae and tendons (726.10), rheumatoid arthritis (714.0x), partial (726.1x) or massive (727.6x) rotator cuff tears. Patient comorbidities were identified from a query of the secondary ICD-9-CM codes, and included chronic respiratory insufficiency (518.83), anemia (280.x, 281.x, 282.x, 283.x, 284.x), asthma (493.x), diabetes mellitus (250.x, 249.x), obstructive sleep apnea (327.23), obesity (278.01), overweight (278.02), coagulopathy (286.x), atrial fibrillation (427.31) or tobacco use disorder (305.1).
In-Hospital Complications
We identified in-hospital procedure related complications by searching for any ICD-9 code specifying a complication of surgical care (ICD-9-CM: 996.x to 999.x). In addition, we evaluated several specific adverse diagnoses, including in hospital mortality from surgical causes (995.4, 968.4, 348.8, 798.2, 798.1, 798.9), spinal cord or nerve injury (952–956), venous thrombosis (453.4), respiratory distress following surgery (518.5), and acute post hemorrhagic anemia (285.1). All indications, comorbidities and complications within the dataset are reliant on medical documentation and in-hospital coding practices.
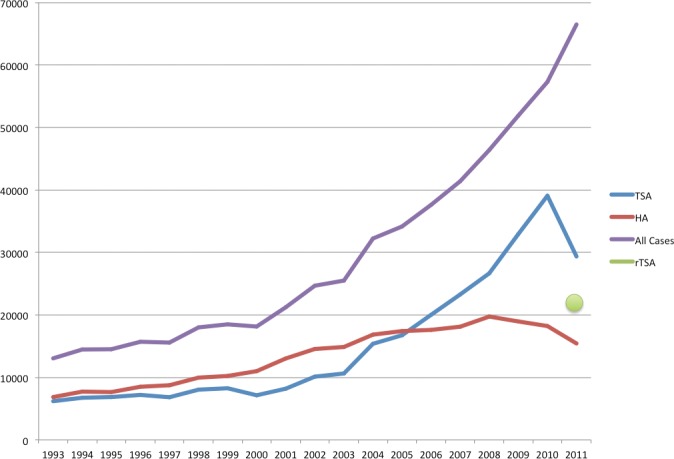
Figure I. Trends in shoulder arthroplasty within the US 1993–2011. This data was obtained from the NIS-HCUP database for HA: 81.81; TSA: 81.80; or RSA: 81.88.
Statistical Analysis
All data was analyzed with SAS (Version 9.3; SAS Institute, Cary, NC). Analysis of variance (ANOVA) statistical analysis was used to identify differences across groups and significance was defined as p value <0.05.
Source of Funding
There was no external source of funding for this study.
Results
Volume & Utilization
In 2002, 24,677 patients underwent primary shoulder arthroplasty, of which 10,125 (41%) were TSA and 14,552 (59%) were HA. In 2011, 66,485 patients underwent shoulder arthroplasty procedures, of which 21,692 (32.6%) were RSA, 29,359 (44%) were TSA, and 15,434 (23%) were HA [Figures 1 & 2]. Between 2002 and 2011, the US population between the ages of 40–95 increased by 21.37% (100,637,078 in 2002 versus 122,142,979 in 2011)23,24. The per-capita utilization of shoulder arthroplasty increased from 24.5 arthroplasties per 100,000 population in 2002 to 54.4 arthroplasties per 100,000 population in 2011. The utilization of HA during this same period decreased from 14.5 arthroplasties per 100,000 population in 2002 to 12.6 arthroplasties per 100,000 population 2011. The utilization of TSA increased froml4.5 per 100,000 population in 2002 to 24.0 arthroplasties per 100,000 population in 2011. The utilization of RSA for patients between the ages of 40–95 in 2011 was 17.8 arthroplasties per 100,000 population.
Demographics
The mean age of patients undergoing RSA in 2011 was 72.71 years. This is significantly higher than that of patients undergoing TSA (67.44 years) or HA (66.84 years) (p<0.001) [Table I]. Patients of white race were less likely to undergo HA (p=0.001) [Table I]. Patients with Medicaid insurance were more likely to undergo HA (p<0.001) [Table I]. The presence of certain patient comorbidities varied between the three procedures. Patients undergoing RSA were more likely to carry a diagnosis of atrial fibrillation (p<0.001) and chronic respiratory insufficiency (p<0.001). Chronic anemia, diabetes mellitus, and tobacco use disorder were more common in HA cases (p<0.001). Patients undergoing TSA were more commonly diagnosed with obstructive sleep apnea or obesity (p<0.001) [Table I]. When co-morbid conditions were pooled, patients undergoing HA were significantly more comorbid (p<0.001).
