This study investigated whether nulliparity and the time interval since pregnancy among parous women affected the breast cancer phenotype in young women. Distribution of breast cancer molecular phenotype did not differ among parous young women by time interval since pregnancy. The implication of these findings for clinical practice suggests that pregnancy-associated breast cancers may be seen up to 5 years beyond parturition.
Keywords: Breast cancer, Molecular phenotypes, Young women, Pregnancy-associated breast cancer
Abstract
Background.
The increase in breast cancer risk during pregnancy and postpartum is well known; however, the molecular phenotype of breast cancers occurring shortly after pregnancy has not been well studied. Given this, we investigated whether nulliparity and the time interval since pregnancy among parous women affects the breast cancer phenotype in young women.
Materials and Methods.
We examined molecular phenotype in relation to time since pregnancy in a prospective cohort of 707 young women (aged ≤40 years) with breast cancer. Parity was ascertained from study questionnaires. Using tumor histologic grade on central review and biomarker expression, cancers were categorized as luminal A- or B-like, HER2 enriched, and triple negative.
Results.
Overall, 32% were luminal A-like, 41% were luminal B-like, 9% were HER2 enriched, and 18% were triple negative. Although, numerically, patients diagnosed >5 years after pregnancy had more luminal A-like subtypes than women with shorter intervals since pregnancy, there was no evidence of a relationship between these intervals and molecular subtypes once family history of breast cancer and age at diagnosis were considered.
Conclusion.
Distribution of breast cancer molecular phenotype did not differ significantly among young women by parity or time interval since parturition when important predictors of tumor phenotype such as age and family history were considered.
Implications for Practice:
Distribution of breast cancer molecular phenotype did not differ among parous young women by time interval since pregnancy. The implication of these findings for clinical practice suggests that pregnancy-associated breast cancers may be seen up to 5 years beyond parturition.
Introduction
The increase in breast cancer risk during pregnancy and postpartum is well documented [1–3]. Studies have also demonstrated poorer prognoses for younger women with breast cancer compared with older women and for women with pregnancy-associated breast cancer (PABC) compared with nulliparous women, likely due to multiple factors including stage at presentation and the biology of breast cancers occurring in younger women and in pregnant women [4–12]. The molecularly distinct breast cancer subgroups identified through gene microarray technology and unsupervised cluster analysis that have been the most reproducibly identified to date include luminal subtypes A and B; the HER2-enriched subtype; and a triple-negative group, the basal-like cancers [13–16]. Studies have examined the distribution of these molecular phenotypes in young women [17–20]; however, the molecular phenotype of breast carcinoma occurring shortly after pregnancy has not been studied widely [21–23].
At the molecular level, a genomic signature specific to the parous cancer-free breast, which remains present postmenopausally, has been identified [24–26]. This signature shows upregulation of genes associated with differentiation and anchoring of cells to basement membrane, upregulation of genes associated with inflammation, and downregulation of genes associated with proliferation, stem cell maintenance, apoptosis, estrogen receptor α (ER-α), and progesterone receptors (PRs) [24–26], which would support the protective effect of pregnancy seen well beyond the postpartum period (“crossover effect”).
Given this aforementioned “protective” genomic signature found in postmenopausal parous women, it is somewhat paradoxical that PABC is associated with many poor prognostic features. Consequently, we sought to investigate whether the time interval since pregnancy affects the molecular phenotype of breast carcinomas, classified using clinically obtained biomarkers, in parous young women with breast cancer. Specifically, we investigated whether women who were diagnosed within a shorter time period since pregnancy were found to have more aggressive molecular phenotypes of breast cancer compared with those diagnosed at greater time intervals since pregnancy and compared with the molecular phenotypes of breast cancer occurring in nulliparous young women (aged ≤40 years).
