Abstract
Background
Increasing concern over bisphenol A (BPA) as an endocrine-disrupting chemical and its possible effects on human health have prompted the removal of BPA from consumer products, often labeled “BPA-free.” Some of the chemical replacements, however, are also bisphenols and may have similar physiological effects in organisms. Bisphenol S (BPS) and bisphenol F (BPF) are two such BPA substitutes.
Objectives
This review was carried out to evaluate the physiological effects and endocrine activities of the BPA substitutes BPS and BPF. Further, we compared the hormonal potency of BPS and BPF to that of BPA.
Methods
We conducted a systematic review based on the Office of Health Assessment and Translation (OHAT) protocol.
Results
We identified the body of literature to date, consisting of 32 studies (25 in vitro only, and 7 in vivo). The majority of these studies examined the hormonal activities of BPS and BPF and found their potency to be in the same order of magnitude and of similar action as BPA (estrogenic, antiestrogenic, androgenic, and antiandrogenic) in vitro and in vivo. BPS also has potencies similar to that of estradiol in membrane-mediated pathways, which are important for cellular actions such as proliferation, differentiation, and death. BPS and BPF also showed other effects in vitro and in vivo, such as altered organ weights, reproductive end points, and enzyme expression.
Conclusions
Based on the current literature, BPS and BPF are as hormonally active as BPA, and they have endocrine-disrupting effects.
Citation
Rochester JR, Bolden AL. 2015. Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Perspect 123:643–650; http://dx.doi.org/10.1289/ehp.1408989
Introduction
There is increasing evidence that bisphenol A (BPA)—used in plastics, receipts, food packaging, and other products—might be harmful to human health due to its actions as an endocrine-disrupting chemical (EDC) (Bonefeld-Jørgensen et al. 2007; Richter et al. 2007b; Rochester 2013). Scientists, regulators, and the general public have raised concerns about the use of BPA, especially because of its ubiquitous nature and potential for continuous exposure (Vandenberg et al. 2010). This has prompted industry to seek alternative chemicals. As manufacturers have begun to remove BPA from their products as a result of consumer concern, there has been a gradual shift to using bisphenol analogs. For the purpose of our review, we chose to evaluate two of these analogs—bisphenol S (BPS) and bisphenol F (BPF)—because of their widespread consumer and commercial use. BPS is used for a variety of industrial applications, for example, as a wash fastening agent in cleaning products, an electroplating solvent, and a constituent of phenolic resin (Clark 2012). BPS is also used as a developer in thermal paper, including products marketed as “BPA-free paper” (Liao et al. 2012c). BPF is used to make epoxy resins and coatings, especially for systems needing increased thickness and durability (i.e., high-solid/high-build systems), such as tank and pipe linings, industrial floors, road and bridge deck toppings, structural adhesives, grouts, coatings, and electrical varnishes (Fiege et al. 2000). BPF epoxy resins are also used for several consumer products such as lacquers, varnishes, liners, adhesives, plastics, water pipes, dental sealants, and food packaging (Office of Environmental Health Hazard Assessment 2012). BPS and BPF have been detected in many everyday products, such as personal care products (e.g., body wash, hair care products, makeup, lotions, toothpaste) (Liao and Kannan 2014), paper products (e.g., currency, flyers, tickets, mailing envelopes, airplane boarding passes) (Liao et al. 2012c), and food (e.g., dairy products, meat and meat products, vegetables, canned foods, cereals) (Liao and Kannan 2013). BPS, BPF, and BPA have been detected in indoor dust at the following concentrations: BPS, 0.34 μg/g; BPF, 0.054 μg/g; BPA, 1.33 μg/g (Liao et al. 2012b). BPS and BPF have also been detected in surface water, sediment, and sewage effluent, generally at lower concentrations than BPA, but in the same order of magnitude (Fromme et al. 2002; Song et al. 2014; Yang et al. 2014). In humans, BPS and BPF have been detected in urine at concentrations and frequencies comparable to BPA (Liao et al. 2012a; Zhou et al. 2014). In urine samples from 100 American, nonoccupationally exposed adults, Liao et al. (2012a) found BPF in 55% of samples at concentrations up to 212 ng/mL, and BPS in 78% of samples at concentrations up to 12.3 ng/mL. BPA was found in 95% of the samples, with concentrations up to 37.7 ng/mL.
Ideally, substitutes used to replace a chemical of concern would be inert, or at least far less toxic than the original chemical(s). Unfortunately, many chemical replacements are untested before being placed on the market, and in some cases are similar enough to the original chemical to cause concern. For that reason, such chemical analogs should be evaluated before they are used as replacements for toxic chemicals. These chemicals may be just as harmful as the originals—or more so—and have been described as “regrettable substitutions,” as is the case with several perfluorinated chemicals (Howard 2014), pesticides (Coggon 2002), and flame retardants (Bergman et al. 2012). In the case of BPS and BPF, these chemicals are structural analogs to BPA (Figure 1); thus their effects in physiological systems may be similar. BPA is a known endocrine disruptor based on in vitro (Wetherill et al. 2007) and animal laboratory studies (Richter et al. 2007a; Vandenberg 2014b), and exposures to environmental levels of BPA have been associated with adverse health outcomes in children and adults in more than 75 human studies (Rochester 2013). To evaluate the endocrine-disrupting properties of the BPA substitutes BPS and BPF, we conducted a systematic review of the literature using the National Institute of Environmental Health Sciences’ Office of Health Assessment and Translation (OHAT) systematic review protocol (National Toxicology Program 2013; Rooney et al. 2014). In this analysis we summarize in vivo and in vitro literature and compare the hormonal potency of BPS and BPF to BPA using the in vitro studies.
Figure 1.
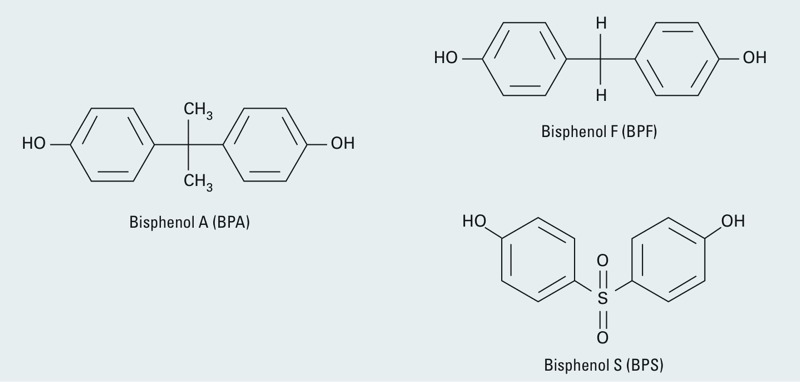
Chemical structures of bisphenol A, bisphenol S, and bisphenol F.
Literature search and review. We performed a comprehensive literature search in order to identify studies describing endocrine and other physiological effects of exposure to BPF and BPS. The search included all articles published and indexed for all years to June 2014. Electronic searches were performed in Web of Science (https://webofknowledge.com/) and PubMed (http://www.ncbi.nlm.nih.gov/pubmed) using CAS registry numbers and common names. Our search logic is summarized in Table 1.
Table 1.
