Abstract
Tomato (Solanum lycopersicum L.) is among the most valuable agricultural products, but Meloidogyne spp. (root-knot nematode) infestations result in serious crop losses. In tomato, resistance to root-knot nematodes is controlled by the gene Mi-1, but heat stress interferes with Mi-1-associated resistance. Inconsistent results in published field and greenhouse experiments led us to test the effect of short-term midday heat stress on tomato susceptibility to Meloidogyne incognita race 1. Under controlled day/night temperatures of 25°C/21°C, ‘Amelia’, which was verified as possessing the Mi-1 gene, was deemed resistant (4.1 ± 0.4 galls/plant) and Rutgers, which does not possess the Mi-1 gene, was susceptible (132 ± 9.9 galls/plant) to M. incognita infection. Exposure to a single 3 hr heat spike of 35°C was sufficient to increase the susceptibility of ‘Amelia’ but did not affect Rutgers. Despite this change in resistance, Mi-1 gene expression was not affected by heat treatment, or nematode infection. The heat-induced breakdown of Mi-1 resistance in ‘Amelia’ did recover with time regardless of additional heat exposures and M. incognita infection. These findings would aid in the development of management strategies to protect the tomato crop at times of heightened M. incognita susceptibility.
Keywords: heat stress, Meloidogyne incognita, Mi-1 gene, resistance, root-knot nematode, Solanum lycopersicum
Tomato (Solanum lycopersicum L.) is among the most valuable agricultural products globally. More than 100 million tons are produced annually, and the USA is among the top five producers (Anonymous, 2012a). However, Meloidogyne spp. (root-knot nematode) infestations cause serious crop losses worldwide. Tomato yield reductions as severe as 40% have been reported (Reddy, 1985), particularly in warm climates such as Florida where 10% of U.S. tomatoes are grown and where conditions allow for high nematode population growth within a season (Lamberti, 1979; Williamson and Hussey, 1996; Koenning et al., 1999; Anonymous, 2012b).
Management of root-knot nematodes is challenging because their wide host range prevents use of crop rotation (Trudgill, 1991; Chen et al., 2006). Soil fumigants, contact and systemic nematicides, resistant cultivars, resistant rootstocks, and cultural practices are commonly employed to control root-knot nematodes (Lopez-Perez et al., 2006; Devran et al., 2010). The highly effective and widely used soil fumigant methyl bromide has been banned or phased out in most countries by international agreement because of its contribution to reducing stratospheric ozone (Rosskopf et al., 2005) and human and animal health concerns (Oka et al., 2000; Ploeg, 2002; Devran et al., 2010). Thus, host-plant resistance to root-knot nematodes is a powerful and sustainable tool for crop protection (Roberts and Thomason, 1986; Devran and Elekçioglu, 2004).
The only commercially available source of resistance to root-knot nematodes in tomatoes is a single, dominant gene named Mi-1, which confers resistance, though not immunity, to three of the most damaging species: Meloidogyne incognita, Meloidogyne javanica, and Meloidogyne arenaria (Milligan et al., 1998). This gene was found in Solanum peruvianum L. and outcrossed into S. lycopersicum L. (Rodriguez, 2013). Resistance is associated with a hypersensitive response, characterized by localized cell death of host tissue near the invading nematode in the root tips (Williamson and Kumar, 2006). For this study, we consider resistance as a reduction in the ability of the nematode to reproduce on the plant, as quantified by the number of galls formed (Sasser et al., 1984). Interestingly, other root-knot nematode resistance genes have been found in the S. peruvianum complex, including Mi-9, a homologue of Mi-1 unaffected by temperature, but these are not yet commercially available (Bleve-Zacheo et al., 2007).
The efficacy of the Mi-1 gene varies with root-knot nematode species and population, tomato cultivar, and environmental conditions, particularly soil temperature (Araújo et al., 1982; Devran et al., 2010; Verdejo-Lucas et al., 2013). Increased gall formation has been shown in plants exposed to soil temperatures above 28°C, with higher temperatures associated with higher gall numbers (Dropkin, 1969; Ammati et al., 1986; Wang et al., 2009; Devran et al., 2010). However, there is some discrepancy in the literature. Several articles have reported complete loss of resistance at temperatures ≥ 32°C (Dropkin, 1969; Williamson, 1998) whereas others have shown that Mi-1-conferred resistance was still effective in some cultivars at soil temperatures ≥ 34°C (Abdul-Baki et al., 1996; Verdejo-Lucas et al., 2013).
