The efficacy of different treatments in elderly patients with epidermal growth factor receptor (EGFR)-mutated lung cancer was studied. The records of pulmonary adenocarcinoma patients treated between 2010 and 2013 were retrospectively reviewed. Elderly patients with disease progression after first-line EGFR-tyrosine kinase inhibitor treatment can receive chemotherapy and have a response rate similar to that of younger patients.
Keywords: Adenocarcinoma, Elderly, Epidermal growth factor receptor, Tyrosine kinase inhibitors
Abstract
Background.
Lung cancer is frequently a disease of elderly patients. However, these patients are often treated less actively owing to a higher comorbidity rate and poor performance status. The efficacy of different treatments in elderly patients with epidermal growth factor receptor (EGFR)-mutated lung cancer is still unknown.
Materials and Methods.
We retrospectively reviewed the records of our pulmonary adenocarcinoma patients treated between 2010 and 2013. Data on patient age, type of tumor EGFR mutation, response to first-line EGFR-tyrosine kinase inhibitor (TKI) treatment, type of salvage chemotherapy, and efficacy of EGFR-TKI and salvage chemotherapy were collected.
Results.
In all, 473 of 1,230 stage IV adenocarcinoma patients had an EGFR mutation, and 330 of them received first-line TKI treatment. Of the 330 patients, 160 were ≥70 years old (elderly group) and 170 were <70 years old (younger group). The response rate and progression-free survival (PFS) with first-line TKI treatment were not significantly different. The elderly group had shorter median survival. A total of 107 patients received salvage chemotherapy after first-line EGFR-TKI treatment: 45 in the elderly group and 62 in the younger group. Their response rate and PFS were not significantly different; however, the younger group had longer median survival. Additional subgroup analysis showed that younger patients who received platinum-based chemotherapy or combination chemotherapy had better median survival than did the elderly patients. The PFS was longer among younger patients receiving a platinum-based regimen than that among the elderly patients.
Conclusion.
Elderly patients with disease progression after first-line EGFR-TKI treatment can receive chemotherapy and have a response rate similar to that of younger patients.
Implications for Practice:
The aim of the present study was to investigate the efficacy of first-line epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) treatment in elderly patients and the outcomes of subsequent salvage chemotherapy after disease progression. The most important finding was that elderly patients with disease progression after first-line EGFR-TKI treatment can receive salvage chemotherapy and have a response rate similar to that of younger patients who received salvage chemotherapy.
Abstract
摘要
背景. 肺癌是老年患者中的常见疾病。然而,这些患者常因共病率较高以及体能状态较差而得不到更积极的治疗。目前还未明确不同治疗在表皮生长因子受体(EGFR)突变肺癌老年患者中的有效性。
材料与方法. 我们对2010年至2013年在本中心接受治疗的肺腺癌患者资料进行回顾性评价。收集数据包括患者年龄、肿瘤EGFR突变类型、一线EGFR酪氨酸激酶抑制剂(TKI)治疗反应、挽救性化疗类型,以及EGFR-TKI和挽救性化疗的有效性。
结果. 总体而言,1 230例IV期腺癌患者中有473例携带EGFR突变,其中330例接受了一线TKI治疗。330例患者中,160例≥ 70岁(老年组),170例˂ 70岁(较年轻组)。两组间一线TKI治疗的缓解率和无进展生存(PFS)的差异无统计学意义。老年组中位生存期更短。共107例患者在一线EGFR-TKI治疗后接受了挽救性化疗,其中老年组45例,较年轻组62例。两组的缓解率和PFS方面的差异均无统计学意义;但较年轻组中位生存期更长。另外的亚组分析显示,曾接受过以铂类为基础的化疗或联合化疗的年轻患者生存期长于老年患者。正在接受以铂类为基础的方案的较年轻患者PFS长于老年患者。
结论. 在一线EGFR-TKI治疗后疾病进展的老年患者可以接受化疗,且缓解率与年轻患者相似。The Oncologist 2015;20:758–766
Introduction
Lung cancer is the leading cause of cancer death worldwide. Non-small-cell lung cancer (NSCLC) accounts for 85% of all lung cancer cases [1]. Despite the advances in NSCLC treatment, such as third-generation chemotherapy, adjuvant chemotherapy, maintenance therapy, epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) therapy, and anaplastic lymphoma kinase-TKI therapy, the 5-year survival rate is only approximately 5%–20% [2–4].
Lung cancer is mostly a disease of the elderly. Previous studies have reported that the lung cancer incidence rate is higher in older individuals, with one third of these patients older than 70 years [2]. In addition, elderly patients with lung cancer are often treated less actively because of their comorbidities [5]. Kuo et al. reported that older patients frequently did not receive treatment that was as aggressive as that given to younger patients and had poorer outcomes [6]. Therefore, clinical studies of elderly lung cancer patients who are intent on improving their survival and/or quality of life are important.
After the Iressa Pan-Asia study and several similar prospective studies showed positive results with EGFR-TKI treatment, it was suggested that patients with tumor EGFR-mutated lung cancer should receive EGFR-TKI treatment instead of chemotherapy as their initial treatment [7]. EGFR-TKI treatment was also a good choice for elderly patients with EGFR mutations, because with this treatment, their quality of life improved, the progression-free survival (PFS) was longer, and life-threatening toxicities were fewer than with traditional chemotherapy. Erlotinib monotherapy was reported recently to be relatively well tolerated by elderly patients with advanced NSCLC [8, 9]. Chen et al. also reported that erlotinib was more effective than oral vinorelbine in elderly patients with an EGFR mutation [10]. Although many studies have focused on first-line EGFR-TKI treatment for elderly patients, the treatment efficacy of second-line salvage chemotherapy for elderly patients remains unknown.
Many studies have discussed which treatment should be the optimal first-line chemotherapy for elderly patients unselected for an EGFR mutation. For example, single-agent vinorelbine treatment showed better overall survival (OS) and quality of life than the best supportive care as first-line treatment of elderly patients; paclitaxel plus carboplatin treatment resulted in better survival than single-agent gemcitabine or vinorelbine treatment; and subgroup analysis of pemetrexed plus carboplatin treatment showed better efficacy than pemetrexed treatment alone [11, 12]. A phase III study of NSCLC patients with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 2 (74 of the 205 study patients [36.1%] were 70 years old or older) also showed pemetrexed plus carboplatin had better efficacy than pemetrexed alone [13]. Also, a subgroup analysis compared the efficacy and toxicity of second-line chemotherapy in elderly NSCLC patients previously treated with chemotherapy [14]. The controversy over which chemotherapy regimen or agent to use has now been extended to elderly patients with acquired resistance after first line EGFR-TKI treatment. The aim of the present study was to investigate the efficacy of first-line EGFR-TKI treatment in elderly patients and the outcomes of subsequent salvage chemotherapy after disease progression.