Patients and Methods
Study Design and Population
The Young Women’s Breast Cancer Study is a multi-institutional prospective cohort study of women diagnosed with breast cancer between 17 and 40 years of age that began enrolling patients in 2006 and has been described in detail previously [20]. In brief, women aged 40 years and younger with newly diagnosed breast cancer at the Dana-Farber/Harvard Cancer Center, including 9 participating institutions within Massachusetts and 3 out of state, were eligible for enrollment provided they were able to respond to questionnaires in English. Participants responding to an invitation by mail provided written informed consent authorizing medical record review that included data abstraction of patient stage, blood sample and pathology specimen collection, and baseline and follow-up participant questionnaires. Institutional review board (IRB) approval for the study was obtained through the Dana-Farber/Harvard Cancer Center IRB; the Newton Wellesley Hospital IRB; the University of Colorado, Colorado Multiple Institutional Review Board; the Sunnybrook Health Sciences Center research ethics board; and the Mayo Clinic IRB.
Pathology Review
Histopathology slides were obtained and reviewed centrally by the study pathologist. When available, both the initial core biopsy and subsequent excision or mastectomy specimens were reviewed. Using a standardized case-reporting form, specific histologic features were recorded for each specimen (detailed description of features captured is shown in [20]).
Classification of Molecular Phenotype
Using the histologic tumor grade obtained by central pathology review by an expert breast pathologist (L.C.C.) and biomarker status as classified in the clinical record (ER, positive or negative; PR, positive or negative; and human epidermal growth factor receptor 2 [HER2], positive/amplified or negative/nonamplified) extracted from pathology reports, cases were classified as one of four molecular subtypes. Use of immunohistochemistry as a surrogate for molecular classification by gene expression profiling has been used in a number of large population-based studies [17, 27–30], has been shown to provide an acceptable level of accuracy for determining molecular phenotype [31], and is supported by the St. Gallen International Expert Consensus Statement [32].
As described previously [20], cases that were ER positive and/or PR positive, HER2 negative, and either histologic grade 1 or 2 were classified as luminal A-like cancers. Cases that were ER positive and/or PR positive and HER2 positive or ER positive and/or PR positive and HER2 negative and that were histologic grade 3 were classified as luminal B-like cancers. Cases that were ER negative, PR negative, and HER2 positive were classified as HER2 enriched. Cases that were negative for ER, PR, and HER2 were classified as triple negative, the clinicopathologic surrogate of basal-like carcinoma [32]. HER2 was considered positive if immunohistochemical stains were reported as 2+ (9 cases) or 3+ and/or if HER2 fluorescence in situ hybridization showed gene amplification.
Statistical Analyses
Among 892 women in the cohort with completed baseline surveys, 707 were included in this analysis; 150 were excluded because pathology review had not yet been completed, 33 were pregnant at diagnosis, and 2 were missing children's birthdates such that pregnancy intervals could not be calculated.
Statistical analyses were carried out with SAS version 9.2 (SAS Institute, Cary, NC, http://www.sas.com). Pearson chi-square statistics were calculated to assess the differences between nulliparous women and parous women and between women whose most recent pregnancies prior to diagnosis were 1–5 years earlier versus those whose pregnancies were >5 years prior to diagnosis. Multinomial logistic regression models were used to assess the impact of potential confounders (age at diagnosis, age at first birth, and family history of breast cancer). No corrections were made for multiple comparisons. Women with a pregnancy that was <1 year prior to diagnosis were not included in the comparative analyses of time interval since pregnancy (n = 47).
