Abstract
High quality clinical biospecimens are vital for biomarker discovery, verification, and validation. Variations in blood processing and handling can affect protein abundances and assay reliability. Using an untargeted LC-MS approach, we systematically measured the impact of preanalytical variables on the plasma proteome. Time prior to processing was the only variable that affected the plasma protein levels. LC-MS quantification showed that preprocessing times <6 h had minimal effects on the immunodepleted plasma proteome, but by 4 days significant changes were apparent. Elevated levels of many proteins were observed, suggesting that in addition to proteolytic degradation during the preanalytical phase, changes in protein structure are also important considerations for protocols using antibody depletion. As to processing variables, a comparison of single- vs double-spun plasma showed minimal differences. After processing, the impact ≤3 freeze–thaw cycles was negligible regardless of whether freshly collected samples were processed in short succession or the cycles occurred during 14–17 years of frozen storage (−80 °C). Thus, clinical workflows that necessitate modest delays in blood processing times or employ different centrifugation steps can yield valuable samples for biomarker discovery and verification studies.
Keywords: Plasma, Preanalytical variables, Proteomics, Mass spectrometry, Serum
Blood is an accessible and promising source for discovering biomarkers for disease screening and diagnosis as well as monitoring progression and/or therapeutic response. Changes in the protein repertoire of cells, tissues, and organs, which reach the bloodstream by active or passive means, are important clinical tools. The challenges associated with plasma proteomics include the large dynamic range of protein concentrations [1,2], variability in preanalytic and analytic processes, and inherent biological variability. Detection of low abundance proteins is facilitated by depletion or enrichment of peptides or proteins prior to mass spectrometry (MS) analyses [3]. The use of sound study designs and system suitability protocols improves analytical reproducibility and statistical power [4–7]. Ultimately, the quality of specimens affects the validity of the data. Preanalytical variables associated with collection, processing, and storage can also be confounding factors. The National Cancer Institute Biospecimen Reporting for Improved Study Quality (BRISQ) workgroup proposed guidelines for reporting specific preanalytical conditions, including factors that might influence the integrity, quality, or composition of samples [8]. However, outstanding questions remain about the most important preanalytical variables in terms of major effects on the validity of biomarker studies.
Protein and peptide integrity in plasma samples can be compromised in multiple ways, including proteolysis, oxidation, loss of posttranslational modifications, and changes in solubility [9]. Biomolecules degrade at different rates under a variety of circumstances. The rate and extent of degradation depend on the time/temperature at which blood is held prior to processing, centrifugation speed, the time/temperature prior to freezing, the number of freeze–thaw cycles, and analyte stability [10]. It is not always feasible to process clinical samples immediately after collection because the clinic and the blood processing facilities are often geographically separate. Prior to and during processing, proteolytic activity or cellular metabolism may alter protein content, which can also be affected by blood cell lysis at higher centrifugation speeds. Postprocessing delays prior to frozen storage or after thawing could afford time for additional ex vivo proteolysis. The use of broad spectrum protease inhibitors during sample collection and processing, which is costly in terms of dollars and effort, is unlikely to occur routinely in the clinical setting. Perhaps more importantly, we lack a global understanding of factors that impact protein stability in blood. Detailed protocols for serum/plasma collection and processing have been published [9,11]. In particular, there are two widely used plasma protocols that differ mainly in the centrifugation step. The Early Detection Research Network (EDRN)1 standard operating procedure (SOP) uses a single centrifugation step no longer than 4 h post collection [11]. The Clinical Proteomic Technologies Assessment for Cancer (CPTAC) SOP entails a second, higher speed centrifugation step to obtain platelet poor plasma [12].
The effects of preanalytical variables on plasma/serum peptide and protein stability over time, assayed by using MS methods, have been reported. Analysis of the low molecular weight (LMW) plasma proteome revealed postprocessing, time-dependent changes in the MALDI TOF profiles over 48 h [13]. At the protein level, changes in a relatively small number of abundant plasma proteins including albumin, hemoglobin, serotransferrin, inter-alpha trypsin inhibitor, and fibrinogen were observed in plasma samples held at room temperature for 1 week before processing [14]. The use of plasma collection tubes containing protease inhibitors to minimize degradation as compared with EDTA has not been shown to have a significant impact on the levels of peptides or proteins [15,16]. Multiple studies have shown changes in the abundance of complement C3, at protein and peptide levels, due to preanalytical variables [13,14,17,18]. However, since the dynamic range in plasma protein concentrations exceeds that of mass spectrometers by at least 5 orders of magnitude, immunoaffinity depletion of abundant proteins is routinely employed in plasma biomarker discovery and verification studies [19]. Preanalytical variables could affect depletion, either directly via antibody binding or indirectly via nonspecific protein interactions with depletion targets. The objective of this study was to analyze the effects of well-defined preanalytical variables on the protein integrity of immunodepleted plasma and serum samples and to compare two widely used methods of plasma preparation.
Materials and methods
Blood collection
Biospecimen Research Network specimens
Donors gave consent for blood collection for research purposes as part of a UCSF Institutional Review Board-approved protocol. Male and female donors (aged 20–40, median 27, Supplemental Table 1) were requested to fast for a minimum of 12 h prior to blood collection. Donors were seated at least 5 min before the draw and the arm was positioned on a slanting armrest in a straight line from the shoulder to the wrist. A tourniquet was applied approximately 2 inches above the antecubital fossa or above area to be drawn with enough pressure to provide adequate vein visibility and the patient was asked to form a fist. The forearm was cleaned with an antiseptic wipe and allowed to dry. Then a butterfly needle was inserted the evacuated tube pushed onto the Luer adapter. The tourniquet was released once blood flow was established, within 1 min. Blood collection was performed by registered nurses at UCSF Moffitt Hospital who were provided with the protocol and briefed on the project, stressing the importance of adherence to the blood collection standard operating protocol (SOP). Two members of the research team were present during blood draws and any deviations from protocol were logged.
Magee–Womens Biorepository specimens
Plasma samples were obtained from an ongoing investigation of preeclampsia (Prenatal Exposures and Preeclampsia Prevention [PEPP]) at the Magee–Womens Research Institute and Hospital (University of Pittsburgh, Pittsburgh, PA, USA). PEPP was approved by the University of Pittsburgh Institutional Review Board, and informed consent was obtained from all participants. The PEPP committee approved the use of these previously frozen samples and deidentified clinical data.
Blood processing
Biospecimen Research Network specimens
Two published SOPS were used to process blood, a double spin protocol [12,20] and a single spin method [11]. Blood was collected in spray-coated K2EDTA Vacutainers (BD, Franklin Lakes, NJ). After blood collection, the tubes were inverted 8 times. For double-spun plasma, one tube was centrifuged within 30 min, in a horizontal rotor, for 15 min at 1500g, 4 °C. The plasma was transferred to a sterile 15 ml conical tube without disturbing the buffy coat and subjected to a second centrifugation step, in a horizontal rotor, at for 15 min at 2000g, 4 °C. The platelet-poor plasma was aliquoted into sterile cryovials (0.75–0.2 ml/tube), immediately transferred to dry ice, and transported within 30 min to a −80 °C freezer. For single-spun plasma, tubes were centrifuged, in a horizontal rotor, for 20 min at 1200g, 20 °C, and then transferred to a sterile 15 ml conical tube without disturbing the buffy coat. Plasma was aliquoted into sterile cryovials (0.75–0.2 ml/tube) and frozen as described for double-spun plasma. Additional biospecimens were prepared by systematically altering several preanalytical variables. (1) Preprocessing holding time was increased to 6 h. (2) Preprocessing holding time was increased to 96 h and the temperature was raised to 37 °C. (3) One to three freeze–thaw cycles were performed: the first thaw was designated “0.” (4) Thawed samples were held for 24 h at room temperature prior to immunodepletion. Exclusion criteria were hemolysis, insufficient sample volume, or SOP deviations.