Materials and Methods
Study Design and Patients
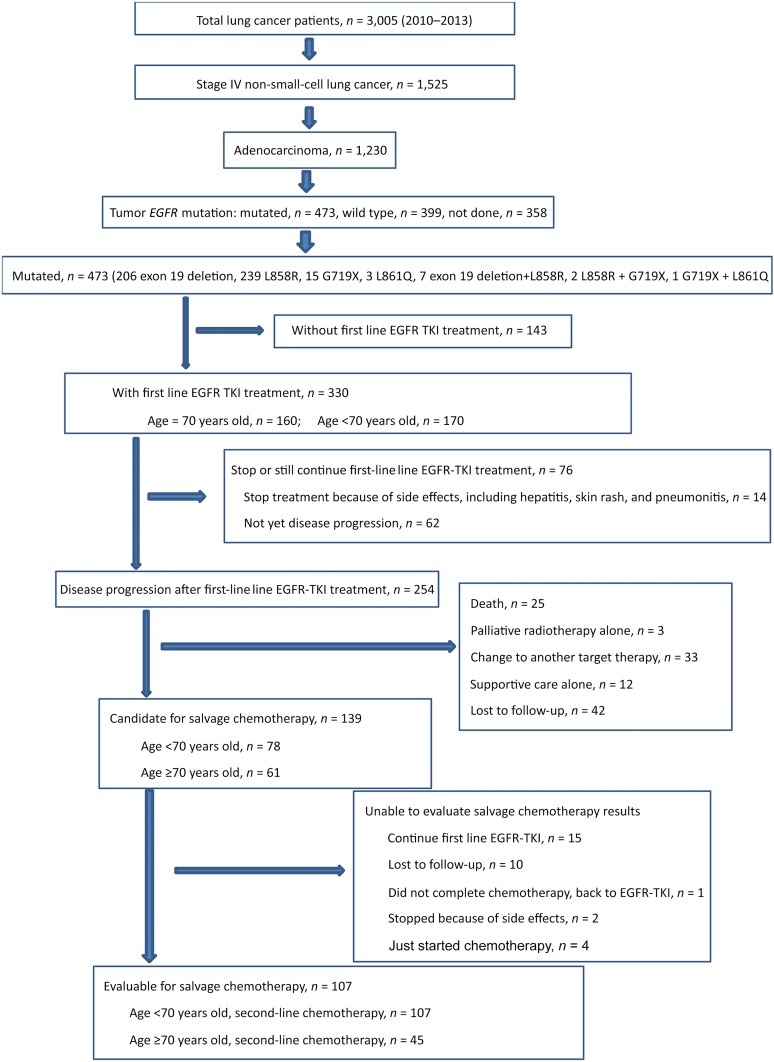
We retrospectively reviewed the medical records and imaging files of lung cancer patients diagnosed and treated between 2010 and 2013 in our hospital. We enrolled those patients who had stage IV adenocarcinoma (American Joint Committee on Cancer staging system, 7th edition) and a documented tumor EGFR mutation who had received first-line EGFR-TKI therapy in our hospital. Patients who stopped first-line EGFR-TKI treatment because of side effects and those still taking an EGFR-TKI without disease progression were included in the EGFR-TKI efficacy analysis but were excluded from the salvage chemotherapy analysis (Fig. 1). We also recorded the treatment strategies used after first-line EGFR-TKI therapy, including chemotherapy and the regimens used, radiotherapy alone, and supportive care alone. Patients who died, were lost to follow-up, who asked to continue or change to another targeted therapy, or who asked to stop salvage chemotherapy were all recorded. Among the patients who received second-line salvage chemotherapy, we excluded those who continued first-line EGFR-TKI, those lost to follow-up, those who stopped because of side effects, and those who had just started chemotherapy. The patients were divided into two groups according to age. One group included patients 70 years old or older (older group) and one included patients younger than 70 years old (younger group). To evaluate the effects of salvage chemotherapy after first-line EGFR-TKI treatment in these two groups, we retrospectively reviewed the cohorts from different perspectives. The institutional ethical review board of Taipei Veterans General Hospital approved the study (VGHIRB No. 2014-05-008AC). The clinical data, including age, gender, ECOG PS, smoking history, type of mutation, first-line EGFR-TKI used, regimens of salvage chemotherapy, treatment cycles, time of disease progression, and OS were recorded.
Figure 1.
CONSORT diagram for the present study.
Abbreviations: EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
Efficacy Evaluation
A chest computed tomography scan (including the liver and adrenal glands) was performed within 3 weeks before starting the targeted therapy and chemotherapy and every 2 to 3 months thereafter or when confirmation of the treatment response or disease progression was required. The type of treatment response was accessed from the chest computed tomography scan using the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) [15]. PFS with targeted therapy and chemotherapy was defined as the duration from the date of initiating the targeted therapy or chemotherapy to the earliest sign of disease progression, as determined using the RECIST or death from any cause. If disease progression had not occurred at the last follow-up visit, PFS was considered to have been censored at that time. OS was defined as the period from the beginning of targeted therapy or chemotherapy to the date of death. OS was censored when the patients were still alive at the last follow-up visit.
EGFR Mutation Analysis
Tumor EGFR mutations were examined using one of two methods. Before the end of 2010, Sanger DNA sequencing was used. All the sequence variations were confirmed by multiple, independent polymerase chain reaction amplifications and repeated sequencing reactions. Beginning in 2011, most specimens were tested using the Scorpion amplification refractory mutation system method.
Statistical Analysis
All categorical variables were analyzed using chi-square tests. Two-sided t tests were used for continuous variables when comparing two groups. The targeted therapy and chemotherapy response rates were compared between the two groups. The median PFS and OS were calculated using the Kaplan-Meier method and compared using the log-rank test. Cox regression analysis was used for multivariate PFS and OS analysis. All statistical analyses were performed using SPSS software, version 19.0 (IBM Corp., Armonk, NY, http://www-01.ibm.com/software/analytics/spss/).
