Abstract
Background
Medial protrusio is a recognized complication of total hip arthroplasty, but it is not known if a medial wall breach during cup implantation increases the risk. We thus investigated the effect of up to a 2 cm defect in the medial acetabular wall in a cadaveric model. Separately, we investigated the ability of acetabular screws to rescue the construct.
Methods
Nine human fresh-frozen hemipelves were reamed medially to create the defect, implanted with acetabular cups, and then loaded to failure. The nine contralateral hemipelves were reamed in a standard fashion and served as controls. Separately, nine hemipelves with a medial defect were augmented with two acetabular screws each, then loaded to failure, with the contralateral side as a control. Load-to-failure, stiffness, and energy were recorded.
Findings
The presence of a medial wall defect decreased the load-to-failure by a mean of 26% (5710 v. 4221 N, p=0.024). The addition of two acetabular screws did not rescue the construct (mean 27% decrease, 4082 v. 2985 N, p=0.024). The majority of specimens failed in a supra-physiologic range of force. Bone density correlated with failure loads (R2 range of 0.54-0.78), and osteoporotic specimens were more likely to fail at a physiologic range, consistent with forces experienced during minor stumbles or falls.
Interpretation
Osteoporotic patients with a medial wall defect after hip arthroplasty may be susceptible to fracture during activities of daily living. Protected weight bearing with an assistive device may be reasonable in order to minimize fall risk until cup ingrowth is achieved.
Introduction
Prosthetic acetabular protrusio is a medial migration of the acetabulum cup past Kohler's line and into the pelvis, and is a known complication of total hip arthroplasty1. The mechanism usually involves a periprosthetic fracture and subsequent pelvic discontinuity, with the incidence reported to be as high as 0.9%2. The defect typically causes pain and dysfunction necessitating revision arthroplasty, and severe cases with disruption of the intra-pelvic vessels and the bladder have been reported3. Furthermore, disruption of the intra-pelvic structures by the acetabular component can lead to sepsis if left untreated4.
The risk factors for this complication include patient factors such as poor medial bone stock, pre-existing protrusio, and rheumatoid arthritis, as well as operative factors including both under and over-reaming of the acetabulum1,5-8. Under-reaming the acetabulum increases the contact stress between the acetabular rim and the prosthesis, thus increasing the risk of iatrogenic fracture at the time of cup impaction6,8. In contrast, excessive medial reaming causes a breach in the medial acetabular wall, and presumably predisposes the patient to fracture during activities of daily living postoperatively3. However, to the best of our knowledge only two prior biomechanical studies have investigated any effect of a medial wall breach5,9, with one being in canine pelves instead of human pelves5, and the results were conflicting. Some authors have proposed that the majority of the stability of the acetabular component comes from contact with the acetabular rim, and thus small medial defects might not have clinical significance9. Overall, the effect of a medial wall breach remains controversial, and very little biomechanical data exists to help guide decision making.
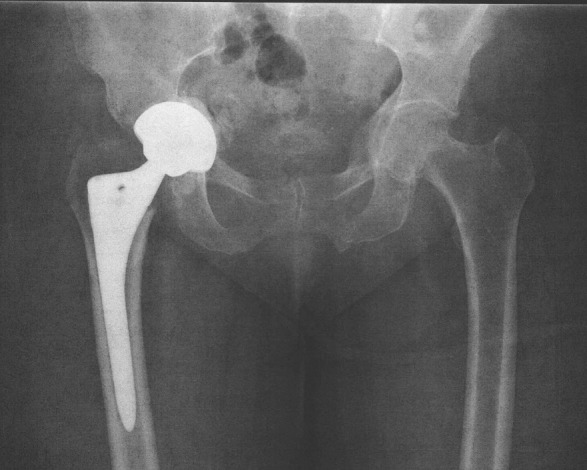
There are multiple treatment modalities available for the treatment of medial acetabular wall defects encountered at the time of a revision total hip arthroplasty, including trabecular metal buttons, revision cages, bone graft, and washers10-15. However, these treatment modalities are usually reserved for massive acetabular bone loss in revision cases. Ideally, if a small medial defect occurred during a primary hip arthroplasty, the surgeon should have an effective treatment modality to prevent the need for revision surgery entirely. At our own institution, a patient was recentiy referred with protrusio acetabuli, secondary to fracture, that developed only two weeks after her primary total hip arthroplasty (Figure 1). The initial treating surgeon had breached the acetabular wall during reaming, causing a two centimeter defect. After reviewing her case, we hypothesized that the medial wall disruption was responsible for the rapid failure, and that the use of well-placed acetabular screws may have strengthened the construct and prevented the subsequent medial displacement. To the best of our knowledge, no study has investigated the use of two prophylactic acetabular screws for restoring the strength of the acetabular construct after a breach in the medial wall has occurred.
Figure 1. Right sided prosthetic protrusio that developed two weeks after a primary total hip arthroplasty.