Results
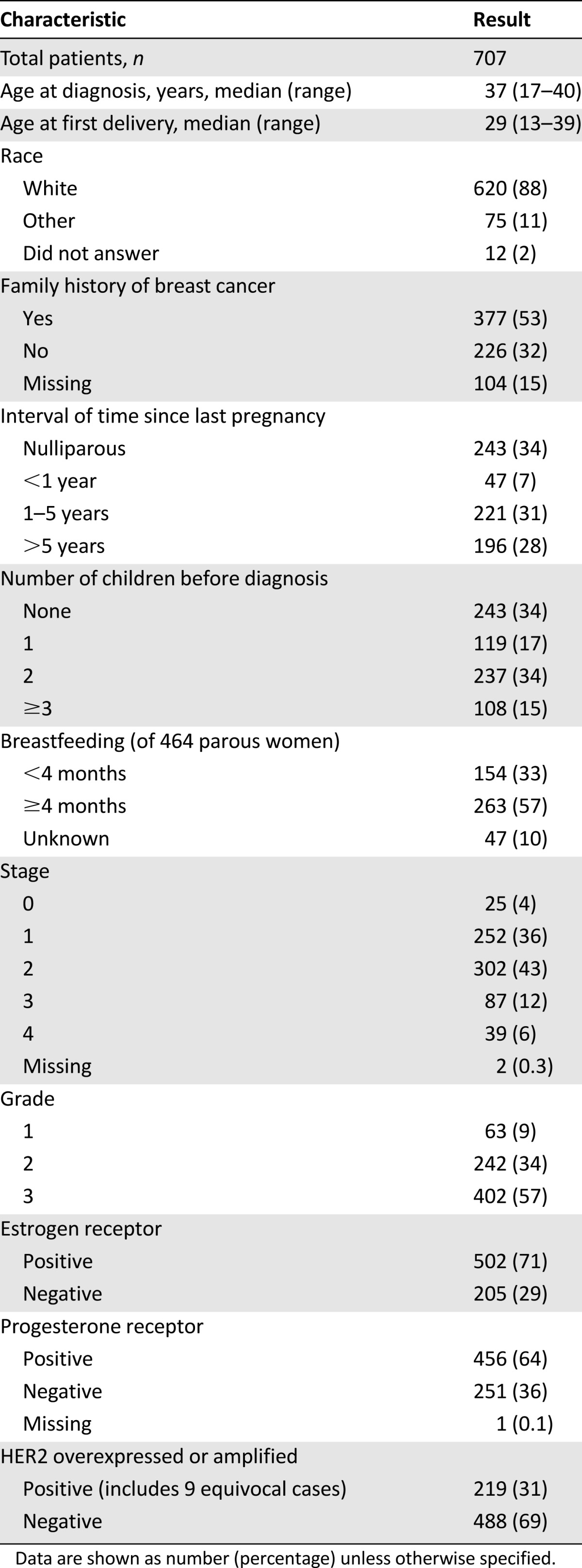
Among the 707 women included in this analysis, the median age at diagnosis was 37 years (range: 17–40 years). The patient characteristics of the overall cohort are summarized in Table 1. Of this population, 88% were white and 53% reported a family history of breast cancer. The majority of patients presented with either stage I or II disease (36% and 43%, respectively). Only 6% presented with stage IV disease, similar to what has been seen in the general population of women with breast cancer. Seven percent of women had a pregnancy within the year prior to diagnosis, 31% had a pregnancy within 1–5 years prior, and 28% had a pregnancy >5 years prior to diagnosis. The majority of women (66%) had had at least 1 pregnancy prior to diagnosis.
Table 1.
Patient and disease characteristics of the study population
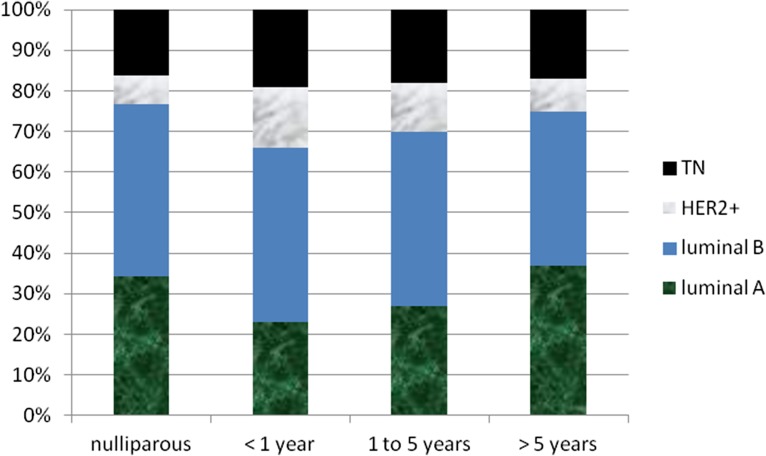
The distribution of pathologic features and molecular phenotypes among nulliparous and parous women according to time interval since pregnancy is presented in Table 2 and Figure 1. Most women had hormone receptor-positive disease. There were no statistically significant differences between nulliparous and parous young women or between women whose most recent pregnancies prior to diagnosis were 1–5 years prior versus those whose pregnancies were >5 years prior to diagnosis with regard to age at presentation, family history of breast cancer, tumor grade and stage at presentation, and molecular phenotype (chi-square test) (Table 2).
