Abstract
Pancreatic ductal adenocarcinoma is the only cancer for which deaths are predicted to increase in 2014 and beyond. Combined radiochemotherapy protocols using gemcitabine and hypofractionated X-rays are ongoing in several clinical trials. Recent results indicate that charged particle therapy substantially increases local control of resectable and unresectable pancreas cancer, as predicted from previous radiobiology studies considering the high tumor hypoxia. Combination with chemotherapy improves the overall survival (OS). We compared published data on X-ray and charged particle clinical results with or without adjuvant chemotherapy calculating the biological effective dose. We show that chemoradiotherapy with protons or carbon ions results in 1 year OS significantly higher than those obtained with other treatment schedules. Further hypofractionation using charged particles may result in improved local control and survival. A comparative clinical trial using the standard X-ray scheme vs. the best current standard with carbon ions is crucial and may open new opportunities for this deadly disease.
Keywords: pancreatic cancer, protontherapy, heavy ion therapy, chemoradiotherapy, gemcitabine
Introduction
Pancreatic cancer (PC), usually ductal adenocarcinoma, is the fourth cause of cancer-related death in USA (1) and the only cancer for which deaths are predicted to increase in Europe for both men and women in 2015 (2). Even after surgery, the mortality from PC is very high. Radiotherapy is used for radical treatment in locally advanced unresectable tumors (LAUPC), generally in combination with chemotherapy, or prior to surgery for potentially resectable malignancies. However, prognosis remains very poor, with <5% of patients surviving for 5 years after diagnosis (3). This makes PC a priority for finding better ways to control it and better treatments. Early tumors usually do not cause symptoms, so that the disease is typically not diagnosed until it has spread beyond the pancreas itself, either with distal metastasis or with infiltration in the neuroplexus. This is one of the reasons for the poor survival rate. Moreover, PC is very hypoxic (4), which makes it radioresistant and promotes epithelial–mesenchymal transition; is resistant to apoptosis; and presents a dense tumor stroma, which acts as a barrier against immune cells, preventing immune suppression (5).
Radiobiology studies suggest that charged particle therapy (CPT) using protons or carbon ions is more effective for treatment of PC than X-rays. In fact, accelerated ions have a reduced oxygen enhancement ratio (OER), and are therefore exquisitely effective against hypoxic tumors (6). Moreover, high doses of densely ionizing radiation elicit a strong immune response, which can be exploited to destroy not only the primary tumor but also distal metastasis (7). Carbon ion radiotherapy (CIRT) is currently performed in only two centers in Europe (HIT in Germany and CNAO in Italy) and none in USA (where many centers use protons only for CPT), but much more experience has been accumulated in Asia, especially at the National Institute for Radiological Sciences (NIRS) in Chiba, Japan. A recent external review of 20 years of CIRT at NIRS highlighted treatment of PC as the most promising application of CIRT, with results clearly superior to any other treatment modalities, especially for LAUPC (8).
Based on these very promising preliminary Japanese results, the US National Cancer Institute (NCI), in his efforts to promote CIRT in USA, issued a solicitation for a prospective randomized phase-III trial comparing CIRT to X-ray therapy for LAUPC in combination with chemotherapy, having survival as main endpoint1. This trial may provide the first evidence of a superiority of CIRT in a common and deadly cancer. Planning of the trial is complicated by the many different variables – not only radiation quality but also chemotherapy regime, fractionation, and treatment plan. Here, we review all the current results in treatment of LAUPC and use a mathematical model to describe the dependence on survival on the biological effective dose (BED) with X-rays and CPT in combination with chemotherapy.
Materials and Methods
Data collection
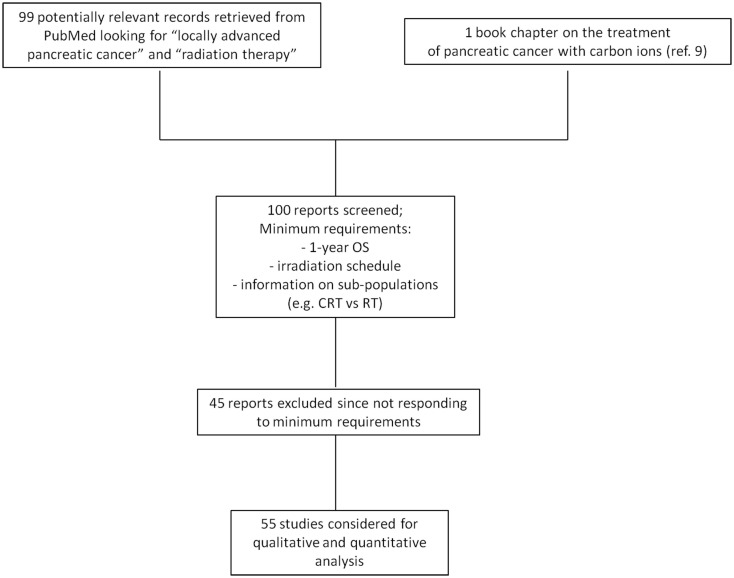
We searched the literature for all data available on radiotherapy, chemotherapy, and combined treatments. The research criteria and outcomes are summarized in the diagram shown in Figure 1. The patient populations generally consist of adults with adenocarcinoma histology, locally advanced tumor presentation, and generally tumors not in direct contact to duodenum and stomach. Radiotherapy included conformal radiotherapy (3DCRT), intensity-modulated radiation therapy (IMRT), stereotactic body radiotherapy (SBRT), protontherapy, and CIRT. Data from CIRT are limited to the NIRS experience and include data as yet only published in the institute annual report and in a recent book (9). Adjuvant, neo-adjuvant, or concomitant chemotherapies were all included in the search, using different drugs. Our data collection was compared with a recent meta-analysis of radiochemotherapy in LAUPC (10), and has been updated on April 2015.
Figure 1.
Diagram summarizing the selection criteria of the studies included in the analysis.
Modeling
To compare the largely variable fractionation and chemotherapy schedules reported in the literature, we used the common quantity of BED (11), which has been extended to chemotherapy to quantify the effect of the drug in terms of radiation-equivalent dose (12). Because many published papers have short follow-up, and not all endpoints are reported, we concentrated on the 1-year overall survival (OS). We assumed that the overall 1-year survival probability OS is a combination of the survival probability following the radiation (RS) and chemotherapy (CS) treatment, i.e.,
| (1) |
Equation 1 implies a purely additive effect of chemotherapy and radiotherapy in the treatment of LAUPC. The dose–response for the OS probability can be expressed with the same functions used for the tumor control probability: Poisson, logistic, or probit models (13). We elected to use the logistic function, which is based on the linear-quadratic model, following the recent model of chemoradiation treatment in bladder cancer (14). Thus, we wrote:
| (2) |
where γ50 is the normalized dose-response gradient and D50 the BED corresponding to a survival in a radiotherapy only treatment of 50% at 1 year.
Combining Eqs 1 and 2, we finally obtain
| (3) |
In a recent analysis of chemoradiation therapy in LAUPC, Moraru et al. (15) used a radiosensitization factor in the BED formula and fitted the LAUPC 1 year OS data with a modified linear-quadratic formula. In general, it is very hard to distinguish additive from synergistic model in chemoradiation data (16). In vitro experiments can provide some information, but do not necessarily reflect the complex in vivo microenvironment. Some chemotherapy drugs used for LAUPC treatment apparently sensitize cell cultures to X-rays (17, 18), but simple additive effects are observed when the drugs are given in vitro concomitantly to charged particles (19, 20). Moreover, in many clinical protocols, chemotherapy is given as adjuvant or neo-adjuvant, and even when concomitant is often continued after the radiotherapy cycle. We therefore assumed, in our analysis, that the simple additive model of Eqs 1 and 3.
The BED was calculated using the Fowler formula (11):
| (4) |
with:
-
–
n: number of fractions
-
–
d: dose/fraction
-
–
T: overall treatment time
-
–
α = 0.393 Gy−1, β = 0.058 Gy−2, α/β = 6.77 Gy (21)
-
–
Td: tumor doubling time, fixed to 42 days (15).
The dose/fraction d was given in Gy for X-ray data, and Gy(RBE) (or GyE) for CPT. For protontherapy, 1 Gy(RBE) = 1.1 Gy (22). In CIRT, Gy(RBE) was calculated according to the NIRS model (23), whose results can be different, depending on the dose and target size, from those that would be obtained using the LEM model (24), implemented in the European CIRT facilities.
Fitting
Clinical data extracted from the published papers were weighted with a vertical error bar, given by the SD of the OS using Poisson statistics:
| (5) |
where ns and ntot indicate the number of surviving patients at 1 year and the total number of patients included in the study, respectively. When possible, a horizontal error bar was also included, corresponding to the range of the doses used. A first weighed fit of the radiotherapy-alone data was performed using Eq. 2 to estimate the two parameters γ50 and D50. The chemoradiation data were then fitted using Eq. 3 having CS as only fitting parameter: γ50 and D50 were indeed taken from the radiotherapy fit. Many different chemotherapy drugs were used in old and new studies. Gemcitabine is one of the most successful and currently adopted, also in the CIRT trials. We have therefore divided the data into gemcitabine only, other drugs, and gemcitabine plus other drugs. Overall, no statistically significant differences were noted among the three groups. We have therefore fitted the data together, even if we plotted the points in different colors. Finally, for fitting the CPT data, we expressed the BED in Gy(RBE) as described above, and used Eq. 3 with a fixed CS and γ50 taken from the fit of the chemoradiation data with X-rays. In fact, we assume that CPT has an impact on the D50 due to the putative improved dose distribution in the target and to the radiobiological properties beyond the calculated RBE used in the Gy(RBE).