Results
Patients
Between 2010 and 2013, 1,230 patients in our hospital had stage IV adenocarcinoma. The results of tumor EGFR analysis were available for 872 patients. The 473 patients with EGFR mutations had classic mutations (exon 19 deletion and L858R) and atypical mutations (G719X, L861Q). Of the 473 patients, 330 received first-line EGFR-TKI treatment. Of these patients, 160 were ≥70 years old (older group) and 170 were <70 years old (younger group). Of the 330 patients, 254 experienced disease progression; 139 patients received salvage chemotherapy, of whom, 107 were eligible for chemotherapy efficacy evaluations. Of these 107 patients, 45 were in the older group and 62 in the younger group (Fig. 1).
First-Line EGFR-TKI Treatment
Of the 330 patients who received first-line EGFR-TKI treatment, those in the younger group (p = .001) had a better performance status. However, no difference in gender, smoking prevalence, or EGFR mutation type was noted between the 2 age groups (Table 1). The response rate for the older patients was 72.6% and was 80.0% for the younger patients (p = .209). Among the patients who received first-line EGFR-TKIs, the rate of completing treatment (defined as receiving first-line EGFR-TKIs for more than 3 months) was not significantly different (81.8% vs. 84.5%; p = .555). The mortality rate during EGFR-TKI treatment or before salvage therapy did not differ between the 2 groups (9.4% vs. 5.9%; p = .231). In addition, 15.6% of the older patients and 10.6% of the younger patients were lost to follow-up (p = .174). Our results also showed that 3.1% of the older patients and 5.3% of the younger patients stopped treatment because side effects from the EGFR-TKI (p = .329). Approximately 38.1% of the older patients and 45.9% of the younger patients who had received EGFR-TKI treatment could receive second-line salvage therapy after their disease had progressed.
Table 1.
First-line epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor response in 330 EGFR-mutated patients
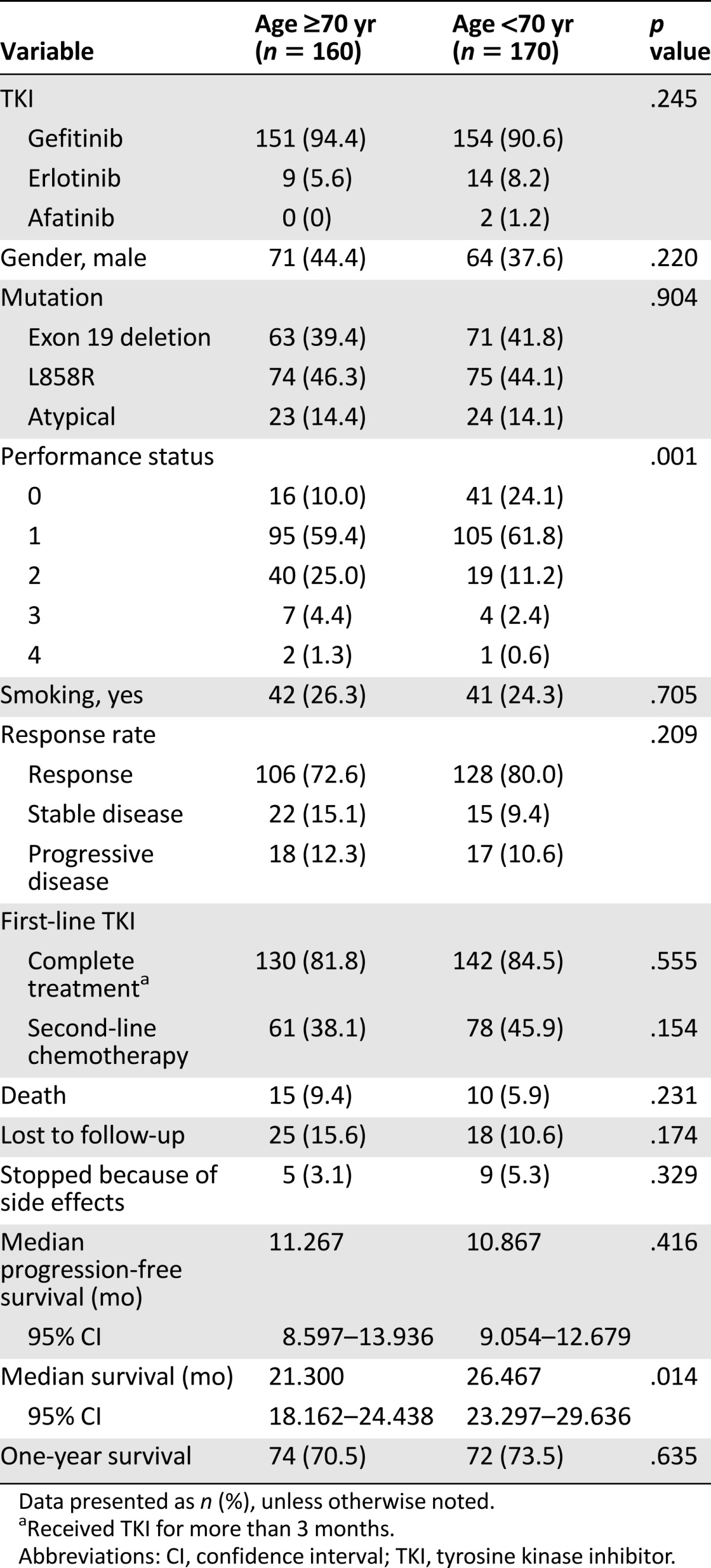
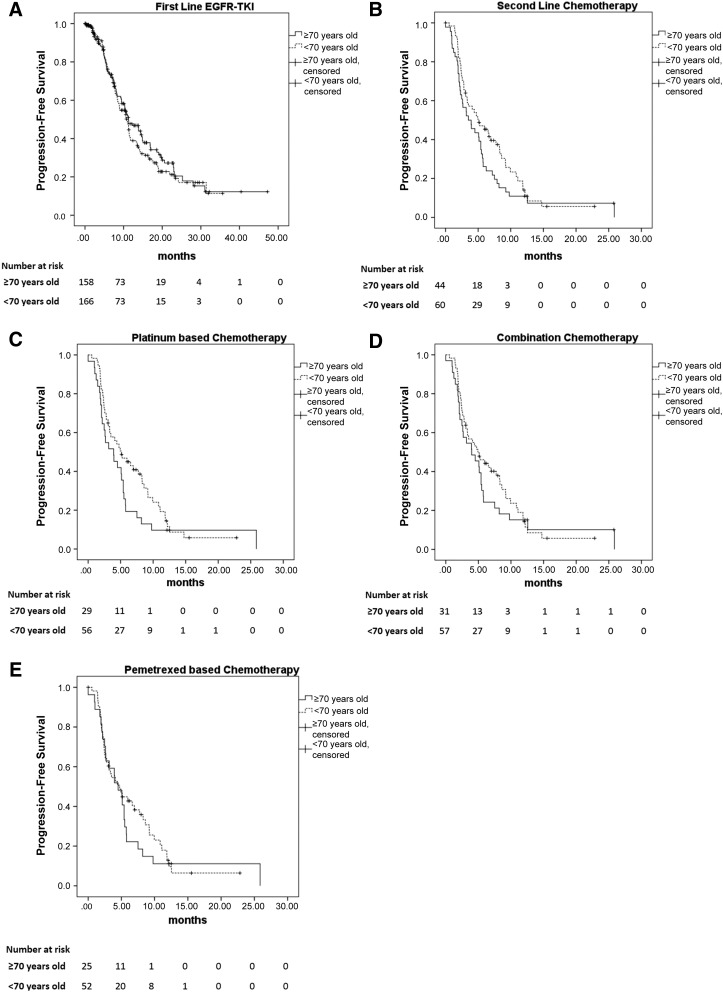
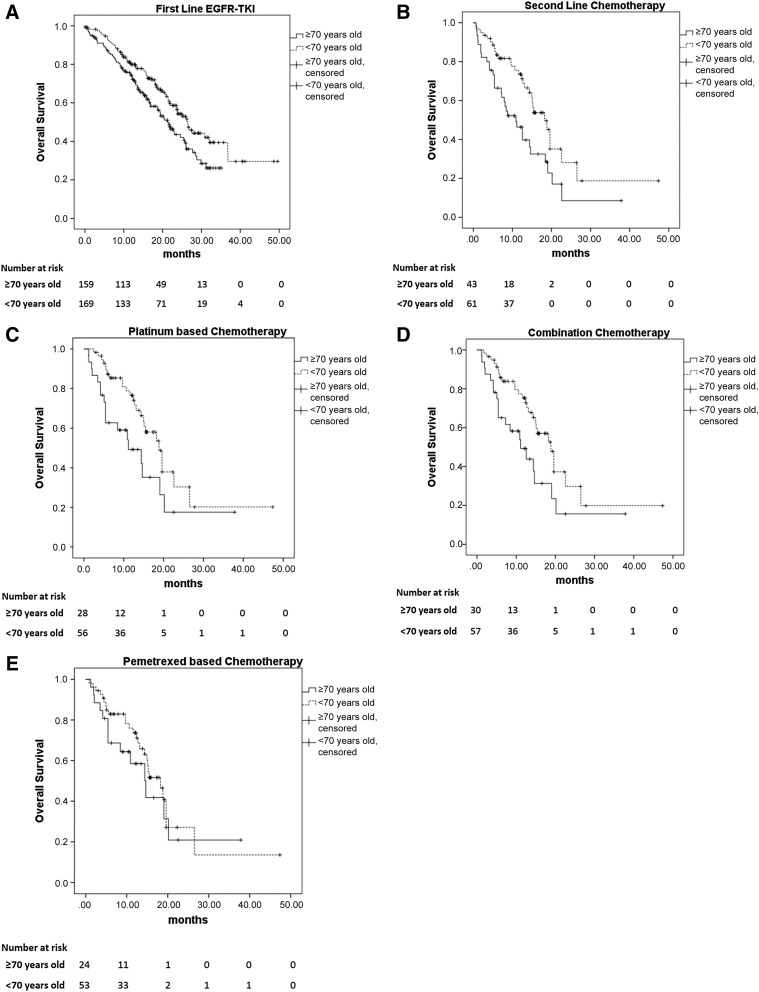