Table 2.
Distributions of molecular phenotype and clinicopathological features by intervals between last pregnancy and diagnosis
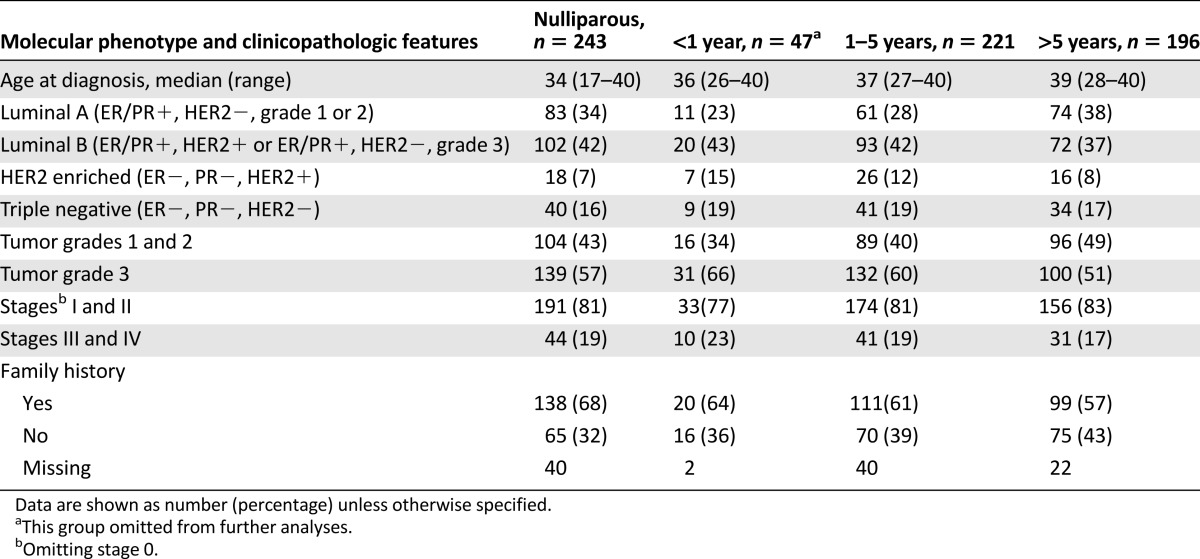
Figure 1.
Distribution of molecular phenotypes by intervals between last pregnancy and diagnosis.
Abbreviation: TN, triple negative.
Multinomial logistic regression modeling was used to assess the association between pregnancy intervals (shorter vs. longer) and the four molecular subtypes. A univariate model showed significant increases in luminal B-like and HER2-enriched subtypes compared with luminal A-like tumors for shorter versus longer intervals. The unadjusted odds ratios of shorter versus longer pregnancy intervals comparing luminal B-like, HER2-enriched, and triple-negative subtypes with the reference subtype of luminal A-like were 1.57 (p = .05), 1.97 (p = .06), and 1.46 (p = .19), respectively. Adjusting for the potential confounders of family history of breast cancer and age at diagnosis reduced the significance of the relationship between shorter and longer pregnancy intervals and the subtype distribution. The odds ratios in the adjusted model were 1.38 (p = .20) for luminal B-like, 1.55 (p = .25) for HER2-enriched, and 1.34 (p = .34) for triple-negative subtypes. A smaller proportion of women with luminal B-like tumors compared with those with luminal A-like had a family history of breast cancer. There was also a smaller proportion of luminal B-like (p = .06) and HER2-enriched (p = .05) tumors compared with luminal A-like for women diagnosed at older ages compared with younger ages.