The purpose of this study was to test the biomechanical effect of a medial wall breach in a human cadaveric pelvis model, and to investigate the use of acetabular screws as an intra-operative treatment for this complication. We hypothesized that the presence of a medial breach would significantly decrease the load-to-failure strength. Furthermore, we hypothesized that adding two points of acetabular screw fixation would restore the stability of the construct.
Materials and Methods
The devices and treatments described in this article have been approved by the Food and Drug Administration, and our article is compliant with the IRB requirements at our institution.
Specimens
We obtained 20 cadaveric pelvis specimens (40 hemipelves from Anatomic Gifts Registry (Hannover, MD)) with information regarding donor age, gender, and cause of death. Patients with known metastatic disease to the pelvis, or known traumatic pelvis injury were excluded. We also excluded two specimens after testing. The first was a 47 year-old female with a bone mineral density (BMD) of 643 mg/cm3, which was 185% higher than the mean BMD for the overall cohort. No results could be obtained because this specimen's load at failure exceeded the maximum force that could be applied by our materials testing machine (15,000 N). The second excluded specimen was incorrectly potted, causing mechanical testing to prematurely abort. Overall, this left 18 specimens (36 hemipelves) that formed our study cohort. The average age of the included specimens was 75 years and the mean BMD was 327 mg/ cm3 (Table 1).
Table 1.
Description of Pelvic Specimens.
| Specimen Name | Side | Sex | Race | Age (yr) | Cause of Death | BMD (mg/cm3) | Testing Group | Medial Wall Defect Size | Cup Size (mm) | Screws Implanted? |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | R | F | C | 74 | COPD | 547.3 | A | 18×18 | 50 | no |
| L | 518 | A | Control | 50 | no | |||||
| 2 | R | M | C | 84 | CHF | 548.6 | A | 15×18 | 46 | no |
| L | 474.1 | A | Control | 46 | no | |||||
| 3 | R | F | C | 65 | Lung Cancer | 387 | A | 20×15 | 50 | no |
| L | 417.9 | A | Control | 50 | no | |||||
| 4 | R | F | C | 74 | Lung Cancer | 319.4 | A | Control | 54 | no |
| L | 347.2 | A | 20×23 | 54 | no | |||||
| 5 | R | F | C | 79 | MI | 205.6 | A | 15×18 | 52 | no |
| L | 207.2 | A | Control | 52 | no | |||||
| 6 | R | F | C | 76 | Failure to Thrive | 299.9 | A | Control | 50 | no |
| L | 258.3 | A | 20×15 | 50 | no | |||||
| 7 | R | F | C | 79 | Respiratory Failure | 254.3 | A | Control | 50 | no |
| L | 225.5 | A | 20×18 | 50 | no | |||||
| 8 | R | F | C | 77 | Congestive Heart | 230.4 | A | 15×19 | 50 | no |
| L | Failure | 230 | A | Control | 50 | no | ||||
| 9 | R | F | C | 77 | Sepsis | 274.2 | A | Control | 46 | no |
| L | 275 | A | 22×18 | 46 | no | |||||
| 10 | R | F | B | 74 | Alzheimer's | 406.4 | B | 15×15 | 54 | yes |
| L | 450.2 | B | Control | 54 | no | |||||
| 11 | R | F | C | 62 | Liver Failure | 414.2 | B | Control | 50 | no |
| L | 391.5 | B | 16×19 | 50 | yes | |||||
| 12 | R | F | C | 78 | ESRD | 394.8 | B | Control | 50 | no |
| L | 329.3 | B | 15×20 | 50 | yes | |||||
| 13 | R | F | C | 67 | Lung Cancer | 356.6 | B | Control | 46 | No |
| L | 334.5 | B | 20×20 | 46 | yes | |||||
| 14 | R | F | C | 69 | Renal Failure | 323.9 | B | 15×17 | 46 | yes |
| L | 307.9 | B | Control | 46 | no | |||||
| 15 | R | F | I | 67 | Lung Cancer | 296.2 | B | 22×20 | 54 | yes |
| L | 291.1 | B | Control | 54 | no | |||||
| 16 | R | F | C | 71 | Pulmonary | 291.4 | B | Control | 54 | no |
| L | Hypertension | 248.9 | B | 18×14 | 54 | yes | ||||
| 17 | R | F | C | 100 | CAD/MI | 278.6 | B | Control | 50 | no |
| L | 233.5 | B | 20×20 | 50 | yes | |||||
| 18 | R | F | C | 77 | ESRD | 216.2 | B | Control | 50 | no |
| L | 201 | B | 20×20 | 50 | yes |
The specimens were dissected free of all soft tissue attachments and wrapped in saline-soaked gauze to keep them moist when not being tested. All specimens were stored in plastic bags at −5 degrees Celsius, and were allowed to thaw overnight to room temperature prior to use. After being separated from the contralateral half, each hemi-pelvis was inspected with a lateral radiograph in order to look for fractures, tumors, malformations, or pre-existing deformity. No specimen had an obvious radiographic deformity. In addition, each specimen was scanned with a peripheral quantitative computed tomography scanner, which allowed precise measurements of bone mineral density (BMD).