Table I.
Demographics and Comorbidities of patients undergoing RSA (Reverse Shoulder Arthroplasty), TSA (Total Shoulder Arthroplasty), and HA (Hemiarthroplasty) within the US in 2011.
| RSA | TSA | HA | p | |
|---|---|---|---|---|
| Mean Patient Age (years) | 72.71 | 67.44 | 66.84 | <0.0001 |
| Female Sex (%) | 63.86 | 50.65 | 62.48 | <0.0001 |
| Race (%) | 0.001 | |||
| Black | 4.75 | 4.73 | 4.77 | |
| White | 89.36 | 89.66 | 87.28 | |
| Other | 5.9 | 5.6 | 7.95 | |
| Type of Insurance (%) | <0.0001 | |||
| Private | 14.96 | 31.28 | 28.42 | |
| Medicaid | 1.45 | 1.79 | 4.25 | |
| Other | 83.59 | 66.94 | 67.33 | |
| Comorbidities | ||||
| Chronic Respiratory Insufficiency (%) | 0.13 | 0.07 | 0.4 | 0.0005 |
| Chronic Anemia (%) | 2.77 | 2.35 | 4.36 | <0.0001 |
| Asthma (%) | 8.95 | 9.11 | 8.72 | 0.8215 |
| Diabetes Mellitus (%) | 21.32 | 19.39 | 22.16 | 0.0033 |
| Obstructive Sleep Apnea (%) | 7.27 | 9.32 | 6.66 | <0.0001 |
| Obesity (%) | 11.69 | 14.97 | 13.09 | <0.0001 |
| Overweight (%) | 0.22 | 0.26 | 0.47 | 0.1253 |
| Coagulopathy (%) | 0.27 | 0.25 | 0.36 | 0.6668 |
| Atrial fibrillation (%) | 8.81 | 5.15 | 7.07 | <0.0001 |
| Tobacco use disorder (%) | 6.32 | 7.24 | 10.33 | <0.0001 |
| Any Comorbidity (%) | 47.52 | 47.13 | 51.20 | <0.0001 |
Indications
Osteoarthritis (OA) was the primary indication for 88.63% of TSA cases in 2011. This was significantly higher than the percentage of patients who underwent RSA (43.67%) or HA (40.51%) (p<0.001) [Table III]. 35.19% of HA cases were performed for proximal humerus fractures compared to 9.36% of RSA and 0.99% of TSA cases (p<0.001). Similarly, posttraumatic sequelae including nonunion or malunion were more commonly indicated in RSA and HA (p<0.001). RSA was performed more often for rotator cuff arthropathy, or shoulder bursa and tendon disorders (p<0.001) [Table III]. The mean age of patients who underwent RSA was significantly higher than TSA or HA for the indications of osteoarthritis, proximal humerus fractures, aseptic necrosis and rotator cuff tear arthropathy (p<0.001).
Table III.
Indications for patients undergoing shoulder arthroplasty in 2011; RSA (Reverse Shoulder Arthroplasty), TSA (Total Shoulder Arthroplasty), and HA (Hemiarthroplasty).
| (ICD-9-CM) Primary Diagnosis | RSA | TSA | HA | p |
|---|---|---|---|---|
| (715.xx) Osteoarthrosis and allied disorders | 43.67 | 88.63 | 40.51 | <0.0001 |
| (812.0x) Fracture of proximal end of humerus, closed | 9.36 | 0.99 | 35.19 | <0.0001 |
| (73341) Aseptic necrosis of head of humerus | 0.98 | 1.9 | 6.46 | <0.0001 |
| (716.91) Unspecified arthropathy shoulder (cuff tear arthropathy) | 11.83 | 3.58 | 2.37 | <0.0001 |
| (726.10) Disorders of bursae and tendons in shoulder | 14.03 | 0.54 | 1.86 | <0.0001 |
| (733.8x) Nonunion or Malunion | 3.27 | 0.39 | 3.17 | <0.0001 |
| (714.0) Rheumatoid arthritis | 1 | 0.95 | 0.48 | 0.0311 |
| (726.1x) Partial RTC Tear | 0.12 | 0.06 | 0 | na |
| (727.6x) Massive RTC Tear | 2.55 | 0.16 | 0.23 | <0.0001 |
In-Hospital Complications
The incidence of any in-hospital morbidity or mortality was higher in RSA (27.38%) compared to TSA (16.64%) or HA (23.96%) (p<0.001) [Table II]. The incidence of in-hospital mortality was higher in RSA (0.2%) and HA (0.26%) when compared to TSA (0.04%) (p=0.014). Acute respiratory distress (p<0.001), post-hemorrhagic anemia (p<0.001), general complications of surgical care (p<0.001), post-operative hypotension (p<0.001) and pulmonary embolism (p=0.019) were more common in RSA [Table II].