Discussion
Women with PABC and younger women with breast cancer have been shown to have poorer prognosis compared with nulliparous and older women, likely due to multiple factors including delays in diagnosis, stage at presentation, the biology of breast cancers occurring in younger women, and treatment-related factors [4–12, 33, 34]. It is also well documented that pregnancy confers a dual effect on breast cancer risk, with a promotional effect during pregnancy and the early postpartum period [1–3] followed by a crossover to a protective effect in postmenopausal women with an age at first pregnancy of <25 years [35–37].
Given this dual effect, we sought to determine whether there was a difference in the molecular phenotype and tumor features of breast carcinoma occurring in young women according to time since last pregnancy and in comparison to nulliparous young women. We found no difference in molecular phenotype or clinicopathologic characteristics according to time intervals since pregnancy among parous and nulliparous young women. Similar to our study, a recent report by Callihan et al. found no difference in the distribution of molecular phenotype, grade, and stage among 619 young (aged ≤45 years) women with PABC (<5 years) compared with nulliparous young women [22]. In contrast, two other groups demonstrated that among parous women with breast cancer, those who were 0–2 years from last parity at the time of diagnosis were the only group more likely than nulliparous women to have grade 3 tumors, positive lymph nodes, and a tumor with a triple-negative phenotype [21, 23], although these differences were no longer present among women who were diagnosed 2–5 or 3–5 years after pregnancy, which is in keeping with the findings of our study. Family history does not appear to have been considered as a confounding variable in these studies. The study by Pilewskie et al. considered age as a categorical variable, but multivariate models were adjusted for “potential confounders other than age” [21], and in the study by Nagatsuma et al., age at diagnosis was not significantly associated with outcome in multivariate models [23]. The study by Callihan et al. reported that there were no differences in age at diagnosis between each of the time-interval groups (although their table 1 appears to demonstrate otherwise), and thus age was not included in multivariable models in that study [22].
It is possible that we did not detect an increase in triple-negative breast carcinomas because we analyzed women who were 1–5 years beyond pregnancy. Our group of women diagnosed <1 year since pregnancy was small (n = 47) and was excluded from further analyses. Another possible explanation is the change in definition of ER positivity since 2010 [38]. The aforementioned studies accrued patients retrospectively (from time periods 1998–2010) when the definition of ER positivity was 10% of tumor cells or greater (or even 20% in some institutions), whereas the current definition of an ER-positive tumor is ≥1% of tumors cells [38]. The current study is accruing patients prospectively; therefore, it is likely that some patients who would have been classified as ER negative before 2010 are now classified as ER (low) positive, rendering our population with slightly fewer triple-negative (and HER2-enriched) patients and shifting those patients to the luminal B-like group.
Another possible explanation proposed for the poorer prognosis of breast carcinoma diagnosed within 5 years of pregnancy is the hypothesis that there are differences in host biology as a result of the involution process that occurs following parturition rather than enrichment for a poor prognostic subtype per se. Our study was not designed to address this hypothesis but may provide some indirect support because enrichment for the triple-negative subtype was not seen in these young parous women, suggesting that it is perhaps host biology rather than the epithelial features of the carcinoma alone conferring the poor prognosis reported in these young, recently pregnant women [39].
Of note, we found that nulliparous young women (aged ≤40 years) were slightly more likely to develop luminal A-like cancers than parous women (34% for nulliparous vs. 28% for women 1–5 years since pregnancy). This was similarly demonstrated in the work from Nagatsuma et al. (70% for nulliparous [aged < 44 years] vs. 64% for women 3–5 years since pregnancy) [23]. Given that nulliparous women are at increased risk for the development of postmenopausal ER-positive breast cancer [40], it is possible that this relative increase in the risk of luminal A-like cancers begins in the premenopausal setting in nulliparous women and remains over their lifetime. This is particularly notable given the observation that, compared with older women, younger women are more likely to develop higher grade, HER2-positive tumors [4–9, 41–44], and basal-like carcinomas [17–19, 44–47].