Experimental Groups
Specimens were stratified according to BMD and assigned to one of two treatment groups (Table 2), with assignment done in a block fashion in order normalize BMD between both groups. After stratification, the mean BMD in treatment group A was 320 mg/cm3 and in treatment group B was 334 mg/cm3, with no significant difference between groups (p=0.67). In both experimental groups, the treatment side was over-reamed medially until up to a two cm defect was created in the medial acetabular wall. The exact dimensions of the defect were measured for each specimen prior to testing (Table 1). The mean area of the created defect was similar between the two groups (299 mm2 v. 331mm2, p=0.52). A goal defect of two cm in diameter was chosen because that size of defect has previously been reported in patients with this complication16, and is consistent with the size of the defect seen in our own recent cases. On the contralateral, control side, the acetabulum was reamed line-to-line up to the medial acetabular wall with careful visual inspection and manual palpation to verify that no breach had occurred. Reaming was generally 1mm greater than the total diameter of the cup. The contralateral hemipelvis was chosen as the control for each experimental specimen in order to minimize variations in specimen age, bone mineral density, or bone morphology, and is a commonly used method of control5,17-19. In group A, no additional points of fixation were added on the over-reamed side. In group B, we used two acetabular screws to strengthen the construct on the over-reamed side. The screws were placed into the posterior-superior acetabular safe zone as defined by Wasielewski et al20. Screw depth was measured at the time of implantation and an appropriate length was chosen for each specimen. No additional points of fixation were added on the control side for either experimental group. The acetabular cups were inserted with 45 degrees of abduction and 15 degrees of anteversion, with local anatomic landmarks used to verify cup positioning. Wright Medical acetabular implants were used (Dynasty implant system, Wright Medical Technology, Inc., Arlington, TN). Cups ranging in size from 46mm to 60mm were available, and each specimen was custom fit with an appropriately sized implant (Table 1).
Table 2.
Description of Treatment Groups
| Treatment Groups | Defect Side | Control Side |
|---|---|---|
| Group A | Over Reamed, No Acetabular Screws | Normal Acetabular Cup Placement |
| Group B | Over Reamed, Two Acetabular Screws | Normal Acetabular Cup Placement |
Biomechanical Testing
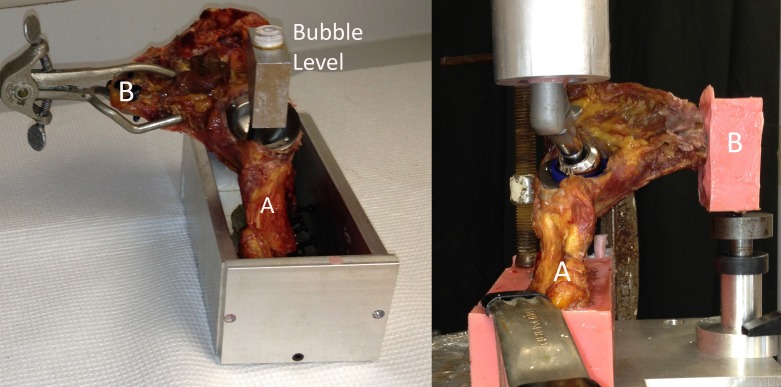
For each hemipelvis, the table of the ilium and the pubic symphysis were separately potted with polymethylmethacrylate (PMMA), with the bones supplemented with multiple screws to increase PMMA purchase (Figure 2). To obtain uniform orientation of each specimen, a bubble level was screwed into the acetabular component (Figure 2 Left), to direct the potting of the ilium such that the ultimate force vector was positioned with the hip in 15 degrees of abduction and 20–30 degrees of flexion, which correlates with the direction of the maximum load experienced during regular walking as defined by Bergman et al21. The pubic symphysis was then potted, with a stainless steel ball bearing on the undersurface to allow for multi-planar motion and rotation of the symphysis, separate from the ilium, during testing (Figure 2 Right).
Figure 2. (Left) A right sided specimen is shown prior to potting, with the positioning measured by the bubble level. (Right) A left sided specimen is shown in the MTS machine, with the ilium (A) and symphysis (B) both potted in PMMA.