Table II.
In-hospital morbidity and mortality in patients undergoing shoulder arthroplasty in 2011 for RSA (Reverse Shoulder Arthroplasty), TSA (Total Shoulder Arthroplasty), and HA (Hemiarthroplasty).
| RSA | TSA | HA | p | |
|---|---|---|---|---|
| Incidence of Any Morbidity or Mortality (%) | 27.38 | 16.64 | 23.96 | <0.0001 |
| Mortality (%) | 0.2 | 0.04 | 0.26 | 0.0137 |
| Neurologic Injury (%) | 0.17 | 0.11 | 0.4 | 0.012 |
| Venous Thrombosis (%) | 0.19 | 0.05 | 0.15 | 0.0797 |
| Acute Respiratory Distress (%) | 1.19 | 0.43 | 1.07 | <0.0001 |
| Post-hemorrhagic Anemia (%) | 16.73 | 9.75 | 15.77 | <0.0001 |
| General Complications of Surgical Care (%) | 7.54 | 3.56 | 6.11 | <0.0001 |
| Paralytic Ileus (%) | 0.24 | 0.23 | 0.31 | 0.7586 |
| Post-Operative Hypotension (%) | 2.17 | 1.18 | 0.96 | <0.0001 |
| Hypovolemia (%) | 0.37 | 0.21 | 0.26 | 0.3358 |
Discussion
Reverse total shoulder arthroplasty has emerged as an alternative to TSA and HA with rapid acceptance in the United States over the past decade. Previously, the national volume of RSA in the United States has been largely unknown. Changes in administrative coding practices in 2011 have allowed for separation of RSA and TSA procedures. In this study we have analyzed patient data from 66,485 total shoulder arthroplasties queried from a national discharge database. Relative to the growth of TSA over the last decade, RSA volume represents a significant portion. The patient demographics, inpatient complications, and indications of patients undergoing RSA and TSA also differ significantly. Several of these findings merit further discussion.
This study has several limitations. First, indications and comorbidities within the dataset are reliant on medical documentation and in-hospital coding practices. Secondly, short term and long-term patient outcomes after discharge are not available in the NIS-HCUP database as all data is collected prior to discharge; we are therefore unable to address differences in certain complications including infections and dislocations that more commonly occur after discharge. Third, the data presented represents hospital discharged from a single year; only one year of complete data (2011) presently exists that distinguishes RSA and conventional TSA. Also, due to the size of data collection the NIS-HCUP database, availability of comprehensive data is generally delayed two years. Last, this study is limited to the population in the United States.
Since the FDA approval of RSA in 2003, overall shoulder arthroplasty volume has been sharply increasing18,20. Between 2002 and 2011, the utilization of shoulder arthroplasty more than doubled. The utilization of conventional TSA increased by 66% between 2002 and 2011. While use of RSA was limited prior to 2003, RSA comprised one-third of all shoulder arthroplasty cases in 2011. RSA, therefore, may be a principle factor driving the increased utilization of shoulder arthroplasty. Our study is the first to report decreasing rates of hemiarthroplasty on a national level. Rates of hemiarthroplasty had been previously reported to be steadily increasing between 1990 and 200418,20. Between 2002 and 2011, the utilization of hemiarthroplasty decreased by 12.5%. We believe the growing acceptance of RSA may be contributing to coincidentally declining HA volumes. Patient populations, especially the elderly, who were previously HA candidates may now be better treated with RSA25.
Our findings also demonstrate significant differences in patient characteristics between patients undergoing RSA, TSA, and HA. Previous reports suggest RSA should be reserved for elderly patient (>70 years old) with low functional demands8,17. The mean age of patients undergoing RSA in the US in 2011 was 72.71 years. This was significantly higher than patients undergoing TSA or HA. Then mean ages of patients who underwent shoulder arthroplasty in our cohort are comparable to previous reports18,26,27. The United States has an aging population28. The RSA is ideal for low demand, elderly patients who previously may not have been candidates for HA or TSA. In our series, RSA was more commonly performed in females (63.86%), however this was not true for TSA (50.65%) p<0.001 and this is consistent with previous reports11.