Magee–Womens Biorepository specimens
For investigating the effects of long-term frozen storage (−80 °C), samples banked at the Magee Women’s Research Institute (MWRI, Pittsburgh, PA) for 14–17 years (median = 15.3) were employed (Supplemental Table 1). Records documented the number of times that the samples were frozen and thawed (median = 3). Paired plasma and serum samples were collected from the same pregnant woman (n = 20), median gestational age [GA] = 40 weeks). The subjects were controls, women with uncomplicated pregnancies, for studies of pregnancy complications. Serum was collected into silicon-coated tubes without additives (BD) and plasma into spray-coated K2EDTA tubes (BD). Tubes that contained serum were maintained upright; Tubes that contained plasma were inverted several times immediately after collection before they were also placed in an upright position. After 1–1.5 h at room temperature, samples were centrifuged at 2000g for 20 min at 25 °C. Sterile transfer pipets were used to aliquot (0.1–0.25 mL/tube) the plasma or serum into sterile cryovials prior to storage at −80 °C. An additional 10 paired control samples (median GA = 35.9 weeks) were collected and processed at MWRI under the same protocols and stored at −80 °C for <3 months prior to analyses.
Protein quantification, immunodepletion, and iTRAQ labeling
All samples were thawed at 37 °C in a water bath and removed immediately thereafter. The BCA assay (Pierce, Rockford, IL) was used to determine the protein concentrations. Samples were immunodepleted by using a MARS-Hu14 column (4.6 × 100 mm, Agilent Technologies, Santa Clara, CA). A control plasma sample was depleted prior to study samples to monitor the efficiency of depletion and column performance. Depleted proteins were buffer-exchanged using Zeba spin columns into 50 mM triethylammonium bicarbonate (Sigma, St. Louis, MO). For each sample, 50 μg of immunodepleted proteins was denatured by the addition of SDS to 0.1%. Cysteines were reduced in 5 mM tris(2-carboxyethyl)phosphine hydrochloride for 60 min at 60 °C followed by alkylation in 10 mM iodoacetamide for 10 min at room temperature. Proteins were digested with trypsin for 16 h at 37 °C. To each iTRAQ-8plex (AB Sciex, Framingham, MA) reagent vial 50 μl of >99.5% isopropanol was added and the entire contents were then added to the protein digest. The mass tag assigned to each sample was randomized using the RAND function in Excel. The pH was confirmed to be 8.0–8.5 and the tubes were incubated for 2 h at room temperature. The iTRAQ reaction was quenched by adding 1 μL of 1 M Tris HCl, pH 8.0. Next, samples were mixed to form multiplexes. For Study 1, samples (4 time points and 2 freeze/thaw cycles) from the same individual were combined for a total of 20 multiplexes. For Study 2 samples (2 processing methods and 3 time/temperature points) from the same individual were combined to form 9 multiplexes. For Study 3, plasma and serum were analyzed separately. For the biorepository samples, a pool comprising newly collected control plasma or serum was used as a reference standard in each multiplex (n = 6 each plasma and serum), each of which comprised samples from 6 individual subjects. Following mixing to form multiplexes, organic solvent was evaporated on a SpeedVac and SDS removed using detergent removal spin columns (Fisher Scientific).
Offline fractionation and LC-MS/MS
For Study 1, peptides were analyzed on a QSTAR Elite (AB Sciex) equipped with a nanoLC-2D (Eksigent, Redwood City, CA) using HPLC buffers A (2% acetonitrile [ACN]/0.1% formic acid) and B (98% ACN/0.1% formic acid). A C18 trap column (Acclaim PepMap300 precolumn; C18, 5 μm, 300 Å [Dionex, Sunnyvale, CA]) was used to desalt the peptides for 5 min with 98% buffer A. Then they were eluted onto an Acclaim PepMap100 analytical column (C18, 3 μm; 75 μm × 15 cm, 100 Å, Dionex) and separated at a flow rate of 300 nL/min over 120 min using a linear gradient of 2–35% Buffer B. MS/MS spectra were obtained in a data-dependent manner for the 6 most intense ions in the survey scan. An exclusion window of 15 s was used after 2 repeated acquisitions of the same precursor ion.
For Studies 2 and 3, peptides were fractionated offline using alkaline reversed phase HPLC [21] on a Michrom Paradigm equipped with a CTC PAL autosampler. Peptides were separated on a Zorbax 300 Extend-C18 column (4.6 × 1 50 mm, 5 μm, Agilent) with a linear gradient of 4–40% B over 32 min (A, 0.1% ammonium hydroxide; B, 0.1% ammonium hydroxide in 80% ACN). To assess column performance, 1 nmol of a tryptic digest of bovine serum albumin was analyzed. Eighteen fractions were collected and analyzed on a TripleTOF 5600 (AB Sciex) equipped with a nanoLC-2D (Eksigent). Each peptide fraction was separated with a linear gradient of 2–35% Buffer B over 60 min using a PepMap100 analytical column (C18, 3 μm; 75 μm × 15 cm, 100 Å, Dionex) at a flow rate 300 nL/min. MS/MS spectra were obtained in a data-dependent manner for the 20 most intense ions in the survey scan, with an exclusion window set as described above.
Prior to LC-MS sample analyses and at approximately 24 h intervals, a standard protein digest was analyzed to assess chromatography and mass spectrometer function. One femtomole of a beta-galactosidase tryptic digest (AB Sciex) was analyzed by using a 15 min linear gradient of 2–35% Buffer B. Retention times, peak shapes, and intensities of extracted ion chromatograms were monitored to verify column performance and mass spectrometer sensitivity.
Database searching and statistical analyses
Protein identification and quantification were performed using ProteinPilot v.4.0. Data were searched against the human Swiss-Prot database 201206 using thorough search effort and false discovery rate (FDR) analysis using a reversed sequences database. Quantification of relative protein ratios utilized background and bias correction. Results from multiple iTRAQ 8plexes were aligned using the Protein Alignment Template from AB Sciex [22]. The number of proteins detected at 5% FDR were reported. Quantitative data were analyzed as ratios of the reporter ion peak for each experimental sample to the control reference pool. To determine significant changes in protein abundances, log-transformed iTRAQ ratios were analyzed using linear regression and P values adjusted for multiple comparisons with the Benjamini-Hochberg method using R software and q ≤ 0.05.
