The primary BC incidence and prevalence data were obtained from the Chinese National Central Cancer Registry. The 2009 to 2011 screening program targeting women aged 35–59 years had a low detection rate that resulted in a second-generation screening program that extended the cohort size and ages screened to 35–64 years.
Keywords: Breast cancer, Incidence, Mortality, Mapping, Screening
Abstract
Background.
As one of its responses to the increasing global burden of breast cancer (BC), China has deployed a national registration and BC screening campaign. The present report describes these programs and the initial results of these national BC control strategies, highlighting the challenges to be considered.
Materials and Methods.
The primary BC incidence and prevalence data were obtained from the Chinese National Central Cancer Registry. MapInfo software was used to map the geographic distribution and variation. The time trends were estimated by the annual percentage of change from 2003 to 2009. The description of the screening plans and preliminary results were provided by the Ministry of Health.
Results.
Chinese cancer registries were primarily developed and activated in the East and Coastal regions of China, with only 12.5% of the registries located in West China. Geographic variation was noted, with the incidence of BC higher in North China than in South China and in urban areas compared with rural areas. Of great interest, these registries reported that the overall BC incidence has been increasing in China, with an earlier age of onset compared with Western countries and a peak incidence rate at age 50. In response to this increasing incidence and early age of onset, BC screening programs assessed 1.46 million women aged 35–59 years, using clinical breast examinations and ultrasound as primary screening tools between 2009 and 2011. The diagnostic rate for this screening program was only 48.0/105 with 440 cases of early stage BC. Early stage BC was detected in nearly 70% of screened patients. Subsequently, a second-generation screening program was conducted that included older women aged 35–64 years and an additional 6 million women were screened.
Conclusion.
The cancer registration system in China has been uneven, with a greater focus on East rather than West China. The data from these registries demonstrate regional variation, an increasing BC incidence, and an early age of onset. The 2009 to 2011 BC screening program targeting women aged 35–59 years had a low detection rate that resulted in a second-generation screening program that extended the cohort size and ages screened to 35–64 years.
Implications for Practice:
Cancer registration has been active in China for decades; however, a national survey of registries has not been routinely reported. This study used MapInfo to describe the reported data and found asymmetric registration activities, geographic variations in breast cancer (BC) burdens, and an increasing incidence with a peak at age 50. The initial Chinese BC screening programs focused on a relatively young population of women aged 35–59 years and had a low detection rate, but 69.7% of patients had early stage BC. Older women were included in the second-generation screening programs, and an additional 6 million women were screened. Consideration of regional variations and age is necessary to optimize the efficiency and utility of BC screening in China, with the ultimate goal to reduce BC mortality.
Abstract
摘要
背景. 作为对全球乳腺癌(BC)负担持续上升的回应措施之一,中国已经部署了全国性乳腺癌登记和乳腺癌筛查工作。本报告对这些项目进行了说明,并且给出了这些国家级乳腺癌控制策略的初步成果,强调了一些应该考虑到的挑战。
材料与方法. 原发性乳腺癌的发病率与患病率数据来自中国肿瘤登记中心。使用MapInfo软件绘制地理分布和变化。根据2003年至2009年的年度频率改变估计时间趋势。筛查计划的说明和初步结果由卫生部提供。
结果. 中国肿瘤登记项目主要在华东和沿海地区开展和进行,只有12.5%的登记点位于华西地区。乳腺癌发病率的地理差异很明显,华北高于华南,城市地区高于农村地区。最值得注意的是,这些登记报告了中国总体乳腺癌发病率正在上升,并且发病年龄早于西方国家,发病率在50岁达到最高峰。鉴于这一发病率升高及发病提早的情况,乳腺癌筛查项目在2009年至2011年使用临床乳腺癌检查和超声作为主要筛查工具,对146万名35 ∼ 59岁的女性进行了评估。该筛查项目发现了440例早期乳腺癌,诊断率仅为48.0/105。而在接受筛查的患者中,近70%发现早期乳腺癌。随后,在年龄较大的35 ∼ 64岁女性以及另外600万名女性中开展了二代筛查项目。
结论. 中国肿瘤登记系统分布不均衡,华东地区比重高于华西地区。来自这些登记中心的数据表明存在地区差异、乳腺癌发病率升高,以及发病时间提早。2009年至2011年乳腺癌筛查项目的目标人群为35 ∼ 59岁女性,由于检出率低,故二代筛查项目扩大了队列样本,并且将筛查年龄调整为35 ∼ 64岁。The Oncologist 2015;20:773–779
Introduction
Breast cancer (BC) has become the most common female cancer worldwide [1]. In 2012, 1.7 million new cases were diagnosed and 522,000 deaths occurred [1]. Although the BC incidence in China is lower than in Western countries, it has been increasing annually [2], with an earlier age of onset [2]. From 2003 to 2009, the number of cancer registries with qualified data reporting to the Chinese National Central Cancer Registry (NCCR) has increased from 35 to 72 [2, 3]. In 2009, 18,132 new BC cases were diagnosed, accounting for 16.8% of cancer cases among 42 million women [3]. Furthermore, BC has become the most common cause of cancer mortality in Chinese women since 2003 [2].
In response to this increasing burden, the Chinese government launched a BC screening program in 2009 in 200 counties, intending to screen 1.2 million women aged 35–59 years within 3 years. The screening consisted of a clinical breast examination (CBE) as the primary detection method, followed by breast ultrasound (US) imaging for women with clinical findings highly suggestive of malignancy (HSM) on the CBE and women with other high risk factors. Positive US findings were evaluated further using mammographic imaging and breast biopsy. After completion of this 3-year program, a second-generation screening program was started in 2012, with modification of the ages of the women screened, the screening methods, and the cohort size.