Our findings demonstrate varying indications between RSA, TSA, and HA. In general, RSA carried a wider array of indications when compared to TSA and HA. RSAs are more commonly performed in the setting of rotator cuff associated conditions due to mechanical advantages of implant design29,30. Our data suggests 28.5% of patients underwent RSA with a primary diagnosis of rotator cuff disease. In 2007, Wall et al. reported good outcomes for patients undergoing RSA for massive rotator cuff tears, primary rotator cuff arthropathy, and osteoarthritis11. These indications represent of approximately 72.2% of the volume of RSA observed in the 2011. In addition, RSA has been described for treatment of proximal humerus fractures4,15,31. Prosthetic replacement of the proximal humerus may be appealing in certain fracture patterns where the incidence of avascular necrosis of the humeral head is high with open reduction and internal fixation; also, tuberosity resorption is common in hemiarthroplasty. In 2011, surgeons in the US were 7-times more likely to choose RSA over a conventional TSA for prosthetic treatment of a proximal humerus fractures. HA remains the most common shoulder arthroplasty option for management of proximal humerus fractures (5,402 cases). Furthermore, surgeons were more likely to choose RSA or HA to address posttraumatic sequelae after proximal humerus fractures (malunion or nonunion). Therefore broad indications for RSA in the 2011 cohort may be due in part to an aging population, and relatively increased utilization in the setting of rotator cuff disease, fracture and post-traumatic sequelae.
Reports of complications after reverse total shoulder arthroplasty vary greatly8,11,17,21,27. According to our findings, RSA was associated with higher rates of in-hospital complications overall. Approximately half of these complications were post-operative anemia. In-patient perioperative mortality was nearly five times higher after RSA and HA compared with TSA (0.2%, 0.26% versus 0.04%, p=0.014). Previous reports indicate that in-hospital morbidity and mortality is highly dependent on patient comorbidities in arthroplasty populations32. Also, patient age has been well established as an independent risk factor for complications in the acute perioperative period33. Patients undergoing RSA were found to be significantly older than those undergoing TSA or HA (72.71 versus 67.44 and 66.84 years, p<0.001). As we did not control for patient factors, we are unable to tell if the true incidence of complications differs between groups.
Our findings represent the first national estimates of RSA within the United Sates. The national volume of RSA was 21,692 cases in 2011. Overall, the volume of shoulder arthroplasty in the US continues to rise; a phenomenon driven, likely in part, by the expanding use of RSA. Conditions traditionally managed with HA in older populations appear to now be more commonly managed with RSA. Importantly, patients undergoing RSA and TSA differ significantly in their demographics, comorbidities, surgical indications and inpatient complications. Given the burden of shoulder disease within the United States, this data will help define a baseline for studying the role of RSA. Future epidemiological studies should continue analyzing the temporal trends of shoulder arthroplasty.
References
- 1.Neer CS, 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319–37. [PubMed] [Google Scholar]
- 2.Norris T.R., Iannotti J.P. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130–5. doi: 10.1067/mse.2002.121146. [DOI] [PubMed] [Google Scholar]
- 3.Franklin JL, et al. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39–46. doi: 10.1016/s0883-5403(88)80051-2. [DOI] [PubMed] [Google Scholar]
- 4.Boileau P, et al. Neer Award 2005: The Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527–40. doi: 10.1016/j.jse.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Post M. Constrained arthroplasty: its use and misuse. Semin Arthroplasty. 1990;1(2):151–9. [PubMed] [Google Scholar]
- 6.Boileau P, et al. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1) Suppl S:147S–161S. doi: 10.1016/j.jse.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Favard L, et al. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res. 2011;469(9):2469–75. doi: 10.1007/s11999-011-1833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guery J, et al. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742–7. doi: 10.2106/JBJS.E.00851. [DOI] [PubMed] [Google Scholar]
