The imaging radiation dose and imaging radiation-induced malignancy (IRIM) risk in subjects participating in breast cancer (BC) trials were estimated. In adjuvant trials, aligning the protocol requirements with the clinical guidelines’ surveillance recommendations and substituting radiating procedures with equivalent nonradiating ones would reduce IRIM risk. No significant risk was observed in metastatic trials, and potential concerns on IRIMs are not justified.
Keywords: Breast cancer trials, Imaging radiation, Radiation dose, Radiation risks, Radiation-induced cancers, Imaging radiation-induced malignancy, Imaging radiation-induced cancer
Abstract
Background.
Medical imaging is commonly required in breast cancer (BC) clinical trials to assess the efficacy and/or safety of study interventions. Despite the lack of definitive epidemiological data linking imaging radiation with cancer development in adults, concerns exist about the risks of imaging radiation-induced malignancies (IRIMs) in subjects exposed to repetitive imaging. We estimated the imaging radiation dose and IRIM risk in subjects participating in BC trials.
Materials and Methods.
The imaging protocol requirements in 10 phase III trials in the adjuvant and advanced settings were assessed to estimate the effective radiation dose received by a typical and fully compliant subject in each trial. For each study, the excess lifetime attributable cancer risk (LAR) was calculated using the National Cancer Institute’s Radiation Risk Assessment Tool, version 3.7.1. Dose and risk calculations were performed for both imaging intensive and nonintensive approaches to reflect the variability in imaging performed within the studies.
Results.
The total effective imaging radiation dose was 0.4–262.2 mSv in adjuvant trials and 26–241.3 mSv in metastatic studies. The dose variability resulted from differing protocol requirements and imaging intensity approaches, with computed tomography, multigated acquisition scans, and bone scans as the major contributors. The mean LAR was 1.87–2,410/100,000 in adjuvant trials (IRIM: 0.0002%–2.41% of randomized subjects) and 6.9–67.3/100,000 in metastatic studies (IRIM: 0.007%–0.067% of subjects).
Conclusion.
IRIMs are infrequent events. In adjuvant trials, aligning the protocol requirements with the clinical guidelines’ surveillance recommendations and substituting radiating procedures with equivalent nonradiating ones would reduce IRIM risk. No significant risk has been observed in metastatic trials, and potential concerns on IRIMs are not justified.
Implications for Practice:
Medical imaging is key in breast cancer (BC) clinical trials. Most of these procedures expose patients to ionizing radiation, and the risk of second cancer development after imaging has prompted recent concerns and controversy. Using accepted calculation models, the number of malignancies were estimated that were potentially attributable to the imaging procedures performed during a patient’s participation in BC clinical trials. The results show that for patients participating in metastatic trials, the risk of imaging radiation-induced malignancies is negligible. In adjuvant trials, some second cancers due to imaging could be expected, and measures can be taken to reduce their risk.
Introduction
Progress in cancer treatment is accomplished through the conduct of well-designed interventional clinical trials. Medical imaging is critical in most cancer trials, and the primary and/or secondary endpoints are often linked to imaging findings. No meaningful trial of advanced breast cancer (BC) could be conducted without serial imaging requirements, because most trials assess the response and/or progression using response criteria that rely on serial scans. In the adjuvant setting, although the intensity of imaging is usually lower than that in advanced trials, some procedures are required for the baseline assessment and to assess for second primaries, relapses, and/or safety.
An average healthy subject in the United States is exposed to an effective dose of ∼3.0 mSv annually of naturally occurring background ionizing radiation, and medical imaging is the main source of non-natural radiation in adults in the United States, with carcinogenesis the most relevant biologic effect of radiation exposure [1]. The frequency of most cancer types appears to be increased after irradiation, although body organs vary in their sensitivity [2]. The latency period from exposure to the development of a radiation-induced malignancy depends on the tissue of origin. Leukemia typically develops within 5 years of exposure, and solid tumors are commonly observed after 10 years [2].
The radiation risks associated with a single imaging procedure are minimal. However, in patients undergoing serial imaging, the risk of imaging radiation-induced malignancies (IRIMs) has been a topic of recent scrutiny by the general public, media, and scientists [3–5]. Some calculations predict several thousands of fatal cancer cases for patients undergoing imaging each year [6–9]. These projections have stirred debate and even been suggested to have negative health consequences by some investigators [10].
In recent trials conducted by the Translational Research in Oncology group (TRIO), the IRIM risk from protocol-required imaging has been a repetitive concern of investigators and subjects. We conducted the present study with the aim of estimating the imaging radiation doses in subjects participating in BC trials and the associated risks and benefits.
Materials and Methods
To estimate the imaging radiation dose received by subjects participating in BC trials and the associated IRIM risk, we assessed the imaging protocol requirements in some of the most relevant phase III trials in adjuvant and advanced settings. The studies were selected based on their large sample size, long follow-up, availability of data on imaging requirements and second primary malignancies (the latter applicable for adjuvant studies), and on their relevance for the oncology community.
To quantify the radiation exposure in each trial, we estimated the per time point and total imaging effective dose (considering the average doses reported in Table 1) for a typical trial participant, assuming full protocol compliance. For our dose and risk calculations, we assumed that the protocol-required imaging was performed for 10 years after study entry in the adjuvant trials and for a period equivalent to the progression-free survival (PFS) or time to progression (TTP) of the arm, with the longest PFS/TTP in the metastatic studies. Because within a trial variability will exist in the imaging studies performed with the same intent (e.g., in some studies, subjects will undergo computed tomography [CT] or ultrasonography to assess liver parenchyma), for each trial we assumed two scenarios: one in which the subject undergoes an imaging radiation-intensive approach (maximum possible dose according to the protocol requirements) and one in which the same subject undergoes a less intensive approach, if allowed per the protocol (e.g., by substituting multigated acquisition [MUGA] with echocardiography and/or chest-abdomen CT with liver ultrasonography and chest radiography).
Table 1.