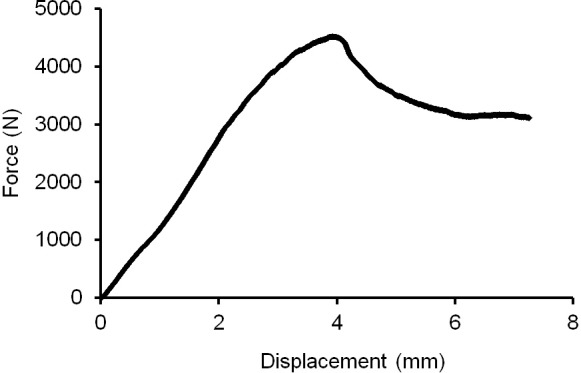
Each hemipelvis was attached to the load cell of an MTS Bionix 858 Materials Testing Machine (MTS Systems Corp., Eden Prairie, MN), through an x-y table. The acetabular cup was loaded through a femoral component attached to the actuator of the MTS (Figure 2 Right). We used a size 28mm femoral head with a Dynasty (Wright Medical Technology, Inc., Arlington, TN) femoral implant to deliver the load to the acetabulum, and the same femoral component was used in each test. Each specimen was first preconditioned with five cycles of axial loading at 0.5 Hz from 5 to 100 N, to ensure seating of all components. Each specimen was then loaded to failure at a rate of 0.2 mm/s; this loading rate was consistent with the rate chosen by other biomechanical studies of the acetabulum5,22-24. The tests were video recorded to assist with determination of failure modes. A load–displacement curve was generated for each specimen, and the maximum (failure) load was recorded (Figure 3). The displacement measurement of the MTS actuator was used in our calculations of energy-to-failure and initial stiffness for each test.
Figure 3. Sample Load to failure curve from experimental group A.
Statistical Analysis
Specimens were compared against their control side with a paired-sample Wilcoxon signed rank test. Statistical significance was considered to be p<0.05.
Funding Source
The cost of purchasing the cadaveric specimens was supported by the Orthopaedic Research and Education Foundation. Supplemental funding was provided through a general donation to biomechanical research at the University of Iowa, made by Dr. Dan Fitzpatrick. Wright Medical (Wright Medical Technology, Inc., Arlington, TN) donated the implants.
Results
In treatment group A, the creation of a 2 cm medial wall defect resulted in a 26% decrease in mean load-to-failure (5710 v. 4221 N, p=0.024), and a 23% mean decrease in the energy (13 v. 10 Nm, p=0.033). There was also a 25% decrease in mean stiffness, but this did not achieve statistical significance (1630 v. 1220 N/mm, p=0.124) (Table 3).
Table 3.
Experimental Results
| GROUP A (No screws) | Control Failure Load (N) | Defect Failure Load (N) | Percent Control | Control Stiffness (N/mm) | Defect Stiffness (N/mm) | Percent Control | Control Energy (J) | Defect Energy (J) | Percent Control | |
| Mean | 5710 | 4221 | 74 | 1630 | 1220 | 75 | 13 | 10 | 77 | |
| Std Dev | 3565 | 2477 | 907 | 524 | 7 | 6 | ||||
| P value | 0.024 | 0.124 | 0.033 | |||||||
| GROUP B (Two screws on the defect side) | Control Failure Load (N) | Defect Failure Load (N) | Percent Control | Control Stiffness (N/mm) | Defect Stiffness (N/mm) | Percent Control | Control Energy (J) | Defect Energy (J) | Percent Control | |
| Mean | 4082 | 2985 | 73 | 1239 | 903 | 73 | 10 | 9 | 90 | |
| Std Dev | 1251 | 1351 | 296 | 401 | 4 | 6 | ||||
| P value | 0.024 | 0.124 | 0.554 |
In treatment group B, the addition of two acetabular screws to posterior-superior safe zone on the defect side did not successfully restore the load-to-failure of the construct relative to the control (mean 27% decrease, 4082 v. 2985 N, p=0.024). Similar to treatment group A, the mean stiffness and energy trended lower when a defect was present, but these did not achieve statistical significance (Table 3).
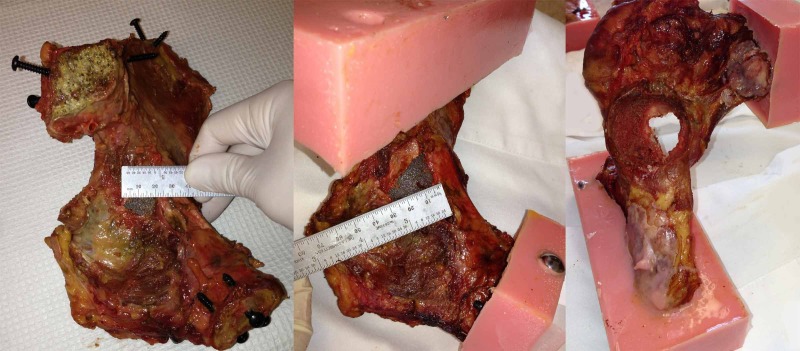
There was a moderate linear correlation between the specimen's bone density and its ultimate failure load in both group A (R2 of 0.78 for defect side, and R2 of 0.74 for the control side) and in group B (R2 of 0.55 for defect side, and R2 of 0.55 for the control side), as measured by Pearson's correlation coefficient (Figure 4). Mechanistically, the acetabulum appeared to fracture before the cup displaced and the screws engaged. Two failure modes were observed. Specimens without a defect developed a transverse type acetabular fracture (Figure 5). In specimens with a defect, the cup failed through the defect itself, resulting in a fracture with significant medial cup displacement and enlargement of the defect (Figure 6). In both cases, the fracture appeared to go directly through the medial wall, which is outside the posterior-superior zone in which screws were placed.