Dropkin (1969) found that Mi-1 resistance was lost after 4 d at temperatures ≥ 33°C in 1- to 3-d-old seedlings exposed to heat treatment in constant-temperature tanks after inoculation with nematodes, and subsequently held at 27°C for 1 mo. In contrast, the Mi-1 gene was still effective at soil temperatures ≥34°C in excised plant roots in constant-temperature growth chambers (Abdul-Baki et al., 1996) and in greenhouses kept at ambient temperature, where heat occurred before and after inoculation (Verdejo-Lucas et al., 2013).
These studies differed in length of time of heat exposure and temporal sequence of heat and inoculation leading us to consider the possibility of resistance recovery. We hypothesized that Mi-1-resistant tomatoes exposed to spikes of high temperature will become susceptible, but resistance may return with time. Thus, the goal of our study was to (i) compare the level of M. incognita infection in a resistant tomato cultivar at ambient temperature (25°C) to the level of infection following a midday heat spike, (ii) evaluate the potential recovery of Mi-1-resistance post-heat stress, (iii) determine the impact of multiple consecutive days of midday heat spikes on resistance, and (iv) assess the susceptibility of infected plants to secondary nematode infection.
Materials and Methods
Plant material and growth condition:
All experiments were conducted using two commercial tomato cultivars: Solanum lycopersicum cv. Amelia, which is heterozygous for the Mi-1 gene for resistance (Desaeger and Csinos, 2006), and S. lycopersicum cv. Rutgers, which does not have the Mi-1 gene and is considered susceptible to M. incognita infection (Melakeberhan, 1998; Bendezu, 2004). ‘Amelia’ is a determinate hybrid with resistance to M. incognita, M. javanica, and M. arenaria; races 1, 2, and 3 of Fusarium wilt, Verticillium wilt, tomato spotted wilt; high temperatures; and cracking (Riley et al., 2011; Srinivasan et al., 2012). It was bred primarily for the southeast United States (Gardner and Panthee, 2009a, 2009b). Level of zygosity and, in general, genotypic background have been shown to affect the level of resistance provided by the Mi-1 gene, and may also affect heat stability (Abdul-Baki et al., 1996; Jacquet et al., 2005).
Plants were grown from seed in autoclaved, sifted 80:20 sand:growing medium (Playsand No. 1113; QuickCrete Intl. Inc. Atlanta, GA and MetroMix 910; Sun Gro Horticulture Distribution, Inc., Bellevue, WA) in 0.5-liter plastic pots. Plants were watered daily with Hoagland’s complete nutrient solution (Gibeaut et al., 1997; Melakeberhan, 1998) and maintained at 25°C ± 2°C (0700–1800 hr; day): 21°C ± 1°C (1900–0600 hr; night), with 55% to 60% relative humidity for 3 wk in an environmentally controlled chamber. Before experimentation with heat treatments, the plants were transferred to two independent environmentally controlled rooms of an eight-room polycarbonate greenhouse in Gainesville, FL, as previously described (Zhang et al., 2014) and were allowed to acclimate for at least 2 hr. Temperature conditions are described in the heat treatment section below.
Meloidogyne incognita nematode inoculation:
The root-knot nematodes, M. incognita race 1, came from a culture maintained on the susceptible tomato cv. Rutgers, originally started from a single egg mass by Dr. J. Brito (Division of Plant Industry, Florida Department of Agriculture and Consumer Services, Gainesville, FL). Nematode eggs were extracted from infected roots using a 10% NaOCl solution (Hussey and Barker, 1973). The plants were inoculated with 500 J2 of M. incognita, a quantity of nematodes commensurate with the small size of the 3-wk-old plants (Cooper et al., 2005). A suspension of J2 nematodes in water was distributed among three holes in the soil (0.5 cm deep), 2 to 3 cm from the stem. In all cases, inoculation occurred after heat treatment, such that nematodes were not exposed to the heat (Figs. 1A; 2A). Also, within an experiment, planting dates and heat treatments were manipulated to enable inoculation to occur on the same day, using the same inoculum.