Results
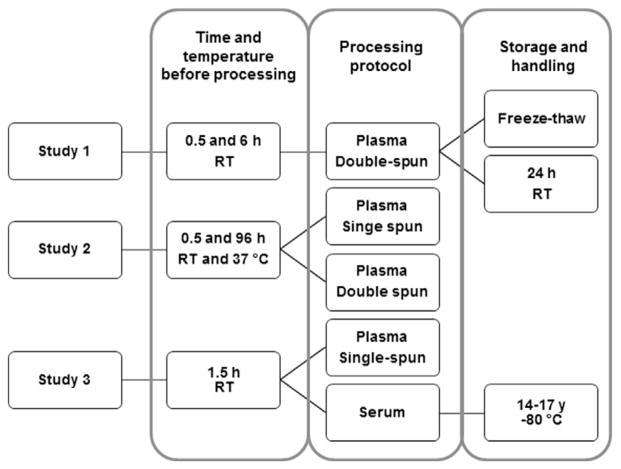
We evaluated the effects of several preanalytical variables related to handling, processing, and storage on the stability of plasma and serum proteomes. To this end, we examined the following variables: time and temperature from specimen collection to processing, plasma centrifugation method, number of freeze–thaw cycles, and time in storage at −80 °C. Effort was made to closely control, during blood collection, all parameters known to impinge on specimens’ properties, i.e., variations in the process of venipuncture and phlebotomy and collection tubes. In order to minimize analytical variables, standard operating procedures were developed that included quality assurance practices and system suitability checks. As shown in Fig. 1, two types of specimens were employed: samples that were collected specifically for this project and those that were part of a biorepository that were collected 14–17 years ago.
Fig. 1.
Study Design. iTRAQ quantification was used to measure the effects of several preanalytical variables on relative plasma and serum protein abundances. Two types of specimens were employed: samples collected specifically for this project (Studies 1 and 2) and those that are part of a biorepository that were collected ~15 years ago (Study 3). RT, room temperature.
Study 1
To measure the effects of delay from the time of blood collection to processing, we collected blood from 20 healthy volunteers into two EDTA tubes. One tube was processed within 30 min (control) and the second tube was held at room temperature for 6 h prior to processing into plasma using the double-spin protocol, aliquoting, and storage at −80 °C. Initially, we asked whether the protein concentrations of undepleted plasma samples were different among donors or experimental conditions. Average plasma protein concentration of control samples was 73.3 ± 3.7(SD) mg/ mL and 6 h time point was 72.4 ± 4.0 mg/mL. Since all samples were frozen prior to analysis, the first thaw was termed freeze–thaw 0. Vials of control plasma removed from the freezer and subjected to 2 additional freeze–thaw cycles averaged 69.3 ± 2.9 mg/ mL. Finally, one vial of plasma from each time point was removed from the freezer and allowed to stand at room temperature for 24 h prior to immunodepletion (control, 71.9 ± 3.0 mg/mL; 6 h, 74.2 ± 6.3 mg/mL). Protein concentration was not found to be significantly different among donors or treatments.
Plasma from each condition (n = 6) from each individual was immunodepleted, labeled with iTRAQ reagent, and analyzed by LC-MS. Proteins detected at FDR <5% and found in at least 5 multiplexes were used for quantification. A total of 121 proteins were identified in all samples, 85 of which were used for statistical analyses (Supplemental Table 2). In the experimental samples that were held 6 h after collection, one protein—serum paraoxonase/arylesterase 1 (PON1)—was significantly increased in abundance (2.0-fold, q = 7.2 × 10−4) relative to the control (held for 30 min) and no proteins decreased. Next we performed an experiment designed to mimic a case where cold storage fails and plasma remains at room temperature for an extended period. This comparison of control and experimental samples that were thawed and held at room temperature for 24 h prior to immunodepletion showed only a single change—increases in PON1 abundance, 4.4-fold (q = 1.4 × 10−9) and 4.3-fold (q = 2.4 × 10−9), respectively. No proteins were observed with decreased abundances. In the freeze thaw study, no significant changes in protein abundances were observed in any plasma samples after 3 cycles.
Study 2
Next, we extended the analyses of preprocessing variables to a longer holding time point and an elevated temperature in a separate set of nine samples. A total of 303 proteins were identified (FDR <5%), 264 of which were detected in at least 5 multiplexes and used for analyses (Supplemental Table 3). Holding plasma for 96 h before processing had profound effects on protein abundances as compared with control samples processed within 30 min. In samples held at room temperature, 41 proteins were altered; 39 were detected at higher levels as compared to controls (Fig. 2A and Supplemental Table 3). In samples held at 37 °C, 83 proteins were significantly altered; 56 increased in abundance (Fig. 2B). Significantly different proteins with fold changes ≥3 are shown in Table 1. The majority of observed protein changes in samples incubated at room temperature were also detected in samples held at 37 °C. The shared differences were approximately equal or up to 4-fold greater in the 37 °C samples.
Fig. 2.
Effects of preprocessing holding time on the plasma proteome. Relative protein abundances of plasma held for (A) 96 h at room temperature or (B) 37 °C prior to processing. Box plots of the iTRAQ ratios for each of the 264 proteins detected in ≥5 multiplexes, ranked by q value, are shown. The proteins are ordered by q value and proteins to the left of the vertical line (q = 0.05) were detected in significantly altered abundances. Proteins, fold changes, P and q values are listed in Supplemental Table 3.
Table 1.
Relative protein abundance changes in plasma held for 96 h prior to processing (Study 2). Proteins with fold changes >3 in at least one experimental condition are shown. The full data set is in Supplemental Table 3.