Effective radiation doses from imaging procedures commonly performed in breast cancer clinical trials
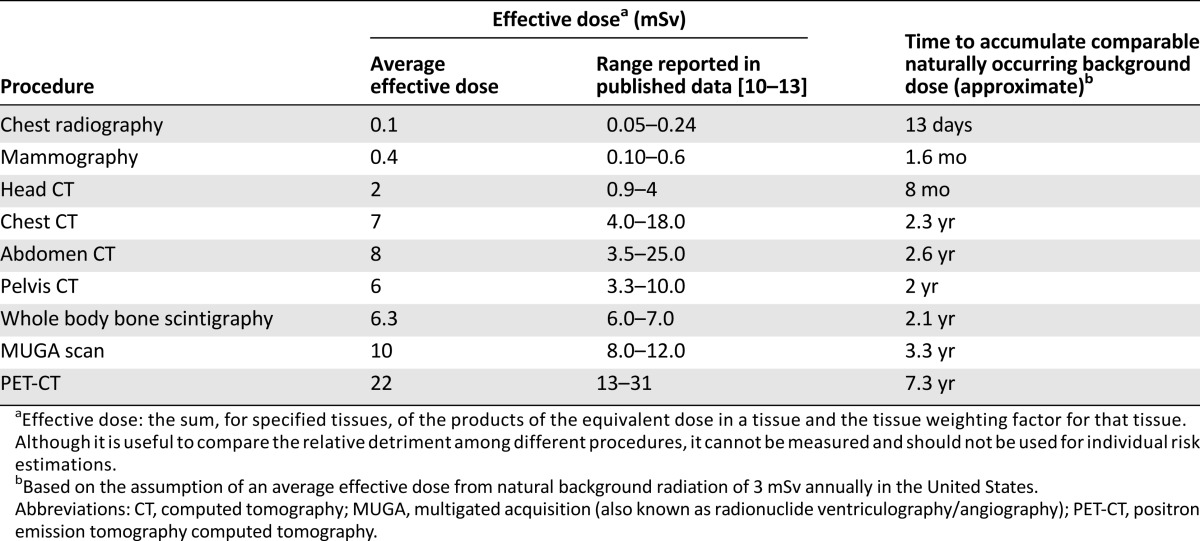
We estimated the excess lifetime attributable cancer risk using the U.S. National Cancer Institute’s Radiation Risk Assessment Tool (RadRAT, version 3.7.1; National Cancer Institute, Bethesda, MD) [14]. The excess lifetime risk is a summary statistic for capturing the total potential detriment of an exposure, calculated as the sum of the age-specific risks adjusted for the probability of surviving to that age (i.e., survival function) [15]. RadRAT was used as recommended by the developers [14, 15]. For each trial, the RadRAT inputs for demographic information were female gender and year of birth (calculated as last calendar year of recruitment minus the median age in the trial). Each imaging study during subject participation was recorded as a single exposure event, considering all organs as acutely exposed. The absorbed dose of each imaging procedure was entered in milligrays after converting the average effective dose reported in Table 1 and applying radiation and tissue weighting factors of 1 [16].
RadRAT estimates the excess lifetime cancer risk considering the life expectancy reported in the U.S. Centers for Disease Control and Prevention National Vital Statistics Reports [17]. Because patients with metastatic BC have a median life expectancy of 2–3 years [18] and this is the same expectancy for a 95-year-old woman in the United States, the year of birth entered when estimating risks in advanced trials was calculated as the last calendar year of recruitment minus 95.
Results
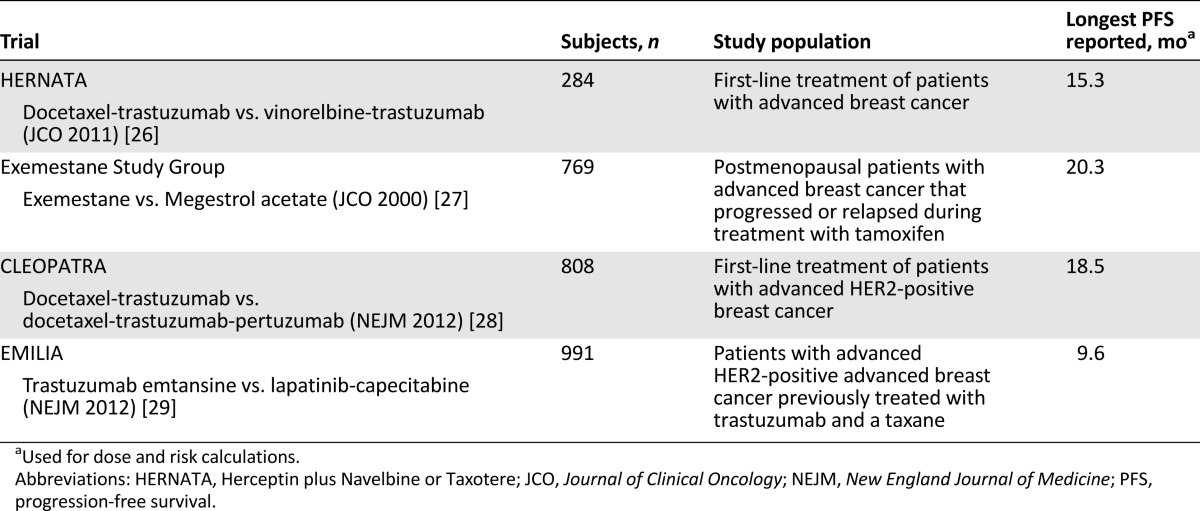
The adjuvant trials selected were Breast Cancer International Research Group (BCIRG)-001, BCIRG-006, Cancer and Leukemia Group B (CALGB)-9344/Intergroup-0148, French Adjuvant Study Group (FASG)-01, FASG-05, and Breast International Group (BIG) 1-98, and the studies in the advanced setting were the Herceptin plus Navelbine or Taxotere (HERNATA) trial, the Exemestane Study Group, the CLEOPATRA trial, and the EMILIA trial [19–29]. The main characteristics of these studies are listed in Tables 2 and 3 [19–24, 26–29].
Table 2.
Main characteristics of selected adjuvant clinical trials
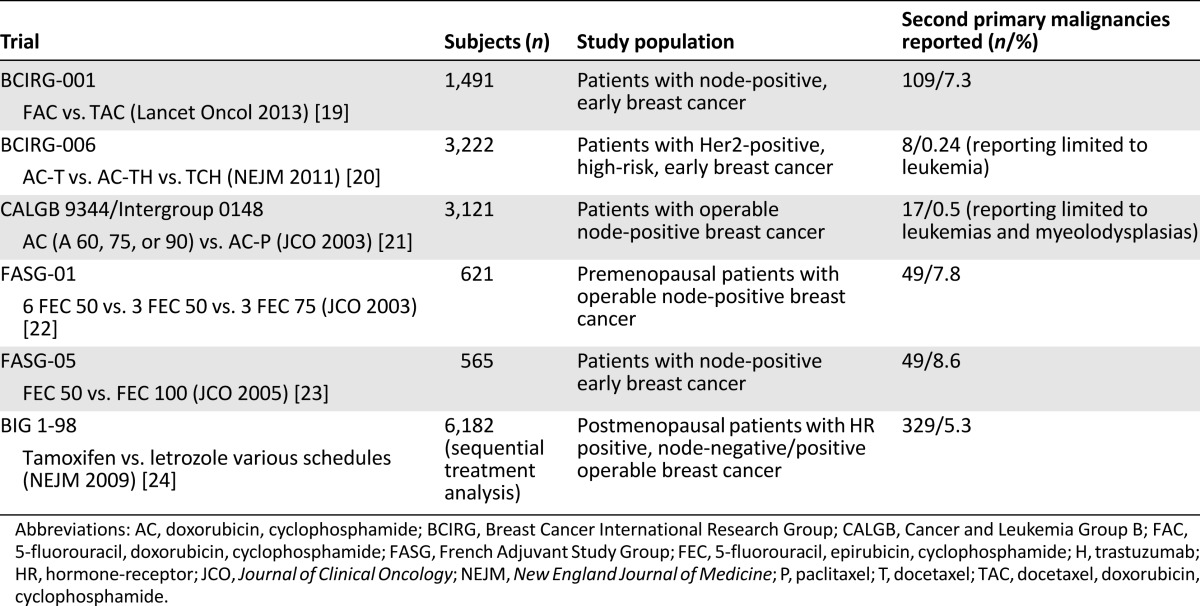
Table 3.