In the present report, we describe the Chinese screening programs in detail and present the primary results. In addition, we used geographic information system analysis to illustrate and compare the BC burden among the regional registries across China. The geographic distribution of the BC burden in China has not been previously reported, and the variations noted suggest that the ongoing screening strategies conducted in China should consider age and geographic location in their design.
Materials and Methods
The annual incidence and mortality data from 2003 to 2009 were obtained from the NCCR. The cancer categories followed the International Classification of Diseases, version 10, rules. In 2009, 104 cancer registries were required to report both incidence and mortality data to the NCCR, which then was committed to checking the data for completeness, validity, and logical consistency. The percentage of cases pathologically verified, the percentage of cases registered from a death certificate only, and the ratio of the number of deaths from a particular cancer to the number of cases registered during the same period were three key indicators of the completeness and quality of data. NCCR rejected incomplete and unqualified data from 32 regional registries. Ultimately, 72 regional registries submitted complete quality data, 31 from urban areas and 41 from rural areas. The 2009 registry data incorporated information from 43 million males and 42 million females in China.
The present study used the reported data from these 72 registries, which did not include unique identifiers. The Beijing Shijitan Hospital ethical review committee, Capital Medical University, reviewed and approved the present study.
The crude incidence and mortality were estimated as the number of new cases of BC and the number of deaths from BC, respectively, divided by the average population size of the same year. The age-standardized rate was estimated with reference to the Segi world population [4]. The age-standardized mortality-to-incidence (M/I) ratio was calculated as age-standardized mortality divided by the age-standardized incidence. For the largest qualified registries, the geographic variation of age-standardized incidence, mortality and M/I ratio in 2009 was estimated using MapInfo software (Pitney Bowes Software, Stamford, CT, http://www.mapinfo.com), and the National Geomatics Centre of China provided the county-level maps (National Geomatics Centre of China, Beijing, China, http://ngcc.sbsm.gov.cn/).
The time trend of the age-standardized incidence and mortality was estimated using data from 2003, 2004, 2005, 2006, 2007, 2008, and 2009, with the same data inclusive criteria. The Joinpoint Regression Program (National Cancer Institute, Bethesda, MD, http://www.surveillance.cancer.gov/joinpoint) was used to calculate the time trends of incidence and mortality with a model based on the assumption of a minimal number of join points at which statistically significant changes in time trends occur. It was a logarithmic linear model that calculated the difference up to a statistically significant value, using the Monte Carlo permutation test [5]. Thus, the annual percentage of change was calculated to illustrate the time trends of incidence and mortality. The east, west, north, and south regions were defined by the traditional geographic classification in China [6, 7], and the BC burden difference was estimated.
For the initial 2009 to 2011 official BC screening program, the target population of woman and the preliminary results were obtained from the Ministry of Health. In 2009, the Chinese Ministry of Health initiated a 1.2-million subject BC screening program of women aged 35–59 years from rural areas. Among 31 provinces, 200 counties had healthcare workers who met the technical qualification for the proposed screening techniques and had the targeted economic status. Surgeons and nurses provided health education for BC self-examination, obtained written informed consent, and collected data (supplemental online Fig. 1). Subsequently, the women received a CBE and breast US examination, as indicated. Women found to have a suspicious lesion on the CBE received a standardized US examination (supplemental online Fig. 1). In addition, high-risk women (age ≥50 years or positive family BC history) also underwent an US examination, independent of their CBE findings. All women with a suspicious lesion found on the BCE or US study underwent additional diagnostic mammography (supplemental online Fig. 1). Women with a clinically suspicious or mammographically suspicious finding underwent biopsy for histologic evaluation (supplemental online Fig. 1). Women with biopsy findings indicating either premalignant or malignant lesions were encouraged to undergo additional appropriate clinical treatment. Women with biopsy results indicating benign findings were followed up at 1 year (supplemental online Fig. 1). Women with benign findings could enroll in the next round of screening 3 years later. Early stage was defined as BC in stage 0, I, and II.
Statistical Analysis
SPSS, version 15.0, software (SPSS, IBM Corp., Armonk, NY, http://www-01.ibm.com/software/analytics/spss/) was used to conduct the analyses. The differences in the incidence, mortality, and M/I ratio among the areas were estimated using the t test. All analyses were 2-tailed tests, with a significance level of .05.