Figure 4. Relationship between bone density and ultimate failure load.
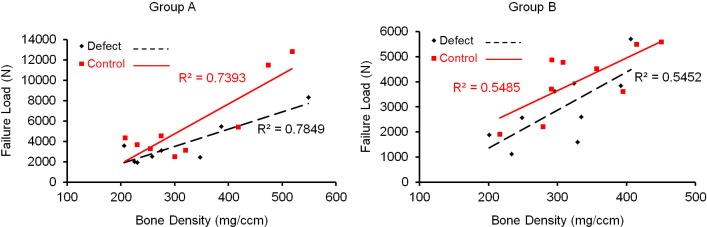
Figure 5. Post-testing photograph of a control specimen showing a transverse acetabular fracture.
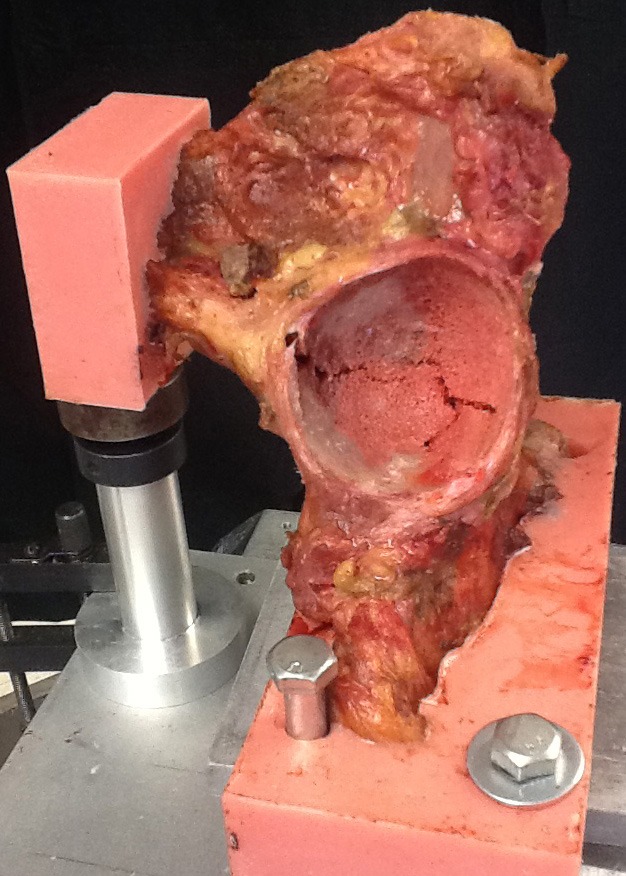
Figure 6. (Left) Pre-test image showing a 2cm medial wall defect. (Center and Right) Post-test images showing a fracture through the medial wall with enlargement of the defect to 3cm.
Discussion
Medial acetabular prosthesis migration is a rare, but known complication of total hip arthroplasty. A medial wall breach has been proposed to be a significant risk factor, but few studies have investigated either the biomechanical effect of a medial breach, or treatment methods to prevent medial prosthesis migration once the defect has occurred. Thus, the purpose of this study was to investigate the effect of up to a two centimeter medial wall defect on load-to-failure in a cadaveric model, and to determine the ability of acetabular screws to restore strength to the construct. Overall, we found that the presence of a breach decreased the load-to-failure by a mean of 26% relative to controls, and that the addition of two acetabular screws in the posterior-superior safe zone did not successfully restore the strength of the construct.
The primary aim of this study was to determine the biomechanical effect of a medial acetabular wall defect caused by over-reaming. Previously, the risks related to creation of an isolated medial wall defect have generally not been well recognized. Hadjari et al reported that isolated central acetabular defects had no effect on initial implant stability under axial loads up to 1000 N9. These authors reported that a medial wall defect allowed placement of a large implant with good rim coverage and excellent initial stability. In contrast, Desai et al reported one case of early pelvic discontinuity resulting from a transverse fracture in a patient with a 2 cm medial wall defect caused by over-reaming the pelvis16. Similarly, Springer et al reported a series of 7 patients who developed transverse acetabular fractures and pelvic discontinuity early in the post-operative period after revision arthroplasty. The authors speculated that excessive reaming to allow the placement of a large revision type implant had weakened the medial bone stock25. In this study, central defects of up to 2 cm in diameter decreased the load to failure strength by a mean of 26%. Thus, excessive removal of medial acetabular bone stock clearly weakened the construct.