Results
Single treatment data
Chemoradiation is generally considered the best standard of cure for LAUPC. For this reason, only a few studies are available with radiotherapy alone, and some of them are old (Table 1). Some recent studies using SBRT have been excluded. An initial trial in Stanford using high-dose (25 Gy) single-fraction reports a 100% survival at 1 year, but this was limited to six patients (25). Later results from Stanford using SBRT are included in Table 2. On the other hand, a Danish study using 45 Gy in three fractions gave very low OS and high toxicity (26). This study was also excluded in our analysis, because these poor outcomes were likely a result of inaccurate positioning, lack of effective motion management techniques, and lack of dose constraints for OARs (27).
Table 1.
Clinical data for treatment of LAUPC using X-ray radiotherapy alone.
| Reference | Year | Total dose (Gy) | Fractions | Sample size | 1 year OS | 2 years OS | Median OS |
|---|---|---|---|---|---|---|---|
| Moertel et al. (38) | 1969 | 35–40 | 20 | 28 | 7% | N/A | N/A |
| Moertel et al. (39) | 1981 | 60 | 30 | 25 | 10% | N/A | 5.3 months |
| Ceha et al. (40) | 2000 | 70–72 | 35–36 | 44 | 39% | N/A | 10 months |
| Cohen et al. (41) | 2005 | 59.4 | 33 | 49 | 20% | N/A | 7.1 months |
| Wang et al. (42) | 2015 | 46 | 23 | 14 | 35% | 14% | 7.4 months |
Table 2.
Clinical data for treatment of LAUPC using X-ray therapy plus gemcitabine.
| Reference | Year | Total dose (Gy) | Fractions | Chemotherapy | Sample size | 1 year OS | 2 years OS | Median OS (months) |
|---|---|---|---|---|---|---|---|---|
| Wolff et al. (43) | 2001 | 30 | 10 | Gem, 350–500 mg/m2/week for 7 weeks | 18 | 66% | N/A | 6 |
| Epelbaum et al. (44) | 2002 | 50.4 | 28 | Gem, 1000 mg/m2 weekly before and after RT, Gem 400 mg/m2 weekly during RT | 20 | 30% | N/A | N/A |
| Joensuu et al. (45) | 2004 | 50.4 | 28 | Gem, 20/50/100 mg/m2 twice weekly before RT | 28 | 55% | N/A | 25 |
| Okusaka et al. (46) | 2004 | 50.4 | 28 | Gem, 250 mg/m2 weekly + maintenance 1000 mg/m2 weekly for 3 weeks every 4 weeks | 38 | 28% | 23% | 9.5 |
| Murphy et al. (47) | 2007 | 36 | 15 | Gem, 1000 mg/m2 on days 1, 8, and 15 | 74 | 46% | 13% | 11.2 |
| Small et al. (48) | 2008 | 36 | 15 | Gem, 1000 mg/m2 2–3 times/week before, during, and after RT treatment | 14 | 47% | N/A | N/A |
| Igarashi et al. (49) | 2008 | 40–50.4 | 20–28 | Gem, 40 mg/m2 twice/week + maintenance 1000 mg/m2 for 3 weeks | 15 | 60% | N/A | 15 |
| Schnellenberg et al. (50) | 2008 | 25 | 1 | Gem, 1000 mg/m2 weekly for 3 weeks before RT + maintenance weekly | 16 | 50% | N/A | 11.4 |
| Polistina et al. (51) | 2010 | 30 | 3 | Gem, 1000 mg/m2 weekly for 6 weeks before RT + maintenance weekly | 23 | 39.1% | 0% | 10.6 |
| Loehrer et al. (52) | 2011 | 50.4 | 28 | Gem, 600 mg/m2 weekly before and during RT | 34 | 50% | 12% | 11.1 |
| Schnellenberg et al. (53) | 2011 | 25 | 1 | Gem, 1000 mg/m2 weekly before and after RT | 20 | 50% | 20% | 11.8 |
| Cardenes et al. (54) | 2011 | 50.4 | 28 | Gem, 600 mg/m2 weekly before and during RT + maintenance 1000 mg/m2 weekly | 28 | 30% | 11% | 10.3 |
| Shibuya et al. (55) | 2011 | 54 | 30 | Gem, 250 mg/m2 weekly during RT + maintenance 1000 mg/m2 every 4 weeks (discretional) | 21 | 74% | N/A | 16.6 |
| Mahadevan et al. (56) | 2011 | 24–36 | 3 | Gem, 1000 mg/m2 weekly before, during, and after RT (at least 6 cycles) | 39 | 72% | 33% | 20 |
| Huang et al. (57) | 2011 | 50.4–63 | 28–35 | Gem, 1000 mg/m2 weekly during RT + induction/adjuvant (discretional) | 55 | 51% | N/A | 12.5 |
| Mukherjee et al. (58) | 2013 | 50.4 | 28 | Gem, induction 300 mg/m2 + concurrent 1000 mg/m2 | 38 | 64.2% | N/A | 13.4 |
| Gurka et al. (59) | 2013 | 25 | 5 | Gem, 1000 mg/m2 weekly before and after RT | 10 | 50% | N/A | 12.2 |
| Herman et al. (60) | 2014 | 33 | 5 | Gem, 1000 mg/m2 weekly before and after RT | 49 | 59% | 18% | 13.9 |
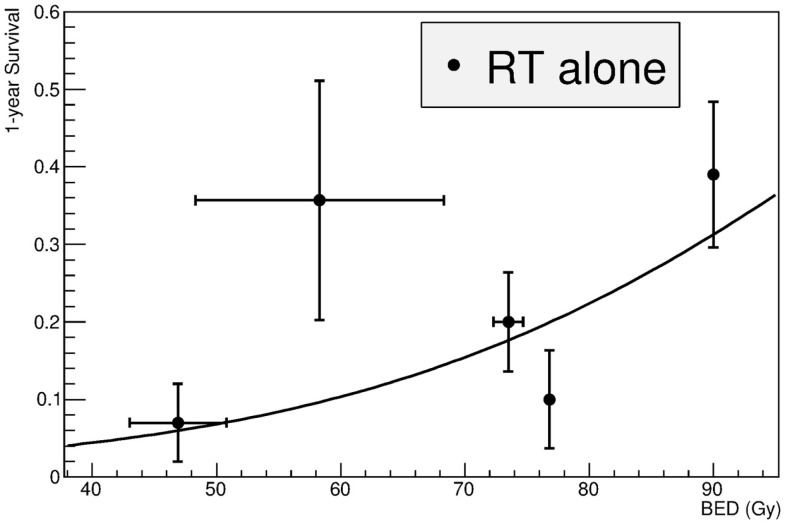
The data are plotted in Figure 2, along with the fit using Eq. 2. Fitting parameters are reported in Table 2. The D50 = 107 Gy clearly shows how impractical is the treatment of LAUPC with X-rays alone. For comparison, Dale et al. (16) estimated a BED at 50% complete response for bladder cancer of 54.4 Gy. From the analysis of the trials using chemotherapy alone (10), an average 1-year survival below 20% can be estimated.
Figure 2.
Fit of the clinical data for treatment of LAUPC with X-ray radiotherapy alone. Studies are listed in Table 1. BED is calculated by Eq. 4. Fitting was performed by Eq. 2 and fitting parameters are in Table 3.
Chemoradiation
Meta-analysis of the clinical data has already shown an advantage in chemoradiation compared to radiotherapy or chemotherapy alone (10). Most clinical trials for LAUPC resort to chemoradiation protocols. Gemcitabine (Table 3) is often regarded as the standard treatment. Several other drugs, such as capecitabine, fluorouracil (5-FU), cisplatin, docetaxel, cetuximab, and fluoropyrimidine prodrug S-1, have been used in the past or in new trials (Table 4), and often combination of gemcitabine and any of the other drugs (Table 5) are applied. The standard X-ray course is 50.4 Gy in 1.8 Gy/fraction, giving a BED of 63 Gy. We did not find significant differences in the groups treated with different drugs, considering the very high scatter of the data also due to the completely different protocols adopted. Figure 3 shows, for example, a comparison of the data in Tables 3 and 4, pointing only to a slight trend for better results in protocols using gemcitabine compared to other drugs. Figure 4 shows the fit of all the data compared to X-rays alone. Having fixed the γ50 and D50 parameters, we estimated the only parameter CS = 0.36 ± 0.01 (Table 2). The radiation dose corresponding to this survival probability RS = CS can be estimated by Eq. 2 as
| (6) |
leading to a chemo-equivalent dose of 94 Gy. This high value underlines the large improvement that chemotherapy gives on the survival of LAUPC patients. Dale and co-workers (16) estimated 43.6 Gy for the BED chemo-equivalent in bladder cancer. They also demonstrated that the chemo-equivalent dose is not a constant and will be of course much lower if we calculate it for a higher survival level.