BPS and BPF search logic.
| PubMed and Web of Science search logic | |
|---|---|
| BPF | 620-92-8[EC/RN] OR bisphenol-F OR (bisphenol* AND BPF) OR bis(4-hydroxyphenyl)methane OR bis(p-hydroxyphenyl)methane OR bis(4-hydroxyphenyl)-methane OR bis(p-hydroxyphenyl)-methane OR p-(p-hydroxybenzyl)phenol OR p-(p-hydroxybenzyl)-phenol OR 4-(4-hydroxybenzyl)phenol OR 4-(4-hydroxybenzyl)-phenol OR “4,4’-methylenebis(phenol)” OR “p,p’-bis(hydroxyphenyl)methane” OR “p,p’-bis(hydroxyphenyl)-methane” OR “4,4’-bis(hydroxyphenyl)methane” OR “4,4’-bis(hydroxyphenyl)-methane” OR “4,4’-dihydroxydiphenylmethane” OR “4,4’-dihydroxydiphenyl-methane” OR “4,4’-methylenediphenol” OR “4,4’-methylene-diphenol” OR “4,4’-methylenebisphenol” OR “4,4’-methylene-bisphenol” |
| BPS | 80-09-1[EC/RN] OR bisphenol-S OR [(bisphenol OR bisphenols) AND BPS] OR bis(4-hydroxyphenyl)-sulfone OR bis(4-hydroxyphenyl)sulfone OR bis(4-hydroxyphenyl)-sulphone OR bis(4-hydroxyphenyl)sulphone OR bis(p-hydroxyphenyl)-sulfone OR bis(p-hydroxyphenyl)sulfone OR bis(p-hydroxyphenyl)-sulphone OR bis(phydroxyphenyl)sulphone OR 4,4’-dihydroxydiphenyl-sulfone OR 4,4’-dihydroxydiphenylsulfone OR 4,4’-dihydroxydiphenyl-sulphone OR 4,4’-dihydroxydiphenylsulphone OR p,p’-dihydroxydiphenyl-sulfone OR p,p’-dihydroxydiphenylsulfone OR p,p’-dihydroxydiphenyl-sulphone OR p,p’-dihydroxydiphenylsulphone OR 4,4’-sulfonyldiphenol OR 4,4’-sulfphonyldiphenol OR p,p’-sulfonyldiphenol OR p,p-sulfphonyldiphenol OR 4,4’-sulfonylbisphenol OR 4,4’-sulfphonylbisphenol OR p,p’-sulfonylbisphenol OR p,p-sulfphonylbisphenol OR 4,4’-sulfonylbiphenol OR 4,4’-sulfphonylbiphenol OR p,p’-sulfonylbiphenol OR p,p’-sulfphonylbiphenol OR 4-hydroxyphenyl-sulfone OR 4-hydroxyphenylsulfone OR 4-hydroxyphenyl-sulphone OR 4-hydroxyphenylsulphone OR p-hydroxyphenyl-sulfone OR p-hydroxyphenylsulfone OR p-hydroxyphenyl-sulphone OR p-hydroxyphenylsulphone |
For inclusion, the studies had to be primary literature and assess any in vitro or in vivo physiological effects of BPS or BPF exposure. Two independent reviewers (J.R.R. and A.L.B.) screened all titles and abstracts for relevancy, using Distiller SR® software (Evidence Partners), and resolved any conflicts or discrepancies. Data from the studies were extracted, and were cross-checked by the two reviewers. When needed, data were extracted from figures or graphs using Universal Desktop Ruler® software (version 3.6; AVPSoft), with measurements taken in triplicate by a single reviewer.
Study quality for in vivo studies was assessed using a protocol developed by OHAT. Briefly, risk of bias (RoB) in experimental methodology was assessed by answering 14 questions. The RoB questions covered biases in subject selection, protocol performance, attrition/exclusion of subjects, detection of outcomes, selective reporting of outcomes, and statistical methodology. Questions were rated as “definitely low RoB,” “probably low RoB,” “probably high RoB,” or “definitely high RoB” depending on standardized responses. The individual RoB questions are provided in Figure 2. Next, “key” study quality questions, identified a priori, were used to determine the initial quality of each study, then ratings of the remaining questions were used to determine the overall study quality: “low,” “moderate,” or “high.” If any study received a “low” rating, it was removed from analysis. This protocol has been described in detail elsewhere (National Toxicology Program 2013; Rooney et al. 2014).
Figure 2.
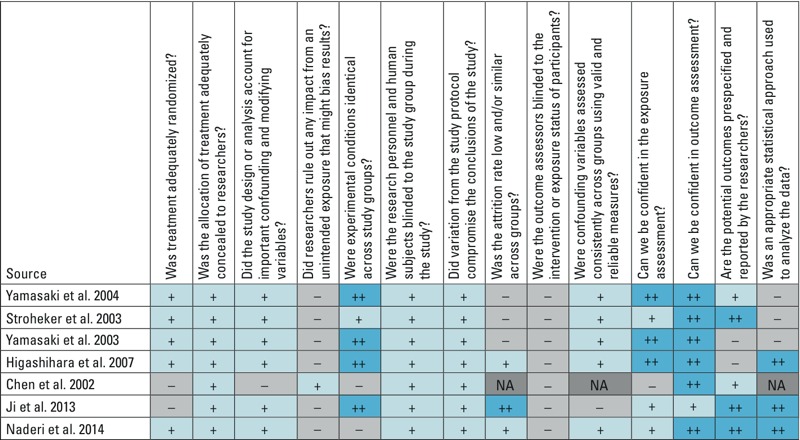
Risk of bias (RoB) ratings for BPS and BPF in vivo studies. Abbreviations: ++, definitely low risk of bias; +, probably low risk of bias; –, probably high risk of bias; NA, not applicable.
As specified in the OHAT protocol (National Toxicology Program 2013; Rooney et al. 2014), in vitro studies were not assessed for quality, but were used to support specific in vivo end points. For example, estrogen receptor (ER) binding or activation studies support the biological plausibility of increased uterine growth, an in vivo estrogenic response. Where there were at least three in vitro studies, the strength of support was rated on the following factors: relevance of biological process or pathway to human disease, consistency across model systems (where there were more than two systems), physiological relevance of the dose concentration, potency (magnitude of response compared with positive control), dose response (monotonic or nonmonotonic), and publication bias. These factors were integrated for a final rating of “weak,” “moderate,” or “strong” in vitro support of the biological plausibility of in vivo observations, but they were not used to exclude studies. In vitro observations that had fewer than three studies per end point, or did not relate to any observed in vivo end points, are described in the text.
Results
Our search identified 1,370 studies; of these, 32 studies (25 in vitro only and 7 in vivo) were identified as relevant for inclusion. Figure 2 shows the study quality ratings for the in vivo studies. All studies were rated moderate quality or better; therefore, no in vivo studies were removed because of low quality.
BPS. The literature reporting the physiological effects of BPS exposure consisted of 4 in vivo studies and 18 in vitro studies. The in vivo studies are presented in Table 2. BPS exposure caused acute toxicity in Daphnia magna (Chen et al. 2002). Yamasaki et al. (2004) found that postnatal BPS exposure in rats caused an induction of uterine growth, a marker of estrogen exposure (Owens and Ashby 2002), at the lowest and highest doses. The authors also found that BPS bound to the nuclear ER at 0.0055% relative binding affinity (Yamasaki et al. 2004). Ji et al. (2013) studied BPS exposure in zebrafish (Danio rerio) and found decreases in gonad weight, alterations in plasma estrogen and testosterone, and disrupted reproduction (i.e., decreased egg production and hatchability, increased time to hatch, increased embryo malformations). Another study in zebrafish showed that BPS exposure increased female to male sex ratio; decreased body length; altered testosterone, estradiol, and vitellogenin concentrations; and led to reproductive disruption (i.e., decreased egg production, increased time to hatch, decreased sperm count) (Naderi et al. 2014).
Table 2.