Results
Breast Cancer Burden in China
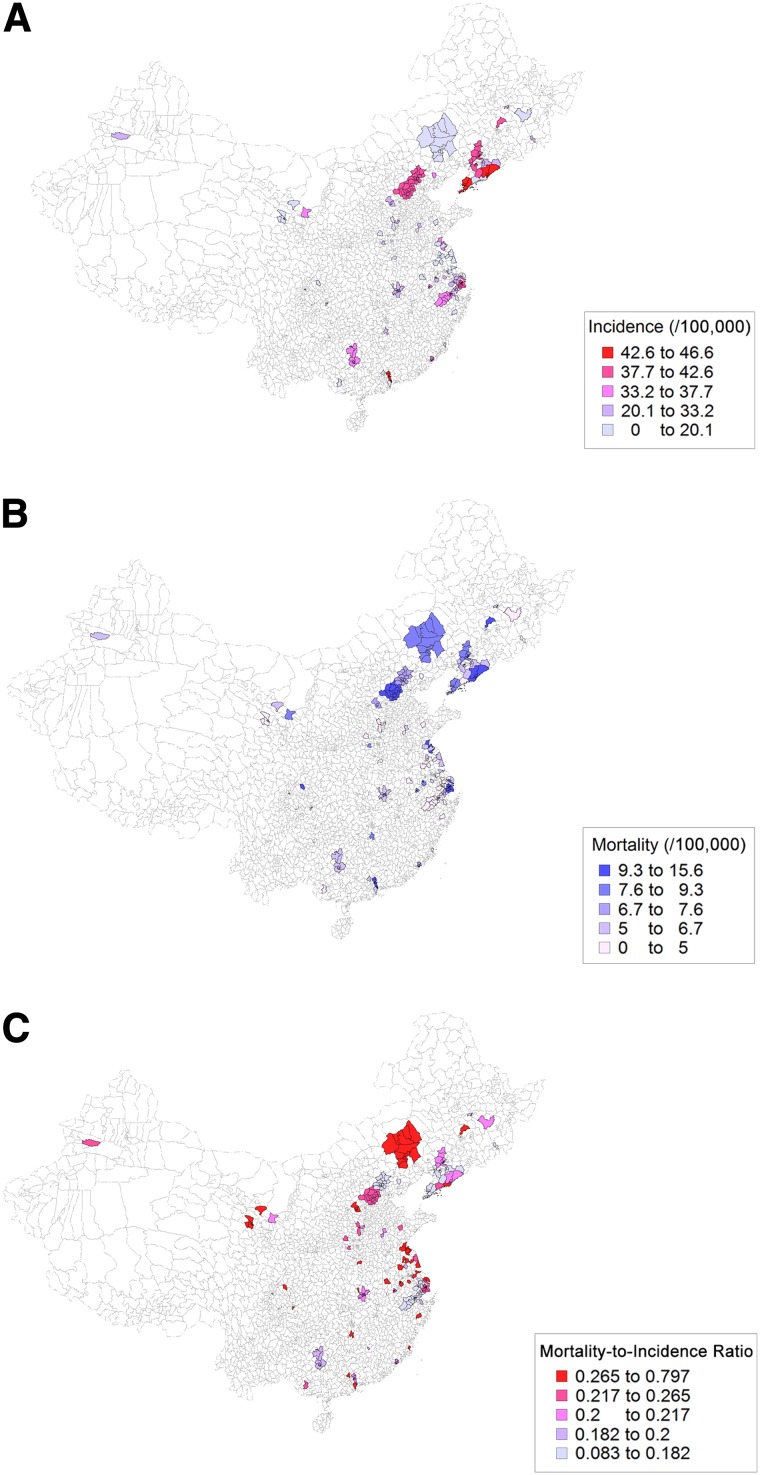
In 2009, 72 cancer registry centers reported the annual incidence and mortality data of female BC in China. Most of the registry centers were located in the East and Coastal China cities, and only 12.5% and 23.6% of the registries were located in West and Central China, respectively (Table 1). Despite the asymmetric distribution of the registry centers, a notable geographic variation was observed in the BC incidence and mortality (Fig. 1A, 1B). The BC incidence ranged from 7.94/105 to 46.55/105, and the mortality ranged from 2.41/105 to 15.51/105. The incidence and mortality were greater in the urban areas than in the rural areas (Table 1). The northern regions had higher BC occurrence than the southern regions (Table 1). In 2009, the age-standardized M/I ratio was 0.25 in the urban areas and 0.30 in the rural areas. Although no difference was found between the urban and rural areas (Table 1), the age-standardized M/I ratio had a remarkable geographic variation, ranging from 0.08 to 0.80 in China (Fig. 1C). The incidence, mortality, and M/I ratio were not significantly different among East, Central, and West China (Table 1).
Table 1.
Breast cancer burdens in women: comparison between geographic areas
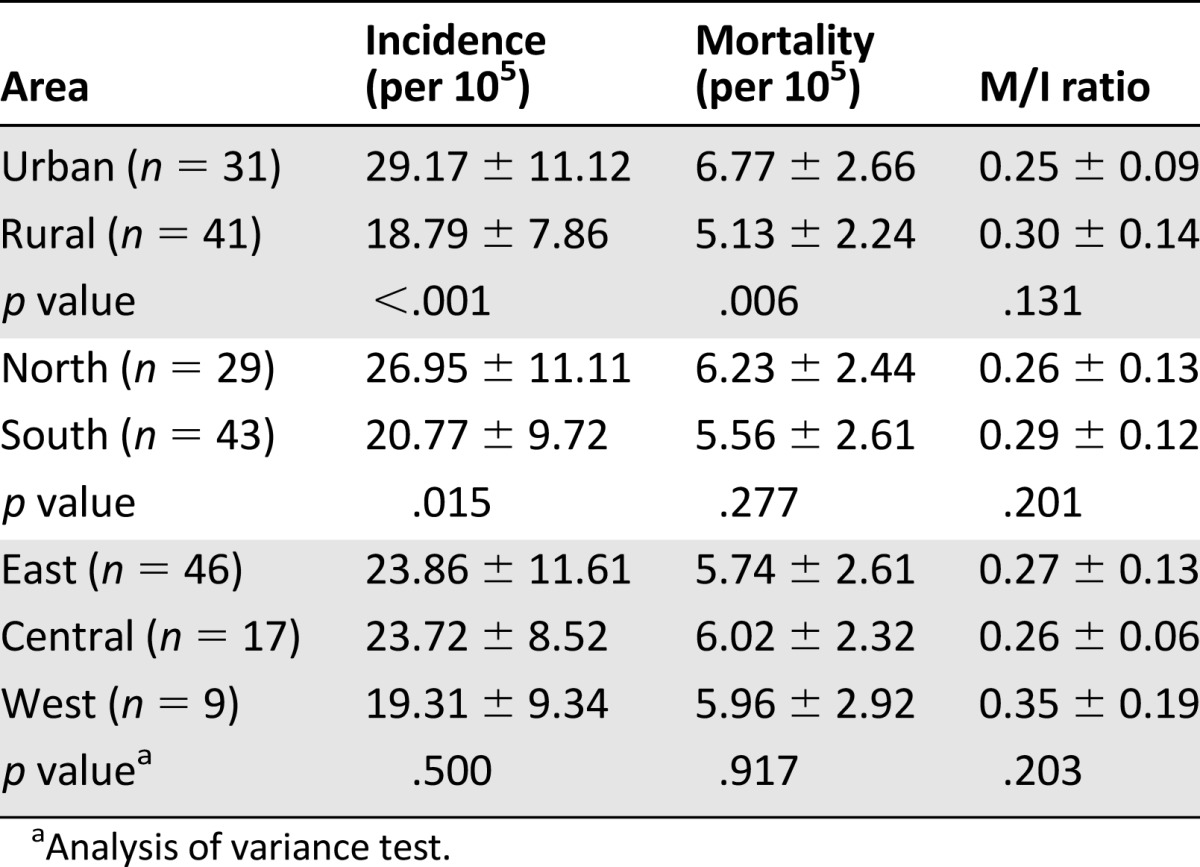
Figure 1.