Table 3.
| Dataset | Table | γ50 | D50 [Gy or Gy(RBE)] | Chemotherapy survival rate (CS) | Figure |
|---|---|---|---|---|---|
| Radiotherapy (X-rays) alone | 1 | 1.2 ± 0.5 | 107 ± 16 | N/A | 2 |
| Radiotherapy (X-rays) + gemcitabine | 2 | 1.2 (fixed) | 107 (fixed) | 0.39 ± 0.03 | 3 |
| Radiotherapy (X-rays) + chemotherapy other than gemcitabine | 4 | 1.2 (fixed) | 107 (fixed) | 0.32 ± 0.02 | 3 |
| Radiotherapy (X-rays) + chemotherapy (all protocols combined) | 3–5 | 1.2 (fixed) | 107 (fixed) | 0.36 ± 0.01 | 4 |
| CPT + chemotherapy | 6 | 1.2 (fixed) | 75 ± 9 | 0.36 (fixed) | 5 |
Table 4.
Clinical data for treatment of LAUPC using X-ray therapy plus chemotherapy, excluding the trials with gemcitabine.
| Reference | Year | Total dose (Gy) | Fractions | Chemotherapy | Sample size | 1 year OS | 2 years OS | Median OS (months) |
|---|---|---|---|---|---|---|---|---|
| Moertel et al. (39) | 1981 | 40 | 20 | 5-FU 500 mg/m2 3 days/week during RT, maintenance 5-FU 500 mg/m2 weekly | 83 | 46% | N/A | 11.4 |
| 60 | 30 | 85 | 35% | N/A | 8.4 | |||
| Wagener et al. (61) | 1996 | 40 | 20 | Epirubicin + Cisplatin + 5-FU | 53 | 49% | N/A | 10.8 |
| Ishii et al. (62) | 1997 | 50.4 | 28 | 5-FU 500 mg/m2 3 days/week during RT | 20 | 41.8% | N/A | 10.3 |
| Fisher et al. (63) | 1999 | 45 | 25 | 5-FU 150–250 mg/m2 continuous infusion 24 h/day during RT | 25 | 32% | N/A | 9 |
| Andre et al. (64) | 2000 | 45 | 25 | 5-FU 375 mg/m2 + Cisplatin 15 mg/m2 daily during RT (first and last week) + maintenance after RT | 32 | 31% | 12.5% | 9 |
| Boz et al. (65) | 2001 | 59.4 | 33 | 5-FU 150–300 mg/m2 continuous infusion 24 h/day during RT | 42 | 30% | N/A | 9.1 |
| Safran et al. (66) | 2001 | 50.4 | 28 | Paclitaxel 50 mg/m2 weekly during RT | 44 | 30% | N/A | 8 |
| Li et al. (67) | 2003 | 50.4–61.2 | 28–34 | 5-FU 500 mg/m2 for 3 days every 2 weeks during RT, Gem 1000 mg/m2 after RT | 16 | 31% | 0% | 6.7 |
| Morganti et al. (68) | 2004 | 39.6–59.4 | 22–33 | 5-FU 1000 mg/m2 during RT at days 1–4 and 21–24 | 50 | 31.3% | N/A | N/A |
| Cohen et al. (41) | 2005 | 59.4 | 33 | 5-FU 1000 mg/m2 at days 1–4 and 21–24 + Mitomycin 10 mg/m2 at day 2 during RT | 55 | 31% | N/A | 8.4 |
| Park et al. (69) | 2006 | 20 | 10 | 5-FU 500 mg/m2 for 3 days twice during RT with 2 weeks break | 56 | 37% | 14.6% | 10.4 |
| Chauffert et al. (70) | 2008 | 60 | 30 | 5-FU 300 mg/m2 5 days/week for 6 weeks + Cisplatin 20 mg/m2 5 days/week on weeks 1 and 5, maintenance Gem 1000 mg/m2 weekly | 59 | 32% | N/A | 8.6 |
| Crane et al. (71) | 2009 | 50.4 | 28 | Capecitabine 825 mg/m2 twice daily + Bevacizumab 5 mg/kg on days 1, 15, and 29; maintenance Gem 1000 mg/m2 weekly + Bevacizumab 5 mg/kg every 2 weeks | 82 | 47% | N/A | 11.9 |
| Sudo et al. (72) | 2011 | 50.4 | 28 | S-1 80 mg/m2 daily during and after RT | 34 | 70.6% | N/A | 16.8 |
| Oberic et al. (73) | 2011 | 54 | 30 | Docetaxel 20 mg/m2 weekly + 5-FU 200 mg/m2 daily during RT | 20 | 40% | N/A | 10 |
| Brunner et al. (74) | 2011 | 55.8 | 33 | 5-FU 1000 mg/m2 on days 1–5 and 29–33 + Mitomycin 10 mg/m2 on days 1–29 during RT | 35 | 40% | N/A | 9.7 |
| Huang et al. (57) | 2011 | 50.4–63 | 28–35 | 5-FU 200–300 mg/m2 5 days/week or 5-FU 500 mg/m2 on days 1–3 and 29–31 or capecitabine 1300–1600 mg/m2 daily during RT | 38 | 24% | N/A | 10.2 |
| Malik et al. (75) | 2012 | 50.4 | 28 | 5-FU based during RT* | 84 | 52.6% | N/A | 10.9 |
| Ikeda et al. (76) | 2012 | 50.4 | 28 | S-1 80 mg/m2 twice daily during RT, maintenance S-1 80 mg/m2 daily after RT | 60 | 72% | N/A | 16.2 |
| Schinchi et al. (77) | 2012 | 50 | 40 | S-1 80 mg/m2 twice daily during and after RT | 50 | 62% | 27% | 14.3 |
| Mukherjee et al. (58) | 2013 | 50.4 | 28 | Capecitabine 830 mg/m2 5 days/week induction and concurrent to RT | 36 | 79.2% | N/A | 13.4 |
| Herman et al. (78) | 2013 | 50.4 | 28 | 5-FU 200 mg/m2 daily during RT, maintenance Gem 1000 mg/m2 weekly | 90 | 36.7% | 10.3% | 10 |
| 5-FU 200 mg/m2 daily + TNFerade weekly during RT, maintenance Gem 1000 mg/m2 weekly | 187 | 41% | 11.3% | 10 | ||||
| Ducreaux et al. (79) | 2014 | 54 | 30 | Docetaxel 20 mg/m2 + Cisplatin 20 mg/m2 weekly during RT | 51 | 41% | 31% | 9.6 |
| Rembielak et al. (80) | 2014 | 50.4 | 28 | Cetuximab loading dose 400 mg/m2 + 250 mg/m2 weekly during RT | 21 | 33% | 11% | 7.5 |
| Kwak et al. (81) | 2014 | 50.4 | 28 | 5-FU 600–1000 mg/m2 during RT, maintenance Gem 200 mg/m2 weekly | 34 | 40% | 10% | 9 |
*Limited information about chemotherapy.
Table 5.
Clinical data for treatment of LAUPC using X-ray therapy plus a chemotherapy cocktail including gemcitabine.