No difference was seen in the sites of disease progression between the older and younger patients (p = .736). Also, no significant difference was found in PFS between the 2 age groups (median PFS 11.3 months in the older group vs. 10.9 months in the younger group; p = .416; Fig. 2A). The median survival was significantly longer for the younger patients than for the older patients (median survival, 26.5 vs. 21.3 months; p = .014; Fig. 3A). The 1-year survival rate was 70.5% for the older patients and 73.5% for the younger patients (p = .635; Table 1).
Figure 2.
Progression-free survival (PFS) of patients who received first-line EGFR-TKI and salvage chemotherapy after failure of first-line EGFR-TKI treatment. (A): A total of 330 patients received first-line EGFR-TKI therapy. No significant difference was seen in PFS between the two age groups (median PFS, 11.3 months in the older group and 10.9 months in the younger group; p = .416). (B): No significant difference was found in PFS between the older and younger patients receiving second-line salvage chemotherapy (3.5 vs. 5.1 months; p = .156). (C): PFS was significantly longer for the younger patients than for the older patients receiving platinum-based chemotherapy (5.1 vs. 3.2 months; p = .033). (D): No significant difference was seen in PFS between the two age groups that received combination therapy (4.9 vs. 3.9 months; p = .474). (E): No significant difference was seen in PFS between the 2 age groups that received pemetrexed-based therapy (4.0 vs. 4.7 months; p = .233).
Abbreviation: EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor.
Figure 3.
The median survival of patients who received first-line EGFR-TKI therapy and salvage chemotherapy after failure of first-line EGFR-TKI treatment. (A): A total of 330 patients received first-line EGFR-TKI therapy. The median survival was significantly longer for the younger patients than for the older patients (median survival, 26.5 vs. 21.3 months; p = .014). (B): The median survival was longer for the younger patients than for the older patients receiving second-line salvage chemotherapy (18.2 vs. 10.9 months; p = .002). (C): The overall survival (OS) was longer in the younger group than in the older group with platinum-based chemotherapy (18.8 vs. 11.1 months; p = .004). (D): The OS was longer in the younger group than in the older group when receiving combination therapy (18.8 vs. 11.1 months; p = .003). (E): No significant difference was found in the median survival between the 2 age groups that received pemetrexed-based therapy (14.4 vs. 18.2 months; p = .162).
Abbreviation: EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor.