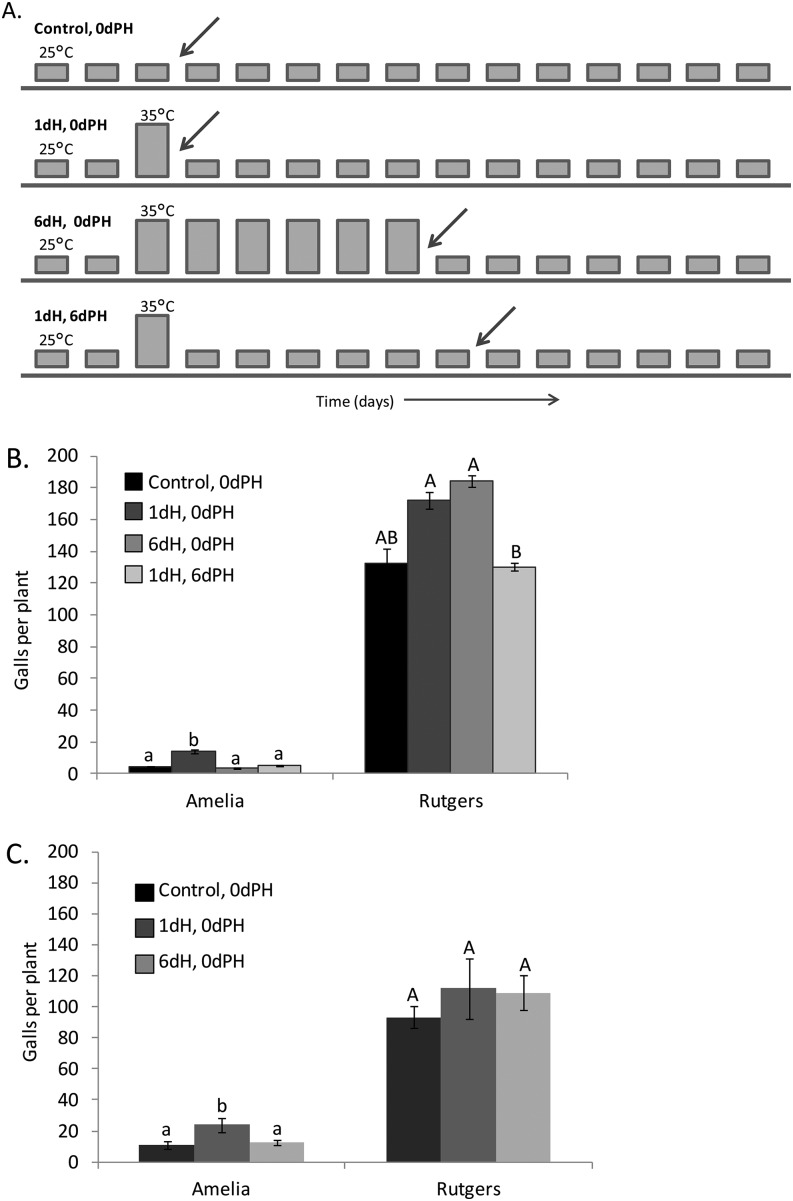
Fig. 1.
Effect of heat treatment on the number of Meloidogyne incognita galls. A. Graphical representation of treatments, where each square represents 1 d, and treatment name is in bold above the sequence of days with heat spike (taller bars) and nematode inoculation (diagonal arrows) indicated. Heat spike consisted of 3 hr at 35°C. Inoculation with 500 J2 nematodes occurred after heat treatment. Roots were collected and stained for gall quantification 2 weeks after nematode inoculation. B. Tomato cv. Amelia (N = 8 plants) and cv. Rutgers (N = 3 plants, except the control, which had 5 plants). Different letters indicate statistical differences (P < 0.05) using a nonparametric distribution. C. Tomato cv. Amelia (N = 8 plants) and Rutgers (N = 5 plants). dH = days of heat treatment, dPH = days post-heat treatment. Different letters indicate statistical differences (P < 0.033) using a nonparametric distribution and different letter case indicates a separate statistical analysis for ‘Amelia’ and ‘Rutgers’. Errors bars indicate ± standard error.