In vivo BPS and BPF hormonal/physiological effect studies.
| Chemical | Study | Model | Exposure duration | Age at exposure | Route of exposure | Doses | LOELa | Results |
|---|---|---|---|---|---|---|---|---|
| BPS | Chen et al. 2002 | Daphnia magna | 2 or 4 days | Juvenile | Culture | NA | NA | BPS was acutely toxic in Daphnia magna; EC50, 76 mg/L (24 hr); EC50 55 mg/L (48 hr). BPS showed estrogenic activity and did not show mutagenic activity in vitro. |
| BPS | Yamasaki et al. 2004 | Rat | 3 days | 20 days | Injection | 0, 20, 100, 500 mg/kg/day | 20 mg/kg | BPS exposure was estrogenic in rats via increases in uterine weight. BPS was also found to bind the estrogen receptor. |
| BPS | Ji et al. 2013 | Danio rerio | 21 days | 3–5 months | Water | 0, 0.5, 5, 50 μg/L | 0.5 μg/L | BPS exposure in zebrafish showed decreases in gonad weight with respect to body weight in males and females. No changes were observed in liver or brain weight with respect to body weight. E2 levels were increased in males and in females, T levels were decreased in males, and E2/T ratios were increased in males and females. Reproduction was impaired as evidenced by decreased egg production and hatchability, and by increased time to hatch and embryo malformation rates. Gene expression in the brain and gonads of several genes involved in the hypothalamic–pituitary–gonadal axis were altered in males and females. |
| BPS | Naderi et al. 2014 | Danio rerio | 75 days | 4–6 months | Water | 0, 0.1, 1, 10, 100 μg/L | 1 μg/L | BPS exposure in zebrafish showed decreased body length and weight in males, increased female to male sex ratio, decreased gonad weight, increased liver weight, decreased T3 and T4, decreased T in males, increased E2 in males and females, and increased VTG in males and females. BPS also caused disrupted reproduction, with decreased number of eggs produced, decreased hatching rate, increased time to hatch, and decreased sperm count. |
| BPF | Chen et al. 2002 | Daphnia magna | 2 or 4 days | Juvenile | Culture | NA | NA | EC50, 80 mg/L (24 hr); and EC50 56 mg/L (48 hr). BPF showed estrogenic activity and did not show mutagenic activity in vitro. |
| BPF | Yamasaki et al. 2003 | Rat | 10 days | 19 days | Gavage | 0, 50, 200, 1,000 mg/kg/day | 100 mg/kg | BPF co-administered with TP increased the weight of the Cowper’s gland. BPF alone and combined with TP decreased body weight. |
| BPF | Yamasaki et al. 2004 | Rat | 3 days | 20 days | Injection | 0, 100, 300, 1,000 mg/kg/day | 100 mg/kg | BPF induced uterine growth in immature rats. BPF was positive for relative binding affinity (E2). |
| BPF | Higashihara et al. 2007 | Rat | 28 days | 8 weeks | Gavage | 0, 20, 100, 500 mg/kg/day | 20 mg/kg | There were decreases in body weight and food consumption in males and females treated with BPF. Hematological and biochemical parameters were altered, including decreased cholesterol and glucose in males and females. BPF treatment decreased T3 and increased T4 levels. BPF increased testes, liver, thyroid, brain, and kidney weights. |
| BPF | Stroheker et al. 2003 | Rat | 4 days | 22 days | Gavage | 0, 25, 50, 100, 200 mg/kg/day | 100 mg/kg | BPF was shown to increase uterine weight in rats. |
| Abbreviations: EC50, half-maximal effective concentration; NA, not available; T, testosterone; T3, triiodothyronine; T4, thyroxin; TP, testosterone propionate; VTG, vitellogenin. aThe dose at the end point of the lowest observed effect. | ||||||||
In vitro data from 12 studies assessing estrogenicity provided strong evidence supporting the estrogenic responses observed in in vivo studies (Table 3), based on relevance of the end point to human health [e.g., interaction with human ERα and G-protein coupled receptor 30 (GPR30)], consistent response across eight cell lines, and physiologically relevant concentrations assessed (micromolar range) (Chen et al. 2002; Grignard et al. 2012; Hashimoto and Nakamura 2000; Hashimoto et al. 2001; Kitamura et al. 2005; Kuruto-Niwa et al. 2005; Molina-Molina et al. 2013; Rajasärkkä et al. 2014; Rosenmai et al. 2014; Teng et al. 2013; Viñas and Watson 2013a, 2013b). Several of these studies showed that BPS had weaker estrogenic potency than estradiol (E2) when assayed in nuclear receptor models (Chen et al. 2002; Grignard et al. 2012; Hashimoto and Nakamura 2000; Hashimoto et al. 2001; Kitamura et al. 2005; Kuruto-Niwa et al. 2005; Molina-Molina et al. 2013; Teng et al. 2013). However, two studies (Viñas and Watson 2013a, 2013b) showed that BPS had equivalent or greater estrogenic potency to E2 when assayed in membrane receptor models; BPS induced membrane receptor–mediated pathways typically up-regulated by E2. Four studies showed that BPS bound to the ER in competitive binding assays (Grignard et al. 2012; Hashimoto et al. 2001; Molina-Molina et al. 2013; Yamasaki et al. 2004). There was also one study showing androgenic activity of BPS (Molina-Molina et al. 2013) and one study showing antiandrogenic activity (Kitamura et al. 2005). In addition, in other in vitro experiments BPS exposure induced caspase 8 production, which indicates that BPS may alter cellular apoptotic and survival signaling (Salvesen and Walsh 2014; Viñas and Watson 2013a, 2013b). BPS also had effects on hepatic cells (Peyre et al. 2014); it bound to serum albumins (Mathew et al. 2014), and it caused DNA damage (Fic et al. 2013; Hashimoto and Nakamura 2000; Lee et al. 2013).
Table 3.
Studies assessing BPS and BPF activity in vitro.
| Study | Chemical(s) tested | End point measured | Concentrations tested |
|---|---|---|---|
| Audebert et al. 2011 | BPF | Cytotoxicity, genotoxicity | 1 to 100 μM |
| Cabaton et al. 2006 | BPF/BPS | Antiandrogenicity, estrogenicity, genotoxicity | 10–11 to 10–5 M and 36.4 to 170 μM |
| Chen et al. 2002 | BPF/BPS | Acute toxicity, estrogenicity | 0.01 to 100 mg/L |
| Fic et al. 2013 | BPF/BPS | Cytotoxicity, genotoxicity, mutagenicity | 12.5 to 100 μM, 0.1 to 10 μM, and 4 to 500 μg/plate |
| Grignard et al. 2012 | BPS | Estrogenicity | 10–12 to 10–4 M |
| Hashimoto and Nakamura 2000 | BPF/BPS | Estrogenicity | 10–7 to 10–3 M |
| Hashimoto et al. 2001 | BPF/BPS | Estrogenicity | 10–9 to 10–3 M |
| Kidani et al. 2010 | BPF | Adiponectin | 80 μM |
| Kitamura et al. 2003 | BPF | Estrogenic, estrogen CBA | 10–8 to 10–4 M |
| Kitamura et al. 2005 | BPF/BPS | Antiandrogenicity, estrogenicity | 10–7 to 10–4 M |
| Kuruto-Niwa et al. 2005 | BPS | Estrogenicity | 10–7 to 10–4 M |
| Lee et al. 2013 | BPF/BPS | Cytotoxicity, genotoxicity | 10 to 250 μM |
| Mathew et al. 2014 | BPS | Serum albumin binding | 0.2 to 4 μM |
| Molina-Molina et al. 2013 | BPF/BPS | Androgenicity, antiandrogenicity, estrogenicity, estrogen CBA | 10–8 to 10–5 M |
| Nakagawa and Tayama 2000 | BPF | Cytotoxicity, mitochondrial function | 0.25 to 1 mM |
| Ogawa et al. 2006 | BPF | Estrogenicity | 10–7 to 10–3 M |
| Perez et al. 1998 | BPF | Estrogenicity | 10–8 to 10–5 M |
| Peyre et al. 2014 | BPS | Hepatic cell function | 1 to 500 μM |
| Pisapia et al. 2012 | BPF | Estrogenicity | 10–7 to 10–5 M |
| Rajasärkkä et al. 2014 | BPF/BPS | BPA activity, estrogenicity | 10–7 to 10–2 M |
| Rosenmai et al. 2014 | BPF/BPS | Antiandrogenicity, estrogenicity, steroidogenesis, AhR activity | 10–4 to 102 μM |
| Satoh et al. 2004 | BPF | Antiandrogenicity, cytotoxicity, estrogenicity, estrogen and androgen CBA | 10–9 to 10–3 M |
| Stroheker et al. 2004 | BPF | Antiandrogenicity, antiestrogenicity, estrogenicity, estrogen CBA | 10–10 to 10–5 M |
| Teng et al. 2013 | BPS | Androgenicity, estrogenicity | 10–13 to 10–4 M |
| Viñas and Watson 2013a | BPS | Estrogenicity | 10–15 to 10–7 M |
| Viñas and Watson 2013b | BPS | Estrogenicity | 10–14 M |
| Yamasaki et al. 2004 | BPS | Estrogen CBA | 10–11 to 10–4 M |
| CBA, competitive binding assay. | |||
BPF. Of the five in vivo studies, four showed that BPF was estrogenic, androgenic, and thyroidogenic (Table 2). Nineteen in vitro studies showed estrogenic, androgenic, and other physiological/biochemical effects (Table 3). BPF was acutely toxic in Daphnia magna (Chen et al. 2002). Two studies showed that BPF exposure induced uterine growth in rats, indicating estrogenic activity (Stroheker et al. 2003; Yamasaki et al. 2004). There were also two studies that showed evidence of androgenic activity: One study indicated that BPF increased the weight of the testes (Higashihara et al. 2007), and the other showed a cumulative effect of BPF when co-administered with testosterone propionate that increased Cowper’s gland weight (Yamasaki et al. 2003). The cumulative effect indicates that BPF may augment other androgens, if indeed it acts synergistically. BPF exposure also increased thyroid weight and altered thyroid hormone concentrations, as well as caused changes to hematological parameters and enzyme expression (Higashihara et al. 2007).