| UniProt ID | Name | Room temperature
|
37 °C
|
||
|---|---|---|---|---|---|
| Fold change | q value | Fold change | q value | ||
| S10A8_HUMAN | Protein S100-A8 | 12.3 | 5.6E-15 | 15.2 | 8.2E-17 |
| S10A9_HUMAN | Protein S100-A9 | 15.4 | 3.8E-14 | 18.5 | 1.7E-15 |
| PON1_HUMAN | Serum paraoxonase/arylesterase 1 | 17.2 | 1.3E-11 | 25.1 | 1.0E-13 |
| CO3_HUMAN | Complement C3 | 4.9 | 3.0E-09 | 14.2 | 8.2E-17 |
| COF1_HUMAN | Cofilin-1 | 9.6 | 1.7E-08 | 5.4 | 4.2E-06 |
| TAGL2_HUMAN | Transgelin-2 | 8.3 | 3.7E-08 | 11.6 | 3.4E-10 |
| CXCL7_HUMAN | Platelet basic protein | 7.3 | 4.2E-08 | 4.4 | 8.3E-06 |
| TSP1_HUMAN | Thrombospondin-1 | 7.5 | 1.9E-07 | 2.2 | 2.6E-02 |
| PPIA_HUMAN | Peptidyl-prolyl cis-trans isomerase A | 9.7 | 6.8E-07 | 7.9 | 2.5E-06 |
| PROF1_HUMAN | Profilin-1 | 7.0 | 1.7E-06 | 6.2 | 3.2E-06 |
| PGK1_HUMAN | Phosphoglycerate kinase 1 | 4.7 | 2.1E-06 | 4.0 | 6.4E-06 |
| ACTB_HUMAN | Actin, cytoplasmic 1 | 8.7 | 2.5E-06 | 5.8 | 3.3E-05 |
| RL40_HUMAN | Ubiquitin-60S ribosomal protein L40 | 4.8 | 3.6E-05 | 8.4 | 6.9E-08 |
| TLN1_HUMAN | Talin-1 | 3.5 | 4.9E-05 | 2.6 | 8.8E-04 |
| TPM4_HUMAN | Tropomyosin alpha-4 chain | 4.7 | 4.9E-05 | 10.0 | 6.6E-09 |
| CD5L_HUMAN | CD5 antigen-like | 3.9 | 7.3E-05 | 7.7 | 7.6E-09 |
| SH3L3_HUMAN | SH3 domain-binding glutamic acid-rich-like protein 3 | 3.1 | 8.1E-05 | 5.0 | 6.9E-08 |
| ANXA1_HUMAN | Annexin A1 | 5.2 | 9.6E-05 | 6.8 | 4.2E-06 |
| PLF4_HUMAN | Platelet factor 4 | 12.8 | 1.1E-04 | 7.9 | 6.6E-04 |
| C1R_HUMAN | Complement C1r subcomponent | −1.6 | 2.5E-04 | −8.6 | 1.4E-24 |
| TRFL_HUMAN | Lactotransferrin | 3.2 | 6.6E-04 | 1.9 | 4.8E-02 |
| G3P_HUMAN | Glyceraldehyde-3-phosphate dehydrogenase | 3.6 | 9.8E-04 | 5.2 | 1.1E-05 |
| PARK7_HUMAN | Protein DJ-1 | 3.2 | 1.0E-03 | 6.8 | 7.4E-07 |
| CAH1_HUMAN | Carbonic anhydrase 1 | 2.8 | 1.7E-03 | 10.3 | 4.5E-11 |
| 6PGD_HUMAN | 6-phosphogluconate dehydrogenase | 3.7 | 3.1E-03 | 3.9 | 1.1E-03 |
| S10A6_HUMAN | Protein S100-A6 | 4.1 | 3.1E-03 | 5.7 | 1.7E-04 |
| GPV_HUMAN | Platelet glycoprotein V | 3.0 | 6.8E-03 | 3.6 | 6.3E-04 |
| FLNA_HUMAN | Filamin-A | 2.5 | 7.0E-03 | 3.2 | 3.3E-04 |
| TPIS_HUMAN | Triosephosphate isomerase | 2.2 | 7.5E-03 | 3.4 | 2.1E-05 |
| ANXA3_HUMAN | Annexin A3 | 3.7 | 9.2E-03 | 3.8 | 4.8E-03 |
| CAH2_HUMAN | Carbonic anhydrase 2 | 2.3 | 2.4E-02 | 5.2 | 2.4E-06 |
| THIO_HUMAN | Thioredoxin | 2.4 | 2.9E-02 | 3.3 | 9.0E-04 |
| MOES_HUMAN | Moesin | 3.8 | 4.6E-02 | 3.8 | 2.4E-02 |
| CAP1_HUMAN | Adenylyl cyclase-associated protein 1 | 2.3 | 6.4E-02 | 4.1 | 3.9E-04 |
| ANXA5_HUMAN | Annexin A5 | 3.3 | 1.0E-01 | 4.5 | 1.3E-02 |
| CATA_HUMAN | Catalase | 1.6 | 1.9E-01 | 3.5 | 6.4E-06 |
| TPM3_HUMAN | Tropomyosin alpha-3 chain | 2.3 | 1.9E-01 | 3.2 | 1.7E-02 |
| PRDX6_HUMAN | Peroxiredoxin-6 | 2.2 | 2.0E-01 | 3.5 | 8.5E-03 |
| FIBB*_HUMAN | Fibrinogen beta chain | −1.7 | 3.5E-01 | 4.7 | 2.7E-05 |
| HBB_HUMAN | Hemoglobin subunit beta | 1.7 | 3.5E-01 | 12.0 | 7.6E-10 |
| APOC4_HUMAN | Apolipoprotein C-IV | −1.7 | 3.6E-01 | −4.2 | 1.7E-04 |
| PRDX2_HUMAN | Peroxiredoxin-2 | 1.6 | 3.6E-01 | 6.9 | 3.4E-08 |
| APOA1_HUMAN | Apolipoprotein A-I | 1.5 | 4.1E-01 | 12.5 | 2.2E-12 |
| HBA_HUMAN | Hemoglobin subunit alpha | 1.7 | 4.2E-01 | 14.6 | 5.9E-10 |
| FIBG_HUMAN | Fibrinogen gamma chain | −1.5 | 5.7E-01 | 5.0 | 1.7E-05 |
| C1S_HUMAN | Complement C1s subcomponent | −1.1 | 6.6E-01 | −3.3 | 1.3E-12 |
| APOE_HUMAN | Apolipoprotein E | −1.2 | 8.4E-01 | −5.5 | 6.8E-09 |
| APOC2_HUMAN | Apolipoprotein C-II | −1.6 | 8.9E-01 | −11.2 | 2.3E-04 |
| CNDP1_HUMAN | Beta-Ala-His dipeptidase | −1.1 | 9.5E-01 | −3.1 | 8.9E-05 |
| FIBA_HUMAN | Fibrinogen alpha chain | −1.1 | 9.8E-01 | 4.1 | 5.3E-04 |
| APOC3_HUMAN | Apolipoprotein C-III | −1.1 | 9.9E-01 | −4.1 | 3.8E-02 |
| HBD_HUMAN | Hemoglobin subunit delta | 1.1 | 9.9E-01 | 8.7 | 1.7E-04 |
In addition, we quantified proteins from these samples that were prepared according to a second common plasma processing SOP. The main differences in the processing protocols were the holding temperature prior to centrifugation (wet ice vs 4 °C) and the number of centrifugation steps (2 vs 1). A comparison of the proteomes of control samples from the two protocols showed that one protein, thrombospondin-1 (TSP1), was 6.7-fold higher in abundance in single-spun plasma (q = 0.02, Supplemental Table 3). Box plots with the ratios for all quantified proteins, ranked by q value, are shown in Fig. 3A. Four additional proteins tended to be elevated in single-spun plasma, but did not reach significance: platelet basic protein (3.6-fold), platelet factor 4 (2.9-fold), platelet glycoprotein V (2.1-fold), and IgG Fc receptor II-a (3.6-fold). PON1 levels were unchanged. Relative protein abundances of single-spun vs double-spun plasmas that were subjected to the same experimental conditions (holding time and temperature) showed no significant differences (Fig. 3B and C), indicating that delay-related effects are not compounded by the centrifugation method.
Fig. 3.
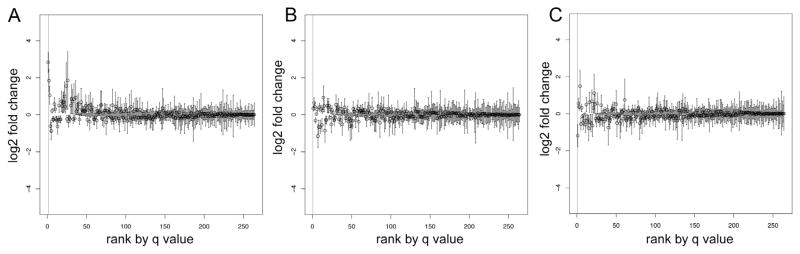
Relative protein abundances in single- compared to double-spun plasma under various processing conditions. Box plots of the iTRAQ ratios for each of the 264 proteins detected in ≥5 multiplexes are shown. The proteins are ordered by q value and proteins to the left of the vertical line (q = 0.05) were detected in significantly altered abundance. (A) Plasma processed according to the SOPs; (B) plasma held for 96 h at room temperature and (C) 37 °C prior to processing. Proteins, fold changes, and q values listed in Supplemental Table 3.