| Reference | Year | Total dose (Gy) | Fractions | Chemotherapy | Sample size | 1 year OS | 2 years OS | Median OS |
|---|---|---|---|---|---|---|---|---|
| Chung et al. (82) | 2004 | 45 | 25 | Gem 1000 mg/m2 weekly + Doxifluoridine 600 mg/m2 daily during and after RT | 22 | 50% | N/A | 12 |
| Haddock et al. (83) | 2007 | 45 | 25 | Gem 30 mg/m2 + Cisplatin 10 mg/m2 twice weekly during first 3 weeks of RT, Gem 1000 mg/m2 weekly after RT | 48 | 40% | N/A | 10.2 |
| Hong et al. (84) | 2008 | 45 | 25 | Gem 1000 mg/m2 weekly + Cisplatin 70 mg/m2 two times during RT, maintenance Gem 1000 mg/m2 weekly + Cisplatin 70 mg/m2 every 4 weeks | 38 | 63.3% | 27.9% | 16.7 |
| Mamon et al. (85) | 2011 | 50.4 | 28 | Gem 200 mg/m2 weekly + 5-FU 200 mg/m2 5 days/week during RT, maintenance Gem 1000 mg/m2 weekly | 78 | 51% | N/A | 12.2 |
| Crane et al. (86) | 2011 | 50.4 | 28 | Gem 1000 mg/m2 + Oxaliplatin 100 mg/m2 before RT + Capecitabine 825 mg/m2 twice daily on RT days, cetuximab 500 mg/m2 every 2 weeks before and during RT | 69 | 66% | 25% | 19.2 |
| Brunner et al. (74) | 2011 | 55.8 | 31 | Gem 300 mg/m2 + Cisplatin 30 mg/m2 weekly during RT | 58 | 53% | N/A | 12.7 |
| Ch’Ang et al. (87) | 2011 | 50.4 | 28 | Gem 800 mg/m2 + Oxaliplatin 85 mg/m2 + 5-FU/Leucovorin 3000/150 mg/m2 twice/week before RT, Gem 400 mg/m2 weekly during RT | 50 | 68% | 20.6% | 14.5 |
| Tozzi et al. (88) | 2013 | 45 | 6 | Gem-based before RT | 30 | 47% | N/A | 11 |
| Ke et al. (89) | 2014 | 50.4 | 28 | Gem 1000 mg/m2 weekly + S-1 40 mg/m2 twice daily before RT, S-1 80 mg/m2 twice daily during RT, S-1 80 mg/m2 twice daily 1 month after RT | 32 | 75% | 34.4% | 15.2 |
| Wang et al. (42) | 2015 | 46 | 23 | Gem-based (sub-groups) | 16 | 71.1% | 40.6% | 19.5 |
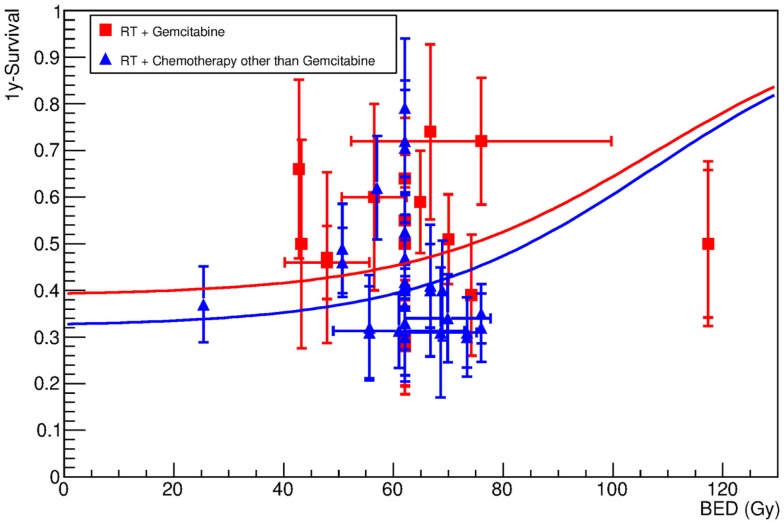
Figure 3.
One-year survival as a function of the BED for patients undergoing X-ray radiotherapy plus gemcitabine (red symbols), or other chemotherapy drugs (blue symbols). Data are reported in Tables 2 and 4. The lines show the result of the fit (Eq. 3), which was performed assuming that γ50 and D50 are obtained by fitting the data in treatments using radiotherapy only (Figure 1). The only free fitting parameter is the chemotherapy survival CS (see Table 3). The results suggest that the final outcome does not strongly depend on the specific chemotherapy treatment, although some advantage seems to be associated to the use of gemcitabine.
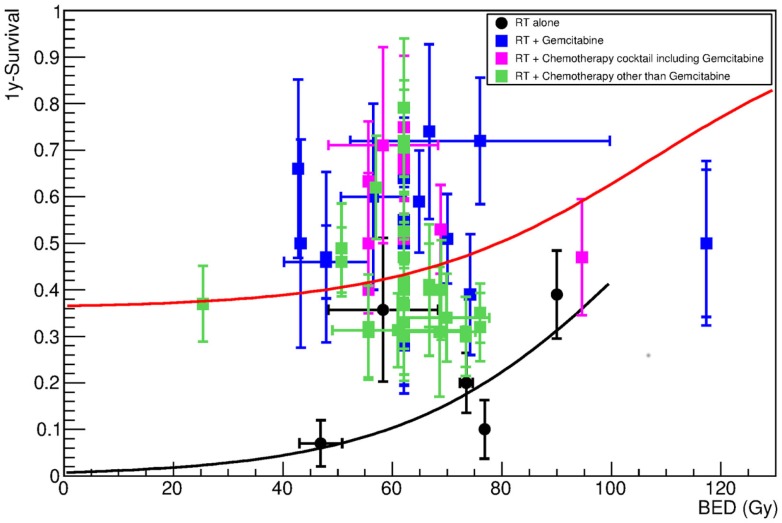
Figure 4.
One-year survival as a function of the BED for patients undergoing X-ray radiotherapy alone (black symbols), or in combination with any chemotherapy treatments. Details about chemotherapy regimen are reported in Tables 4–6. The lines show the result of the fit (black for radiotherapy-alone data, red for all chemotherapy data pooled together), which was performed assuming that γ50 and D50 are obtained by fitting RT-alone data. Fitting parameters with Eq. 3 are in Table 3.
Charged particle therapy
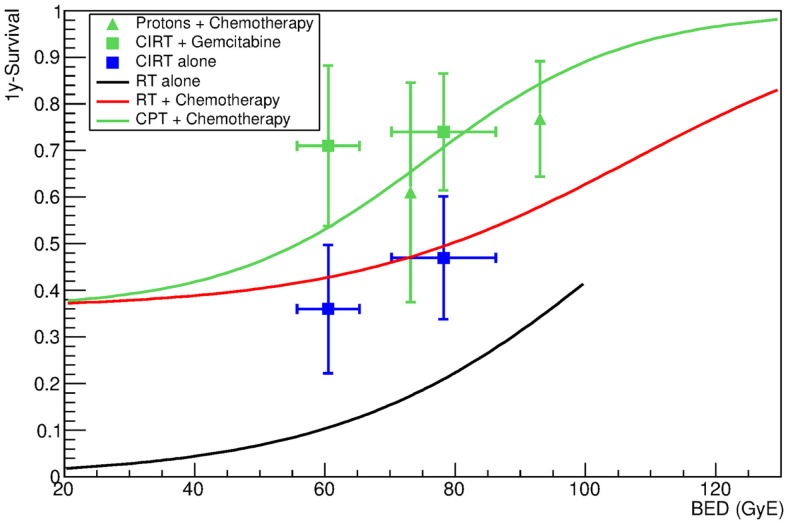
Although only a few studies are available with CPT, the data in Table 6 show that they are the best current options for LAUPC. A 2-year survival rate around 50% was reached with protons (28) or C-ions (9) in combination with gemcitabine, a value far exceeding any other chemoradiation trial using X-rays and any cocktail of drugs. The data with CIRT alone (no chemotherapy) are clearly superior to those with X-rays alone and comparable to the results with chemoradiation at the same X-rays BED. The best 1-year OSs for combined chemotherapy (gemcitabine) and CPT are those from Hyogo (28) using protons up to 70.2 Gy(RBE) in 26 fractions, but they came at a cost of grade 3–5 toxicity in 10% of the patients, especially gastric ulcer and hemorrhage. CIRT toxicity was much more mild, with 17% of the patients experiencing grade 3 GI toxicity, in the form of appetite loss. Low toxicity was observed for the duodenum, both for protons and 12C-ions. The fit of the chemoradiation with CPT, using the same CS and γ50 parameters calculated for X-rays + chemotherapy, is shown in Figure 5. This fit assumes that CPT does not change the effect of the chemotherapy compared to X-rays, but results in a lower D50 due to biological and/or physical improvements compared to X-rays. Should these improvements be already included in the RBE model used to calculate the equivalent dose in Gy(RBE), we should see the same effect at the same BED [see Ref. (23) for CIRT in Japan; RBE = 1.1 for protons]. Instead, the best fit is reduced to D50 = 75 ± 9 Gy(RBE) for CPT (Table 2). This 50% improvement is caused either by a better physics, enabling treatment of infiltrations in the neuroplexus, or to a better biology, especially to a reduced OER (6) or to a stronger immune response (7) using CPT compared to X-rays.
Table 6.
Clinical data for treatment of LAUPC using CPT.
| Reference | Year | Radiation quality | Total dose in Gy (RBE) | Fractions | Chemotherapy | Sample size | 1 year OS | 2 years OS | Median OS |
|---|---|---|---|---|---|---|---|---|---|
| Terashima et al. (28) | 2012 | Protons | 67.5 | 25 | Gem, 800 mg/m2/week for 3 weeks | 50 | 76.8% | 50% | N/A |
| Sachsman et al. (90) | 2014 | Protons | 59.4 | 33 | Capecitabine, 1000 mg twice/day; 5 days/week on radiation treatment days only | 11 | 61% | 31% | 18.4 |
| Yamada et al. (9) | 2014 | Carbon ions | 38.4–43.2 | 12 | – | 19 | 36% | 5% | N/A |
| Gem 1000 mg/m2/week for 3 weeks | 24 | 71% | 21% | N/A | |||||
| 45.6–52.8 | 12 | – | 27 | 47% | 16% | N/A | |||
| Gem 1000 mg/m2/week for 3 weeks | 47 | 74% | 54% | N/A |
Figure 5.
One-year survival as a function of the BED for patients undergoing CPT with or without additional chemotherapy. Blue symbols refer to patients receiving radiotherapy with C-ions without additional chemotherapy. Green symbols refer to data obtained with proton (triangles) and carbon ions (full squares) in combination with chemotherapy. Data are given in Table 6. The green line shows the result of the fit of data for chemotherapy combined with proton or carbon ions. The fit was performed using γ50 and CS from X-ray + chemotherapy data. The only free parameter is therefore D50. The black and red lines show the results of the fit for X-rays alone and X-rays plus chemotherapy, and are reported for comparison. Fitting parameters are in Table 3.