Main characteristics of selected clinical trials in advanced setting
Imaging Radiation Doses and Risks in Adjuvant Trials
For each adjuvant trial, the imaging protocol requirements and per time point and total effective radiation doses received by a typical subject are listed in Table 4 [19–24, 30]. The total effective imaging radiation dose was between 0.4 mSv (BIG 1-98, with only mammography at baseline performed) and 262.2 mSv (FASG trials, in which an imaging intensive approach was required). In BCIRG-001, BCIRG-006, CALGB-9344, and BIG 1-98, the dose levels with the less-intensive approach were around the 10-mSv threshold for which no evidence has shown an increased risk of cancer.
Table 4.
Imaging studies performed in selected adjuvant clinical trials and estimated radiation-induced cancer risks
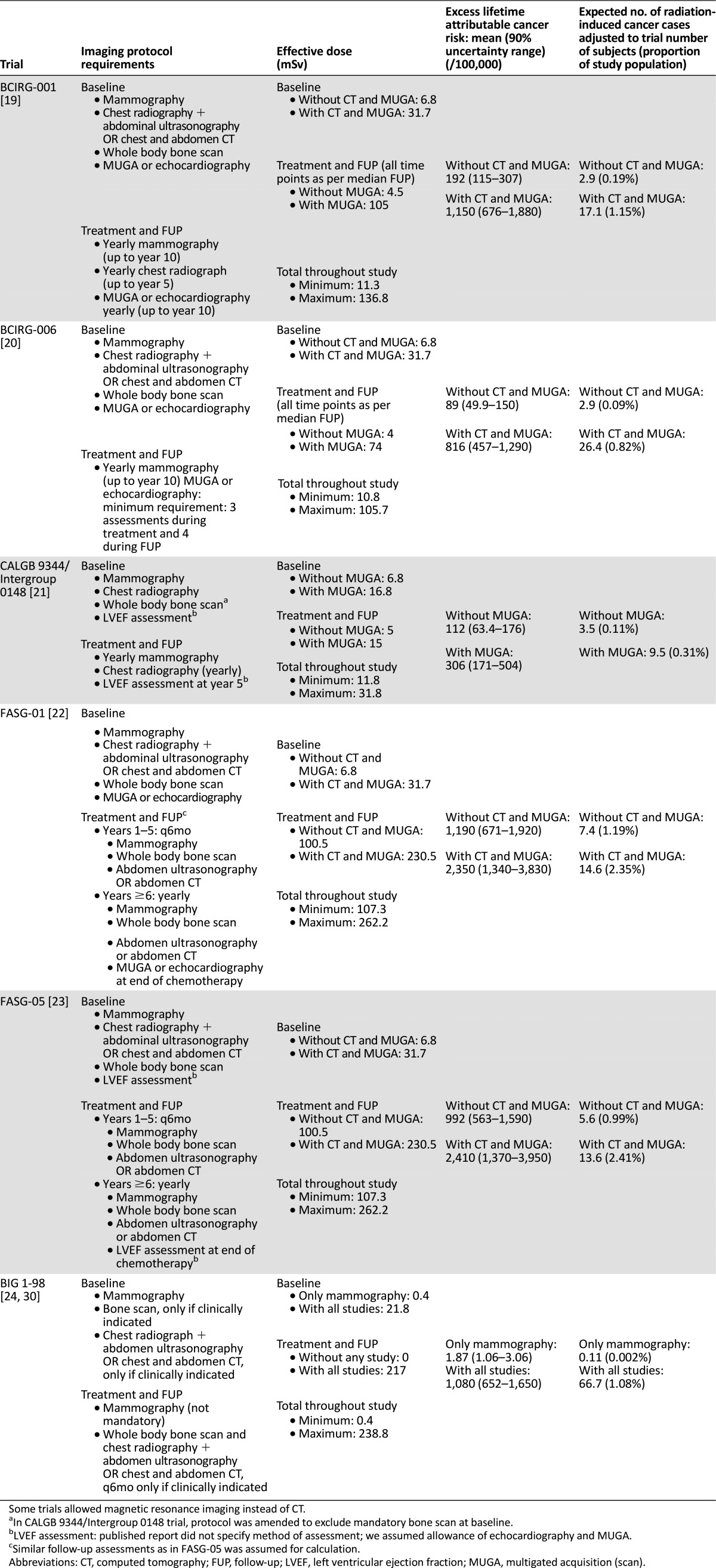
The baseline requirements were fairly homogenous across the trials, with effective doses ranging from 0.4 to 6.8 mSv with the less-intensive approach and 16.8 and 31.7 mSv with the intensive one. Greater variability across the trials was observed during the treatment and follow-up phases, in which the requirements ranged from no mandatory imaging (BIG 1-98) to intensive radiologic surveillance with doses up to 262.2 mSv (FASG trials).
The corresponding excess lifetime attributable risk and the expected number of IRIMs during the trials are listed in Table 4. Depending on the trial and the imaging intensity, 0.002%– 2.41% of subjects could develop an IRIM (average, 0.4% with the less-intensive approach and 1.35% with the intensive one). The risk variability within and across trials is high when subjects undergo an intensive versus a nonintensive approach. The major contributors to the risk are the studies performed during the follow-up phase, in particular, CT, MUGA, and bone scans. In FASG-05, the estimated IRIM cases ranged from 0.99% to 2.41% of the study subjects, depending on whether they underwent follow-up with chest radiography, ultrasonography, and echocardiography versus CT and MUGA. In addition, in CALGB 9344, the estimated IRIM cases were threefold higher when the left ventricular ejection fraction (LVEF) is assessed with MUGA compared with echocardiography (0.31% vs. 0.11% of subjects).
Imaging Radiation Doses and Risks in Metastatic Trials
For each metastatic trial, the imaging protocol requirements and per time point and total effective radiation dose received by a typical participant are listed in Table 5. The total effective doses are clearly more homogeneous across metastatic trials than among adjuvant ones, because most trials have similar imaging requirements to assess efficacy or safety. The total effective imaging radiation dose from tumor assessments in metastatic trials was between 111.3 mSv (HERNATA; not shown in Table 5) and 141.3 mSv. The study by the Exemestane Study Group allowed for chest radiography and ultrasonography as alternatives to CT, and in such cases, the total dose decreased to 26 mSv. The choice of MUGA to assess the LVEF resulted in a 42% and 70% increase in the total effective dose in the CLEOPATRA and EMILIA trials, respectively, compared with the subjects who had undergone identical tumor assessments but had had LVEF assessed with echocardiography.
Table 5.