Age-standardized incidence (A), mortality (B), and mortality-to-incidence ratio (C) of female breast cancer at registries in China.
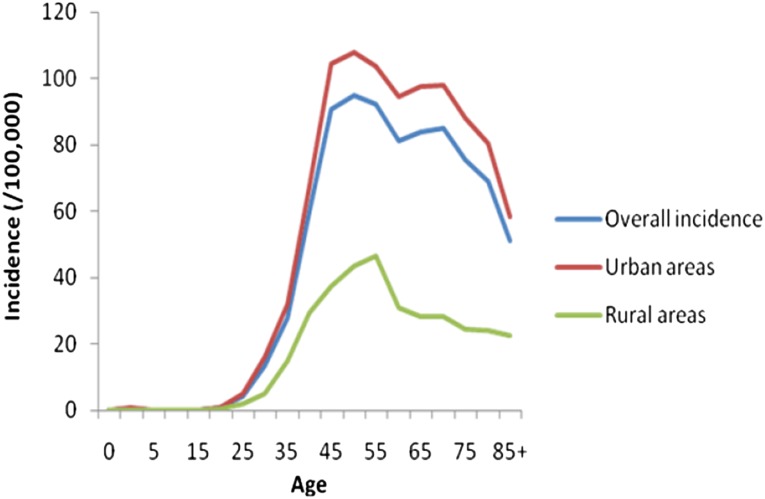
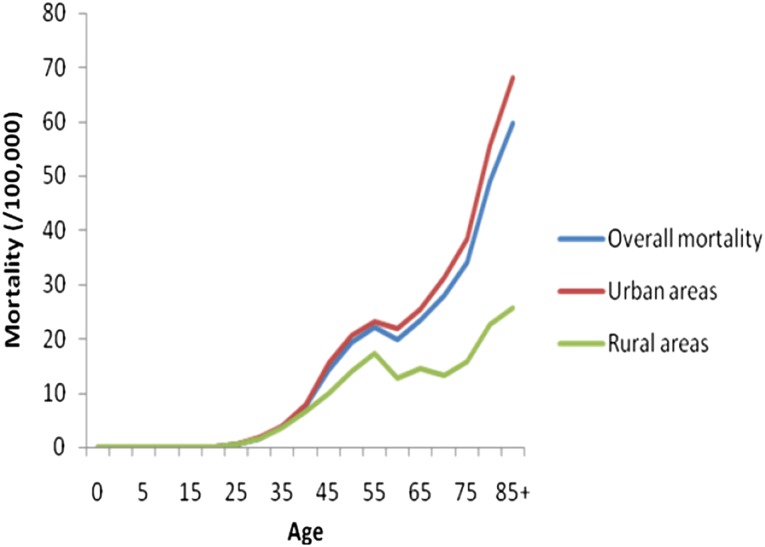
The age-specific incidence of BC increased with age until age 50 years and then declined (Fig. 2). In both urban and rural areas, the age-specific BC incidence peaked at approximately 50 years of age (Fig. 2). However, the age-specific mortality uniformly increased with age, in both urban and rural areas (Fig. 3), without demonstrating any decline.
Figure 2.
Age-specific incidence of female breast cancer in China.
Figure 3.
Age-specific mortality of female breast cancer in China.
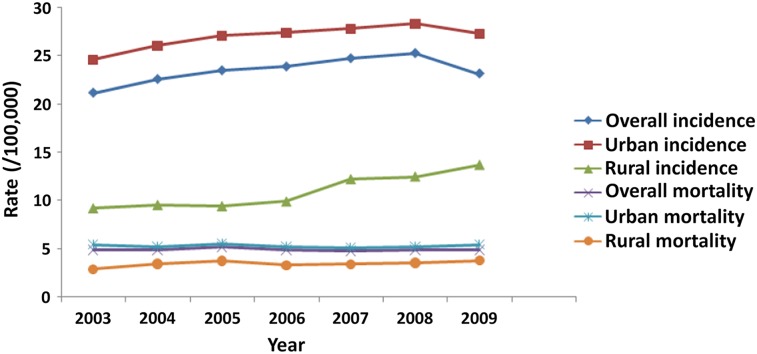
The overall BC incidence demonstrated an upward trend over time, increasing from 21.17/105 in 2003 to 23.16/105 in 2009, with an annual increase of 2% (p = .07) (Fig. 4). The increase in incidence was observed in both urban and rural areas. The incidence in urban areas increased from 24.61/105 to 27.32/105 in 2009 and increased at a rate of 2% annually (p = .02) (Fig. 4). The annual increase in the incidence rate was 7% in rural areas, increasing from 9.22/105 to 13.69/105 in 2009 (p = .002) (Fig. 4). BC mortality did not change significantly in 7 years, at approximately 4.9/105 in urban areas and 5.3/105 in rural areas (Fig. 4).
Figure 4.
Time trends of age-standardized incidence and mortality of breast cancer in females.