Discussion
The large interest for the use of CPT in LAUPC comes from the exceptional clinical results (8), supported by our clinical data analysis in Figure 5. These results reflect the biological rationale of reduced OER for high-LET radiation and possible dose escalation with limited side effects exploiting the Bragg peak. The high GI toxicity observed in the Hyogo trial (28) seems to set a threshold at a BED around 100 Gy(RBE). The question is whether the same threshold applies to CIRT, where the sharper dose edges of the treatment plan may reduce the exposure of the critical organs compared to protons, whose lateral scattering is much higher than for heavy ions (6). An example of a treatment plan of a pancreatic head cancer with carbon ions is shown in Figure 6. It is possible to give a high-dose against tumor and neuroplexus with acceptable doses to stomach or duodenum. The dose distribution can further improve using raster scanning instead of passive modulation, as shown in Figure 7. The new NIRS facility is now equipped with raster scanning, and so are the HIT and CNAO facilities now treating the first LAUPC patients with C-ions. Under these optimal conditions, it appears feasible to exceed a BED of 100 Gy(RBE) with acceptable toxicities.
Figure 6.
A typical treatment plan used at NIRS for a locally advanced pancreatic head cancer. The beam is shaped with passive modulation and four opposite fields are applied with respiratory gating. GTV includes the primary tumor and lymph nodes involved. CTV = PTV + neuroplexus infiltration (periarterial area) + proximal lymph nodes. PTV = CTV + 5 mm, excluding GI tract.
Figure 7.
Comparison of the current passive beam modulation treatment plan with a spot scanning treatment plan for LAUPC. In the right panel, the dose–volume histogram for different organs is shown for passive modulation (dotted line) and raster scanning (solid line). Dose to the spinal cord and kidney are highly reduced. Potential reduction is also clear for stomach and duodenum, whose movements are, however, critical.
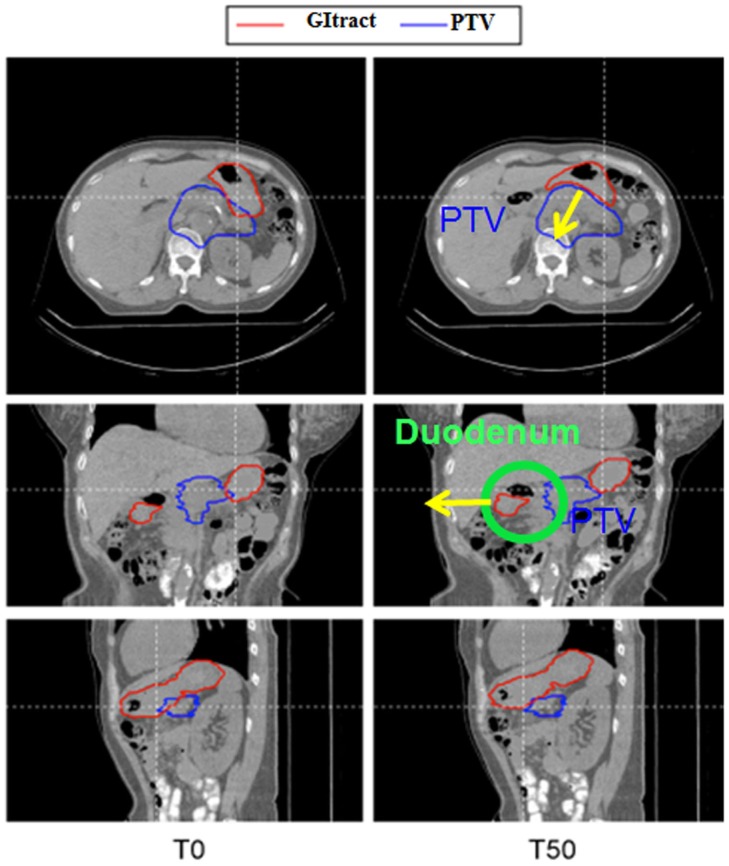
Modeling chemotherapy in terms of equivalent radiation dose is an effective method to predict outcomes of dose-escalation trials (12, 16). The large scatter in the chemoradiation data leads, however, to a poor goodness-of-fit in Figures 3 and 4. This is due in part to the many different protocols used in chemotherapy for LAUPC, and to inclusion of data published in over 30 years using very different methods both for drug and radiation delivery. In this paper, we have decided to analyze all the data available in the literature, without including the treatment year as a function in the model. We have also assumed no synergistic interaction between chemicals and radiation. Finally, Eq. 4 should be modified for protons or carbon ions, where α/β is higher than for X-rays leading to a lower dependence on fractionation. Due to the lack of sufficient information leading to an educated guess for other parameters and models, we decided to stick to the conventional logistic function, replacing Gy with Gy(RBE) in Table 6. The basic assumption remains that a higher BED will result in a higher OS in LAUPC patients, an assumption clearly supported by the analysis of the several trials included in our data mining. Our analysis supports the concept that a dose escalation will improve OS, and toxicity is the limiting factor. In Table 7, we have calculated with the logistic model (Eq. 3) the expected survival in hypofractionated dose-escalation trials and compared with the standard chemoradiation treatment and other schedules proposed for SBRT using X-rays (15, 27). The standard at NIRS is 12 fractions in 3 weeks, and with the current maximum dose/fraction the OS at 1 year is expected to improve from 40 to 70% compared to the standard X-ray regime (50.4 Gy in 28 fractions). Reaching 18 fractions with the same dose/fraction, it could be possible to double the survival. Further hypofractionation, down to a single dose of 25 Gy(RBE) is very attractive in terms of expected survival, but raises concerns for the GI toxicity. C-ions delivered by raster scanning should provide the optimal dose distributions (Figure 7) compared to CIRT with passive scattering and protons, where the lateral scattering unavoidably leads to a dose penumbra around the PTV. However, for beam scanning, the issue of motion mitigation must be tackled very carefully, because of the known problem of the interplay. Currently, NIRS is using respiratory gating to compensate especially the movements of stomach and duodenum in the PTV (Figure 8). A treatment with high number of fractions compensates the interplay between beam scanning and organ motion, but this compensation is lost in radiosurgery (29). In the treatment of hepatocellular carcinoma with 12C-ions at the HIT facility in Heidelberg, it has been shown that the simple increase from 1 to 4 fractions substantially improved the dose target coverage and reduced overdosage (V107 from 32 to 4%) (30), this means that keeping the hypofractionation schemes above 4 fractions, major inhomogeneities should be avoided. Nevertheless, the range uncertainties due to bowel movement, stomach peristalsis, and breathing, have to be solved to reduce toxicity to the many critical organs surrounding the pancreas. Motion mitigation strategy include respiratory gating or layer stacking boost irradiation, such as used at NIRS for treating PC (31), and 4D optimization of the plan based on 4DCT (32). Patients with tumors in a favorable location, preferably >1 cm from the closest luminal organ, should be selected for the dose escalation.
Table 7.
Expected improvement in survival according to our model in chemoradiation trials using CPT.
| Dose/fraction inGy or Gy(RBE) | Radiation quality | Fractions | Total dose in Gy or Gy(RBE) | BED in Gy or Gy(RBE) | Expected 1 year survival rate | Comments |
|---|---|---|---|---|---|---|
| 1.8 | X-rays | 28 | 50.4 | 62.9 | 42% | Current standard fractionation scheme |
| 2.25 | X-rays | 33 | 74.3 | 97.8 | 61% | Proposed dose-escalation trial at Medical College of Winsconsin (15) |
| 6.6 | X-rays | 5 | 33 | 65.2 | 45% | Standard for SBRT in adjuvant settings (27) |
| 2.7 | Protons | 26 | 70.2 | 97.4 | 75% | Maximum dose reached at Hyogo |
| 4.6 | C-ions | 12 | 55.2 | 92.5 | 71% | Maximum dose reached at NIRS |
| 5.85 | C-ions or protons | 12 | 70.2 | 130.6 | 82% | Maximum total dose reached with protons in Hyogo using the number of fraction from NIRS |
| 25 | C-ions or protons | 1 | 25 | 117.5 | 76% | Maximum dose used in single-fraction X-ray radiosurgery for LAUPC (27) |
| 4.6 | C-ions or protons | 18 | 82.8 | 138.6 | 84% | Expected doubling of the OS with conventional X-ray fractionation scheme, using the dose/fraction from NIRS |
Figure 8.
4DCT analysis of the movement of the critical organs during treatment of LAUPC at NIRS with C-ions. T0 is the peak inhalation and T50 the peak exhalation phases. Stomach and duodenum move in and out the PTV in the two phases.