Second-Line Salvage Chemotherapy
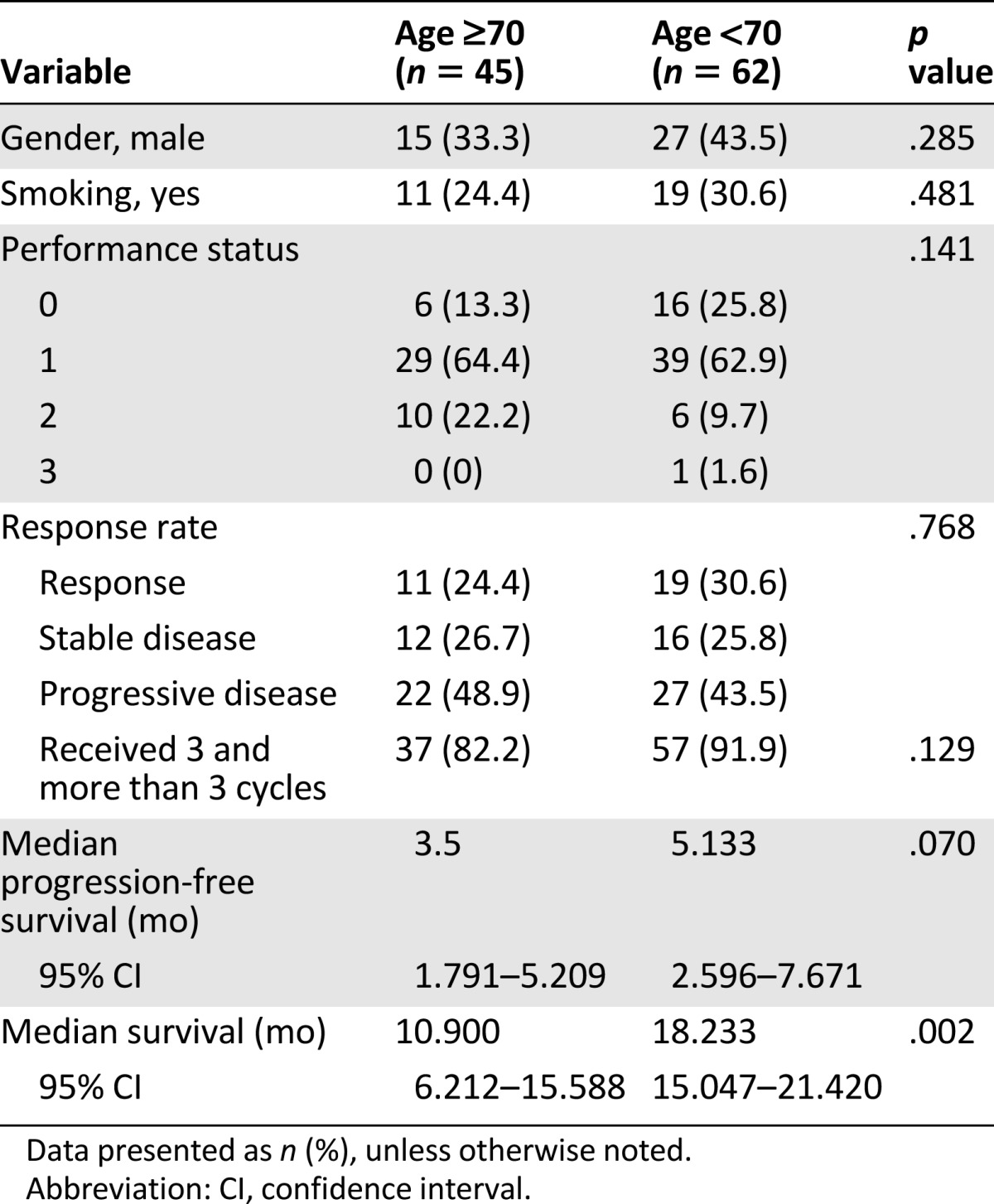
A total of 45 older patients and 62 younger patients received second-line salvage chemotherapy (Table 2). No differences were found in gender, smoking habit, or performance status between these two groups of patients. The tumor response rate was 24.4% for the older patients and 30.6% for the younger patients. No significant difference was seen in PFS between the older and younger groups of patients (3.5 vs. 5.1 months; p = .156; Fig. 2B). However, the OS was longer for the younger patients than for the older patients (18.2 vs. 10.9 months; p = .002; Fig. 3B).
Table 2.
Second-line chemotherapy status of patients with disease progression after first-line epidermal growth factor receptor-tyrosine kinase inhibitor treatment
Platinum-Based Chemotherapy
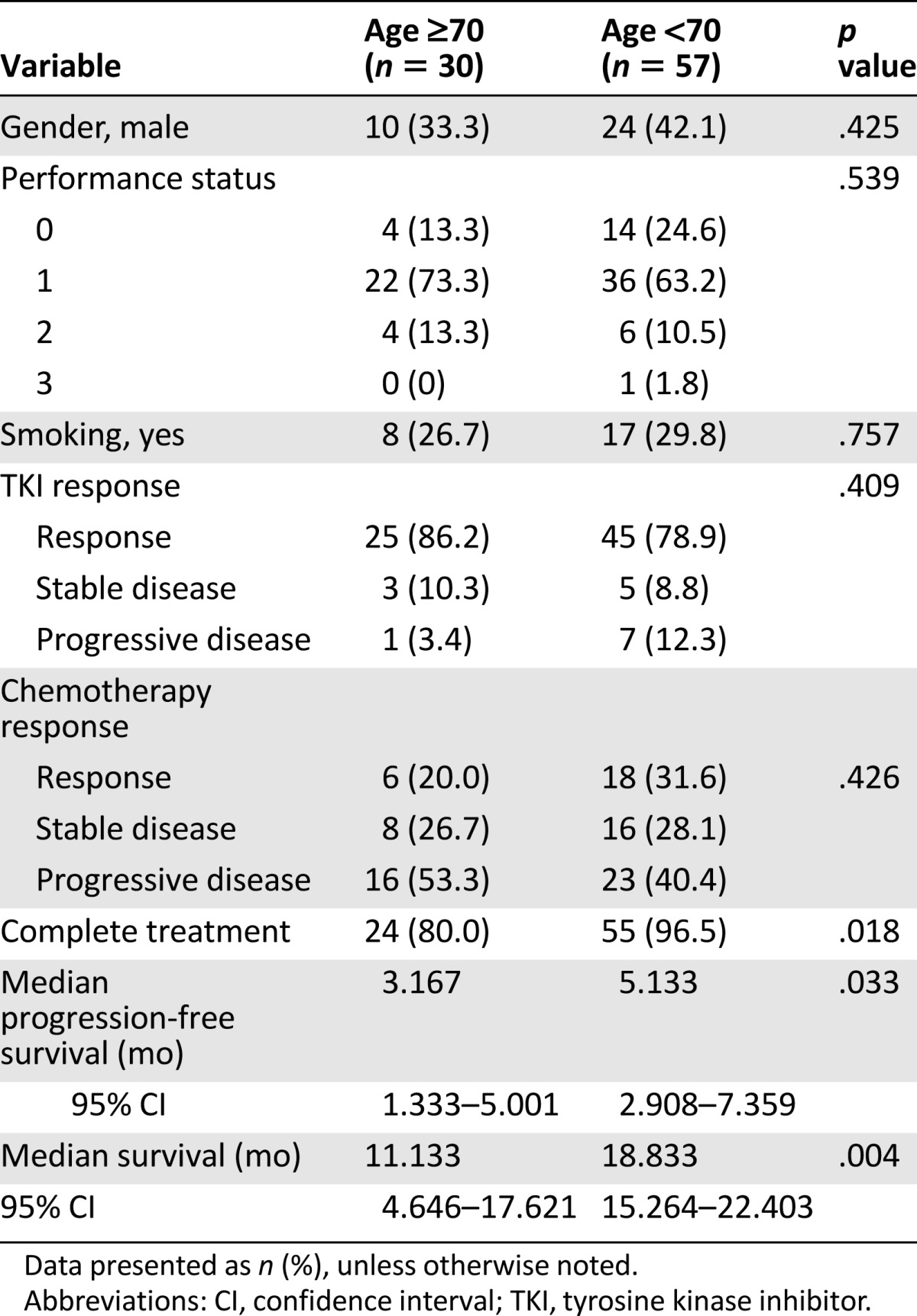
A total of 30 older patients and 57 younger patients received platinum-based therapy (Table 3). No differences in gender, smoking habits, or performance status were noted between the 2 age groups. The tumor response rate in the older patients was 20.0% and was 31.6% in the younger patients (p = .426). Younger patients had a significantly higher treatment completion rate (96.5% vs. 80.0%; p = .018). PFS was significantly longer for the younger patients than for the older patients (5.1 vs. 3.2 months; p = .033; Fig. 2E). Also, the younger patients had significantly longer OS than did the older patients (18.8 vs. 11.1 months; p = .004; Fig. 3E).