Study 3
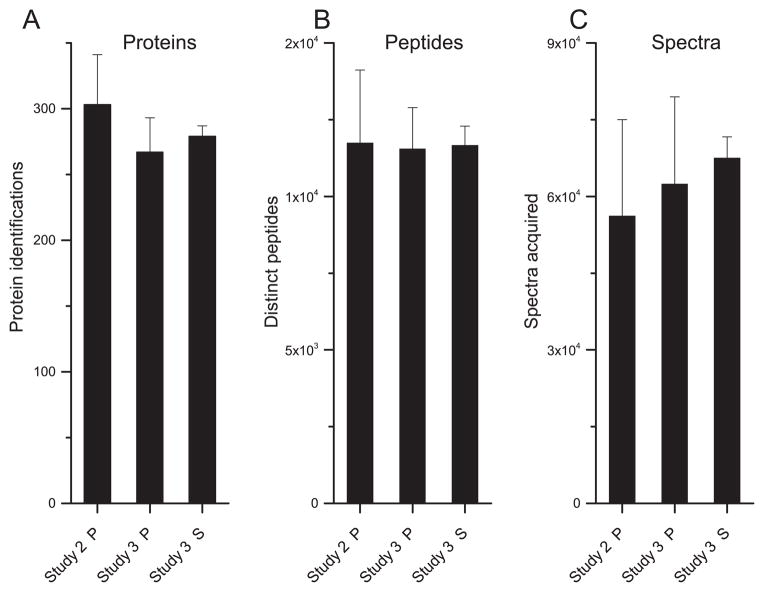
Finally, we undertook a study of plasma and serum (n = 20 each) that had been stored at −80 °C for an extended period (14–17 years). The records documented the number of times each sample was thawed and refrozen, an average of 3. For comparison, we used plasma and serum samples (n = 10 each) collected at MWRI for the purpose of this study using the same collection and processing protocols as the banked specimens. These samples were stored continuously at −80 °C for less than 3 months. A total of 267 plasma proteins and 279 serum proteins were detected at <5% FDR (Supplemental Table 4). The total number of plasma proteins detected was not significantly different from the number of plasma proteins identified in Study 2, which employed the same LC-MS approach (Fig. 4A). Similarly, the number of spectra acquired and distinct peptides observed showed no significant differences (Fig. 4B and C). As in Study 2, the selection criterion for statistical analyses was proteins detected in at least 5 multiplexes, which resulted in quantification data for 212 serum proteins and 183 plasma proteins. The lower fraction of plasma proteins used for quantification (69%) as compared to Study 2 (87%) was likely due to the fact that fewer multiplexes were analyzed in this study (n = 6) vs Study 2 (n = 9). Comparison of the plasma data from Studies 2 and 3 showed that 165 (90%) proteins were quantified in both. The abundance of a single protein, alpha-2-macroglobulin, was significantly different (Supplemental Table 4) in both plasma (decreased 13-fold) and serum (decreased 17-fold) samples that were stored long term. This protein was detected in Studies 1 and 2 with no change in relative abundance under any of the experimental conditions. Two proteins were more abundant in plasma stored long term, complement C1q subcomponent subunit B (2.9-fold; q = 0.02) and plasma protease C1 inhibitor (9-fold; q = 0.03).
Fig. 4.
LC-MS analysis metrics of plasma and serum from Studies 2 and 3. (A) Number of proteins detected (5% FDR), (B) distinct peptides, and (C) product ion spectra of immunodepleted plasma (P) and serum (S) samples. All values are the mean ± standard deviation.
To investigate the potential effects of immunodepletion on changes in protein abundances due to preanalytical processing variables, we compiled the proteins that are reported in the literature as nonspecifically retained during antibody depletion [23–27]. We chose studies that employed the MARS-Hu14 column used here or IgY-12, which has similar affinities. For the purpose of this analysis, we eliminated MARS-Hu14 targets, immunoglobulin variants, and keratins. Comparison of this list of 65 proteins to those that were quantified in Studies 2 and 3 (Supplemental Table 5) showed that 54 were in common. Of these, 21 proteins changed in abundance in one or more of the experimental conditions tested. MARS-Hu14 immunodepletion targets were also detected, four with altered abundances in at least one experimental sample (alpha-2-macroglobulin, apolipoprotein A-I, complement C3, fibrinogen). This occurred despite strict adherence to the manufacturer’s protocol, including the lifetime of the column in terms of the number of samples processed.
Discussion
We conducted a systematic analysis to determine the relationships among blood processing and storage variables and the quality and reproducibility of quantitative proteomics data. Multiple processing and storage variables were assayed: (1) time and temperature before processing; (2) time after thawing prior to immunodepletion; (3) centrifugation steps (single vs double); (4) number of freeze–thaw cycles; and (5) time in frozen storage. An untargeted comparative proteomics approach applied to EDTA plasma samples held at room temperature for 6 h prior to centrifugation showed a single change—an increase in PON1 abundance. The same phenomenon was observed in processed plasma held at room temperature for 24 h prior to immunodepletion. At the level of this analysis, these results suggested that plasma proteins are stable for hours at room temperature, before and after processing, without the addition of protease inhibitors.
Since enzyme activity is temperature dependent, we reasoned that increasing the temperature or extending the holding time to multiple days would exacerbate proteolysis and could uncover sentinels of protein degradation. These indicator proteins could have utility in more sensitive, targeted assays designed to assess plasma quality. We identified many proteins that differed significantly in abundance when they were held at 96 h prior to processing. At room temperature, 41 proteins differed in abundance. The majority were detected at increased levels. Even at body temperature, which in principle would facilitate proteolytic activity, the majority of proteins (56/83) also increased. Thus, in addition to proteolysis, changes in protein conformation that affect antibody depletion and/or trypsin digestion are likely to impact protein detection. Zimmerman et al., who also using an MS-based approach, reported few significant protein changes in plasma samples held for up to a week at room temperature or 4 °C [14]. In that study, whole blood was incubated in 96-well plates and the work-flow did not include immunodepletion. The few significant changes that were observed generally trended toward increased protein abundances over time. Schenk et al. found that the addition of protease inhibitors, after plasma processing and prior to immunodepletion, resulted in fewer proteins identified by LC-MS [28]. Yi et al. thawed nondepleted plasma and serum samples and incubated them at room temperature for up to 72 h [13]. MALDI TOF-MS showed that some spectral peaks decreased in abundance over time while others increased. The authors concluded that their results were consistent with proteolysis over time. However, it is also possible that, in some cases, changes in protein conformations or interactions were involved.