- 9.Nam D, et al. Reverse total shoulder arthroplasty: current concepts, results, and component wear analysis. J Bone Joint Surg Am. 2010;92(Suppl 2):23–35. doi: 10.2106/JBJS.J.00769. [DOI] [PubMed] [Google Scholar]
- 10.Gerber C, Pennington SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17(5):284–95. doi: 10.5435/00124635-200905000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Wall B, et al. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476–85. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 12.Levy JC, et al. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189–95. doi: 10.1302/0301-620X.89B2.18161. [DOI] [PubMed] [Google Scholar]
- 13.Levy J, et al. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg Am. 2007;89(2):292–300. doi: 10.2106/JBJS.E.01310. [DOI] [PubMed] [Google Scholar]
- 14.Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17–22. doi: 10.1067/mse.2001.110515. [DOI] [PubMed] [Google Scholar]
- 15.Bufquin T, et al. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly: a prospective review of 43 cases with a short-term follow-up. J Bone Joint Surg Br. 2007;89(4):516–20. doi: 10.1302/0301-620X.89B4.18435. [DOI] [PubMed] [Google Scholar]
- 16.Brorson S, et al. Reverse shoulder arthroplasty in acute fractures of the proximal humerus: A systematic review. Int J Shoulder Surg. 2013;7(2):70–8. doi: 10.4103/0973-6042.114225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohsali KI, Wirth MA, Rockwood CA., Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279–92. doi: 10.2106/JBJS.F.00125. [DOI] [PubMed] [Google Scholar]
- 18.Kim SH, et al. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249–54. doi: 10.2106/JBJS.J.01994. [DOI] [PubMed] [Google Scholar]
- 19.Jain NB, et al. Trends in the epidemiology of total shoulder arthroplasty in the United States from 1990–2000. Arthritis Rheum. 2006;55(4):591–7. doi: 10.1002/art.22102. [DOI] [PubMed] [Google Scholar]
- 20.Day JS, et al. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115–20. doi: 10.1016/j.jse.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Werner CM, et al. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476–86. doi: 10.2106/JBJS.D.02342. [DOI] [PubMed] [Google Scholar]
- 22.HCUP. Overview of the Nationwide Inpatient Sample (NIS) [February 2013 4/14/13]]. Available from: www.hcup-us.ahrq.gov/nisoverviewisp.
- 23.United States Census Bureau. 2002. data, a.a. http://www.census.gov/popest/data/historical/2000s/vintage 2002/index.html.
- 24.United States Census Bureau. 2011. data, a.a. http://www.census.gov/popest/data/historical/2010s/vintage2011/index.html.
- 25.Young SW, et al. Comparison of functional outcomes of reverse shoulder arthroplasty with those of hemiarthroplasty in the treatment of cuff-tear arthropathy: a matched-pair analysis. J Bone Joint Surg Am. 2013;95(10):910–5. doi: 10.2106/JBJS.L.00302. [DOI] [PubMed] [Google Scholar]
- 26.Lyman S, et al. Prevalence and risk factors for symptomatic thromboembolic events after shoulder arthroplasty. Clin Orthop Relat Res. 2006;448:152–6. doi: 10.1097/01.blo.0000194679.87258.6e. [DOI] [PubMed] [Google Scholar]
- 27.Sirveaux F, et al. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388–95. doi: 10.1302/0301-620x.86b3.14024. [DOI] [PubMed] [Google Scholar]
- 28.Kinsella KG, et al. An aging world 2008, in International population reports Series P-95 09-1. Washington, DC: U.S. Department of Health and Human Services, National Institutes of Health, National Institute on Aging: U.S. Department of Commerce, Economics and Statistics Administration, U.S. Census Bureau; 2009. p. 1. online resource (203 p.) [Google Scholar]
- 29.Matsen FA, III, et al. The reverse total shoulder arthroplasty. Instr Course Lect. 2008;57:167–74. [PubMed] [Google Scholar]
- 30.Sanchez-Sotelo J. Reverse total shoulder arthroplasty. Clin Anat. 2009;22(2):172–82. doi: 10.1002/ca.20736. [DOI] [PubMed] [Google Scholar]
- 31.Cazeneuve JF, Cristofari DJ. [Grammont reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up]. Rev Chir Orthop Reparatrice Appar Mot. 2006;92(6):543–8. doi: 10.1016/s0035-1040(06)75911-6. [DOI] [PubMed] [Google Scholar]
- 32.Jain NB, et al. Comorbidities increase complication rates in patients having arthroplasty. Clin Orthop Relat Res. 2005;(435):232–8. doi: 10.1097/01.blo.0000156479.97488.a2. [DOI] [PubMed] [Google Scholar]
- 33.Polanczyk CA, et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med. 2001;134(8):637–43. doi: 10.7326/0003-4819-134-8-200104170-00008. [DOI] [PubMed] [Google Scholar]