Imaging studies performed in selected clinical trials in advanced setting and estimated radiation-induced cancer risks
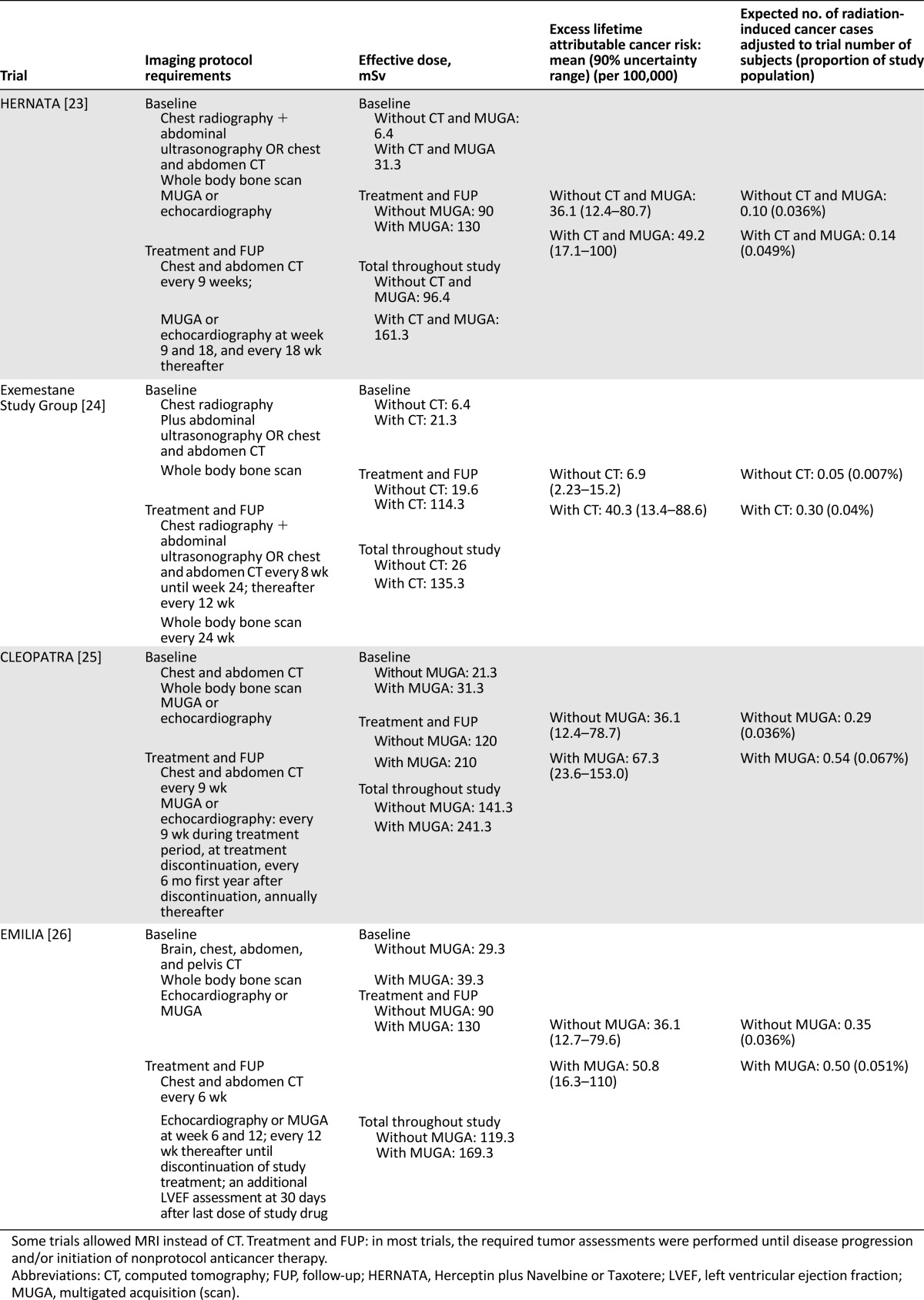
The corresponding excess lifetime attributable risk and the expected number of IRIMs during trials are listed in Table 5. Considering all the trials selected and the most intensive approach (worst case scenario), the average IRIM risk is only 0.05%.
Discussion
Most of the evidence on radiation-induced malignancies comes from four distinct groups: medically, occupationally, and environmentally exposed populations and atomic bomb survivors [31, 32]. Data from Japanese survivors show that exposures greater than 100 mSv have a proved increased malignancy risk [33]. In contrast, no epidemiological data support an increased risk at doses less than 10 mSv (dose level in most single imaging procedures). However, controversy exists regarding establishing a definitive risk at doses between 10 and 100 mSv, the dose commonly received by subjects undergoing multiple scans [34].
Definitive evidence linking diagnostic imaging with cancer development in adults is lacking. A recent population-based study that included 4,874 curatively treated non-Hodgkin lymphoma patients showed that those receiving more than 8 CT scans after diagnosis had a twofold risk of developing a second primary malignancy than those with ≤8 CT scans (hazard ratio, 2.23; 95% confidence interval, 1.60–3.11; p < .001) [35]. The malignancies were typically located in regions where the radiation fields of CT overlap. Although provocative, these findings cannot be considered definitive. Epidemiological studies including thousands or even millions of subjects followed for long periods would be needed to demonstrate a significant risk related to exposure to low-dose radiation [36]. Thus, the radiation-induced malignancy risk estimates for patients undergoing medical imaging has come mainly from extrapolation of atomic bomb survivor data. The most widely accepted extrapolation model is the linear no-threshold (LNT), which indicates that no dose is without carcinogenic risk and that the cancer risk increases linearly with the dose [1, 9, 31, 32].
Radiation-induced malignancies in BC patients are infrequent events, and no definitive epidemiological data relating imaging radiation and IRIMs are available. In recent trials conducted by TRIO, concerns from investigational sites regarding the risks of protocol imaging requirements have been repetitive. Various explanations for this are possible. First, patient and physician knowledge about medical imaging risks is generally low. The lack of understanding of imaging radiation doses and corresponding risks has been shown to be very common among patients in several studies [37–39]. Additionally, a systematic review showed that only a few physicians are well informed about CT radiation doses and the associated risks [40]. This was also shown in a recent study in which only 17.3% of physicians from 14 major Australian hospitals correctly estimated the radiation dose from CT [41].
Second, the risks of medical radiation have been a topic of recent scrutiny by the general public, media, and the scientific community, after some reports predicting thousands of future fatal cancers for patients imaged during a given year [6–9]. The data from these studies, although controversial, could be beneficial if properly interpreted and used with the goal of enhancing awareness about medical imaging and optimizing indications and techniques. However, given the complexity of the data and the uncertainty and controversy around the models used by the investigators, the results of these studies have sometimes been misinterpreted and invalidly used for individual risk predictions or by the media, who in some cases have transformed hypothesis into facts [3–5, 42–44].
The lack of knowledge about radiation from medical imaging and its risks/benefits, combined with the misinterpretation of the referenced studies, could affect BC research efforts. Overestimation of risk from imaging required in a trial could affect a patient’s willingness to participate, retention, and/or on protocol compliance, and underestimation can lead to subjects not being well informed about the risk/benefit ratio or sponsors and investigators not fully adopting the “as low as reasonably achievable” principle.