Our study is one of the largest to date evaluating the pathology of breast carcinoma in young parous women and incorporating central pathology review to document histologic grade. However, as reported previously [20], a potential limitation of our study was that ER, PR, and HER2 results abstracted from pathology reports were used as a surrogate for molecular subtype, and the time period of accrual bridges a period of change in the cutoff levels for positivity in ER testing. As discussed, use of immunohistochemistry is generally accepted as a reasonable surrogate for molecular classification by gene expression profiling [32]. It is also appropriate to note that this is not a population-based study, despite identifying patients through pathology record review at nine community and academic sites, and there may be both participation bias (women who participate in our study may differ by tumor phenotype) and referral bias (women referred to the academic sites, in particular, may vary by tumor phenotype). Importantly, although we adjusted for a family history of breast cancer, we did not consider BRCA1 or BRCA2 mutation status for the present analysis. Given the known relationship between tumor phenotype and BRCA1 mutation status in particular, future evaluation of BRCA status in consideration of the relationship between tumor phenotype and parity in young women is warranted and may be feasible as the size of our cohort increases and genetic analyses are completed.
Conclusion
Among our large cohort of young women, distribution of breast cancer molecular phenotype did not differ among parous young women with respect to time interval since parturition or compared with nulliparous women once important predictors of tumor phenotype such as age and family history were considered. Future research to determine potential unique cancer initiation and promotion factors related to the early postpartum period is warranted.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgment
Funding was provided by a grant from the Susan G. Komen Foundation.
Author Contributions
Conception/Design: Laura C. Collins, Shari Gelber, Jonathan D. Marotti, Rulla M. Tamimi, Eric P. Winer, Ann H. Partridge
Provision of study material or patients: Laura C. Collins, Kathryn Ruddy, Lidia Schapira, Steven E. Come, Virginia F. Borges, Ellen Warner, Eric P. Winer, Ann H. Partridge
Collection and/or assembly of data: Laura C. Collins, Shari Gelber, Jonathan D. Marotti, Sarah White, Kathryn Ruddy, Elena F. Brachtel, Taylor Wensley, Ann H. Partridge
Data analysis and interpretation: Laura C. Collins, Shari Gelber, Jonathan D. Marotti, Sarah White, Kathryn Ruddy, Elena F. Brachtel, Lidia Schapira, Steven E. Come, Virginia F. Borges, Pepper Schedin, Ellen Warner, Rulla M. Tamimi, Eric P. Winer, Ann H. Partridge
Manuscript writing: Laura C. Collins, Shari Gelber, Jonathan D. Marotti, Sarah White, Kathryn Ruddy, Elena F. Brachtel, Lidia Schapira, Steven E. Come, Virginia F. Borges, Pepper Schedin, Ellen Warner, Rulla M. Tamimi, Ann H. Partridge
Final approval of manuscript: Laura C. Collins, Shari Gelber, Jonathan D. Marotti, Sarah White, Kathryn Ruddy, Elena F. Brachtel, Lidia Schapira, Steven E. Come, Virginia F. Borges, Pepper Schedin, Ellen Warner, Taylor Wensley, Rulla M. Tamimi, Eric P. Winer, Ann H. Partridge
Disclosures
Elena F. Brachtel: BioTheranostics, Inc. (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Albrektsen G, Heuch I, Hansen S, et al. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br J Cancer. 2005;92:167–175. doi: 10.1038/sj.bjc.6602302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruzzi P, Negri E, La Vecchia C, et al. Short term increase in risk of breast cancer after full term pregnancy. BMJ. 1988;297:1096–1098. doi: 10.1136/bmj.297.6656.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambe M, Hsieh C, Trichopoulos D, et al. Transient increase in the risk of breast cancer after giving birth. N Engl J Med. 1994;331:5–9. doi: 10.1056/NEJM199407073310102. [DOI] [PubMed] [Google Scholar]