However, it is not immediately clear whether or not this loss in load-to-failure is enough to be clinically significant or to produce fractures during activities of daily living in an in vivo setting. Results from in vivo investigations have reported that average hip joint reactive forces during common activities ranged from 238–260% body-weight21. For a 70 kg (154 1b) patient this corresponds to 1,632–1,783 N and for a 100 kg (220 1b) patient this rises to 2,334–2,550 N. The mean failure loads in this study were well above these ranges, even in specimens with a defect. However, during stumbling, joint reactive forces have been reported to rise to up to 720% body-weight26,27, which would correspond to 4,939 N and 7,063 N, respectively for the patient weights indicated. The body weights of our cadaveric specimens were not known, and we cannot definitively say whether the forces observed in our study would have resulted in fractures in vivo. However, 14 of 18 (77%) specimens with a medial wall defect failed at forces below 4,900 N, and 16 of 18 (88%) failed below 7,000 N. Thus, a medial wall defect seems likely to place the patient into a range where fracture could occur following a stumble or other mild trauma. Furthermore, there may be a particular subset of patients in whom a medial wall defect is a particular risk for fracture. Springer et al25 and Desai et al21 separately speculated that osteoporotic patients were at particular risk for this complication. In this study, the ultimate failure loads correlated with the bone density of the specimens, thus supporting the speculations of the prior authors. Overall, patients with low bone density combined with a medial wall defect may be the group most susceptible to fracture during activities of daily living, and particularly during stumbling or after a minor trauma.
The second aim of this study was to investigate the ability of acetabular screws to prophylactically treat the medial wall defect. No significant benefit was seen in terms of ultimate load-to-failure, energy, or stiffness with the addition of screws. This finding is similar to that from previous studies on the impact of acetabular screws on initial cup stability. Perona et al reported that two dome screws had a small effect on preventing micromotion between the implant and the ilium, but no effect on motion at either the pubis or ischium28. Similarly, Kwong et al reported that the addition of screws did not substantially reduce cup micromotion under press-fit conditions29. Mechanistically we observed that, due to the tight rim fit, the force was primarily delivered through the implant and into the acetabular rim. Thus, the acetabulum fractured and the cup began to displace before the screws engaged, and acetabular screws placed into the posterior-superior safe zone were not sufficient to prevent fracture from occurring. In this study, we chose to mimic the most common clinical pattern of screw placement with two screws placed in the posterior-superior safe zone. However, it is possible that an alternative screw pattern would have had more success. In pelvic discontinuity cases screws are placed both into the posterior-superior safe zone, and also down into the ischium in order to bridge the defect. A construct that bridged the defect may have been more successful and would be an interesting area for further study.
Our study has several limitations. First, cadaveric bone does not have the same properties as living bone in terms of viscoelasticity and ability to heal or respond to local stressors, and this could feasibly limit the generalizability of our conclusions. Second, we attempted to control for variations between specimens (in terms of bone quality, amount of osteoarthritis, age of the specimen, and size of the acetabulum relative to the implant) by having each contralateral hemipelvis serve as its own internal control. Thus, our results are presented as a percent of the control side. Nonetheless, it is possible that small differences remained that we were unable to account for. Third, our study design allowed the investigation of only a single direction of force applied with increasing magnitude at a quasi-static loading rate in a single laboratory setting. Living patients are subjected to multiple axial and torsional loads in a cyclic fashion, and at higher loading rates. Thus, our experimental model best approximates the application of a large force, such as during a stumble or fall, but cannot accurately represent the results of cumulative stresses over time. Fourth, the ability of acetabular screws to resist torsional strains, seat the cup down to the acetabulum, or to resist micromotion which might prevent cup ingrowth was not studied. Clinically, the failures we had observed were transverse fractures resulting in medial cup displacement, and we thus designed the study to reproduce that mechanism. Prior authors have argued that acetabular screws increase the initial implant stability in response to torsional forces, and help to seat the cup, thus minimizing the distance between the bone and the implant9,30. Thus, acetabular screws may have benefits beyond what we are able to comment on in this study, and our data should not be interpreted as generally discouraging their use. Fifth, the ultimate failure load of the specimens is likely dependent, in part, on the direction in which the force is applied. We implanted the cups using anatomic landmarks, and then directed the force in reference to the acetabular implants. Thus, it is possible that there was variability in the way the cups were implanted, and in the ultimate force direction. However, the use of anatomic landmarks for cup positioning is common during in vivo surgeries, and all of the surgeries were performed in a uniform fashion by a single individual. Thus, we feel that this is not a substantial source of bias.