Our observation that a subset of proteins is reproducibly increased in abundance and others are decreased suggests that multiple processes affect protein quantification in immunodepleted plasma. Plasma has protein concentrations spanning more than 10 orders of magnitude [1]. About 95% of the proteome is composed of 10–12 proteins, which can interfere with MS-based approaches for identifying and quantifying less abundant proteins. The removal of albumin and other highly abundant proteins via antibody depletion has become standard practice [3,25]. Recently the immunodepletion of abundant blood-derived proteins from solid tissue specimens has been recommended [29]. This approach, which has the advantage of removing the most abundant proteins, also has disadvantages. For example, depletion targets can be carriers for other proteins and small molecules [30–34], which may variably travel with them in removal strategies, affecting quantification [26,27]. Conformational changes in antibody targets presumably affect epitope binding, resulting in altered depletion efficiencies. Many groups have reported retention of nontargeted proteins, which may be due to homologous epitopes, nonspecific interactions with column matrices, or protein–protein interactions [13,26,31,35,36]. The closely controlled trypsin digestion protocol used here, employing single lots of reagents and enzyme and consistent incubation times, makes this step in and of itself unlikely to be a significant contributor to the variations observed. However, progressive denaturation of proteins could improve trypsin digestion, which in turn, could affect quantification. Evaluation of digestion efficiencies has been notoriously difficult (reviewed in [37]) and more research is needed to explain the underlying mechanisms that increase detection of certain proteins. Here, we observed elevated levels of many proteins in plasma samples held for extended time periods prior to immunodepletion, which could be related to any or all of the above phenomena.
As to other variables, we found that ≤3 freeze–thaw cycles during long-term storage or in short succession of recently collected samples had little effect. This result is in concordance with shotgun proteomics analyses performed on undepleted plasma, which also examined this variable [14]. A study of MALDI TOF-MS peak intensities (1000–40,000 m/z) showed that continuous long-term storage of plasma had minimal effects. Additionally, up to 5 freeze–thaw cycles led to variable, albeit small, changes [38]. Conversely, serum peptide profiling using reverse phase magnetic particles and MALDI TOF-MS (700–4000 m/z) showed that 4 freeze–thaw cycles affected the observed MS patterns, with decreases in both the number and the intensities of the peaks [39]. Thus, freeze–thaw cycles may have effects on native peptides, a possibility that our study did not address. In summary, minimal degradation of larger polypeptides/proteins after several freeze–thaw cycles or long-term storage at −80 °C is encouraging for the use of valuable samples from archival biorepositories for biomarker research.
Processing EDTA plasma by using established protocols [11,12] that differ primarily in the number of centrifugation steps, i.e., one or two, resulted in very few effects when monitored at the level of relative protein abundances. This held true for samples prepared off-protocol; i.e., the changes observed at an extended holding time and an elevated temperature were not distinguishable based on the centrifugation method. Thus, plasma processed using different methods could be useful for proteomics experiments, especially in the verification phase when high sample numbers from disparate sources are required.
A high confidence human plasma proteome, compiled from multiple sources, has 1929 nonredundant proteins (1% FDR) [36]. Our quantitative proteomics analyses with limited fractionation allowed for the detection of ~300 proteins. While this set of proteins can be considered a proxy for the plasma proteome in its entirety, these experiments did not directly address the effects of preanalytical variables on the lower abundance plasma proteins. The concentrations of proteins that were detected in immunode-pleted plasma ranged over 5 orders of magnitude, from 1.2 ng/mL to 7.5 × 105 ng/mL (Supplemental Tables 2 and 3, [36,40–42]). Fundamentally, the question of the integrity of biospecimens in regard to their potential utility in proteomics studies can be answered accurately only in the context of a specific set of protein targets and the type of assay used for their analyses. However, cost often prohibits these types of surveys, the rationale for studies such as ours. In a global context, when targets of interest are not known, a range of potential scenarios can affect biospecimen integrity. At one end of the spectrum lies significant degradation of a relatively large subset of proteins and representative changes in abundances of proteins are likely to be detected by shotgun analyses, e.g., relative protein quantification using isobaric tags, our approach. On the other hand, protein-specific changes, such as those that could affect unusual structural features, lower abundance proteins, and/or post-translational modifications are unlikely to be detected using the nontargeted analytical strategies employed here. Ultimately, once a biomarker candidate is identified, verification studies using a robust, targeted assay and sample sets collected from multiple sources is required prior to moving targets to the validation phase [20].
Progress in the components of proteomics workflows—immunoenrichment strategies, system suitability/quality assurance protocols, software applications and hardware—is increasing the utility of MS-based assays in clinical settings (reviewed in [43]). Ultimately, accurate quantification of analytes rests on a foundation of high quality, well-annotated specimens. Several organizations provide best practices guidelines covering various aspects of the biospecimen lifecycle [9,11,44,45]. The International Society for Biological and Environmental Repositories (ISBER) recently compiled the literature concerning potential plasma/serum protein markers for preanalytical variation assessment; the bulk were single analytes measured by using immunoassays [46]. The National Cancer Institute established the Biorepositories and Biospecimen Research Branch, which recently published evidence-based guidance for the snap freezing of human tissue biospecimens [47]. There are a plethora of biomarker discovery, verification, and validation sample preparation workflows, and it is unrealistic to expect that a single quality assay will prove useful for all types of quantitative analyses. The work presented here adds to the body of knowledge driving evidence-based protocols and documentation of preanalytical variables that could affect blood-based protein measurements in biomarker studies. In this regard, we suggest that samples collected with modest delays in plasma processing times, one vs two centrifugation steps, limited freeze–thaw cycles, or extended long-term storage should not necessarily be excluded from protein biomarker studies.
Supplementary Material
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E (UCSF) and PPG Grants P01HD30367, UL1RR024153, and UL1TR000005 (University of Pittsburgh Clinical and Translational Science Institute). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ab.2015.03.003.
Footnotes
Abbreviations used: CPTAC, Clinical Proteomic Technologies Assessment for Cancer; EDRN, Early Detection Research Network; SOP, standard operating procedure.