Table 3.
Patients who received second-line chemotherapy with a platinum-based regimen according to age
Combination Chemotherapy
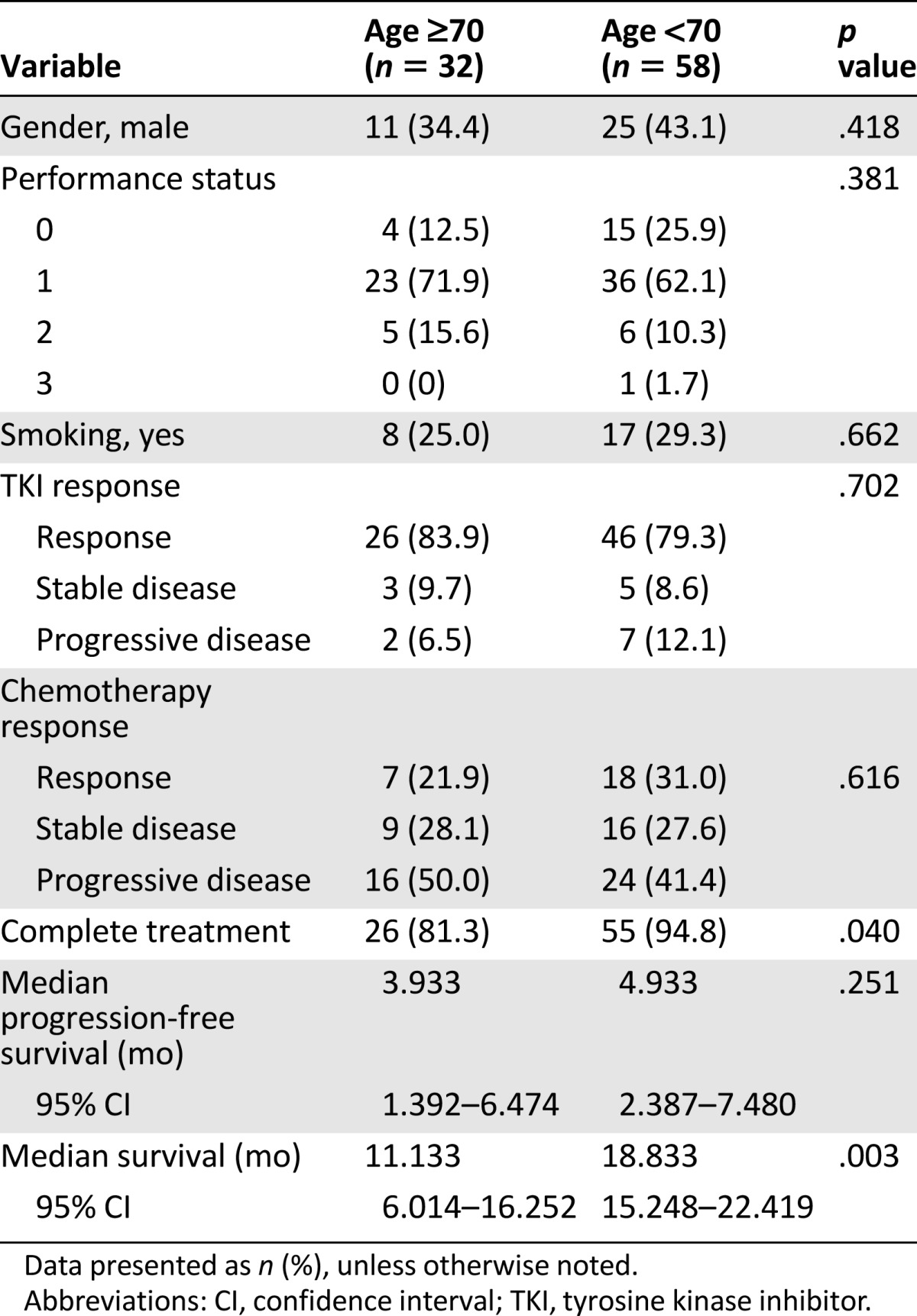
A total of 32 older patients and 58 younger patients received combination chemotherapy (Table 4). No differences in gender, smoking habits, or performance status were noted among the patients. The treatment response rate was not different between the 2 age groups (21.9% in the older group vs. 31.0% in the younger group; p = .616). The younger patients had a higher rate of treatment completion than did the older patients (94.8% vs. 81.3%; p = .040; Table 4). The PFS was insignificantly longer for the younger patients than for the older patients (4.9 vs. 3.9 months; p = .474; Fig. 2D), and the OS was longer in the younger group than in the older group (18.8 vs. 11.1 months; p = .003; Fig. 3D).
Table 4.