The solution of this problem is an important step to push toward higher doses and fewer fractions thus leading to a substantial improvement in survival can be expected using chemoradiation protocols with CPT rather than X-rays. The first clinical CIRT vs. IMRT trial for LAUPC should compare the standard chemoradiation treatment (Table 7, row 1), with the NIRS most advanced protocol (Table 7, row 5). The additional advantage of using the standard protocols is that at the dose/fraction of 4.6 Gy(RBE) reached in the escalation trial at NIRS, there is practically no difference between the biological dose calculated at NIRS and those predicted by LEM (24) and implemented in European CIRT facilities. However, in a multi-centric trial, it will be unavoidable to have different systems for dose delivery, motion management, patient selection, etc. For instance, NIRS is using passive modulation, CNAO raster scanning, and HIT can use the gantry. Nevertheless, a comparative trial for LAUPC is absolutely necessary to support the use of CIRT and to confirm the very promising data in the phase I–II trials at NIRS (8). The lack of comparative, phase-III clinical trials is generally considered as a major hindrance to a more widespread use of CPT in the clinics (33). A trial on LAUPC may definitely clarify the clinical advantage of CPT in such a lethal tumor.
Apart from the international comparative trial, further developments of phase-II trials with CPT should point to two directions. First, several molecular markers, such as mutations in SMAD4/DPC4, have been validated as prognostic factors in PCs (34). Whole-genome sequencing and copy number variation analysis suggest that PCs can be divided into four genetic subtypes, with potential clinical utility (35). Trials with CPT combined with molecular analysis of these genes are highly needed, because CPT may elicit different molecular pathways than conventional X-rays (36). Combined CIRT + gemcitabine may be especially effective against pancreatic stem-like cells, as suggested by a recent in vitro study (37), and hence, study of stem cells markers and genetic pathways will be highly desirable. In addition, further hypofractionation is desirable if the problems of the organ movements are tackled as described above. For instance, the use of 12 fractions (such as done at NIRS) with the total dose used for protons in Hyogo is expected to push the 1-year survival over 80% (Table 7, row 6). A careful motion mitigation strategy should be rapidly implemented to allow this further escalation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The work was partly supported by the Portfolio Technologie und Medizin, Helmholtz Gemeinschaft and INFN-TIFPA (Trento).
Footnotes
1Solicitation number BAA-N01CM51007-51 (April 17, 2015) available online through FedBizOpps at http://www.fbo.gov
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin (2015) 65:5–29. 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 2.Malvezzi M, Bertuccio P, Rosso T, Rota M, Levi F, La Vecchia C, et al. European cancer mortality predictions for the year 2015: does lung cancer have the highest death rate in EU women? Ann Oncol (2015) 26:779–86. 10.1093/annonc/mdv001 [DOI] [PubMed] [Google Scholar]
- 3.Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, et al. Recent progress in pancreatic cancer. CA Cancer J Clin (2013) 63:318–48. 10.3322/caac.21190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koong AC, Mehta VK, Le QT, Fisher GA, Terris DJ, Brown JM, et al. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys (2000) 48:919–22. 10.1016/S0360-3016(00)00803-8 [DOI] [PubMed] [Google Scholar]
- 5.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science (2009) 324:1457–61. 10.1126/science.1171362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loeffler JS, Durante M. Charged particle therapy – optimization, challenges and future directions. Nat Rev Clin Oncol (2013) 10:411–24. 10.1038/nrclinonc.2013.79 [DOI] [PubMed] [Google Scholar]
- 7.Durante M, Reppingen N, Held KD. Immunologically augmented cancer treatment using modern radiotherapy. Trends Mol Med (2013) 19:565–82. 10.1016/j.molmed.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 8.Kamada T, Tsujii H, Blakely EA, Debus J, De Neve W, Durante M, et al. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol (2015) 16:e93–100. 10.1016/S1470-2045(14)70412-7 [DOI] [PubMed] [Google Scholar]
- 9.Yamada S, Terashima K, Shinoto M, Yasuda S, Shiomi M, Isozaki T. Pancreatic cancer. In: Tsujii H, Kamada T, Shirai K, Noda K, Tsuji H, Karasawa K, editors. Carbon Ion Radiotherapy. Berlin: Springer-Verlag; (2014). p. 221–8. [Google Scholar]
- 10.Chen Y, Sun X-J, Jiang T-H, Mao A-W. Combined radiochemotherapy in patients with locally advanced pancreatic cancer: a meta-analysis. World J Gastroenterol (2013) 19:7461–71. 10.3748/wjg.v19.i42.7461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler JF. 21 years of biologically effective dose. Br J Radiol (2010) 83:554–68. 10.1259/bjr/31372149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones B, Dale RG. The potential for mathematical modelling in the assessment of the radiation dose equivalent of cytotoxic chemotherapy given concomitantly with radiotherapy. Br J Radiol (2005) 78:939–43. 10.1259/bjr/40226390 [DOI] [PubMed] [Google Scholar]
- 13.Bentzen SM, Tucker SL. Quantifying the position and steepness of radiation dose-response curves. Int J Radiat Biol (1997) 71:531–42. 10.1080/095530097143860 [DOI] [PubMed] [Google Scholar]
- 14.Plataniotis G, Dale RG. Radio-chemotherapy for bladder cancer: contribution of chemotherapy on local control. World J Radiol (2013) 5:267–74. 10.4329/wjr.v5.i8.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moraru IC, Tai A, Erickson B, Li XA. Radiation dose responses for chemoradiation therapy of pancreatic cancer: an analysis of compiled clinical data using biophysical models. Pract Radiat Oncol (2014) 4:13–9. 10.1016/j.prro.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 16.Plataniotis G, Dale RG. Assessment of the radiation-equivalent of chemotherapy contributions in 1-phase radio-chemotherapy treatment of muscle-invasive bladder cancer. Int J Radiat Oncol Biol Phys (2014) 88:927–32. 10.1016/j.ijrobp.2013.11.242 [DOI] [PubMed] [Google Scholar]
- 17.Pauwels B, Vermorken JB, Wouters A, Ides J, Van Laere S, Lambrechts HAJ, et al. The role of apoptotic cell death in the radiosensitising effect of gemcitabine. Br J Cancer (2009) 101:628–36. 10.1038/sj.bjc.6605145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wouters A, Pauwels B, Burrows N, Baay M, Deschoolmeester V, Vu TN, et al. The radiosensitising effect of gemcitabine and its main metabolite dFdU under low oxygen conditions is in vitro not dependent on functional HIF-1 protein. BMC Cancer (2014) 14:594. 10.1186/1471-2407-14-594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Shafie RA, Habermehl D, Rieken S, Mairani A, Orschiedt L, Brons S, et al. In vitro evaluation of photon and raster-scanned carbon ion radiotherapy in combination with gemcitabine in pancreatic cancer cell lines. J Radiat Res (2013) 54:i113–9. 10.1093/jrr/rrt052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlaich F, Brons S, Haberer T, Debus J, Combs SE, Weber K-J. Comparison of the effects of photon versus carbon ion irradiation when combined with chemotherapy in vitro. Radiat Oncol (2013) 8:260. 10.1186/1748-717X-8-260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapman JD. Can the two mechanisms of tumor cell killing by radiation be exploited for therapeutic gain? J Radiat Res (2014) 55:2–9. 10.1093/jrr/rrt111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tommasino F, Durante M. Proton radiobiology. Cancers (Basel) (2015) 7:353–81. 10.3390/cancers7010353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanai T, Matsufuji N, Miyamoto T, Mizoe J, Kamada T, Tsuji H, et al. Examination of GyE system for HIMAC carbon therapy. Int J Radiat Oncol Biol Phys (2006) 64:650–6. 10.1016/j.ijrobp.2005.09.043 [DOI] [PubMed] [Google Scholar]
- 24.Steinsträter O, Grün R, Scholz U, Friedrich T, Durante M, Scholz M. Mapping of RBE-weighted doses between HIMAC- and LEM-based treatment planning systems for carbon ion therapy. Int J Radiat Oncol Biol Phys (2012) 84:854–60. 10.1016/j.ijrobp.2012.01.038 [DOI] [PubMed] [Google Scholar]
- 25.Koong AC, Le QT, Ho A, Fong B, Fisher G, Cho C, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys (2004) 58:1017–21. 10.1016/j.ijrobp.2003.11.004 [DOI] [PubMed] [Google Scholar]
- 26.Hoyer M, Roed H, Sengelov L, Traberg A, Ohlhuis L, Pedersen J, et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol (2005) 76:48–53. 10.1016/j.radonc.2004.12.022 [DOI] [PubMed] [Google Scholar]