Risk-Benefit in Adjuvant Trials
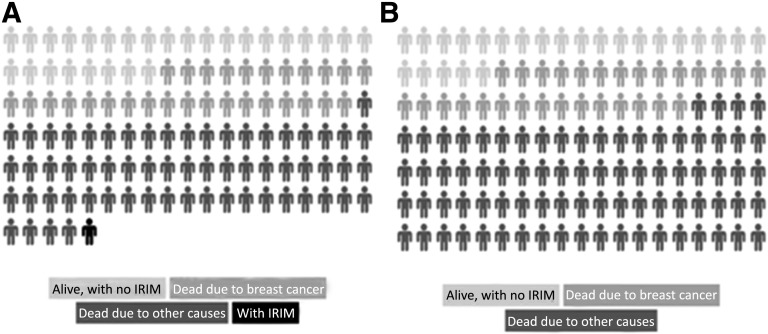
Owing to improved early detection efforts and more effective adjuvant treatments, the number of BC long-term survivors is increasing [45]. Consequently, most women are living long enough for the late consequences of treatment and procedures to become apparent. The expected survival of most women is longer than the latency period needed for the appearance of second malignancies. Studies on second malignancies in BC patients treated curatively have emphasized the effect of chemotherapy, hormonal therapy, and radiotherapy on their development [46–48]. These studies have not considered medical imaging as a relevant contributing factor. From our results, the estimated IRIM risk in adjuvant trials ranged from 0.4% to 1.35%, depending on the intensity of the imaging approach. Hence, a number of second cancer cases could be derived from imaging performed during a trial, in a population with high chances of cure. However, given the low frequency of IRIMs and the significantly higher probability of dying from BC or other causes, the risks are comparatively low [49]. Figure 1 shows a visual representation of the possible outcomes (including IRIM) in a population of early BC patients participating in an adjuvant trial.
Figure 1.
Visual representation of the possible outcomes, including imaging radiation-induced malignancy in a population of early breast cancer patients (age 50–59 years) participating in an adjuvant clinical trial (outcome at 28 years) (A) and a population of advanced breast cancer patients (age 50–59 years) participating in a metastatic clinical trial (outcome at 5 years) (B). Based on data from Schairer et al. [49]. In adjuvant trials, the risk depends on the imaging protocol requirements (see text). In metastatic trials, no cases of imaging radiation-induced tumors are expected.
Abbreviation: IRIM, imaging radiation-induced malignancy.
The trials selected in our study reported a variable number of second malignancies. In all cases, as expected, the number of IRIMs calculated by us was lower than the total number of second cancers reported. Estimation of the risk of second cancers due to radiotherapy, chemotherapy, or any other carcinogenetic treatment factor was beyond the scope of our study, but it can be hypothesized that the associated risks would be comparatively higher. Between 58% and 96% of the patients enrolled in the selected adjuvant trials received radiotherapy during the trial, with doses in the treated areas vastly higher than the ones from imaging and also in adjacent areas owing to scatter radiation. This would have contributed to the observed second cancers on a much greater extent than radiation derived from imaging.
Lin et al. postulated as possible methods to reduce the risk from medical imaging not performing a radiating study, using nonradiating alternative methods, or using less radiation to create images [34]. In adjuvant trials, some measures could reduce imaging radiation doses and derived risks. One is the reduction of the dose received during the follow-up phase of trials and the second, the use of echocardiography instead of MUGA when assessing LVEF.
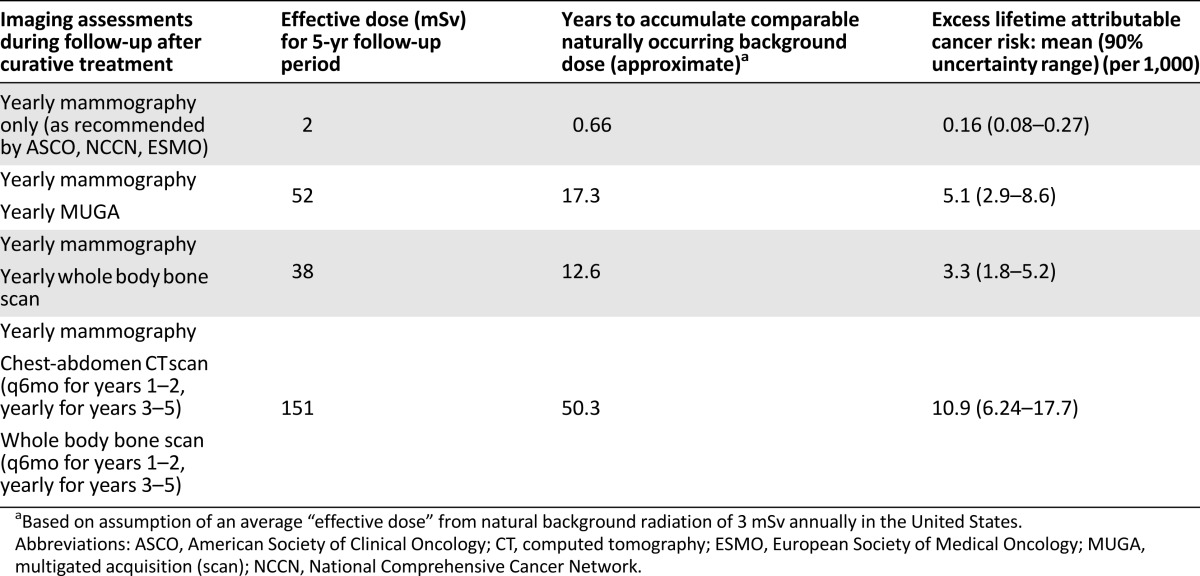
The American Society of Clinical Oncology, National Comprehensive Cancer Network, and European Society of Medical Oncology all recommend against the use imaging studies for metastasis screening during follow-up of curatively treated BC patients [50–52]. However, the use of nonrecommended tests occurs frequently in standard practice [53–55]. All six trials selected in our study required or allowed the use of these nonrecommended tests. The effective dose of several surveillance strategies after BC curative treatment and their corresponding excess lifetime cancer risk in a 55-year-old woman are listed in Table 6. When the clinical guidelines are strictly followed, the IRIM risk is negligible. However, with an imaging-intensive approach, the risk is 11-fold higher, and 11 IRIM cases could be expected of 1,000 subjects according to our estimation model. We acknowledge that in a trial setting, intensive imaging surveillance can eventually detect recurrences earlier, potentially affecting the study endpoints. However, as our results suggest, this is potentially at the cost of an increase in the risk of IRIMs. Considering this and the lack of survival benefit with intensive surveillance, we believe that trials should not systematically require this type of follow-up study [56].
Table 6.
Surveillance strategies after curative treatment of breast cancer and corresponding estimated imaging radiation-induced malignancy risks
Both MUGA and echocardiography are accepted methods for assessing LVEF in BC trials with cardiotoxic drugs. Verma and Ewer showed that MUGA was the most common LVEF assessment technique used in BC studies with cardiac endpoints [57]. From our estimations, MUGA can be a significant contributor to the risk of IRIM, with a threefold risk compared with assessing LVEF using echocardiography in CALGB 9344.