Breast Cancer Screening in China
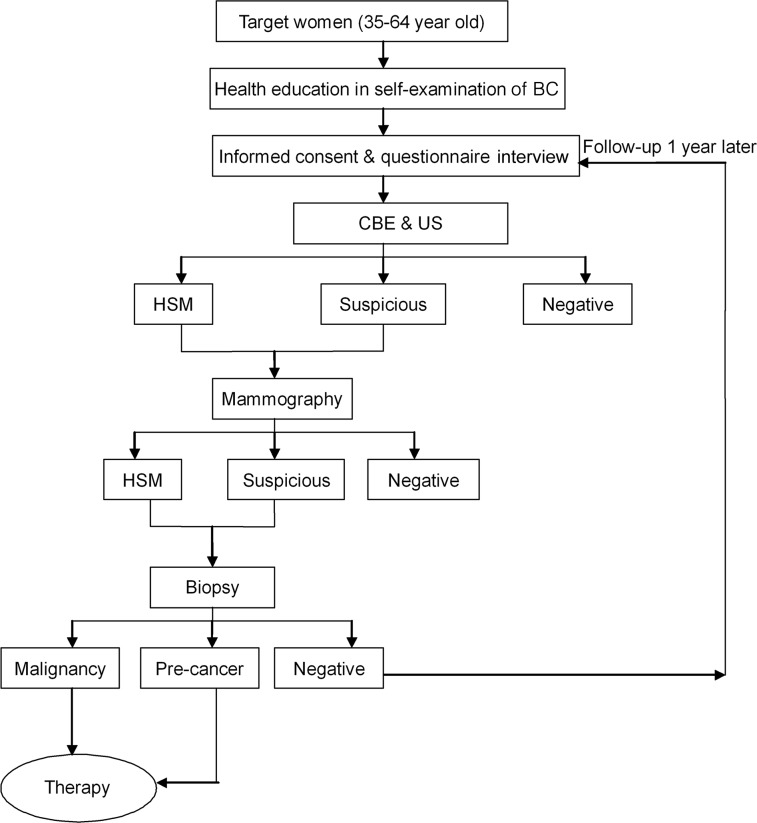
From 2009 to 2011, 1.46 million asymptomatic women participated in the first-generation BC screening program. Of these 1.46 million women, 631 had BC. The detection rate was 48.0/105, including 440 cases in early stage, and the proportion of early-stage detection was 69.7%. The results from the 2009 to 2011 screening program had a lower detection rate than anticipated. Therefore, a second-generation screening program was initiated in 2012. The second-generation screening program modified the screening methods and cohort size and the target population, which included women aged 35–64 years (Fig. 5). The new screening procedures included parallel CBE and breast US (Fig. 5). Women with suspicious findings from either examination were recommended to undergo mammography imaging (Fig. 5). Also, the population to be screened was increased to 6 million.
Figure 5.
Breast cancer screening program in China, 2012. Abbreviations: BC, breast cancer; CBE, clinical breast examination; HSM, highly suggestive malignancy; US, ultrasound.
Discussion
BC is the second most common cancer in the world and the most frequent cancer among women, with an estimated 1.67 million new cases in 2012 (25% of all cancers) [1]. In GLOBOCAN 2012 (International Agency for Research on Cancer, World Health Organization, Lyon, France, http://www.globocan.iarc.fr), the global age-standardized incidence and mortality of BC were 43.3/105 and 12.9/105, respectively, twice as high as the data in China [1]. Nonetheless, the large Chinese population, and the impact of aging in this large population, most likely will result in an increasing incidence and mortality of BC in the future.
As expected, a geographic variation was observed in the incidence and mortality of BC, which has also been previously observed in China, as well as in other Asian countries [8] and the United States [9]. For example, the Mongolia and Jiangsu Provinces in China have reported a higher incidence in urban areas [10, 11]. Similarly, a higher mortality rate in urban areas compared with rural areas was reported from the studies in Brazil [12] and Hubei Province [13]. The maldistribution of cancer registries was a possible explanation for this unbalanced distribution of reported cases; however, other data have suggested a true variation in BC incidence exists. For example, a south-north gradient of variation in BC incidence also exists [8, 9], which is not affected by the variation of geographic distribution of registries. These geographic differences in BC incidence have been previously reported to be related to variations in lifestyle risks. From immigration studies, Asian immigrants to Western countries were reported to have a greater incidence of BC than native Asian people but a lower incidence than Western whites [14, 15]. Asian women who were born and raised in the West had a 60% greater risk than immigrant women born in the East; migrants who lived in the West for a decade or longer had a risk that was 80% higher than that for more recent migrants [16].
Exposure to Western lifestyles had a particularly substantial impact on BC risk in Asian migrants to the United States during their lifetime. One migration study indicated oral contraceptive consumption increased with the time since migration [17]. Zhang et al. [18] reported a 74% decreased risk among women consuming the highest quartile of the vegetable, fruit, soy milk, poultry, and fish dietary pattern relative to the lowest quartile (odds ratio [OR] 0.26, 95% confidence interval [CI] 0.17–0.42) and the refined grain, meat, and pickle pattern was positively associated with risk (OR 2.58, 95% CI 1.53–4.34). The risk exposure changes might play a role in the geographic variation in BC incidence.
Although the incidence is lower, the mortality rate for BC is higher in China. The M/I ratio was 0.21 in China compared with 0.16 in the United States [1]. The higher mortality rate in urban Chinese women might be driven by the higher risk exposure. The higher M/I ratio in rural China might have resulted from unevenness in the access to, or the quality of, the healthcare system. Compared with urban patients, Chinese rural BC patients tended to be diagnosed at an advanced stage and to have tumors with a more aggressive histologic type (p = .0251), larger size (p < .0001), and more metastatic lymph nodes (p < .0001) [19, 20]. In our previous multicenter study, patients from low socioeconomic areas were also more likely to be diagnosed with later stage tumors [21]. These data suggest that a disparity in healthcare services exists between rural and urban China. Nonetheless, the geographic distribution of the cancer registry system was another possible explanation for the skewed incidence and mortality, and a more uniform and consistent reporting system would help address this.