- 4.Fisher ER, Anderson S, Tan-Chiu E, et al. Fifteen-year prognostic discriminants for invasive breast carcinoma: National Surgical Adjuvant Breast and Bowel Project Protocol-06. Cancer. 2001;91(suppl):1679–1687. [PubMed] [Google Scholar]
- 5.Gajdos C, Tartter PI, Bleiweiss IJ, et al. Stage 0 to stage III breast cancer in young women. J Am Coll Surg. 2000;190:523–529. doi: 10.1016/s1072-7515(00)00257-x. [DOI] [PubMed] [Google Scholar]
- 6.Han W, Kim SW, Park IA, et al. Young age: an independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer. 2004;4:82. doi: 10.1186/1471-2407-4-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kroman N, Jensen MB, Wohlfahrt J, et al. Factors influencing the effect of age on prognosis in breast cancer: population based study. BMJ. 2000;320:474–478. doi: 10.1136/bmj.320.7233.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rapiti E, Fioretta G, Verkooijen HM, et al. Survival of young and older breast cancer patients in Geneva from 1990 to 2001. Eur J Cancer. 2005;41:1446–1452. doi: 10.1016/j.ejca.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 9.Adami HO, Malker B, Holmberg L, et al. The relation between survival and age at diagnosis in breast cancer. N Engl J Med. 1986;315:559–563. doi: 10.1056/NEJM198608283150906. [DOI] [PubMed] [Google Scholar]
- 10.Bonnier P, Romain S, Dilhuydy JM, et al. Influence of pregnancy on the outcome of breast cancer: a case-control study. Societe Francaise de Senologie et de Pathologie Mammaire Study Group. Int J Cancer. 1997;72:720–727. doi: 10.1002/(sici)1097-0215(19970904)72:5<720::aid-ijc3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 11.Olson SH, Zauber AG, Tang J, et al. Relation of time since last birth and parity to survival of young women with breast cancer. Epidemiology. 1998;9:669–671. [PubMed] [Google Scholar]
- 12.Johansson AL, Andersson TM, Hsieh CC, et al. Stage at diagnosis and mortality in women with pregnancy-associated breast cancer (PABC) Breast Cancer Res Treat. 2013;139:183–192. doi: 10.1007/s10549-013-2522-1. [DOI] [PubMed] [Google Scholar]
- 13.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 14.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenton JD, Carey LA, Ahmed AA, et al. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005;23:7350–7360. doi: 10.1200/JCO.2005.03.3845. [DOI] [PubMed] [Google Scholar]
- 17.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 18.Carvalho FM, Bacchi LM, Santos PP, et al. Triple-negative breast carcinomas are a heterogeneous entity that differs between young and old patients. Clinics (Sao Paulo) 2010;65:1033–1036. doi: 10.1590/S1807-59322010001000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarado-Cabrero IV-CR, Barroso-Bravo S. Breast cancer in Mexican women younger than age 45 years. A clinicopathologic study of 1,320 cases. Mod Pathol. 2011;24(suppl 1s):26A. [Google Scholar]
- 20.Collins LC, Marotti JD, Gelber S, et al. Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Cancer Res Treat. 2012;131:1061–1066. doi: 10.1007/s10549-011-1872-9. [DOI] [PubMed] [Google Scholar]
- 21.Pilewskie M, Gorodinsky P, Fought A, et al. Association between recency of last pregnancy and biologic subtype of breast cancer. Ann Surg Oncol. 2012;19:1167–1173. doi: 10.1245/s10434-011-2104-6. [DOI] [PubMed] [Google Scholar]
- 22.Callihan EB, Gao D, Jindal S, et al. Postpartum diagnosis demonstrates a high risk for metastasis and merits an expanded definition of pregnancy-associated breast cancer. Breast Cancer Res Treat. 2013;138:549–559. doi: 10.1007/s10549-013-2437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagatsuma AK, Shimizu C, Takahashi F, et al. Impact of recent parity on histopathological tumor features and breast cancer outcome in premenopausal Japanese women. Breast Cancer Res Treat. 2013;138:941–950. doi: 10.1007/s10549-013-2507-0. [DOI] [PubMed] [Google Scholar]