Finally, we propose two additional measures for adjuvant trials. First, we consider that radiology manuals and/or trial protocols should systematically include information about the radiological protection of the study subjects. Second, trial participants should be provided with the information needed to understand the potential risk/benefit of protocol-required imaging. Currently, informed consent forms include detailed information about the risks and discomforts from study treatments and procedures; however, commonly this information about imaging is lacking. Finding a balance between the ICH-GCP (International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice) requirement to inform subjects about the risks from trial participation and the chance of overwhelming participants with difficult to understand information remains a challenge. Studies that have assessed a patient’s understanding of medical radiation have shown that many have a limited ability to make well-informed decisions about imaging involving radiation [38]. Also, physicians commonly lack knowledge about the imaging risks and thus might be poorly equipped to discuss this with trial candidates. Overcoming these limitations will certainly be a challenge, but the imaging radiation doses in adjuvant trials and their associated risks might be sufficiently large to warrant the inclusion of corresponding information during the informed consent process.
Risk-Benefit in Trials in the Advanced Setting
Based on our results, concerns about IRIM risk in subjects participating in trials in the advanced setting are not justified. Given the latency period for the development of IRIM, the risk in these patients is negligible because of their shortened life expectancy, even for short-latency malignancies such as leukemia. As shown by Brenner et al., the radiation risk estimates are derived almost entirely from individuals with a normal life expectancy and therefore might not be fully applicable for individuals with reduced life expectancy [58]. These investigators showed that the risk of IRIM was 92% lower in subjects with metastatic colon cancer relative to the corresponding lifetime risk in healthy individuals undergoing the same CT examinations. Even in BC patients with the longest survival (e.g., bone-only disease), our results are aligned with the conclusions from Brenner et al. and from a recent review about imaging in cancer patients, and indicate that the competing risk of death from advanced cancer significantly diminishes the IRIM risk, which should not factor into imaging decisions [59].
Most BC trials in the advanced setting rely on standardized imaging criteria to assess the tumor response, being the Response Evaluation Criteria in Solid Tumors (RECIST) the most widely accepted. Protocol deviations are common and introduce uncertainty in the assessment of treatment effect. This effect is minimized in randomized trials by conducting analysis according to the intention-to-treat (ITT) principle [60]. However, in trials in which the ITT analysis was positive, the study results can be questioned if inconsistency is present between the ITT and the per-protocol sensitivity analysis (in which subjects who deviate from the protocol are excluded from the analysis) [61]. Therefore, compliance with the imaging schedule and the protocol-specified response criteria in trials in which the primary endpoint is linked to response assessment can be critical for the scientific validity of a trial. Given the lack of a clinically significant risk of IRIMs in metastatic BC patients, study candidates/participants, investigators, ethics committees, advocacy groups, and regulators should be reassured that the benefits of imaging procedures on the proper assessment of tumor response and, therefore, on the accurate determination of efficacy endpoints in phase II and III trials, outweigh any sort of risk for subjects. We consider that the risks of lowering the scientific validity of a trial in which a new potentially practice-changing agent is being tested by noncompliance with imaging requirements is higher than any unproved risk from imaging procedures.
Our study was not without limitations. We have estimated the lifetime attributable cancer risk using RadRAT, whose reported risks are based primarily on the methods used in the Biological Effects of Ionizing Radiation VII (BEIR VII) report, which assumes the LNT model. Consequently, our reported estimates derive from data coming from the consensus opinion of a committee and are not readily empirically proven. Although the validity of the LNT model when estimating imaging risks has been questioned [10, 62–64] and the analysis of its appropriateness is beyond the scope of our study, no alternative model is as widely accepted and the estimates on which RadRAT are based are the most widely adopted and consistent with current standards.
Another potential limitation was that RadRAT is most appropriate for individuals with life expectancy and cancer rates similar to those in the United States. Most of the trials analyzed were multinational studies conducted in various regions where the life expectancy and cancer rates might not be similar to those in the United States, potentially leading to a risk of over- or underestimation. Furthermore, we assumed that each imaging procedure produced a uniform whole-body radiation dose, ignoring organ-specific radiosensitivity and that the doses received by different organs and in different areas of an organ vary during imaging procedures [65]. We also assumed identical dose per imaging procedure across all subjects in a trial, though interindividual and interinstitutional variability is expected [30].
When estimating the IRIM risk in the metastatic trials we entered in RadRAT a patient’s age as 95 years old given that the life expectancy for a woman of that age is similar to that of a patient diagnosed with metastatic BC. Because age at exposure has an influence on the IRIM risk estimations [15, 66], it is acknowledged that this approach might have underestimated the risk for younger women. However, given the low risk, the IRIM latency period, and the life expectancy for these patients, our conclusions would remain unchanged.
Finally, it is not possible to directly attribute any malignancy to imaging radiation, because genetic, environmental, and treatment factors can also contribute (and in some cases to a greater extent) to second malignancy risk.
Reaching a definitive and incontestable conclusion regarding the relationship between imaging procedures and radiation-induced malignancies would require large-scale, well-designed, and well-conducted epidemiological studies, which would seem to be infeasible to be conducted. In the absence of this definitive evidence, approaches such as the one in our study are able to provide valuable information and adds to the currently available evidence, specifically in the BC clinical trials setting.
Conclusion
We conducted the first study estimating the cancer risks from imaging radiation received by subjects during their participation in BC clinical trials. Our RadRAT estimations suggest that in adjuvant studies, aligning the protocol requirements during follow-up with the recommendations from clinical guidelines and substituting radiating procedures with nonradiating ones could potentially lower the IRIM risk. In metastatic trials, our results showed that no significant risk of IRIM exists, and, therefore, potential concerns on this regard are unjustified.
Acknowledgments
We thank Alison Schmidt and Dr. Valeria González for editorial review and the Centro de Documentación e Información en Cáncer from the Comisión Honoraria de Lucha Contra el Cáncer (Montevideo, Uruguay) and, especially, Mercedes Achard for support with the bibliographic search and article provision.
Author Contributions
Conception/Design: Rodrigo Fresco, Gonzalo Spera, Carlos Meyer, John Mackey
Provision of study material or patients: Rodrigo Fresco, Gonzalo Spera, Carlos Meyer
Collection and/or assembly of data: Rodrigo Fresco, Gonzalo Spera, Pablo Cabral, John Mackey
Data analysis and interpretation: Rodrigo Fresco, Gonzalo Spera, Carlos Meyer, Pablo Cabral, John Mackey
Manuscript writing: Rodrigo Fresco, Gonzalo Spera, Carlos Meyer, Pablo Cabral, John Mackey
Final approval of manuscript: Rodrigo Fresco, Gonzalo Spera, Carlos Meyer, Pablo Cabral, John Mackey
Disclosures
John Mackey: Roche, Pfizer, Sanofi (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Loftus DML, Sanelli PPC, Frush PDP, et al. Radiation exposure from medical imaging. In: Medina LS, Sanelli PC, Jarvik JG, editors. Evidence-Based Neuroimaging Diagnosis and Treatment. New York: Springer; 2013. pp. 63–79. [Google Scholar]
- 2.Boice JD. Cancer following medical irradiation. Cancer. 1981;47(suppl):1081–1090. doi: 10.1002/1097-0142(19810301)47:5+<1081::aid-cncr2820471305>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Gee A. As Medical Imaging Rises, Radiation Concerns Follow. The New York Times 2012. Available at http://www.nytimes.com/2012/06/13/health/as-medical-imaging-rises-radiation-concerns-follow.html. Accessed November 11, 2013.