In the present study, the age-specific BC incidence had an age plateau, with a peak at approximately 50 years. This dual-dimensional pattern in China is different from the incidence patterns seen in the United States and European Union. The age-specific BC incidence increased with age in the West [1]. However, among the Asian countries, Japan, Korea, Mongolia, and India all had a younger peak age for BC incidence [1, 10]. Asian women, and Chinese women in particular, were reported to have an earlier age of BC onset [22–24]. In China, the proportion of patients at diagnosis younger than 50 years old was 46%, onefold higher than that in the United States and European Union [1]. The proportion of premenopausal cases was 62.9% in China, much greater than that in the United States and European Union [24]. The higher proportion of younger patients might drive the earlier onset of BC in China. Mousavi-Jarrrahi et al. [25] proposed that the earlier onset in Asia was possibly the result of a cohort effect in the population and the age-specific distribution of BC would follow the West pattern.
From 2003 to 2009, the BC incidence increased in both urban and rural areas; however, the mortality data did not show a significant difference. A similar pattern of change in BC incidence was also reported from the Beijing 1998 to 2007 cancer registry database [26] and China 1998 to 2002 registry database [27]. However, these longer term cancer mortality data have shown an increasing mortality rate among Chinese women [28]. In addition, BC treatment has improved over the past 10-year period, which could have affected mortality [29]. The nonsignificant change in BC mortality might have resulted from the limited period of observation. In addition, the unevenness of reporting to cancer registrations is another possible explanation for the unchanged mortality.
During the past 5 decades, China has undergone significant development and remarkable change in socioeconomic status, altering lifestyles and risk factor exposures to populations. The economic development in China has resulted in a shift from a predominately rural lifestyle to a more western/urban style. These lifestyle changes have correlated with an increase in the body mass index in children and adolescents over these decades [30, 31]. As expected, the prevalence of central obesity among Chinese adults has increased from 1993 to 2009 [32]. The average age of menarche onset in Chinese women also declined [33, 34]. The earlier onset of menarche among Chinese girls was reported to be related to economic development and an increase in body mass index [33]. With the improvement in the economy, the consumption of soy, vegetables, and grains has gradually declined, with a concomitant increase in the consumption of meat and oil among the Chinese people [35]. These lifestyle shifts could possibly have introduced additional risks to Chinese women, resulting in the increasing burden of BC.
In the United States and some European countries, both the BC incidence and the mortality have decreased [1]. In contrast, in Asian regions, BC mortality has had different patterns, increasing in Taiwan, Japan, and Korean and decreasing in Hong Kong and Singapore [36]. The inconsistent trends might be related to the awareness of BC, BC prevention, and the implementation of screening, rather than true differences in incidence.
Although the Chinese government has launched BC screening, the program was applied to only a limited number of women and a substantial number of women remained unscreened, with more than 450 million women at risk of breast cancer in China. The 2009 to 2011 screening programs in China targeted younger women and used CBEs and US studies for BC detection, rather than mammography, which is the primary detection method used in other countries. Using these methods to screen 1.2 million women at 200 screening sites, the detection rate was 48.0/105, and early-stage lesions were detected in 440 cases. A site variation was observed in the screening detection rate [37]. The general detection rate in these 35- to 59-year-old screened rural women was lower than the reported occurrence rate in the same general population of women. In the Shanghai screening program, the detection rate was 194/105, and the detection of early-stage disease was 60% [37]. In the 2009 to 2011 screening program of rural women, the detection rate of early BC was 33.5/105. Among Chinese rural women, the age-specific incidence for those aged 35–59 years ranged from 14.9/105 to 46.7/105 [2]. Among the clinically diagnosed BC cases, the early-stage rate was 74.2% and the rate of stages III and IV was 25.8%. [21]. Thus, the early detection rate in tertiary hospitals of early stage BC among rural women was much lower than urban women. This screening program should be further evaluated for detecting earlier lesions, but reducing overall BC mortality must be the ultimate endpoint for screening.
The screening program chose CBE and US methods rather than mammography for screening for two major reasons. First, the proportion of breast cancer in young and premenopausal women in China is higher, with a peak incidence at age 50. Additionally, the average breast density in Chinese women is higher than that in many Western populations [38]. The sensitivity of US in BC screening has been reported to be higher than that for mammography among women with dense breasts, a small breast volume, and premenopausal status [39]. US imaging, as an adjunct to mammography, in women with dense breasts had an additive beneficial effect [40].
Despite this rationale, 50.8% of Chinese women had fatty breasts [41], and US imaging might not be an ideal screening tool among these women. US was more sensitive than mammography among Chinese women younger than 55 years [39]. Nevertheless, the age of women in the second-generation screening programs was extended to 64 years, and mammography might be a better screening tool for these older women [37]. It is necessary to create a practical guideline for screening site selection and to take actions to support the selected sites to conduct high-quality screening programs. The detection methods warrant investigation in the future. A lower detection rate of screening possibly resulted from the lower incidence of BC in rural Chinese women. It may be necessary to propose a risk assessment tool for selection of those at high risk for screening. Comprehensive and prioritized strategies are needed to improve the participation of high-risk women.
The present study had some potential limitations. Most cancer registry centers located in East and Coastal China, which might have influenced the generalizability of the national incidence and mortality. The registry disparity was possibly related to the geographic inequity of BC burden. In addition, the number of regional cancer registries increased annually; thus, the data in the time trend analysis were derived from the unrestricted registries. Moreover, the screening program was implemented among a limited population, including a large proportion of rural subjects; therefore, more active attention should be given in the forthcoming program.
Conclusion
The cancer registry system in China is currently uneven, and a more balanced distribution of registries is needed, including registries in West and Central China. The BC burden in China showed geographic variations, including differences between rural and urban populations. The incidence of BC is increasing, and the age-specific incidence of BC had a peak at approximately 50 years old. Despite these concerns, the Chinese BC control programs, using CBE and breast US imaging in women aged 35–59 years, had detection rates that were relatively low. The detection tools and strategies for screening BC in China are works in progress and warrant monitoring and additional investigation.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgment
We thank Lin Zhao, who contributed to the data preparation.