As shown in Table 3, in vitro data from 12 studies provided strong evidence that BPF had estrogenic activity, supporting in vivo observations. This rating was based on relevance to human health (MCF-7 cell and human ER), consistency across five cell models, and the use of relevant concentrations (micromolar range) (Cabaton et al. 2009; Chen et al. 2002; Hashimoto and Nakamura 2000; Hashimoto et al. 2001; Kitamura et al. 2003, 2005; Molina-Molina et al. 2013; Perez et al. 1998; Pisapia et al. 2012; Rajasärkkä et al. 2014; Rosenmai et al. 2014; Satoh et al. 2004). One study showed that BPF was not estrogenic in a yeast two-hybrid assay (Ogawa et al. 2006). One study indicated that BPF was antiestrogenic (Stroheker et al. 2004). Moderate evidence from 6 studies showed that BPF was antiandrogenic based on relevance to human health [i.e., human androgen receptor (AR)], consistency across four cell models, and potency (i.e., within 100 orders of magnitude of positive control) (Cabaton et al. 2009; Kitamura et al. 2005; Molina-Molina et al. 2013; Rosenmai et al. 2014; Satoh et al. 2004; Stroheker et al. 2004). BPF also showed other in vitro effects such as cytotoxicity, cellular dysfunction, DNA damage, and chromosomal aberrations (Audebert et al. 2011; Cabaton et al. 2009; Lee et al. 2013; Nakagawa and Tayama 2000; Pisapia et al. 2012), and decreased adiponectin production and secretion in vitro (Kidani et al. 2010).
Potency of BPS and BPF compared with BPA. BPS and BPF are already being used as alternatives for BPA; thus, it is important to understand whether these substitutes possess endocrine-disruptive/active properties similar to those of BPA. Seventeen studies tested BPS and/or BPF along with BPA in the same assays, allowing the potencies and mechanisms of action to be directly compared. Table 4 presents these results, comparing the hormonal potencies of BPF and/or BPS to BPA. The average estrogenic potency (mean ± SD) for BPF compared with BPA was 1.07 ± 1.20, with a range of 0.10–4.83. The average estrogenic potency for BPS compared with BPA was 0.32 ± 0.28, with a range of 0.01–0.90. These results indicate that the potencies of BPS and BPF are in the same order of magnitude as the potency of BPA, and BPF may be just as potent (or more potent) than BPA. Further, BPS and BPF have potencies in the same order of magnitude as BPA in regard to androgenic, antiandrogenic, antiestrogenic, and aryl hydrocarbon activity and inhibitory hormonal signaling in adipocytes (Table 4).
Table 4.
In vitro BPS and BPF hormonal activity compared with BPA.
| Assay (receptor tested) | Chemical potency vs. positive control (control) | BPA potency vs. positive control (control) | Chemical potency compared with BPA potencya | Reference |
|---|---|---|---|---|
| BPS, estrogenic activity | ||||
| MCF-7 GFP (ERα) | 5.54 × 10–6 (E2) | 8.86 × 10–6 (E2) | 0.62 | Kuruto-Niwa et al. 2005 |
| E-screen (ERα) | NA (E2) | NA (E2) | 0.67 | Hashimoto and Nakamura 2000 |
| Yeast 2-hybrid (ERα) | 4.33 × 10–6 (E2) | 2.76 × 10–5 (E2) | 0.16 | Hashimoto and Nakamura 2000 |
| E-screen (ERα) | NA (E2) | NA (E2) | 0.90 | Hashimoto et al. 2001 |
| Yeast 2-hybrid (ERα) | 4.83 × 10–6 (E2) | 2.40 × 10–5 (E2) | 0.20 | Hashimoto et al. 2001 |
| Yeast 2-hybrid (ERα) | NC (E2) | NC (E2) | 0.10 | Chen et al. 2002 |
| MCF-7 luc (ERα) | 7.82 × 10–6 (E2) | 1.37 × 10–5 (E2) | 0.57 | Kitamura et al. 2005 |
| MELN (ERα) | 9.76 × 10–6 (E2) | 1.77 × 10–5 (E2) | 0.55 | Grignard et al. 2012 |
| BG1Luc4E2 (ERα, ERβ) | 2.52 × 10–7 (E2) | 3.14 × 10–6 (E2) | 0.08 | Grignard et al. 2012 |
| E-screen (ERα) | 1.0 × 10–6 (E2) | 3.75 × 10–5 (E2) | 0.03 | Molina-Molina et al. 2013 |
| MELN (ERα) | NR | NR | 0.04 | Molina-Molina et al. 2013 |
| HELN (ERα) | NR | NR | 0.10 | Molina-Molina et al. 2013 |
| HELN (ERβ) | NR | NR | 0.30 | Molina-Molina et al. 2013 |
| CV-1 luc (ERα) | 5.73 × 10–5 (E2) | 4.63× 10–4 (E2) | 0.12 | Teng et al. 2013 |
| GH3/B6/F10 ERK (mER) | 0.68 (E2) | 1.56 (E2) | 0.43 | Viñas and Watson 2013a |
| GH3/B6/F10 ERK (mER) | 1.36 (E2) | 1.91 (E2) | 0.71 | Viñas and Watson 2013b |
| Yeast bioreporter (ERα) | NR | NR | 0.01 | Rajasärkkä et al. 2014 |
| BG1Luc4E2 (ERα) | NC (E2) | NC (E2) | 0.23 | Rosenmai et al. 2014 |
| BPS average estrogenic potency compared with BPA (mean ± SD) | 0.32 ± 0.28 | |||
| BPS, antiandrogenic activity | ||||
| NIH353 + DHT (AR) | 0.18 (Flutamide) | 0.58 (Flutamide) | 0.25 | Kitamura et al. 2005 |
| BPS, androgenic activity | ||||
| MCF-7 AR1 (AR) | 9.00 × 10–7 (R1881) | 2.25 × 10–6 (R1881) | 0.40 | Molina-Molina et al. 2013 |
| PALM (AR) | NR | NR | 0.79 | Molina-Molina et al. 2013 |
| BPS, BPA activity | ||||
| Yeast bioreporter (BPAR) | 2.50 × 10–2 (BPA) | 1.00 (BPA) | 0.03 | Rajasärkkä et al. 2014 |
| BPF, estrogenic activity | ||||
| E-screen (ERα) | 1.0 × 10–3 (E2) | 0.01 (E2) | 0.10 | Perez et al. 1998 |
| E-screen (ERα) | NA (E2) | NA (E2) | 0.89 | Hashimoto and Nakamura 2000 |
| Yeast 2-hybrid (ERα) | 6.69 × 10–6 (E2) | 2.76 × 10–5 (E2) | 2.42 | Hashimoto and Nakamura 2000 |
| E-screen (ERα) | NA (E2) | NA (E2) | 0.99 | Hashimoto et al. 2001 |
| Yeast 2-hybrid (ERα) | 6.39 × 10–5 (E2) | 2.40 × 10–5 (E2) | 2.67 | Hashimoto et al. 2001 |
| Yeast 2-hybrid (ERα) | NC (E2) | NC (E2) | 0.79 | Chen et al. 2002 |
| E-screen (ERα) | 5.31 × 10–5 (E2) | 1.10 × 10–5 (E2) | 4.83 | Stroheker et al. 2004 |
| E-screen (ERα) | 4.67 × 10–6 (E2) | 7.78 × 10–6 (E2) | 0.60 | Satoh et al. 2004 |
| MVLN luc (ERα) | 5.86 × 10–6 (E2) | 1.17 × 10–5 (E2) | 0.50 | Satoh et al. 2004 |
| MCF-7 luc (ERα) | 8.6 × 10–6 (E2) | 1.37 × 10–5 (E2) | 0.63 | Kitamura et al. 2005 |
| E-screen (ERα) | 0.55 (E2) | 0.86 (E2) | 0.64 | Pisapia et al. 2012 |
| E-screen (ERα) | 1.0 × 10–5 (E2) | 3.75 × 10–5 (E2) | 0.27 | Rajasärkkä et al. 2014 |