- 24.Peri S, de Cicco RL, Santucci-Pereira J, et al. Defining the genomic signature of the parous breast. BMC Med Genomics. 2012;5:46. doi: 10.1186/1755-8794-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo J, Balogh GA, Russo IH. Full-term pregnancy induces a specific genomic signature in the human breast. Cancer Epidemiol Biomarkers Prev. 2008;17:51–66. doi: 10.1158/1055-9965.EPI-07-0678. [DOI] [PubMed] [Google Scholar]
- 26.Asztalos S, Gann PH, Hayes MK, et al. Gene expression patterns in the human breast after pregnancy. Cancer Prev Res (Phila) 2010;3:301–311. doi: 10.1158/1940-6207.CAPR-09-0069. [DOI] [PubMed] [Google Scholar]
- 27.Cheang MC, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 28.Cheang MCU, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamimi RM, Baer HJ, Marotti J, et al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res. 2008;10:R67. doi: 10.1186/bcr2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang XR, Sherman ME, Rimm DL, et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev. 2007;16:439–443. doi: 10.1158/1055-9965.EPI-06-0806. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 32.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes—dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keegan TH, Press DJ, Tao L, et al. Impact of breast cancer subtypes on 3-year survival among adolescent and young adult women. Breast Cancer Res. 2013;15:R95. doi: 10.1186/bcr3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Partridge AHHM, Hughes ME, Ottesen RA, et al. The effect of age on delay in diagnosis and stage of breast cancer. The Oncologist. 2012;17:775–782. doi: 10.1634/theoncologist.2011-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyons TR, Schedin PJ, Borges VF. Pregnancy and breast cancer: when they collide. J Mammary Gland Biol Neoplasia. 2009;14:87–98. doi: 10.1007/s10911-009-9119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosner B, Colditz GA. Nurses’ health study: log-incidence mathematical model of breast cancer incidence. J Natl Cancer Inst. 1996;88:359–364. doi: 10.1093/jnci/88.6.359. [DOI] [PubMed] [Google Scholar]
- 37.Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer. 2006;6:281–291. doi: 10.1038/nrc1839. [DOI] [PubMed] [Google Scholar]
- 38.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyons TR, O’Brien J, Borges VF, et al. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med. 2011;17:1109–1115. doi: 10.1038/nm.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colditz GA, Rosner BA, Chen WY, et al. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst. 2004;96:218–228. doi: 10.1093/jnci/djh025. [DOI] [PubMed] [Google Scholar]
- 41.Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 42.Ahn SH, Son BH, Kim SW, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: Nationwide survival data in Korea—a report from the Korean Breast Cancer Society. J Clin Oncol. 2007;25:2360–2368. doi: 10.1200/JCO.2006.10.3754. [DOI] [PubMed] [Google Scholar]
- 43.Colleoni M, Rotmensz N, Robertson C, et al. Very young women (<35 years) with operable breast cancer: features of disease at presentation. Ann Oncol. 2002;13:273–279. doi: 10.1093/annonc/mdf039. [DOI] [PubMed] [Google Scholar]
- 44.Keegan TH, DeRouen MC, Press DJ, et al. Occurrence of breast cancer subtypes in adolescent and young adult women. Breast Cancer Res. 2012;14:R55. doi: 10.1186/bcr3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Brien KM, Cole SR, Tse CK, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16:6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caldarella A, Crocetti E, Bianchi S, et al. Female breast cancer status according to ER, PR and HER2 expression: a population based analysis. Pathol Oncol Res. 2011;17:753–758. doi: 10.1007/s12253-011-9381-z. [DOI] [PubMed] [Google Scholar]
- 47.Livasy CA, Karaca G, Nanda R, et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006;19:264–271. doi: 10.1038/modpathol.3800528. [DOI] [PubMed] [Google Scholar]