Conclusions
Overall, our study has demonstrated that medial acetabular defects caused by medial reaming decreased the ultimate load-to-failure of the pelvis. Osteoporotic patients seem to be the most likely to develop a fracture as a result of this complication. Acetabular screws placed into the posterior-superior safe zone do not appear to be sufficient prophylaxis in our in vitro model. It seems feasible that once cup ingrowth has occurred the importance of a medial wall defect would likely be reduced. Thus, when this complication is encountered clinically, it may be reasonable to institute a period of protected weight bearing with an assistive device in order to minimize fall risk until cup ingrowth can be achieved. Further clinical follow-up is necessary, and future studies should focus on clinical outcomes of patients with medial wall breaches to determine if the incidence of fracture is substantively increased.
Acknowledgments
The authors would like to thank Dr. M. James Rudert for his contributions to the design and implementation of the biomechanical analysis. We would also like to thank Dr. Dan Fitzpatrick and the Orthopaedic Research and Education Foundation for providing funding for this study. Lastly, we would like to thank Wright Medical Technology Inc. for donating the implants used.
References
- 1.Salvati EA, Bullough P, Wilson PD., Jr. Intrapelvic protrusion of the acetabular component following total hip replacement. 1975. Clinical orthopaedics and related research. 2006;453:8–12. doi: 10.1097/01.blo.0000246555.35220.9c. [DOI] [PubMed] [Google Scholar]
- 2.Berry DJ, Lewallen DG, Hanssen AD, Cabanela ME. Pelvic discontinuity in revision total hip arthroplasty. The Journal of bone and joint surgery. American volume. 1999;81:1692–1702. doi: 10.2106/00004623-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Morley DC, Jr., Schmidt RH. Protrusio acetabuli prosthetica. Orthopaedic review. 1986;15:135–141. [PubMed] [Google Scholar]
- 4.Stiehl JB. Acetabular prosthetic protrusion and sepsis: case report and review of the literature. The Journal of arthroplasty. 2007;22:283–288. doi: 10.1016/j.arth.2006.02.170. [DOI] [PubMed] [Google Scholar]
- 5.Margalit KA, Hayashi K, Jackson J, Kim SY, Garcia TC, Wiggans KT, Aiken S, Stover SM. Biomechanical evaluation of acetabular cup implantation in cementless total hip arthroplasty. Veterinary surgery: VS. 2010;39:818–823. doi: 10.1111/j.1532-950X.2010.00726.x. [DOI] [PubMed] [Google Scholar]
- 6.Sharkey PF, Hozack WJ, Callaghan JJ, Kim YS, Berry DJ, Hanssen AD, LeWallen DG. Acetabular fracture associated with cementless acetabular component insertion: a report of 13 cases. The Journal of arthroplasty. 1999;14:426–431. doi: 10.1016/s0883-5403(99)90097-9. [DOI] [PubMed] [Google Scholar]
- 7.Berry DJ. Identification and management of pelvic discontinuity. Orthopedics. 2001;24:881–882. doi: 10.3928/0147-7447-20010901-25. [DOI] [PubMed] [Google Scholar]
- 8.Kim YS, Callaghan JJ, Ahn PB, Brown TD. Fracture of the acetabulum during insertion of an oversized hemispherical component. The Journal of bone and joint surgery. American volume. 1995;77:111–117. doi: 10.2106/00004623-199501000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Hadjari MH, Hollis JM, Hofmann OE, Flahiff CM, Nelson CL. Initial stability of porous coated acetabular implants. The effect of screw placement, screw tightness, defect type, and oversize implants. Clinical orthopaedics and related research. 1994:117–123. [PubMed] [Google Scholar]
- 10.Foster S, Chaudhary H, Assenmacher B. Intrapelvic cementiess component extraction with immediate triflange acetabular reconstruction using the retroperitoneal approach. The Journal of arthroplasty. 2009;24(323):e321–325. doi: 10.1016/j.arth.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Grigoris P, Roberts P, McMinn DJ, Villar RN. A technique for removing an intrapelvic acetabular cup. The Journal of bone and joint surgery. British volume. 1993;75:25–27. doi: 10.1302/0301-620X.75B1.8421027. [DOI] [PubMed] [Google Scholar]
- 12.Eftekhar NS, Nercessian O. Intrapelvic migration of total hip prostheses. Operative treatment. The Journal of bone and joint surgery. American volume. 1989;71:1480–1486. [PubMed] [Google Scholar]
- 13.Sarasin SM, Karthikeyan R, Skinner P, Nassef A, Stockley I. Transperitoneal removal of an intrapelvic acetabular component after total hip replacement and salvage of a destroyed acetabulum. The Journal of bone and joint surgery. British volume. 2011;93:844–846. doi: 10.1302/0301-620X.93B6.26323. [DOI] [PubMed] [Google Scholar]