References
- 1.Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 2.Hortin GL, Sviridov D. The dynamic range problem in the analysis of the plasma proteome. J Proteomics. 2010;73:629–636. doi: 10.1016/j.jprot.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Hakimi A, Auluck J, Jones GD, Ng LL, Jones DJ. Assessment of reproducibility in depletion and enrichment workflows for plasma proteomics using label-free quantitative data-independent LC-MS. Proteomics. 2014;14:4–13. doi: 10.1002/pmic.201200563. [DOI] [PubMed] [Google Scholar]
- 4.Abbatiello SE, Mani DR, Schilling B, Maclean B, Zimmerman LJ, Feng X, Cusack MP, Sedransk N, Hall SC, Addona T, Allen S, Dodder NG, Ghosh M, Held JM, Hedrick V, Inerowicz HD, Jackson A, Keshishian H, Kim JW, Lyssand JS, Riley CP, Rudnick P, Sadowski P, Shaddox K, Smith D, Tomazela D, Wahlander A, Waldemarson S, Whitwell CA, You J, Zhang S, Kinsinger CR, Mesri M, Rodriguez H, Borchers CH, Buck C, Fisher SJ, Gibson BW, Liebler D, Maccoss M, Neubert TA, Paulovich A, Regnier F, Skates SJ, Tempst P, Wang M, Carr SA. Design, implementation and multisite evaluation of a system suitability protocol for the quantitative assessment of instrument performance in liquid chromatography-multiple reaction monitoring-MS (LC-MRM-MS) Mol Cell Proteomics. 2013;12:2623–2639. doi: 10.1074/mcp.M112.027078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Issaq HJ, Waybright TJ, Veenstra TD. Cancer biomarker discovery: opportunities and pitfalls in analytical methods. Electrophoresis. 2011;32:967–975. doi: 10.1002/elps.201000588. [DOI] [PubMed] [Google Scholar]
- 6.Skates SJ, Gillette MA, LaBaer J, Carr SA, Anderson L, Liebler DC, Ransohoff D, Rifai N, Kondratovich M, Tezak Z, Mansfield E, Oberg AL, Wright I, Barnes G, Gail M, Mesri M, Kinsinger CR, Rodriguez H, Boja ES. Statistical design for biospecimen cohort size in proteomics-based biomarker discovery and verification studies. J Proteome Res. 2013;12:5383–5394. doi: 10.1021/pr400132j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ransohoff DF. How to improve reliability and efficiency of research about molecular markers: roles of phases, guidelines, and study design. J Clin Epidemiol. 2007;60:1205–1219. doi: 10.1016/j.jclinepi.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Moore HM, Kelly A, Jewell SD, McShane LM, Clark DP, Greenspan R, Hainaut P, Hayes DF, Kim P, Mansfield E, Potapova O, Riegman P, Rubinstein Y, Seijo E, Somiari S, Watson P, Weier HU, Zhu C, Vaught J. Biospecimen reporting for improved study quality. Biopreserv Biobank. 2011;9:57–70. doi: 10.1089/bio.2010.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rai AJ, Gelfand CA, Haywood BC, Warunek DJ, Yi J, Schuchard MD, Mehigh RJ, Cockrill SL, Scott GB, Tammen H, Schulz-Knappe P, Speicher DW, Vitzthum F, Haab BB, Siest G, Chan DW. HUPO Plasma Proteome Project specimen collection and handling: towards the standardization of parameters for plasma proteome samples. Proteomics. 2005;5:3262–3277. doi: 10.1002/pmic.200401245. [DOI] [PubMed] [Google Scholar]
- 10.Jewell SD, Srinivasan M, McCart LM, Williams N, Grizzle WH, LiVolsi V, MacLennan G, Sedmak DD. Analysis of the molecular quality of human tissues: an experience from the Cooperative Human Tissue Network. Am J Clin Pathol. 2002;118:733–741. doi: 10.1309/VPQL-RT21-X7YH-XDXK. [DOI] [PubMed] [Google Scholar]
- 11.Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE, Rom W, Sanda M, Sorbara L, Stass S, Wang W, Brenner DE. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res. 2009;8:113–117. doi: 10.1021/pr800545q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zawadzka AM, Schilling B, Cusack MP, Sahu AK, Drake P, Fisher SJ, Benz CC, Gibson BW. Phosphoprotein secretome of tumor cells as a source of candidates for breast cancer biomarkers in plasma. Mol Cell Proteomics. 2014;13:1034–1049. doi: 10.1074/mcp.M113.035485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi J, Kim C, Gelfand CA. Inhibition of intrinsic proteolytic activities moderates preanalytical variability and instability of human plasma. J Proteome Res. 2007;6:1768–1781. doi: 10.1021/pr060550h. [DOI] [PubMed] [Google Scholar]
- 14.Zimmerman LJ, Li M, Yarbrough WG, Slebos RJ, Liebler DC. Global stability of plasma proteomes for mass spectrometry-based analyses. Mol Cell Proteomics. 2012;11:M111 014340. doi: 10.1074/mcp.M111.014340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randall SA, McKay MJ, Baker MS, Molloy MP. Evaluation of blood collection tubes using selected reaction monitoring MS: implications for proteomic biomarker studies. Proteomics. 2010;10:2050–2056. doi: 10.1002/pmic.200900517. [DOI] [PubMed] [Google Scholar]
- 16.Aguilar-Mahecha A, Kuzyk MA, Domanski D, Borchers CH, Basik M. The effect of pre-analytical variability on the measurement of MRM-MS-based mid- to high-abundance plasma protein biomarkers and a panel of cytokines. PLoS One. 2012;7:e38290. doi: 10.1371/journal.pone.0038290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall J, Kupchak P, Zhu W, Yantha J, Vrees T, Furesz S, Jacks K, Smith C, Kireeva I, Zhang R, Takahashi M, Stanton E, Jackowski G. Processing of serum proteins underlies the mass spectral fingerprinting of myocardial infarction. J Proteome Res. 2003;2:361–372. doi: 10.1021/pr030003l. [DOI] [PubMed] [Google Scholar]
- 18.Insenser M, Martinez-Garcia M, Nieto RM, San-Millan JL, Escobar-Morreale HF. Impact of the storage temperature on human plasma proteomic analysis: implications for the use of human plasma collections in research. Proteomics Clin Appl. 2010;4:739–744. doi: 10.1002/prca.201000007. [DOI] [PubMed] [Google Scholar]
- 19.Issaq HJ, Xiao Z, Veenstra TD. Serum and plasma proteomics. Chem Rev. 2007;107:3601–3620. doi: 10.1021/cr068287r. [DOI] [PubMed] [Google Scholar]
- 20.Addona TA, Abbatiello SE, Schilling B, Skates SJ, Mani DR, Bunk DM, Spiegelman CH, Zimmerman LJ, Ham AJ, Keshishian H, Hall SC, Allen S, Blackman RK, Borchers CH, Buck C, Cardasis HL, Cusack MP, Dodder NG, Gibson BW, Held JM, Hiltke T, Jackson A, Johansen EB, Kinsinger CR, Li J, Mesri M, Neubert TA, Niles RK, Pulsipher TC, Ransohoff D, Rodriguez H, Rudnick PA, Smith D, Tabb DL, Tegeler TJ, Variyath AM, Vega-Montoto LJ, Wahlander A, Waldemarson S, Wang M, Whiteaker JR, Zhao L, Anderson NL, Fisher SJ, Liebler DC, Paulovich AG, Regnier FE, Tempst P, Carr SA. Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat Biotechnol. 2009;27:633–641. doi: 10.1038/nbt.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dwivedi RC, Spicer V, Harder M, Antonovici M, Ens W, Standing KG, Wilkins JA, Krokhin OV. Practical implementation of 2D HPLC scheme with accurate peptide retention prediction in both dimensions for high-throughput bottom-up proteomics. Anal Chem. 2008;80:7036–7042. doi: 10.1021/ac800984n. [DOI] [PubMed] [Google Scholar]