- 4.Rabin RC. With Rise in Radiation Exposure, Experts Urge Caution on Tests. The New York Times 2007. Available at http://www.nytimes.com/2007/06/19/health/19cons.html. Accessed October 30, 2013.
- 5.Hope J. CAT scan cancer fear: Radiation “could trigger the disease in one in 80 patients.” Mail Online. 2009. Available at http://www.dailymail.co.uk/health/article-1235901/CAT-scan-cancer-fear-Radiation-trigger-disease-80-patients.html. Accessed November 22, 2013.
- 6.Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071–2077. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169:2078–2086. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenner DJ, Hall EJ. Computed tomography—An increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 9.Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. Br J Radiol. 2008;81:362–378. doi: 10.1259/bjr/01948454. [DOI] [PubMed] [Google Scholar]
- 10.Hendee WR, O’Connor MK. Radiation risks of medical imaging: Separating fact from fantasy. Radiology. 2012;264:312–321. doi: 10.1148/radiol.12112678. [DOI] [PubMed] [Google Scholar]
- 11.Wall BF, Hart D. Revised radiation doses for typical X-ray examinations. Report on a recent review of doses to patients from medical X-ray examinations in the UK by NRPB. National Radiological Protection Board. Br J Radiol. 1997;70:437–439. doi: 10.1259/bjr.70.833.9227222. [DOI] [PubMed] [Google Scholar]
- 12.Mettler FA, Jr, Huda W, Yoshizumi TT, et al. Effective doses in radiology and diagnostic nuclear medicine: A catalog. Radiology. 2008;248:254–263. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 13.Huang B, Law MW-M, Khong P-L. Whole-body PET/CT scanning: Estimation of radiation dose and cancer risk. Radiology. 2009;251:166–174. doi: 10.1148/radiol.2511081300. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Radiation Risk Assessment Tool. Available at https://irep.nci.nih.gov/radrat. Accessed November 4, 2013.
- 15.Berrington de Gonzalez A, Iulian Apostoaei A, Veiga LHS, et al. RadRAT: A radiation risk assessment tool for lifetime cancer risk projection. J Radiol Prot. 2012;32:205–222. doi: 10.1088/0952-4746/32/3/205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Task Group on Radiation Quality Effects in Radiological Protection. Committee 1 on Radiation Effects, International Commission on Radiological Protection Relative biological effectiveness (RBE), quality factor (Q), and radiation weighting factor (w(R)). A report of the International Commission on Radiological Protection. Ann ICRP. 2003;33:1–117. doi: 10.1016/s0146-6453(03)00024-1. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. National Vital Statistics Report. Available at http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_04.pdf. Accessed October 30, 2013.
- 18.Andre F, Slimane K, Bachelot T, et al. Breast cancer with synchronous metastases: Trends in survival during a 14-year period. J Clin Oncol. 2004;22:3302–3308. doi: 10.1200/JCO.2004.08.095. [DOI] [PubMed] [Google Scholar]
- 19.Mackey JR, Martin M, Pienkowski T, et al. TRIO/BCIRG 001 investigators Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node-positive breast cancer: 10-Year follow-up of the phase 3 randomised BCIRG 001 trial. Lancet Oncol. 2013;14:72–80. doi: 10.1016/S1470-2045(12)70525-9. [DOI] [PubMed] [Google Scholar]
- 20.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 22.Fumoleau P, Kerbrat P, Romestaing P, et al. Randomized trial comparing six versus three cycles of epirubicin-based adjuvant chemotherapy in premenopausal, node-positive breast cancer patients: 10-Year follow-up results of the French Adjuvant Study Group 01 trial. J Clin Oncol. 2003;21:298–305. doi: 10.1200/JCO.2003.04.148. [DOI] [PubMed] [Google Scholar]
- 23.Bonneterre J, Roché H, Kerbrat P, et al. Epirubicin increases long-term survival in adjuvant chemotherapy of patients with poor-prognosis, node-positive, early breast cancer: 10-Year follow-up results of the French Adjuvant Study Group 05 randomized trial. J Clin Oncol. 2005;23:2686–2693. doi: 10.1200/JCO.2005.05.059. [DOI] [PubMed] [Google Scholar]
- 24.BIG 1-98 Collaborative Group. Mouridsen H, Giobbie-Hurder A, Goldhirsch A, et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009;361:766–776. doi: 10.1056/NEJMoa0810818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breast International Group (BIG) 1-98 Collaborative Group, Thürlimann B, Keshaviah A et al. Comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 2005;353:2747–2757. [DOI] [PubMed]
- 26.Andersson M, Lidbrink E, Bjerre K, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: The HERNATA study. J Clin Oncol. 2011;29:264–271. doi: 10.1200/JCO.2010.30.8213. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann M, Bajetta E, Dirix LY, et al. Exemestane is superior to megestrol acetate after tamoxifen failure in postmenopausal women with advanced breast cancer: Results of a phase III randomized double-blind trial. The Exemestane Study Group. J Clin Oncol. 2000;18:1399–1411. doi: 10.1200/JCO.2000.18.7.1399. [DOI] [PubMed] [Google Scholar]
- 28.Baselga J, Cortés J, Kim S-B, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA. 2012;307:2400–2409. doi: 10.1001/jama.2012.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. United Nations Scientific Committee on the Effects of Atomic Radiation. Effects of ionizing radiation. UNSCEAR 2006 Report to the General Assembly with Scientific Annexes. New York, NY: United Nations, 2008. [Google Scholar]
- 32.Little MP, Wakeford R, Tawn EJ, et al. Risks associated with low doses and low dose rates of ionizing radiation: Why linearity may be (almost) the best we can do. Radiology. 2009;251:6–12. doi: 10.1148/radiol.2511081686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Little MP. Cancer and non-cancer effects in Japanese atomic bomb survivors. J Radiol Prot. 2009;29:A43–A59. doi: 10.1088/0952-4746/29/2A/S04. [DOI] [PubMed] [Google Scholar]
- 34.Lin EC. Radiation risk from medical imaging. Mayo Clin Proc. 2010;85:1142–1146; quiz 1146. doi: 10.4065/mcp.2010.0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chien SH, Liu CJ, Gau JP et al. Frequency of surveillance computed tomography in non-Hodgkin lymphoma and the risk of secondary primary malignancies. Paper presented at: 19th Congress of the European Hematology Association; Milan, Italy; 2014; abstract 3488. [Google Scholar]
- 36.Prasad KN, Cole WC, Hasse GM. Health risks of low dose ionizing radiation in humans: A review. Exp Biol Med (Maywood) 2004;229:378–382. doi: 10.1177/153537020422900505. [DOI] [PubMed] [Google Scholar]