- 27.Moningi S, Marciscano AE, Rosati LM, Ng SK, Teboh Forbang R, Jackson J, et al. Stereotactic body radiation therapy in pancreatic cancer: the new frontier. Expert Rev Anticancer Ther (2014) 14:1461–75. 10.1586/14737140.2014.952286 [DOI] [PubMed] [Google Scholar]
- 28.Terashima K, Demizu Y, Hashimoto N, Jin D, Mima M, Fujii O, et al. A phase I/II study of gemcitabine-concurrent proton radiotherapy for locally advanced pancreatic cancer without distant metastasis. Radiother Oncol (2012) 103:25–31. 10.1016/j.radonc.2011.12.029 [DOI] [PubMed] [Google Scholar]
- 29.Bert C, Durante M. Particle radiosurgery: a new frontier of physics in medicine. Phys Med (2014) 30:535–8. 10.1016/j.ejmp.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 30.Richter D, Saito N, Chaudhri N, Härtig M, Ellerbrock M, Jäkel O, et al. Four-dimensional patient dose reconstruction for scanned ion beam therapy of moving liver tumors. Int J Radiat Oncol Biol Phys (2014) 89:175–81. 10.1016/j.ijrobp.2014.01.043 [DOI] [PubMed] [Google Scholar]
- 31.Mori S, Shinoto M, Yamada S. Four-dimensional treatment planning in layer-stacking boost irradiation for carbon-ion pancreatic therapy. Radiother Oncol (2014) 111:258–63. 10.1016/j.radonc.2014.02.014 [DOI] [PubMed] [Google Scholar]
- 32.Graeff C. Motion mitigation in scanned ion beam therapy through 4D-optimization. Phys Med (2014) 30:570–7. 10.1016/j.ejmp.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 33.Mitin T, Zietman AL. Promise and pitfalls of heavy-particle therapy. J Clin Oncol (2014) 32:2855–63. 10.1200/JCO.2014.55.1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet (2011) 378:607–20. 10.1016/S0140-6736(10)62307-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature (2015) 518:495–501. 10.1038/nature14169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durante M. New challenges in high-energy particle radiobiology. Br J Radiol (2014) 87:20130626. 10.1259/bjr.20130626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sai S, Wakai T, Vares G, Yamada S, Kamijo K, Kamada T, et al. Combination of carbon ion beam and gemcitabine causes irreparable DNA damage and death of radioresistant pancreatic cancer stem-like cells in vitro and in vivo. Oncotarget (2015) 6:5517–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moertel CG, Childs DS, Reitemeier RJ, Colby MY, Holbrook MA. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet (1969) 2(7626):865–7. 10.1016/S0140-6736(69)92326-5 [DOI] [PubMed] [Google Scholar]
- 39.Moertel CG, Frytak S, Hahn RG, O’Connell MJ, Reitemeier RJ, Rubin J, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil. The Gastrointestinal Tumor Study Group. Cancer (1981) 48:1705–10. [DOI] [PubMed] [Google Scholar]
- 40.Ceha HM, van Tienhoven G, Gouma DJ, Veenhof CH, Schneider CJ, Rauws EA, et al. Feasibility and efficacy of high dose conformal radiotherapy for patients with locally advanced pancreatic carcinoma. Cancer (2000) 89:2222–9. [DOI] [PubMed] [Google Scholar]
- 41.Cohen SJ, Dobelbower R, Lipsitz S, Catalano PJ, Sischy B, Smith TJ, et al. A randomized phase III study of radiotherapy alone or with 5-fluorouracil and mitomycin-C in patients with locally advanced adenocarcinoma of the pancreas: Eastern Cooperative Oncology Group Study E8282. Int J Radiat Oncol Biol Phys (2005) 62:1345–50. 10.1016/j.ijrobp.2004.12.074 [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Ren Z-G, Ma N-Y, Zhao J-D, Zhang Z, Ma X-J, et al. Intensity modulated radiotherapy for locally advanced and metastatic pancreatic cancer: a mono-institutional retrospective analysis. Radiat Oncol (2015) 10:4–11. 10.1186/s13014-014-0312-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolff RA, Evans DB, Gravel DM, Lenzi R, Pisters PW, Lee JE, et al. Phase I trial of gemcitabine combined with radiation for the treatment of locally advanced pancreatic adenocarcinoma. Clin Cancer Res (2001) 7:2246–53. [PubMed] [Google Scholar]
- 44.Epelbaum R, Rosenblatt E, Nasrallah S, Faraggi D, Gaitini D, Mizrahi S, et al. Phase II study of gemcitabine combined with radiation therapy in patients with localized, unresectable pancreatic cancer. J Surg Oncol (2002) 81:138–43. 10.1002/jso.10159 [DOI] [PubMed] [Google Scholar]
- 45.Joensuu TK, Kiviluoto T, Kärkkäinen P, Vento P, Kivisaari L, Tenhunen M, et al. Phase I-II trial of twice-weekly gemcitabine and concomitant irradiation in patients undergoing pancreaticoduodenectomy with extended lymphadenectomy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys (2004) 60:444–52. 10.1016/j.ijrobp.2004.03.026 [DOI] [PubMed] [Google Scholar]
- 46.Okusaka T, Ito Y, Ueno H, Ikeda M, Takezako Y, Morizane C, et al. Phase II study of radiotherapy combined with gemcitabine for locally advanced pancreatic cancer. Br J Cancer (2004) 91:673–7. 10.1038/sj.bjc.6602001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy JD, Adusumilli S, Griffith KA, Ray ME, Zalupski MM, Lawrence TS, et al. Full-dose gemcitabine and concurrent radiotherapy for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys (2007) 68:801–8. 10.1016/j.ijrobp.2006.12.053 [DOI] [PubMed] [Google Scholar]
- 48.Small W, Berlin J, Freedman GM, Lawrence T, Talamonti MS, Mulcahy MF, et al. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: a multicenter phase II trial. J Clin Oncol (2008) 26:942–7. 10.1200/JCO.2007.13.9014 [DOI] [PubMed] [Google Scholar]
- 49.Igarashi H, Ito T, Kawabe K, Hisano T, Arita Y, Kaku T, et al. Chemoradiotherapy with twice-weekly administration of low-dose gemcitabine for locally advanced pancreatic cancer. World J Gastroenterol (2008) 14:5311–5. 10.3748/wjg.14.5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schellenberg D, Goodman KA, Lee F, Chang S, Kuo T, Ford JM, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys (2008) 72:678–86. 10.1016/j.ijrobp.2008.01.051 [DOI] [PubMed] [Google Scholar]
- 51.Polistina F, Costantin G, Casamassima F, Francescon P, Guglielmi R, Panizzoni G, et al. Unresectable locally advanced pancreatic cancer: a multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Ann Surg Oncol (2010) 17:2092–101. 10.1245/s10434-010-1019-y [DOI] [PubMed] [Google Scholar]
- 52.Loehrer PJ, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol (2011) 29:4105–12. 10.1200/JCO.2011.34.8904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schellenberg D, Kim J, Christman-Skieller C, Chun CL, Columbo LA, Ford JM, et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys (2011) 81:181–8. 10.1016/j.ijrobp.2010.05.006 [DOI] [PubMed] [Google Scholar]
- 54.Cardenes HR, Moore AM, Johnson CS, Yu M, Helft P, Chiorean EG, et al. A Phase II Study of Gemcitabine in combination with radiation therapy in patients with localized, unresectable, pancreatic cancer. Am J Clin Oncol (2011) 34:460–5. 10.1097/COC.0b013e3181e9c103 [DOI] [PubMed] [Google Scholar]
- 55.Shibuya K, Oya N, Fujii T, Doi R, Nakamura A, Matsuo Y, et al. Phase II study of radiation therapy combined with weekly low-dose gemcitabine for locally advanced, unresectable pancreatic cancer. Am J Clin Oncol (2011) 34:115–9. 10.1097/COC.0b013e3181c4c7a8 [DOI] [PubMed] [Google Scholar]
- 56.Mahadevan A, Miksad R, Goldstein M, Sullivan R, Bullock A, Buchbinder E, et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys (2011) 81:615–22. 10.1016/j.ijrobp.2011.04.045 [DOI] [PubMed] [Google Scholar]
- 57.Huang J, Robertson JM, Margolis J, Balaraman S, Gustafson G, Khilanani P, et al. Long-term results of full-dose gemcitabine with radiation therapy compared to 5-fluorouracil with radiation therapy for locally advanced pancreas cancer. Radiother Oncol (2011) 99:114–9. 10.1016/j.radonc.2011.05.038 [DOI] [PubMed] [Google Scholar]
- 58.Mukherjee S, Hurt CN, Bridgewater J, Falk S, Cummins S, Wasan H, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol (2013) 14:317–26. 10.1016/S1470-2045(13)70021-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gurka MK, Collins SP, Slack R, Tse G, Charabaty A, Ley L, et al. Stereotactic body radiation therapy with concurrent full-dose gemcitabine for locally advanced pancreatic cancer: a pilot trial demonstrating safety. Radiat Oncol (2013) 8:44. 10.1186/1748-717X-8-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herman JM, Chang DT, Goodman KA, Dholakia AS, Raman SP, Hacker-Prietz A, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer (2015) 121:1128–37. 10.1002/cncr.29161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wagener DJT, Hoogenraad WJ, Rougier P, Lusinchi A, Taal BG, Veenhof CHN, et al. Results of a phase II trial of epirubicin and cisplatin (EP) before and after irradiation and 5-fluorouracil in locally advanced pancreatic cancer: an EORTC GITCCG study. Eur J Cancer (1996) 32:1310–3. 10.1016/0959-8049(96)00070-6 [DOI] [PubMed] [Google Scholar]