| MELN (ERα) | NR | NR | 0.48 | Molina-Molina et al. 2013 |
| HELN (ERα) | NR | NR | 0.29 | Molina-Molina et al. 2013 |
| HELN (ERβ) | NR | NR | 0.36 | Molina-Molina et al. 2013 |
| Yeast bioreporter (ERα) | NR | NR | 1 | Rajasärkkä et al. 2014 |
| BG1Luc4E2 (ERα) | NC (E2) | NC (E2) | 0.81 | Rosenmai et al. 2014 |
| BPF average estrogenic potency compared with BPA (mean ± SD) | 1.07 ± 1.20 | |||
| BPF, antiandrogenic activity | ||||
| MDA-MB453+DHT (AR) | NR | NR | 0.78 | Stroheker et al. 2004 |
| AR-EcoScreen+DHT (AR) | 0.03 (Cyproterone acetate) | 0.06 (Cyproterone acetate) | 0.52 | Satoh et al. 2004 |
| NIH353+DHT (AR) | 0.21 (Flutamide) | 0.58 (Flutamide) | 0.36 | Kitamura et al. 2005 |
| PALM (AR) | NR | NR | 0.13 | Molina-Molina et al. 2013 |
| CHO AR (AR) | NC (R1881) | NC (R1881) | 0.94 | Rosenmai et al. 2014 |
| BPF average antiandrogenic potency compared with BPA (mean ± SD) | 0.55 ± 0.32 | |||
| BPF, antiestrogenic activity | ||||
| E-screen+tamoxifin (ERα) | NR | NR | 1.12 | Stroheker et al. 2004 |
| BPF, adiponectin secretion | ||||
| 3T3-L1 | NR | NR | 0.56 | Kidani et al. 2010 |
| BPF, BPA activity | ||||
| Yeast bioreporter (BPAR) | 2.50 × 10–3 (BPA) | 1.00 (BPA) | 0.003 | Rajasärkkä et al. 2014 |
| BPF, AhR activity | ||||
| H4IIE/CALUX (AhR) | NC (TCDD) | NC (TCDD) | 1.2 | Rosenmai et al. 2014 |
| Abbreviations: AhR, aryl hydrocarbon receptor; AR, androgen receptor; BPAR, BPA-targeted receptor; DHT, dihydrotestosterone; GFP, green fluorescent protein; luc, luciferase; mER, membrane estrogen receptor; NA, not available; NC, not able to calculate from the data presented (e.g., the positive control values were not reported); NR, not reported; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin. aPotencies were calculated by dividing the BPS or BPF potency by the BPA potency in the same study. | ||||
Rosenmai et al. (2014) used several assays to assess steroidogenic activity, as well as teratogenicity, genotoxicity, carcinogenicity, and metabolic effects. Similar to the present evaluation, they found that BPS and BPF had estrogen receptor binding, estrogenic activity, and antiandrogenic activity similar to those of BPA, with BPS being the least potent. However, BPS and BPF exhibited the greatest steroidogenic (i.e., progesterone) activity, increasing levels of 17α-hydroxyprogesterone and progesterone levels, whereas BPA did not (Rosenmai et al. 2014). Although the authors did not examine the mechanism of action of progesterone up-regulation, previous work suggested a direct inhibition of the CYP17 (cytochrome P450 17A1) lyase reaction, independent of ER action (Zhang et al. 2011). Thus, BPA analogs may have additional disruptive effects that have not been detected with BPA.
Discussion
Although relatively few studies have examined the hormonal actions of BPS and BPF (especially in vivo), the in vitro literature indicates that BPS and BPF have actions and potencies similar to those of BPA and supports the biological plausibility of their hormonal activity in vivo. This is not surprising because BPF and BPS are structural analogs of BPA and thus mechanisms of action would be expected to be similar. For example, BPF showed cumulative, possibly synergistic, actions in vivo when co-administered with an androgen (Yamasaki et al. 2003), and BPA has also been shown to have these types of effects when combined with other hormones or xenoestrogens (Kang et al. 2002; Silva et al. 2002). Particularly interesting is the fact that BPS seems to have actions on nongenomic signaling similar to those of BPA (Viñas and Watson 2013a, 2013b). BPA is sometimes called a “weak” estrogen because of its relatively weak binding/activation of the nuclear receptors compared with E2, although this is not always the case (Table 3; Kitamura et al. 2005; Perez et al. 1998; Pisapia et al. 2012). However, when the nongenomic estrogenic activity of BPA was measured, it was comparable, if not more potent, than E2. This potent, nongenomic estrogenic activity of BPA has been described in several experimental models (Alonso-Magdalena et al. 2008, 2012; Viñas and Watson 2013a, 2013b; Watson et al. 2014). The potency of BPS in a nongenomic signaling assay was similar to that of BPA. In femtomolar to picomolar concentrations, BPS induced membrane ERα-mediated pathways and actions: MAPK (mitogen-activated protein kinase) signaling, cell proliferation, and activation of caspase 8 (Viñas and Watson 2013a, 2013b). These rapid, nongenomic pathways are important for optimal cell function, mediating proliferation and apoptosis (Viñas and Watson 2013a, 2013b), as well as other actions such as pancreatic cell function (Alonso-Magdalena et al. 2008) and estrogen-mediated brain function and behavior (Laredo et al. 2014; Moenter and Chu 2012).
BPS and BPF had potencies in the same order of magnitude as BPA. The issue of potency is complicated because of the fact that lowest observed effect levels depend on end point, receptor type, pathway, tissues, windows of exposure, and so on. In general, BPS was slightly less potent than BPA. The average BPF potency was similar to BPA, with a fairly wide range of potencies. However, the implications of these differences are not clear. In regard to potency, it is not known whether a compound that is, for example, half as potent as BPA in vitro would have half the effect in vivo, especially because very little is known about the exposure and metabolism of BPS and BPF. Further, even if potencies of BPS and BPF are slightly less than that of BPA, it is unclear if these compounds are safer; many scientists have advocated a “no-threshold” approach to endocrine disruption because thresholds may change during development or may be very difficult to assess (Munn and Goumenou 2013).