- 14.Hansen E, Ries MD. Revision total hip arthroplasty for large medial (protrusio) defects with a rim-fit cementless acetabular component. The Journal of arthroplasty. 2006;21:72–79. doi: 10.1016/j.arth.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 15.McBride MT, Muldoon MP, Santore RF, Trousdale RT, Wenger DR. Protrusio acetabuli: diagnosis and treatment. The Journal of the American Academy of Orthopaedic Surgeons. 2001;9:79–88. doi: 10.5435/00124635-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Desai G, Ries MD. Early postoperative acetabular discontinuity after total hip arthroplasty. The Journal of arthroplasty. 2011;26(1570):e1517–1579. doi: 10.1016/j.arth.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Wilson LJ, Richards CJ, Irvine D, Tillie A, Crawford RW. Risk of periprosthetic femur fracture after anterior cortical bone windowing: a mechanical analysis of shortversus long cemented stems in pigs. Acta orthopaedica. 2011;82:674–678. doi: 10.3109/17453674.2011.636670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hugate RR, Dickey ID, Chen Q, Wood CM, Sim FH, Rock MG. Fixed-angle screws vs standard screws in acetabular prosthesis fixation: a cadaveric biomechanical study. The Journal of arthroplasty. 2009;24:806–814. doi: 10.1016/j.arth.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Demey G, Fary C, Lustig S, Neyret P, si Selmi TA. Does a collar improve the immediate stability of uncemented femoral hip stems in total hip arthroplasty? A bilateral comparative cadaver study. The Journal of arthroplasty. 2011;26:1549–1555. doi: 10.1016/j.arth.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 20.Wasielewski RC, Cooperstein LA, Kruger MP, Rubash HE. Acetabular anatomy and the transac-etabular fixation of screws in total hip arthroplasty. The Journal of bone and joint surgery. American volume. 1990;72:501–508. [PubMed] [Google Scholar]
- 21.Bergmann G, Deuretzbacher G, Heller M, Graichen F, Rohlmann A, Strauss J, Duda GN. Hip contact forces and gait patterns from routine activities. Journal of biomechanics. 2001;34:859–871. doi: 10.1016/s0021-9290(01)00040-9. [DOI] [PubMed] [Google Scholar]
- 22.Babis GC, Trousdale RT, Jenkyn TR, Kaufman K. Comparison of two methods of screw fixation in periacetabular osteotomy. Clinical orthopaedics and related research. 2002:221–227. doi: 10.1097/00003086-200210000-00032. [DOI] [PubMed] [Google Scholar]
- 23.Shazar N, Brumback RJ, Novak VP, Belkoff SM. Biomechanical evaluation of transverse acetabular fracture fixation. Clinical orthopaedics and related research. 1998:215–222. [PubMed] [Google Scholar]
- 24.Khajavi K, Lee AT, Lindsey DP, Leucht P, Bellino MJ, Giori NJ. Single column locking plate fixation is inadequate in two column acetabular fractures. A biomechanical analysis. Journal of orthopaedic surgery and research. 2010;5:30. doi: 10.1186/1749-799X-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Springer BD, Berry DJ, Cabanela ME, Hanssen AD, Lewallen DG. Early postoperative transverse pelvic fracture: a new complication related to revision arthroplasty with an uncemented cup. The Journal of bone and joint surgery. American volume. 2005;87:2626–2631. doi: 10.2106/JBJS.E.00088. [DOI] [PubMed] [Google Scholar]
- 26.Bergmann G, Graichen F, Rohlmann A. Hip joint contact forces during stumbling. Langenbeck's archives of surgery / Deutsche Gesellschaft fur Chirur-gie. 2004;389:53–59. doi: 10.1007/s00423-003-0434-y. [DOI] [PubMed] [Google Scholar]
- 27.Bergmann G, Graichen F, Rohlmann A. Hip joint loading during walking and running, measured in two patients. Journal of biomechanics. 1993;26:969–990. doi: 10.1016/0021-9290(93)90058-m. [DOI] [PubMed] [Google Scholar]
- 28.Perona PG, Lawrence J, Paprosky WG, Patwardhan AG, Sartori M. Acetabular micromotion as a measure of initial implant stability in primary hip arthroplasty. An in vitro comparison of different methods of initial acetabular component fixation. The Journal of arthroplasty. 1992;7:537–547. doi: 10.1016/s0883-5403(06)80076-8. [DOI] [PubMed] [Google Scholar]
- 29.Kwong LM, O'Connor DO, Sedlacek RC, Krushell RJ, Maloney WJ, Harris WH. A quantitative in vitro assessment of fit and screw fixation on the stability of a cementless hemispherical acetabular component. The Journal of arthroplasty. 1994;9:163–170. doi: 10.1016/0883-5403(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 30.Lachiewicz PF, Suh PB, Gilbert JA. In vitro initial fixation of porous-coated acetabular total hip components. A biomechanical comparative study. The Journal of arthroplasty. 1989;4:201–205. doi: 10.1016/s0883-5403(89)80015-4. [DOI] [PubMed] [Google Scholar]