- 22.Tambor V, Hunter CL, Seymour SL, Kacerovsky M, Stulik J, Lenco J. CysTRAQ—a combination of iTRAQ and enrichment of cysteinyl peptides for uncovering and quantifying hidden proteomes. J Proteomics. 2011 doi: 10.1016/j.jprot.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 23.Yadav AK, Bhardwaj G, Basak T, Kumar D, Ahmad S, Priyadarshini R, Singh AK, Dash D, Sengupta S. A systematic analysis of eluted fraction of plasma post immunoaffinity depletion: implications in biomarker discovery. PLoS One. 2011;6:e24442. doi: 10.1371/journal.pone.0024442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tu C, Rudnick PA, Martinez MY, Cheek KL, Stein SE, Slebos RJ, Liebler DC. Depletion of abundant plasma proteins and limitations of plasma proteomics. J Proteome Res. 2010;9:4982–4991. doi: 10.1021/pr100646w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shuford CM, Hawkridge AM, Burnett JC, Jr, Muddiman DC. Utilizing spectral counting to quantitatively characterize tandem removal of abundant proteins (TRAP) in human plasma. Anal Chem. 2010;82:10179–10185. doi: 10.1021/ac102248d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu T, Qian WJ, Mottaz HM, Gritsenko MA, Norbeck AD, Moore RJ, Purvine SO, Camp DG, 2nd, Smith RD. Evaluation of multiprotein immunoaffinity subtraction for plasma proteomics and candidate biomarker discovery using mass spectrometry. Mol Cell Proteomics. 2006;5:2167–2174. doi: 10.1074/mcp.T600039-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gundry RL, Fu Q, Jelinek CA, Van Eyk JE, Cotter RJ. Investigation of an albumin-enriched fraction of human serum and its albuminome. Proteomics Clin Appl. 2007;1:73–88. doi: 10.1002/prca.200600276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schenk S, Schoenhals GJ, de Souza G, Mann M. A high confidence, manually validated human blood plasma protein reference set. BMC Med Genomics. 2008;1:41. doi: 10.1186/1755-8794-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prieto DA, Johann DJ, Jr, Wei BR, Ye X, Chan KC, Nissley DV, Simpson RM, Citrin DE, Mackall CL, Linehan WM, Blonder J. Mass spectrometry in cancer biomarker research: a case for immunodepletion of abundant blood-derived proteins from clinical tissue specimens. Biomarkers Med. 2014;8:269–286. doi: 10.2217/bmm.13.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baczynskyj L, Bronson GE, Kubiak TM. Application of thermally assisted electrospray ionization mass spectrometry for detection of noncovalent complexes of bovine serum albumin with growth hormone releasing factor and other biologically active peptides. Rapid Commun Mass Spectrom. 1994;8:280–286. doi: 10.1002/rcm.1290080311. [DOI] [PubMed] [Google Scholar]
- 31.Carter WA. Binding of human interferons to immobilized albumin. Methods Enzymol. 1981;78:576–582. doi: 10.1016/0076-6879(81)78171-0. [DOI] [PubMed] [Google Scholar]
- 32.Sjobring U, Bjorck L, Kastern W. Streptococcal protein G. Gene structure and protein binding properties. J Biol Chem. 1991;266:399–405. [PubMed] [Google Scholar]
- 33.Carter DC, Ho JX. Structure of serum albumin. Adv Protein Chem. 1994;45:153–203. doi: 10.1016/s0065-3233(08)60640-3. [DOI] [PubMed] [Google Scholar]
- 34.Peters T., Jr Serum albumin. Adv Protein Chem. 1985;37:161–245. doi: 10.1016/s0065-3233(08)60065-0. [DOI] [PubMed] [Google Scholar]
- 35.Colombo G, Clerici M, Giustarini D, Rossi R, Milzani A, Dalle-Donne I. Redox Albuminomics: Oxidized Albumin in Human Diseases. Antioxid, Redox Signal. 2012 doi: 10.1089/ars.2012.4702. [DOI] [PubMed] [Google Scholar]
- 36.Farrah T, Deutsch EW, Omenn GS, Campbell DS, Sun Z, Bletz JA, Mallick P, Katz JE, Malmstrom J, Ossola R, Watts JD, Lin B, Zhang H, Moritz RL, Aebersold R. A high-confidence human plasma proteome reference set with estimated concentrations in PeptideAtlas. Mol Cell Proteomics. 2011;10:M110.006353. doi: 10.1074/mcp.M110.006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hustoft HK, Malerod H, Wilson SR, Reubsaet L, Lundanes E, Greibrokk T. A critical review of trypsin digestion for LC-MS based proteomics. In: Leung H-C, editor. Integrative Proteomics. InTech; 2012. http://www.intechopen.com/books/integrative-proteomics. [Google Scholar]
- 38.Mitchell BL, Yasui Y, Li CI, Fitzpatrick AL, Lampe PD. Impact of freeze-thaw cycles and storage time on plasma samples used in mass spectrometry based biomarker discovery projects. Cancer Inform. 2005;1:98–104. [PMC free article] [PubMed] [Google Scholar]
- 39.Villanueva J, Philip J, Chaparro CA, Li Y, Toledo-Crow R, DeNoyer L, Fleisher M, Robbins RJ, Tempst P. Correcting common errors in identifying cancer-specific serum peptide signatures. J Proteome Res. 2005;4:1060–1072. doi: 10.1021/pr050034b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haab BB, Geierstanger BH, Michailidis G, Vitzthum F, Forrester S, Okon R, Saviranta P, Brinker A, Sorette M, Perlee L, Suresh S, Drwal G, Adkins JN, Omenn GS. Immunoassay and antibody microarray analysis of the HUPO Plasma Proteome Project reference specimens: systematic variation between sample types and calibration of mass spectrometry data. Proteomics. 2005;5:3278–3291. doi: 10.1002/pmic.200401276. [DOI] [PubMed] [Google Scholar]
- 41.Hortin GL, Sviridov D, Anderson NL. High-abundance polypeptides of the human plasma proteome comprising the top 4 logs of polypeptide abundance. Clin Chem. 2008;54:1608–1616. doi: 10.1373/clinchem.2008.108175. [DOI] [PubMed] [Google Scholar]
- 42.Polanski M, Anderson NL. A list of candidate cancer biomarkers for targeted proteomics. Biomarker Insights. 2007;1:1–48. [PMC free article] [PubMed] [Google Scholar]
- 43.Gillette MA, Carr SA. Quantitative analysis of peptides and proteins in biomedicine by targeted mass spectrometry. Nat Methods. 2013;10:28–34. doi: 10.1038/nmeth.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell LD, Betsou F, Garcia DL, Giri JG, Pitt KE, Pugh RS, Sexton KC, Skubitz AP, Somiari SB. Development of the ISBER best practices for repositories: collection, storage, retrieval and distribution of biological materials for research. Biopreserv Biobank. 2012;10:232–233. doi: 10.1089/bio.2012.1025. [DOI] [PubMed] [Google Scholar]
- 45.Robb JA, Gulley ML, Fitzgibbons PL, Kennedy MF, Cosentino LM, Washington K, Dash RC, Branton PA, Jewell SD, Lapham RL. A call to standardize preanalytic data elements for biospecimens. Arch Pathol Lab Med. 2014;138:526–537. doi: 10.5858/arpa.2013-0250-CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Betsou F, Gunter E, Clements J, DeSouza Y, Goddard KA, Guadagni F, Yan W, Skubitz A, Somiari S, Yeadon T, Chuaqui R. Identification of evidence-based biospecimen quality-control tools: a report of the International Society for Biological and Environmental Repositories (ISBER) Biospecimen Science Working Group. J Mol Diagn. 2013;15:3–16. doi: 10.1016/j.jmoldx.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engel KB, Vaught J, Moore HM. National Cancer Institute Biospecimen Evidence-Based Practices: a novel approach to pre-analytical standardization. Biopreserv Biobank. 2014;12:148–150. doi: 10.1089/bio.2013.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.