Patients who received second-line chemotherapy with a combination regimen according to age
Pemetrexed-Based Chemotherapy
A total of 26 older patients and 54 younger patients received pemetrexed-based chemotherapy, including 51 with pemetrexed plus cisplatin, 19 with pemetrexed plus carboplatin, 6 with pemetrexed, cisplatin, and bevacizumab, 2 with pemetrexed, carboplatin, and bevacizumab, and 2 with pemetrexed alone. No differences in gender, smoking habits, or performance status were noted between the 2 age groups. The tumor response rate with pemetrexed-based therapy was 23.1% for the older patients and 29.6% for the younger patients (p = .751). No significant difference was seen in PFS between the 2 age groups that received pemetrexed-based therapy (4.0 vs. 4.7 months; p = .233; Fig. 2C). Also, no difference was seen in OS (14.4 vs. 18.2 months; p = .162; Fig. 3C).
Multivariate Survival Analysis of Patients Who Received Salvage Chemotherapy
The Cox regression analysis test, including age, performance status, receipt of pemetrexed treatment, single-agent or combination chemotherapy, platinum-based or non-platinum-based treatment, response to previous EGFR-TKI treatment, and response to chemotherapy, was used for the multivariate PFS and OS analysis. The PFS and OS of the younger patients was significantly longer than that of the older patients (p = .005 and p = .002, respectively). In addition, those with a response to chemotherapy had statistically significantly longer PFS (p = .015) but not OS (p = .227).
Discussion
In 2007, Jackman et al. [8] treated patients unselected by mutation status who were >70 years old with erlotinib. The reported median PFS was 3.5 months, and the median OS was 10.9 months [8]. In 2008, Crinò et al. enrolled chemotherapy-naïve patients who were >70 years old and divided them into 2 groups. One of the groups received gefitinib and the other vinorelbine. The median PFS of the gefitinib group was 2.7 months and the OS was 5.9 months [9]. Few studies have compared the efficacy of EGFR-TKI treatment and chemotherapy in EGFR-mutated elderly and younger patients. Our data showed that when receiving active treatment that included EGFR-TKI and subsequent salvage chemotherapy, the PFS did not differ significantly between the younger and older patients (10.9 vs. 11.3 months, p = .416, and 5.1 vs. 3.5 months, p = .070, respectively, for EGFR-TKI and subsequent salvage chemotherapy). However, the OS of the younger patients was significantly longer than that of the older group (26.5 vs. 21.3 months, p = .021; and 18.2 vs. 10.9 months, p = .014, respectively, for EGFR-TKI and subsequent salvage chemotherapy).
A previous study suggested that advanced age alone should not preclude the use of chemotherapy. Although elderly patients needed more frequent hospital visits and were more likely to experience cancer symptoms and a deteriorated quality of life, they had similar survival rates [5]. Our data showed that more elderly patients than younger patients died and were lost to follow-up during EGFR-TKI treatment (9.4% vs. 5.9%, p = .231; and 15.6% vs. 10.6%, p = .174, respectively). However, elderly patients could tolerate and complete EGFR-TKI treatment as well as the younger patients did (3.1% vs. 5.3%, p = .329; and 81.8% vs. 84.5%, p = .555, respectively). In addition, our data showed that elderly patients who received second-line salvage chemotherapy after acquired resistance to first-line EGFR-TKI had a median OS of approximately 10.9 months. This is longer than the OS of patients who received the best supportive care in the previous study [11].
Treatment of acquired resistance to EGFR-TKI treatment in EGFR-mutated advanced NSCLC includes continuing EGFR-TKI, local ablation to oligoprogressive disease followed by reinitiation of TKI, combined targeted therapies such as afatinib and cetuximab, chemotherapy, or third-generation EGFR-TKI treatment [16]. Of these, third-generation EGFR-TKIs have been newly launched with promising efficacy in patients with an acquired T790M mutation [17, 18]. Up to now, chemotherapy was most often used as a salvage modality. However, the question remains of which chemotherapy agents to choose after elderly patients acquire resistance to EGFR-TKI. Goldberg et al. compared chemotherapy plus erlotinib with chemotherapy alone in advanced NSCLC patients who had received first-line EGFR-TKI and had acquired resistance. An improved response rate (63% vs. 21%; p = .02) was seen, but no significant difference was found in PFS (4.4 vs. 4.2 months; p = .5) or OS (14.2 vs. 15.0 months; p = .37) [19]. Another study showed that platinum-based combination regimens might be associated with better OS than other chemotherapy regimens or erlotinib as second-line chemotherapy after first-line gefitinib treatment, although the study did not focus on EGFR-mutated patients [20]. Our study results revealed a similar chemotherapy response rate for older and younger patients (24.4% vs. 30.6%; p = .768) and similar PFS (3.5 vs. 5.1 months; p = .070). The OS was longer for the younger patients than for the older patients (18.2 vs. 10.9 months; p = .002).
Although some studies have suggested that chemotherapy should be the first choice for patients with acquired EGFR-TKI resistance, no study has specifically reported on the treatment used for elderly patients [21, 22]. Ours is the first retrospective study to review the different chemotherapy regimens given after acquired resistance to first-line EGFR-TKI treatment in elderly patients and to compare the effects with those in younger patients. The response rate of the elderly in our study was 23.1% for pemetrexed-based chemotherapy, 21.9% for combination chemotherapy, and 20.0% for platinum-based chemotherapy, similar to the results from previous studies that focused on chemotherapy for an EGFR-mutated population not limited to the elderly [23, 24]. In our study, the PFS was not significantly different between the older and younger patients undergoing pemetrexed-based chemotherapy and combination chemotherapy but was longer in younger patients receiving platinum-based chemotherapy than that in the older patients (5.1 vs. 3.2 months; p = .033). The OS was better for the younger than for the older patients when using combination therapy and platinum-based chemotherapy.
Some limitations in our study should be mentioned. First, ours was a retrospective study; thus, undoubtedly, some selection bias was present. Owing to differences in patient characteristics, physicians might choose different chemotherapy regimens, which could confound the outcomes of the study. Second, we did not routinely perform repeat biopsy for patients who developed acquired resistance to first-line EGFR-TKI therapy. Histological changes could have occurred and influenced the treatment outcome. Finally, the population in our study was relatively small. A large, prospective, randomized trial is necessary to achieve definite answers to the questions raised. However, the issues of platinum versus nonplatinum, single versus combination chemotherapy, and pemetrexed versus nonpemetrexed use in first-line NSCLC treatment for elderly patients were not definitively answered, not to mention the issues surrounding EGFR-mutated elderly patients after they have developed acquired resistance to EGFR-TKI.