The metabolism and biological fate of BPS and BPF have not been well studied, but in vitro and in vivo experiments indicated that BPF metabolism and distribution are similar to those of BPA. In vitro, BPA was metabolized by human and rat hepatic cells to many different metabolites, including non-bioactive sulfate and glucuronide conjugates (Cabaton et al. 2008; Dumont et al. 2011). In vivo, BPF administered to pregnant rats via gavage resulted in the excretion of BPF and several metabolites in the urine, including the nonactive sulfate-conjugated BPF. Active BPF was also distributed to many tissues, including the uterus, placenta, amniotic fluid, and fetuses. The ratio of the active parent compound to the metabolites/conjugates was similar to that of BPA (Cabaton et al. 2006; Vandenberg et al. 2013b). The primary route of excretion for BPF appeared to be through the sulfatase conjugate, rather than the glucuronide conjugate (as with BPA). Cabaton et al. (2006) suggested that this may be due to the fact that BPF glucuronide may be more easily deconjugated to its bioactive state and reabsorbed in large quantities, which also appears to occur with BPA (Vandenberg et al. 2013b). No studies have assessed the metabolism of BPS or the bioactivity of the metabolites. Studies determining the metabolism of BPS and the bioactivity of metabolites from BPF and BPS are warranted.
The body of literature on the in vivo effects of BPS and BPF is scant, but it points to these chemicals as endocrine disruptors and reproductive toxicants. BPS induced uterine growth in rodents (indicative of estrogenic action) and disrupted reproduction in fish (Ji et al. 2013; Naderi et al. 2014; Yamasaki et al. 2004), and BPF also had uterotropic (estrogenic) effects in female rodents and gonadotropic (androgenic) effects in male rodents (Higashihara et al. 2007; Stroheker et al. 2003, 2004; Yamasaki et al. 2004). Although most of the in vitro data support estrogenic, and to some extent, antiandrogenic, actions of BPS and BPF (Table 3), one in vitro study showed that BPS has androgenic activity similar to BPA (Molina-Molina et al. 2013). Thus, the in vitro data support the in vivo observations of hormonal and endocrine disruptive activity of these compounds.
Concern over the endocrine-disruptive effects of BPA has resulted in hundreds of laboratory studies, including in vitro (Wetherill et al. 2007) and in vivo (Richter et al. 2007b; Vandenberg 2014b) studies, identifying estrogenic and other effects. Although some regulators have rejected this body of literature because of a lack of standardized protocols, reviews of these studies have indicated strong methodologies and stringent laboratory practices, often of higher quality than studies employing Good Laboratory Practices (Myers et al. 2009). Many in vivo BPA studies have demonstrated adverse outcomes at “low” (i.e., environmentally or physiologically relevant) doses (Vandenberg 2014a; Vandenberg et al. 2012). Many studies also report that BPA has a nonlinear, or nonmonotonic, dose–response curve. Nonmonotonic dose responses are indicative of an endocrine-mediated response and are consistent with natural hormone responses (Vandenberg 2014b; Vandenberg et al. 2012, 2013a; Zoeller et al. 2012). Further, nearly 100 human studies described the relationship between BPA and several endocrine-related health impacts on reproduction, neurodevelopment, thyroid function, and metabolic health (Rochester 2013). Although epidemiological studies are less controlled than laboratory animal experiments, making it difficult to show causation, they are important indicators of potential health effects (Diamanti-Kandarakis et al. 2009; Zoeller et al. 2012). Further, although BPA is quickly metabolized and excreted from the body [with a half life of about 6 hr (Dekant and Völkel 2008)], the fact that it is found in almost all humans sampled at any one time suggests the ubiquitous and constant nature of BPA exposure (Vandenberg et al. 2010), which is disconcerting in light of the animal and human evidence of health effects. Many researchers have raised concern over this overwhelming evidence and have called for stricter regulation of BPA (Vandenberg et al. 2009, 2012). Although this concern has prompted BPA to be phased out of certain products (Food and Drug Administration 2012), the structural analog replacements may not be any safer.
Because BPS and BPF appear to have metabolism, potencies, and mechanisms of action in vitro similar to BPA, including hormonal actions beyond that of BPA, they may pose similar potential health hazards as BPA. Therefore, when evaluating the safety of compounds for consumer use, it may be prudent to consider entire classes instead of individual compounds. In addition, as other researchers have suggested (Viñas and Watson 2013a), future research efforts should focus on designing chemical substitutes that do not have biological or hormonal activity similar to those of BPA. Further, this review demonstrates that systematic reviews may be useful in the process of conducting safety evaluations of chemical classes. The use of the bisphenol class of compounds as replacements for BPA in consumer products with high human contact should be implemented with caution.
Acknowledgments
We dedicate this manuscript to T. Colborn (1927–2014), and we thank C. Kwiatkowski, L. Carroll, C. Ribbens, and T. Colborn for their help with and critical review of this manuscript.
Footnotes
This work was supported by the Arkansas Community Foundation, the Winslow Foundation, the Wallace Genetic Foundation, and the International Chemical Secretariat.
Funders did not have scientific or editorial control over the design, conduct, analysis, or interpretation of data, nor were they involved in producing this work. J.R.R. and A.L.B. are employed by The Endocrine Disruption Exchange (TEDX), a U.S. 501(c)3 organization that occasionally provides consultation, legal assistance, or expert testimony on the topic of endocrine-disrupting chemicals. Neither the authors nor TEDX stand to gain or lose financially through publication of this article.
References
- Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquie M, Gauthier BR, et al. 2008Pancreatic insulin content regulation by the estrogen receptor ERα. PLoS One 3e2069; 10.1371/journal.pone.0002069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Ropero AB, Soriano S, García-Arévalo M, Ripoll C, Fuentes E, et al. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Mol Cell Endocrinol. 2012;355:201–207. doi: 10.1016/j.mce.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Audebert M, Dolo L, Perdu E, Cravedi JP, Zalko D. Use of the γH2AX assay for assessing the genotoxicity of bisphenol A and bisphenol F in human cell lines. Arch Toxicol. 2011;85:1463–1473. doi: 10.1007/s00204-011-0721-2. [DOI] [PubMed] [Google Scholar]
- Bergman A, Rydén A, Law RJ, de Boer J, Covaci A, Alaee M, et al. A novel abbreviation standard for organobromine, organochlorine and organophosphorus flame retardants and some characteristics of the chemicals. Environ Int. 2012;49:57–82. doi: 10.1016/j.envint.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonefeld-Jørgensen EC, Long M, Hofmeister MV, Vinggaard AM.2007Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new data and a brief review. Environ Health Perspect 115suppl 169–76.; 10.1289/ehp.9368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabaton N, Chagnon MC, Lhuguenot JC, Cravedi JP, Zalko D. Disposition and metabolic profiling of bisphenol F in pregnant and nonpregnant rats. J Agric Food Chem. 2006;54:10307–10314. doi: 10.1021/jf062250q. [DOI] [PubMed] [Google Scholar]
- Cabaton N, Dumont C, Severin I, Perdu E, Zalko D, Cherkaoui-Malki M, et al. Genotoxic and endocrine activities of bis(hydroxyphenyl)methane (bisphenol F) and its derivatives in the HepG2 cell line. Toxicology. 2009;255:15–24. doi: 10.1016/j.tox.2008.09.024. [DOI] [PubMed] [Google Scholar]