Footnotes
For Further Reading: Ching-Hung Lin, Po-Ya Chuang, Chun-Ju Chiang et al. Distinct Clinicopathological Features and Prognosis of Emerging Young-Female Breast Cancer in an East Asian Country: A Nationwide Cancer Registry-Based Study. The Oncologist 2014;19:583–591.
Implications for Practice: The emerging young-female breast cancer in Taiwan are predominantly estrogen receptor-positive (ER+), and young patients (aged 40–49 years) with ER+ breast cancer are uniquely associated with better outcome. The findings imply that estrogen–related environmental changes play an important role in the carcinogenesis of YFBC in Taiwan and other East Asian countries. For clinical practice, this study suggests that clinicians may need to perform an adjustment when using Adjuvant! Online (www.adjuvantonline.com) to evaluate the relapse and mortality risks for young patients with ER+ breast cancer in East Asia.
Author Contributions
Conception/Design: Qing-Kun Song, Hua-Bing Yang, Jun Ren, Herbert K. Lyerly
Provision of study material or patients: Qing-Kun Song, Xiao-Li Wang, Xin-Na Zhou, Yu-Chen Li, Jiang-Ping Wu
Collection and/or assembly of data: Qing-Kun Song, Xiao-Li Wang, Xin-Na Zhou, Hua-Bing Yang, Jiang-Ping Wu, Herbert K. Lyerly
Data analysis and interpretation: Qing-Kun Song, Yu-Chen Li, Jun Ren, Herbert K. Lyerly
Manuscript writing: Qing-Kun Song, Jun Ren, Herbert K. Lyerly
Final approval of manuscript: Qing-Kun Song, Xiao-Li Wang, Xin-Na Zhou, Hua-Bing Yang, Yu-Chen Li, Jiang-Ping Wu, Jun Ren, Herbert K. Lyerly
Disclosures
The authors indicated no financial relationships.
References
- 1.Ferlay J, Soerjomataram I, Ervik M et al. Globocan 2012 v1.0, cancer incidence and mortality worldwide. IARC Cancerbase no. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available at http://www.globocan.Iarc.Fr. Accessed July 7, 2014.
- 2.Zhao P, Chen W, Kong L. Cancer Incidence and Mortality in China, 2003–2007. Beijing: Military Medical Science Press; 2011. [Google Scholar]
- 3.He J, Chen W. Chinese Cancer Registry Annual Report 2012. Beijing: Military Medical Science Press; 2013. [Google Scholar]
- 4.Segi M. Cancer Mortality for Selected Sites in 24 Countries (1950-57) Sendai, Japan: Department of Public Health, Tohoku University of Medicine; 1960. [Google Scholar]
- 5.Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Wikipedia. Northern and Southern China; 2014. Available at http://www.en.Wikipedia.Org/wiki/northern_and_southern_china. Accessed January 15, 2015.
- 7.CNKI. How to distinguish east, central and west China; 2000. Available at http://www.Cnki.Com.Cn/article/cjfdtotal-tian200007015.htm. Accessed January 15, 2015.
- 8.Shin HR, Joubert C, Boniol M, et al. Recent trends and patterns in breast cancer incidence among Eastern and Southeastern Asian women. Cancer Causes Control. 2010;21:1777–1785. doi: 10.1007/s10552-010-9604-8. [DOI] [PubMed] [Google Scholar]
- 9.Mandal R, St-Hilaire S, Kie JG, et al. Spatial trends of breast and prostate cancers in the United States between 2000 and 2005. Int J Health Geogr. 2009;8:53. doi: 10.1186/1476-072X-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troisi R, Altantsetseg D, Davaasambuu G, et al. Breast cancer incidence in Mongolia. Cancer Causes Control. 2012;23:1047–1053. doi: 10.1007/s10552-012-9973-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu LZ, Han RQ, Zhou JY, et al. Incidence and mortality of female breast cancer in Jiangsu, China. Asian Pac J Cancer Prev. 2014;15:2727–2732. doi: 10.7314/apjcp.2014.15.6.2727. [DOI] [PubMed] [Google Scholar]
- 12.Gonzaga CM, Freitas-Junior R, Souza MR, et al. Disparities in female breast cancer mortality rates between urban centers and rural areas of Brazil: Ecological time-series study. Breast. 2014;23:180–187. doi: 10.1016/j.breast.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Cheng L, Tan L, Zhang L, et al. Chronic disease mortality in rural and urban residents in Hubei Province, China, 2008-2010. BMC Public Health. 2013;13:713. doi: 10.1186/1471-2458-13-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo W, Birkett NJ, Ugnat AM, et al. Cancer incidence patterns among Chinese immigrant populations in Alberta. J Immigr Health. 2004;6:41–48. doi: 10.1023/B:JOIH.0000014641.68476.2d. [DOI] [PubMed] [Google Scholar]
- 15.Blesch KS, Davis F, Kamath SK. A comparison of breast and colon cancer incidence rates among native Asian Indians, US immigrant Asian Indians, and whites. J Am Diet Assoc. 1999;99:1275–1277. doi: 10.1016/S0002-8223(99)00313-2. [DOI] [PubMed] [Google Scholar]
- 16.Ziegler RG, Hoover RN, Pike MC, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 17.Ursin G, Wu AH, Hoover RN, et al. Breast cancer and oral contraceptive use in Asian-American women. Am J Epidemiol. 1999;150:561–567. doi: 10.1093/oxfordjournals.aje.a010053. [DOI] [PubMed] [Google Scholar]