- 37.Baumann BM, Chen EH, Mills AM, et al. Patient perceptions of computed tomographic imaging and their understanding of radiation risk and exposure. Ann Emerg Med. 2011;58:1–7.e2. doi: 10.1016/j.annemergmed.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Busey JM, Soine LA, Yager JR, et al. Patient knowledge and understanding of radiation from diagnostic imaging. JAMA Intern Med. 2013;173:239–241. doi: 10.1001/2013.jamainternmed.1013. [DOI] [PubMed] [Google Scholar]
- 39.Takakuwa KM, Estepa AT, Shofer FS. Knowledge and attitudes of emergency department patients regarding radiation risk of CT: Effects of age, sex, race, education, insurance, body mass index, pain, and seriousness of illness. AJR Am J Roentgenol. 2010;195:1151–1158. doi: 10.2214/AJR.09.3847. [DOI] [PubMed] [Google Scholar]
- 40.Krille L, Hammer GP, Merzenich H, et al. Systematic review on physician’s knowledge about radiation doses and radiation risks of computed tomography. Eur J Radiol. 2010;76:36–41. doi: 10.1016/j.ejrad.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 41.Brown N, Jones L. Knowledge of medical imaging radiation dose and risk among doctors. J Med Imaging Radiat Oncol. 2013;57:8–14. doi: 10.1111/j.1754-9485.2012.02469.x. [DOI] [PubMed] [Google Scholar]
- 42.Balter S, Zanzonico P, Reiss GR, et al. Radiation is not the only risk. AJR Am J Roentgenol. 2011;196:762–767. doi: 10.2214/AJR.10.5982. [DOI] [PubMed] [Google Scholar]
- 43.Dauer LT, Thornton RH, Hay JL, et al. Fears, feelings, and facts: Interactively communicating benefits and risks of medical radiation with patients. AJR Am J Roentgenol. 2011;196:756–761. doi: 10.2214/AJR.10.5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang S. CT scans linked to cancer. The Wall Street Journal, 2009.Available at http://online.wsj.com/news/articles/SB126082398582691047. Accessed November 11, 2013.
- 45.Cancer of the breast—SEER stat fact sheets. Available at http://seer.cancer.gov/statfacts/html/breast.html. Accessed November 13, 2013.
- 46.Roychoudhuri R, Evans H, Robinson D, et al. Radiation-induced malignancies following radiotherapy for breast cancer. Br J Cancer. 2004;91:868–872. doi: 10.1038/sj.bjc.6602084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matesich SMA, Shapiro CL. Second cancers after breast cancer treatment. Semin Oncol. 2003;30:740–748. doi: 10.1053/j.seminoncol.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 48.Gianni L, Gelber S, Ravaioli A, et al. Second non-breast primary cancer following adjuvant therapy for early breast cancer: A report from the International Breast Cancer Study Group. Eur J Cancer. 2009;45:561–571. doi: 10.1016/j.ejca.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schairer C, Mink PJ, Carroll L, et al. Probabilities of death from breast cancer and other causes among female breast cancer patients. J Natl Cancer Inst. 2004;96:1311–1321. doi: 10.1093/jnci/djh253. [DOI] [PubMed] [Google Scholar]
- 50.Senkus E, Kyriakides S, Penault-Llorca F, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi7–vi23. doi: 10.1093/annonc/mdt284. [DOI] [PubMed] [Google Scholar]
- 51.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer, v.1.2015. Available at http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed February 23, 2015.
- 52.Khatcheressian JL, Hurley P, Bantug E, et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:961–965. doi: 10.1200/JCO.2012.45.9859. [DOI] [PubMed] [Google Scholar]
- 53.Hahn EE, Hays RD, Kahn KL, et al. Use of imaging and biomarker tests for posttreatment care of early-stage breast cancer survivors. Cancer. 2013;119:4316–4324. doi: 10.1002/cncr.28363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sperduti I, Vici P, Tinari N, et al. Breast cancer follow-up strategies in randomized phase III adjuvant clinical trials: A systematic review. J Exp Clin Cancer Res. 2013;32:89. doi: 10.1186/1756-9966-32-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Panageas KS, Sima CS, Liberman L, et al. Use of high technology imaging for surveillance of early stage breast cancer. Breast Cancer Res Treat. 2012;131:663–670. doi: 10.1007/s10549-011-1773-y. [DOI] [PubMed] [Google Scholar]
- 56.Rojas MP, Telaro E, Russo A, et al. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst Rev. 2005;(1):CD001768. doi: 10.1002/14651858.CD001768. [DOI] [PubMed] [Google Scholar]
- 57.Verma S, Ewer MS. Is cardiotoxicity being adequately assessed in current trials of cytotoxic and targeted agents in breast cancer? Ann Oncol. 2011;22:1011–1018. doi: 10.1093/annonc/mdq607. [DOI] [PubMed] [Google Scholar]
- 58.Brenner DJ, Shuryak I, Einstein AJ. Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology. 2011;261:193–198. doi: 10.1148/radiol.11102452. [DOI] [PubMed] [Google Scholar]
- 59.Goske MJ, Frush DP, Brink JA, et al. Curbing potential radiation-induced cancer risks in oncologic imaging: Perspectives from the ‘image gently’ and ‘image wisely’ campaigns. Oncology (Williston Park) 2014;28:232–238, 243. [PubMed] [Google Scholar]
- 60.Nüesch E, Trelle S, Reichenbach S, et al. The effects of excluding patients from the analysis in randomised controlled trials: Meta-epidemiological study. BMJ. 2009;339:b3244. doi: 10.1136/bmj.b3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thabane L, Mbuagbaw L, Zhang S, et al. A tutorial on sensitivity analyses in clinical trials: The what, why, when and how. BMC Med Res Methodol. 2013;13:92. doi: 10.1186/1471-2288-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Averbeck D. Does scientific evidence support a change from the LNT model for low-dose radiation risk extrapolation? Health Phys. 2009;97:493–504. doi: 10.1097/HP.0b013e3181b08a20. [DOI] [PubMed] [Google Scholar]
- 63.Tubiana M, Feinendegen LE, Yang C, et al. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology. 2009;251:13–22. doi: 10.1148/radiol.2511080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siegel JA, Stabin MG. Medical imaging: The challenges of radiation risk assessment. J Nucl Med. 2014;55:16N–17N. [PubMed] [Google Scholar]
- 65.Huda W, Sterzik A, Tipnis S, et al. Organ doses to adult patients for chest CT. Med Phys. 2010;37:842–847. doi: 10.1118/1.3298015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wall B, Haylock R, Jansen J et al. Radiation Risks from Medical X-ray Examinations as a Function of the Age and Sex of the Patient. Report HPA-CRCE-028. United Kingdom: Health Protection Agency–Public Health England, 2011.