- Cabaton N, Zalko D, Rathahao E, Canlet C, Delous G, Chagnon MC, et al. Biotransformation of bisphenol F by human and rat liver subcellular fractions. Toxicol In Vitro. 2008;22:1697–1704. doi: 10.1016/j.tiv.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Chen MY, Ike M, Fujita M. Acute toxicity, mutagenicity, and estrogenicity of bisphenol-A and other bisphenols. Environ Toxicol. 2002;17:80–86. doi: 10.1002/tox.10035. [DOI] [PubMed] [Google Scholar]
- Clark E. In: Kirk-Othmer Encyclopedia of Chemical Technology. New York, NY:John Wiley & Sons; 2012. Sulfolane and sulfones. [Google Scholar]
- Coggon D. Work with pesticides and organophosphate sheep dips. Occup Med (Lond) 2002;52:467–470. doi: 10.1093/occmed/52.8.467. [DOI] [PubMed] [Google Scholar]
- Dekant W, Völkel W. Human exposure to bisphenol A by biomonitoring: methods, results and assessment of environmental exposures. Toxicol Appl Pharmacol. 2008;228:114–134. doi: 10.1016/j.taap.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont C, Perdu E, de Sousa G, Debrauwer L, Rahmani R, Cravedi JP, et al. Bis(hydroxyphenyl)methane-bisphenol F-metabolism by the HepG2 human hepatoma cell line and cryopreserved human hepatocytes. Drug Chem Toxicol. 2011;34:445–453. doi: 10.3109/01480545.2011.585651. [DOI] [PubMed] [Google Scholar]
- Fic A, Žegura B, Sollner Dolenc M, Filipic M, Peterlin Masic L. Mutagenicity and DNA damage of bisphenol A and its structural analogues in HepG2 cells. Arh Hig Rada Toksikol. 2013;64:3–14. doi: 10.2478/10004-1254-64-2013-2319. [DOI] [PubMed] [Google Scholar]
- Fiege H, Voges HW, Hamamoto T, Umemura S, Iwata T, Miki H, et al. Winheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, 643–647; 2000. Phenol derivatives. In: Ullmann’s Encyclopedia of Industrial Chemistry. [Google Scholar]
- Food and Drug Administration. CFR part 177. Indirect food additives polymers. Final rule. Fed Reg. 2012;77:41899–41902. [Google Scholar]
- Fromme H, Küchler T, Otto T, Pilz K, Müller J, Wenzel A. Occurrence of phthalates and bisphenol A and F in the environment. Water Res. 2002;36:1429–1438. doi: 10.1016/s0043-1354(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Grignard E, Lapenna S, Bremer S. Weak estrogenic transcriptional activities of bisphenol A and bisphenol S. Toxicol In Vitro. 2012;26:727–731. doi: 10.1016/j.tiv.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Moriguchi Y, Oshima H, Kawaguchi M, Miyazaki K, Nakamura M. Measurement of estrogenic activity of chemicals for the development of new dental polymers. Toxicol In Vitro. 2001;15:421–425. doi: 10.1016/s0887-2333(01)00046-7. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Nakamura M. Estrogenic activity of dental materials and bisphenol-A related chemicals in vitro. Dent Mater J. 2000;19:245–262. doi: 10.4012/dmj.19.245. [DOI] [PubMed] [Google Scholar]
- Higashihara N, Shiraishi K, Miyata K, Oshima Y, Minobe Y, Yamasaki K. Subacute oral toxicity study of bisphenol F based on the draft protocol for the “Enhanced OECD Test Guideline no. 407.”. Arch Toxicol. 2007;81:825–832. doi: 10.1007/s00204-007-0223-4. [DOI] [PubMed] [Google Scholar]
- Howard GJ. Chemical alternatives assessment: the case of flame retardants. Chemosphere. 2014;116:112–117. doi: 10.1016/j.chemosphere.2014.02.034. [DOI] [PubMed] [Google Scholar]
- Ji K, Hong S, Kho Y, Choi K. Effects of bisphenol S exposure on endocrine functions and reproduction of zebrafish. Environ Sci Technol. 2013;47:8793–8800. doi: 10.1021/es400329t. [DOI] [PubMed] [Google Scholar]
- Kang KS, Cho SD, Lee YS. Additive estrogenic activities of the binary mixtures of four estrogenic chemicals in recombinant yeast expressing human estrogen receptor. J Vet Sci. 2002;3:1–5. [PubMed] [Google Scholar]
- Kidani T, Kamei S, Miyawaki J, Aizawa J, Sakayama K, Masuno H. Bisphenol A downregulates Akt signaling and inhibits adiponectin production and secretion in 3T3-L1 adipocytes. J Atheroscler Thromb. 2010;17:834–843. doi: 10.5551/jat.4051. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Sanoh S, Kohta R, Suzuki T, Sugihara K, Fujimoto N, et al. Metabolic activation of proestrogenic diphenyl and related compounds by rat liver microsomes. J Health Sci. 2003;49:298–310. [Google Scholar]
- Kitamura S, Suzuki T, Sanoh S, Kohta R, Jinno N, Sugihara K, et al. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol Sci. 2005;84:249–259. doi: 10.1093/toxsci/kfi074. [DOI] [PubMed] [Google Scholar]
- Kuruto-Niwa R, Nozawa R, Miyakoshi T, Shiozawa T, Terao Y. Estrogenic activity of alkylphenols, bisphenol S, and their chlorinated derivatives using a GFP expression system. Environ Toxicol Pharmacol. 2005;19:121–130. doi: 10.1016/j.etap.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Laredo SA, Villalon Landeros R, Trainor BC. Rapid effects of estrogens on behavior: environmental modulation and molecular mechanisms. Front Neuroendocrinol. 2014;35:447–458. doi: 10.1016/j.yfrne.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Liu X, Takeda S, Choi K. Genotoxic potentials and related mechanisms of bisphenol A and other bisphenol compounds: a comparison study employing chicken DT40 cells. Chemosphere. 2013;93:434–440. doi: 10.1016/j.chemosphere.2013.05.029. [DOI] [PubMed] [Google Scholar]
- Liao C, Kannan K. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J Agric Food Chem. 2013;61:4655–4662. doi: 10.1021/jf400445n. [DOI] [PubMed] [Google Scholar]
- Liao C, Kannan K. A survey of alkylphenols, bisphenols, and triclosan in personal care products from China and the United States. Arch Environ Contam Toxicol. 2014;67:50–59. doi: 10.1007/s00244-014-0016-8. [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Alomirah H, Loi VD, Mohd MA, Moon HB, et al. Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures. Environ Sci Technol. 2012a;46:6860–6866. doi: 10.1021/es301334j. [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Guo Y, Moon HB, Nakata H, Wu Q, et al. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ Sci Technol. 2012b;46:9138–9145. doi: 10.1021/es302004w. [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Kannan K. Bisphenol S, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol A residues. Environ Sci Technol. 2012c;46:6515–6522. doi: 10.1021/es300876n. [DOI] [PubMed] [Google Scholar]
- Mathew M, Sreedhanya S, Manoj P, Aravindakumar CT, Aravind UK. Exploring the interaction of bisphenol-S with serum albumins: a better or worse alternative for bisphenol A? J Phys Chem B. 2014;118:3832–3843. doi: 10.1021/jp500404u. [DOI] [PubMed] [Google Scholar]
- Moenter SM, Chu Z. Rapid nongenomic effects of oestradiol on gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2012;24:117–121. doi: 10.1111/j.1365-2826.2011.02135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Molina JM, Amaya E, Grimaldi M, Sáenz JM, Real M, Fernández MF, et al. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol Appl Pharmacol. 2013;272:127–136. doi: 10.1016/j.taap.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Munn S, Goumenou M. Thresholds for Endocrine Disrupters and Related Uncertainties, Report of the Endocrine Disrupters Expert Advisory Group. Luxembourg:Publications Office of the European Union. 2013. Available: https://ec.europa.eu/jrc/sites/default/files/lb-na-26-068-en-n.pdf [accessed 23 January 2015]
- Myers JP, vom Saal FS, Akingbemi BT, Arizono K, Belcher S, Colborn T, et al. 2009Why public health agencies cannot depend on good laboratory practices as a criterion for selecting data: the case of bisphenol A. Environ Health Perspect 117309–315.; 10.1289/ehp.0800173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi M, Wong MY, Gholami F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquat Toxicol. 2014;148:195–203. doi: 10.1016/j.aquatox.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Tayama S. Metabolism and cytotoxicity of bisphenol A and other bisphenols in isolated rat hepatocytes. Arch Toxicol. 2000;74:99–105. doi: 10.1007/s002040050659. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program. Draft Protocol for Systematic Review to Evaluate the Evidence for an Association between Bisphenol A (BPA) and Obesity. 2013. Available: http://ntp.niehs.nih.gov/ntp/ohat/evaluationprocess/bpaprotocoldraft.pdf [accessed 26 January 2015]