Conclusion
Elderly patients who have disease progression after first-line EGFR-TKI treatment can receive chemotherapy and have a chemotherapy response rate similar to that of younger patients.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception/Design: Yen-Han Tseng, Yu-Chin Lee, Reury-Perng Perng, Jacqueline Whang-Peng, Yuh-Min Chen
Provision of study material or patients: Yen-Han Tseng, Yi-hsuan Lin, Yu-Chin Lee, Reury-Perng Perng, Jacqueline Whang-Peng, Yuh-Min Chen
Collection and/or assembly of data: Yen-Han Tseng, Yen-Chiang Tseng, Yi-hsuan Lin
Data analysis and interpretation: Yen-Han Tseng, Yen-Chiang Tseng, Yi-hsuan Lin
Manuscript writing: Yen-Han Tseng, Yuh-Min Chen
Final approval of manuscript: Yen-Han Tseng, Yu-Chin Lee, Reury-Perng Perng, Jacqueline Whang-Peng, Yuh-Min Chen
Disclosures
The authors indicated no financial relationships.
References
- 1.Lara MS, Brunson A, Wun T, et al. Predictors of survival for younger patients less than 50 years of age with non-small cell lung cancer (NSCLC): A California Cancer Registry analysis. Lung Cancer. 2014;85:264–269. doi: 10.1016/j.lungcan.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Youlden DR, Cramb SM, Baade PD. The International Epidemiology of Lung Cancer: Geographical distribution and secular trends. J Thorac Oncol. 2008;3:819–831. doi: 10.1097/JTO.0b013e31818020eb. [DOI] [PubMed] [Google Scholar]
- 3.Verdecchia A, Francisci S, Brenner H, et al. Recent cancer survival in Europe: A 2000-02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8:784–796. doi: 10.1016/S1470-2045(07)70246-2. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 5.Chen YM, Perng RP, Shih JF, et al. Chemotherapy for non-small cell lung cancer in elderly patients. Chest. 2005;128:132–139. doi: 10.1378/chest.128.1.132. [DOI] [PubMed] [Google Scholar]
- 6.Kuo CW, Chen YM, Chao JY, et al. Non-small cell lung cancer in very young and very old patients. Chest. 2000;117:354–357. doi: 10.1378/chest.117.2.354. [DOI] [PubMed] [Google Scholar]
- 7.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 8.Jackman DM, Yeap BY, Lindeman NI, et al. Phase II clinical trial of chemotherapy-naive patients > or = 70 years of age treated with erlotinib for advanced non-small-cell lung cancer. J Clin Oncol. 2007;25:760–766. doi: 10.1200/JCO.2006.07.5754. [DOI] [PubMed] [Google Scholar]
- 9.Crinò L, Cappuzzo F, Zatloukal P, et al. Gefitinib versus vinorelbine in chemotherapy-naive elderly patients with advanced non-small-cell lung cancer (INVITE): A randomized, phase II study. J Clin Oncol. 2008;26:4253–4260. doi: 10.1200/JCO.2007.15.0672. [DOI] [PubMed] [Google Scholar]
- 10.Chen YM, Tsai CM, Fan WC, et al. Phase II randomized trial of erlotinib or vinorelbine in chemonaive, advanced, non-small cell lung cancer patients aged 70 years or older. J Thorac Oncol. 2012;7:412–418. doi: 10.1097/JTO.0b013e31823a39e8. [DOI] [PubMed] [Google Scholar]
- 11.Gridelli C. The ELVIS trial: A phase III study of single-agent vinorelbine as first-line treatment in elderly patients with advanced non-small cell lung cancer. Elderly Lung Cancer Vinorelbine Italian Study. The Oncologist. 2001;6(suppl 1):4–7. doi: 10.1634/theoncologist.6-suppl_1-4. [DOI] [PubMed] [Google Scholar]
- 12.Quoix E, Zalcman G, Oster JP, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet. 2011;378:1079–1088. doi: 10.1016/S0140-6736(11)60780-0. [DOI] [PubMed] [Google Scholar]
- 13.Zukin M, Barrios CH, Pereira JR, et al. Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol. 2013;31:2849–2853. doi: 10.1200/JCO.2012.48.1911. [DOI] [PubMed] [Google Scholar]
- 14.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Ettinger DS, Wood DE, Akerley W, et al. Non-small cell lung cancer, version 1.2015. J Natl Compr Canc Netw. 2014;12:1738–1761. doi: 10.6004/jnccn.2014.0176. [DOI] [PubMed] [Google Scholar]
- 17.Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakelee H, Soria J, Camidge R, et al. First-in-human evaluation of Co-1686, an irreversible, highly selective tyrosine kinase inhibitor of mutations of EGFR (activating and T790m) J Thorac Oncol. 2014;9:S38–S38. [Google Scholar]
- 19.Goldberg SB, Oxnard GR, Digumarthy S, et al. Chemotherapy with erlotinib or chemotherapy alone in advanced non-small cell lung cancer with acquired resistance to EGFR tyrosine kinase inhibitors. The Oncologist. 2013;18:1214–1220. doi: 10.1634/theoncologist.2013-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu JY, Shih JY, Yang CH, et al. Second-line treatments after first-line gefitinib therapy in advanced nonsmall cell lung cancer. Int J Cancer. 2010;126:247–255. doi: 10.1002/ijc.24657. [DOI] [PubMed] [Google Scholar]
- 21.West H, Oxnard GR, Doebele RC. Acquired resistance to targeted therapies in advanced non-small cell lung cancer: New strategies and new agents. American Society of Clinical Oncology Educational Book, 2013, ASCO University. Available at http://meetinglibrary.asco.org/content/198-132. Accessed February 6, 2015. [DOI] [PMC free article] [PubMed]
- 22.Mok T, Yang JJ, Lam KC. Treating patients with EGFR-sensitizing mutations: first line or second line—Is there a difference? J Clin Oncol. 2013;31:1081–1088. doi: 10.1200/JCO.2012.43.0652. [DOI] [PubMed] [Google Scholar]
- 23.Gridelli C, Rossi A. EURTAC first-line phase III randomized study in advanced non-small cell lung cancer: Erlotinib works also in European population. J Thorac Dis. 2012;4:219–220. doi: 10.3978/j.issn.2072-1439.2012.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Govindan R, Bogart J, Stinchcombe T, et al. Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small-cell lung cancer: Cancer and Leukemia Group B trial 30407. J Clin Oncol. 2011;29:3120–3125. doi: 10.1200/JCO.2010.33.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]