- 18.Zhang CX, Ho SC, Fu JH, et al. Dietary patterns and breast cancer risk among Chinese women. Cancer Causes Control. 2011;22:115–124. doi: 10.1007/s10552-010-9681-8. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Bu Y, Gao H. Rural-urban disparities of breast cancer patients in China. Med Oncol. 2013;30:387. doi: 10.1007/s12032-012-0387-5. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen-Pham S, Leung J, McLaughlin D. Disparities in breast cancer stage at diagnosis in urban and rural adult women: A systematic review and meta-analysis. Ann Epidemiol. 2014;24:228–235. doi: 10.1016/j.annepidem.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Li J, Zheng S, et al. Breast cancer stage at diagnosis and area-based socioeconomic status: A multicenter 10-year retrospective clinical epidemiological study in China. BMC Cancer. 2012;12:122. doi: 10.1186/1471-2407-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemminki K, Mousavi SM, Sundquist J, et al. Does the breast cancer age at diagnosis differ by ethnicity? A study on immigrants to Sweden. The Oncologist. 2011;16:146–154. doi: 10.1634/theoncologist.2010-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwong A, Cheung P, Chan S, et al. Breast cancer in Chinese women younger than age 40: Are they different from their older counterparts? World J Surg. 2008;32:2554–2561. doi: 10.1007/s00268-008-9589-6. [DOI] [PubMed] [Google Scholar]
- 24.Zheng S, Bai JQ, Li J, et al. The pathologic characteristics of breast cancer in China and its shift during 1999-2008: A national-wide multicenter cross-sectional image over 10 years. Int J Cancer. 2012;131:2622–2631. doi: 10.1002/ijc.27513. [DOI] [PubMed] [Google Scholar]
- 25.Mousavi-Jarrrahi SH, Kasaeian A, Mansori K, et al. Addressing the younger age at onset in breast cancer patients in Asia: An age-period-cohort analysis of fifty years of quality data from the International Agency for Research on Cancer. ISRN Oncol. 2013;2013:429862. doi: 10.1155/2013/429862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang N, Zhu WX, Xing XM, et al. Time trends of cancer incidence in urban Beijing, 1998-2007. Chin J Cancer Res. 2011;23:15–20. doi: 10.1007/s11670-011-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei T, Mao WM, Yang HJ, et al. [Study on cancer incidence through the cancer registry program in 11 cities and counties, China] Zhonghua Liu Xing Bing Xue Za Zhi. 2009;30:1165–1170. [PubMed] [Google Scholar]
- 28.Guo P, Huang ZL, Yu P, et al. Trends in cancer mortality in china: An update. Ann Oncol. 2012;23:2755–2762. doi: 10.1093/annonc/mds069. [DOI] [PubMed] [Google Scholar]
- 29.Zhang B, Song Q, Zhang B, et al. A 10-year (1999 ~ 2008) retrospective multi-center study of breast cancer surgical management in various geographic areas of China. Breast. 2013;22:676–681. doi: 10.1016/j.breast.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Ji CY, Chen TJ, Sun X. Secular changes on the distribution of body mass index among Chinese children and adolescents, 1985-2010. Biomed Environ Sci. 2013;26:520–530. doi: 10.3967/0895-3988.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Dong B, Wang HJ, Wang Z, et al. Trends in blood pressure and body mass index among Chinese children and adolescents from 2005 to 2010. Am J Hypertens. 2013;26:997–1004. doi: 10.1093/ajh/hpt050. [DOI] [PubMed] [Google Scholar]
- 32.Du T, Sun X, Yin P, et al. Increasing trends in central obesity among Chinese adults with normal body mass index, 1993-2009. BMC Public Health. 2013;13:327. doi: 10.1186/1471-2458-13-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin F, Shao H, Tao M. Trends of menarcheal age in women in the reproductive age and menopause in Shanghai. Gynecol Obstet Invest. 2013;76:228–232. doi: 10.1159/000355695. [DOI] [PubMed] [Google Scholar]
- 34.Chen H, Shu HM, Xiong M, et al. Survey on age of menarche in 56,924 women recruited from Pudong district of Shanghai [in Chinese] Zhonghua Fu Chan Ke Za Zhi. 2009;44:500–503. [PubMed] [Google Scholar]
- 35.National Bureau of Statistics of the People’s Republic of China. China Statistics Yearbook 2013. Available at http://www.Stats.Gov.Cn/tjsj/ndsj/2013/indexch.htm. Accessed July 8, 2014.
- 36.Shin HR, Boniol M, Joubert C, et al. Secular trends in breast cancer mortality in five East Asian populations: Hong Kong, Japan, Korea, Singapore and Taiwan. Cancer Sci. 2010;101:1241–1246. doi: 10.1111/j.1349-7006.2010.01519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mo M, Liu GY, Zheng Y, et al. Performance of breast cancer screening methods and modality among Chinese women: A report from a society-based breast screening program (SBSP) in Shanghai. Springerplus. 2013;2:276. doi: 10.1186/2193-1801-2-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maskarinec G, Meng L, Ursin G. Ethnic differences in mammographic densities. Int J Epidemiol. 2001;30:959–965. doi: 10.1093/ije/30.5.959. [DOI] [PubMed] [Google Scholar]
- 39.Wang FL, Chen F, Yin H, et al. Effects of age, breast density and volume on breast cancer diagnosis: A retrospective comparison of sensitivity of mammography and ultrasonography in China’s rural areas. Asian Pac J Cancer Prev. 2013;14:2277–2282. doi: 10.7314/apjcp.2013.14.4.2277. [DOI] [PubMed] [Google Scholar]
- 40.Scheel JR, Lee JM, Sprague BL, et al. Screening ultrasound as an adjunct to mammography in women with mammographically dense breasts. Am J Obstet Gynecol. 2015;212:9–17. doi: 10.1016/j.ajog.2014.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai H, Yan Y, Wang P, et al. Distribution of mammographic density and its influential factors among Chinese women. Int J Epidemiol. 2014;43:1240–1251. doi: 10.1093/ije/dyu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.