- Office of Environmental Health Hazard Assessment. Biomonitoring California: p,p’-Bisphenols and Diglycidyl Ethers of p,p’-Bisphenols. 2012. Available: http://www.oehha.ca.gov/multimedia/biomon/pdf/041113Bisphenols_priority.pdf [accessed 3 February 2015]
- Ogawa Y, Kawamura Y, Wakui C, Mutsuga M, Nishimura T, Tanamoto K. Estrogenic activities of chemicals related to food contact plastics and rubbers tested by the yeast two-hybrid assay. Food Addit Contam. 2006;23:422–430. doi: 10.1080/02652030500482371. [DOI] [PubMed] [Google Scholar]
- Owens JW, Ashby J. Critical review and evaluation of the uterotrophic bioassay for the identification of possible estrogen agonists and antagonists: in support of the validation of the OECD uterotrophic protocols for the laboratory rodent. Crit Rev Toxicol. 2002;32:445–520. doi: 10.1080/20024091064291. [DOI] [PubMed] [Google Scholar]
- Perez P, Pulgar R, Olea-Serrano F, Villalobos M, Rivas A, Metzler M, et al. The estrogenicity of bisphenol A-related diphenylalkanes with various substituents at the central carbon and the hydroxy groups. Environ Health Perspect. 1998;106:167–174. doi: 10.1289/ehp.98106167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyre L, Rouimi P, de Sousa G, Héliès-Toussaint C, Carré B, Barcellini S, et al. Comparative study of bisphenol A and its analogue bisphenol S on human hepatic cells: a focus on their potential involvement in nonalcoholic fatty liver disease. Food Chem Toxicol. 2014;70:9–18. doi: 10.1016/j.fct.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Pisapia L, Del Pozzo G, Barba P, Caputo L, Mita L, Viggiano E, et al. Effects of some endocrine disruptors on cell cycle progression and murine dendritic cell differentiation. Gen Comp Endocrinol. 2012;178:54–63. doi: 10.1016/j.ygcen.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Rajasärkkä J, Koponen J, Airaksinen R, Kiviranta H, Virta M. Monitoring bisphenol A and estrogenic chemicals in thermal paper with yeast-based bioreporter assay. Anal Bioanal Chem. 2014;406:5695–5702. doi: 10.1007/s00216-014-7812-x. [DOI] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007a;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CA, Taylor JA, Ruhlen RL, Welshons WV, vom Saal FS. Estradiol and bisphenol A stimulate androgen receptor and estrogen receptor gene expression in fetal mouse prostate mesenchyme cells. Environ Health Perspect. 2007b;115:902–908. doi: 10.1289/ehp.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA.2014Systematic review and evidence integration for literature-based environmental health science assessments. Environ Health Perspect 122711–718.; 10.1289/ehp.1307972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmai AK, Dybdahl M, Pedersen M, van Vugt-Lussenburg BM, Wedebye EB, Taxvig C, et al. Are structural analogues to bisphenol A safe alternatives? Toxicol Sci. 2014;139:35–47. doi: 10.1093/toxsci/kfu030. [DOI] [PubMed] [Google Scholar]
- Salvesen GS, Walsh CM. Functions of caspase 8: the identified and the mysterious. Semin Immunol. 2014;26:246–252. doi: 10.1016/j.smim.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K, Ohyama K, Aoki N, Iida M, Nagai F. Study on anti-androgenic effects of bisphenol A diglycidyl ether (BADGE), bisphenol F diglycidyl ether (BFDGE) and their derivatives using cells stably transfected with human androgen receptor, AR-EcoScreen. Food Chem Toxicol. 2004;42:983–993. doi: 10.1016/j.fct.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Silva E, Rajapakse N, Kortenkamp A. Something from “nothing”—eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ Sci Technol. 2002;36:1751–1756. doi: 10.1021/es0101227. [DOI] [PubMed] [Google Scholar]
- Song S, Song M, Zeng L, Wang T, Liu R, Ruan T, et al. Occurrence and profiles of bisphenol analogues in municipal sewage sludge in China. Environ Pollut. 2014;186:14–19. doi: 10.1016/j.envpol.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Stroheker T, Chagnon MC, Pinnert MF, Berges R, Canivenc-Lavier MC. Estrogenic effects of food wrap packaging xenoestrogens and flavonoids in female Wistar rats: a comparative study. Reprod Toxicol. 2003;17:421–432. doi: 10.1016/s0890-6238(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Stroheker T, Picard K, Lhuguenot JC, Canivenc-Lavier MC, Chagnon MC. Steroid activities comparison of natural and food wrap compounds in human breast cancer cell lines. Food Chem Toxicol. 2004;42:887–897. doi: 10.1016/j.fct.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Teng C, Goodwin B, Shockley K, Xia M, Huang R, Norris J, et al. Bisphenol A affects androgen receptor function via multiple mechanisms. Chem Biol Interact. 2013;203:556–564. doi: 10.1016/j.cbi.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN. Low-dose effects of hormones and endocrine disruptors. Vitam Horm. 2014a;94:129–165. doi: 10.1016/B978-0-12-800095-3.00005-5. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN. Non-monotonic dose responses in studies of endocrine disrupting chemicals: bisphenol A as a case study. Dose Response. 2014b;12:259–276. doi: 10.2203/dose-response.13-020.Vandenberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G.2010Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 1181055–1070.; 10.1289/ehp.0901716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, et al. Regulatory decisions on endocrine disrupting chemicals should be based on the principles of endocrinology. Reprod Toxicol. 2013a;38:1–15. doi: 10.1016/j.reprotox.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Hunt PA, Myers JP, vom Saal FS. Human exposures to bisphenol A: mismatches between data and assumptions. Rev Environ Health. 2013b;28:37–58. doi: 10.1515/reveh-2012-0034. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viñas R, Watson CS.2013aBisphenol S disrupts estradiol-induced nongenomic signaling in a rat pituitary cell line: effects on cell functions. Environ Health Perspect 121352–358.; 10.1289/ehp.1205826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viñas R, Watson CS.2013bMixtures of xenoestrogens disrupt estradiol-induced non-genomic signaling and downstream functions in pituitary cells. Environ Health 1226; 10.1186/1476-069X-12-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CS, Hu G, Paulucci-Holthauzen AA. Rapid actions of xenoestrogens disrupt normal estrogenic signaling. Steroids. 2014;81:36–42. doi: 10.1016/j.steroids.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, et al. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol. 2007;24:178–198. doi: 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Noda S, Imatanaka N, Yakabe Y. Comparative study of the uterotrophic potency of 14 chemicals in a uterotrophic assay and their receptor-binding affinity. Toxicol Lett. 2004;146:111–120. doi: 10.1016/j.toxlet.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Takeyoshi M, Sawaki M, Imatanaka N, Shinoda K, Takatsuki M. Immature rat uterotrophic assay of 18 chemicals and Hershberger assay of 30 chemicals. Toxicology. 2003;183:93–115. doi: 10.1016/s0300-483x(02)00445-6. [DOI] [PubMed] [Google Scholar]
- Yang Y, Lu L, Zhang J, Yang Y, Wu Y, Shao B. Simultaneous determination of seven bisphenols in environmental water and solid samples by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr A. 2014;1328:26–34. doi: 10.1016/j.chroma.2013.12.074. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chang H, Wiseman S, He Y, Higley E, Jones P, et al. Bisphenol A disrupts steroidogenesis in human H295R cells. Toxicol Sci. 2011;121:320–327. doi: 10.1093/toxsci/kfr061. [DOI] [PubMed] [Google Scholar]
- Zhou X, Kramer JP, Calafat AM, Ye X. Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;944:152–156. doi: 10.1016/j.jchromb.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology. 2012;153:4097–4110. doi: 10.1210/en.2012-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]