- 62.Ishii H, Okada S, Tokuuye K, Nose H, Okusaka T, Yoshimori M, et al. Protracted 5-fluorouracil infusion with concurrent radiotherapy as a treatment for locally advanced pancreatic carcinoma. Cancer (1997) 79:1516–20. [DOI] [PubMed] [Google Scholar]
- 63.Fisher BJ, Perera FE, Kocha W, Tomiak A, Taylor M, Vincent M, et al. Analysis of the clinical benefit of 5-fluorouracil and radiation treatment in locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys (1999) 45:291–5. 10.1016/S0360-3016(99)00197-2 [DOI] [PubMed] [Google Scholar]
- 64.André T, Balosso J, Louvet C, Hannoun L, Houry S, Huguier M, et al. Combined radiotherapy and chemotherapy (cisplatin and 5-fluorouracil) as palliative treatment for localized unresectable or adjuvant treatment for resected pancreatic adenocarcinoma: results of a feasibility study. Int J Radiat Oncol Biol Phys (2000) 46:903–11. 10.1016/S0360-3016(99)00478-2 [DOI] [PubMed] [Google Scholar]
- 65.Boz G, De Paoli A, Innocente R, Rossi C, Tosolini G, Pederzoli P, et al. Proceedings of the 25th National Congress of the Italian Association of Pancreatology Study (AISP). Cernobbio, Como (Italy). September 20-22, 2001. JOP (2001) 2(5 Suppl):330–54. [PubMed] [Google Scholar]
- 66.Safran H, Moore T, Iannitti D, Dipetrillo T, Akerman P, Cioffi W, et al. Paclitaxel and concurrent radiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys (2001) 49:1275–9. 10.1016/S0360-3016(00)01527-3 [DOI] [PubMed] [Google Scholar]
- 67.Li C-P, Chao Y, Chi K-H, Chan W-K, Teng H-C, Lee R-C, et al. Concurrent chemoradiotherapy treatment of locally advanced pancreatic cancer: gemcitabine versus 5-fluorouracil, a randomized controlled study. Int J Radiat Oncol Biol Phys (2003) 57:98–104. 10.1016/S0360-3016(03)00435-8 [DOI] [PubMed] [Google Scholar]
- 68.Morganti AG, Valentini V, Macchia G, Mattiucci GC, Costamagna G, Deodato F, et al. 5-fluorouracil-based chemoradiation in unresectable pancreatic carcinoma: phase I-II dose-escalation study. Int J Radiat Oncol Biol Phys (2004) 59:1454–60. 10.1016/j.ijrobp.2004.01.035 [DOI] [PubMed] [Google Scholar]
- 69.Park JK, Ryu JK, Lee JK, Yoon WJ, Lee SH, Kim YT, et al. Gemcitabine chemotherapy versus 5-fluorouracil-based concurrent chemoradiotherapy in locally advanced unresectable pancreatic cancer. Pancreas (2006) 33:397–402. 10.1097/01.mpa.0000236725.26672.be [DOI] [PubMed] [Google Scholar]
- 70.Chauffert B, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouché O, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2. Ann Oncol (2008) 19:1592–9. 10.1093/annonc/mdn281 [DOI] [PubMed] [Google Scholar]
- 71.Crane CH, Winter K, Regine WF, Safran H, Rich TA, Curran W, et al. Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 0411. J Clin Oncol (2009) 27:4096–102. 10.1200/JCO.2009.21.8529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sudo K, Yamaguchi T, Ishihara T, Nakamura K, Hara T, Denda T, et al. Phase II study of oral S-1 and concurrent radiotherapy in patients with unresectable locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys (2011) 80:119–25. 10.1016/j.ijrobp.2010.01.027 [DOI] [PubMed] [Google Scholar]
- 73.Oberic L, Viret F, Baey C, Ychou M, Bennouna J, Adenis A, et al. Docetaxel- and 5-FU-concurrent radiotherapy in patients presenting unresectable locally advanced pancreatic cancer: a FNCLCC-ACCORD/0201 randomized phase II trial’s pre-planned analysis and case report of a 5.5-year disease-free survival. Radiat Oncol (2011) 6:124. 10.1186/1748-717X-6-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brunner TB, Sauer R, Fietkau R. Gemcitabine/cisplatin versus 5-fluorouracil/mitomycin C chemoradiotherapy in locally advanced pancreatic cancer: a retrospective analysis of 93 patients. Radiat Oncol (2011) 6:88. 10.1186/1748-717X-6-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malik NK, May KS, Chandrasekhar R, Ma WW, Flaherty L, Iyer R, et al. Treatment of locally advanced unresectable pancreatic cancer: a 10-year experience. J Gastrointest Oncol (2012) 3:326–34. 10.3978/j.issn.2078-6891.2012.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ikeda M, Ioka T, Ito Y, Yonemoto N, Nagase M, Yamao K, et al. A multicenter phase II trial of S-1 with concurrent radiation therapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys (2013) 85:163–9. 10.1016/j.ijrobp.2012.03.059 [DOI] [PubMed] [Google Scholar]
- 77.Shinchi H, Maemura K, Mataki Y, Kurahara H, Sakoda M, Ueno S, et al. A phase II study of oral S-1 with concurrent radiotherapy followed by chemotherapy with S-1 alone for locally advanced pancreatic cancer. J Hepatobiliary Pancreat Sci (2012) 19:152–8. 10.1007/s00534-011-0400-y [DOI] [PubMed] [Google Scholar]
- 78.Herman JM, Wild AT, Wang H, Tran PT, Chang KJ, Taylor GE, et al. Randomized phase iii multi-institutional study of tnferade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: final results. J Clin Oncol (2013) 31:886–94. 10.1200/JCO.2012.44.7516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ducreux M, Giovannini M, Baey C, Llacer C, Bennouna J, Adenis A, et al. Radiation plus docetaxel and cisplatin in locally advanced pancreatic carcinoma: a non-comparative randomized phase II trial. Dig Liver Dis (2014) 46:950–5. 10.1016/j.dld.2014.06.006 [DOI] [PubMed] [Google Scholar]
- 80.Rembielak AI, Jain P, Jackson AS, Green MM, Santorelli GR, Whitfield GA, et al. Phase II trial of cetuximab and conformal radiotherapy only in locally advanced pancreatic cancer with Concurrent Tissue Sampling Feasibility Study. Transl Oncol (2014) 7:55–64. 10.1593/tlo.13724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kwak Y, Lee JH, Lee M, Chun H, Kim D, You YK, et al. Definitive concurrent chemoradiotherapy in locally advanced pancreatic cancer. Radiat Oncol J (2014) 32:49–56. 10.3857/roj.2014.32.2.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chung HW, Bang SM, Park SW, Chung JB, Kang JK, Kim JW, et al. A prospective randomized study of gemcitabine with doxifluridine versus paclitaxel with doxifluridine in concurrent chemoradiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys (2004) 60:1494–501. 10.1016/j.ijrobp.2004.05.061 [DOI] [PubMed] [Google Scholar]
- 83.Haddock MG, Swaminathan R, Foster NR, Hauge MD, Martenson JA, Camoriano JK, et al. Gemcitabine, cisplatin, and radiotherapy for patients with locally advanced pancreatic adenocarcinoma: results of the North Central Cancer Treatment Group Phase II Study N9942. J Clin Oncol (2007) 25:2567–72. 10.1200/JCO.2006.10.2111 [DOI] [PubMed] [Google Scholar]
- 84.Hong SP, Park JY, Jeon TJ, Bang S, Park SW, Chung JB, et al. Weekly full-dose gemcitabine and single-dose cisplatin with concurrent radiotherapy in patients with locally advanced pancreatic cancer. Br J Cancer (2008) 98:881–7. 10.1038/sj.bjc.6604247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mamon HJ, Niedzwiecki D, Hollis D, Tan BR, Mayer RJ, Tepper JE, et al. A phase 2 trial of gemcitabine, 5-fluorouracil, and radiation therapy in locally advanced nonmetastatic pancreatic adenocarcinoma: cancer and Leukemia Group B (CALGB) 80003. Cancer (2011) 117:2620–8. 10.1002/cncr.25742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Crane CH, Varadhachary GR, Yordy JS, Staerkel GA, Javle MM, Safran H, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol (2011) 29:3037–43. 10.1200/JCO.2010.33.8038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ch’Ang HJ, Lin YL, Wang HP, Chiu YF, Chang MC, Hsu CH, et al. Induction chemotherapy with gemcitabine, oxaliplatin, and 5-fluorouracil/leucovorin followed by concomitant chemoradiotherapy in patients with locally advanced pancreatic cancer: a Taiwan cooperative oncology group phase II study. Int J Radiat Oncol Biol Phys (2011) 81:e749–57. 10.1016/j.ijrobp.2010.10.034 [DOI] [PubMed] [Google Scholar]
- 88.Tozzi A, Comito T, Alongi F, Navarria P, Iftode C, Mancosu P, et al. SBRT in unresectable advanced pancreatic cancer: preliminary results of a mono-institutional experience. Radiat Oncol (2013) 8:148. 10.1186/1748-717X-8-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ke Q. S-1 plus gemcitabine chemotherapy followed by concurrent radiotherapy and maintenance therapy with S-1 for unresectable pancreatic cancer. World J Gastroenterol (2014) 20:13987. 10.3748/wjg.v20.i38.13987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sachsman S, Nichols RC, Morris CG, Zaiden R, Johnson EA, Awad Z, et al. Proton therapy and concomitant capecitabine for non-metastatic unresectable pancreatic adenocarcinoma. Int J Part Ther (2014) 1:692–701. 10.14338/IJPT.14-00006.1 [DOI] [Google Scholar]