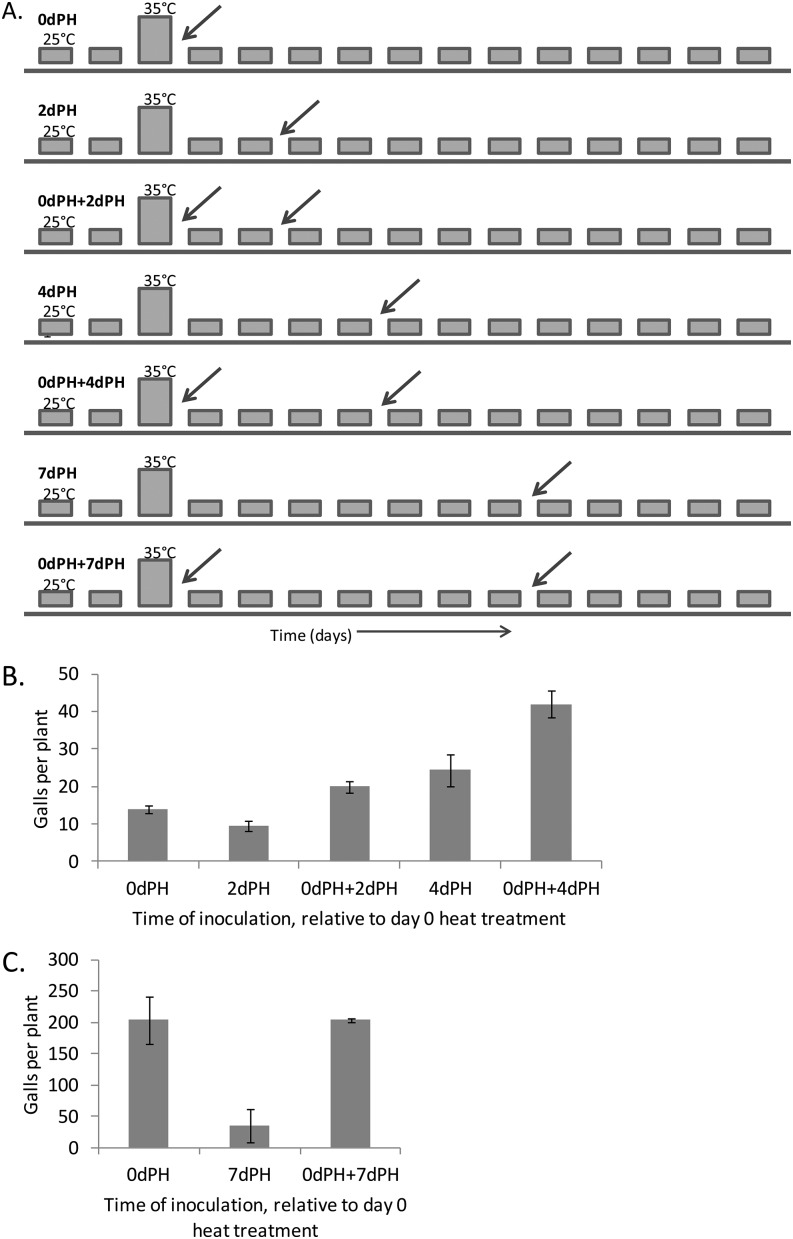
Fig. 2.
Effect of time since heat treatment and of previous inoculation on the number of root-knot nematode galls. A. Graphical representation of treatments, where each square represents 1 d, and treatment name is in bold above the sequence of days with heat spike (taller bars) and nematode inoculation (diagonal arrows) indicated. Heat spike consisted of 3 hr at 35°C. Inoculation with 500 J2 nematodes occurred after heat treatment. Roots were collected and stained for gall quantification 2 wk after last nematode inoculation of each treatment. B. Tomato cv. Amelia (N = 10 plants). Using a nonparametric distribution, 0 dPH and 2 dPH combined are not significantly different from 0 dPH + 2 dPH (P = 0.1122), and 0 dPH and 4 dPH combined are not significantly different from 0 dPH + 4 dPH (P = 1.0362). C. Tomato cv. Amelia (N = 6 plants). Using a nonparametric distribution, 0 dPH and 7 dPH combined are not significantly different from 0 dPH + 7 dPH (P = 0.0802). dH = days of heat treatment, dPH = days post-heat treatment. Errors bars indicate ± standard error.
Verification of Mi-1 gene genotype in tested cultivars:
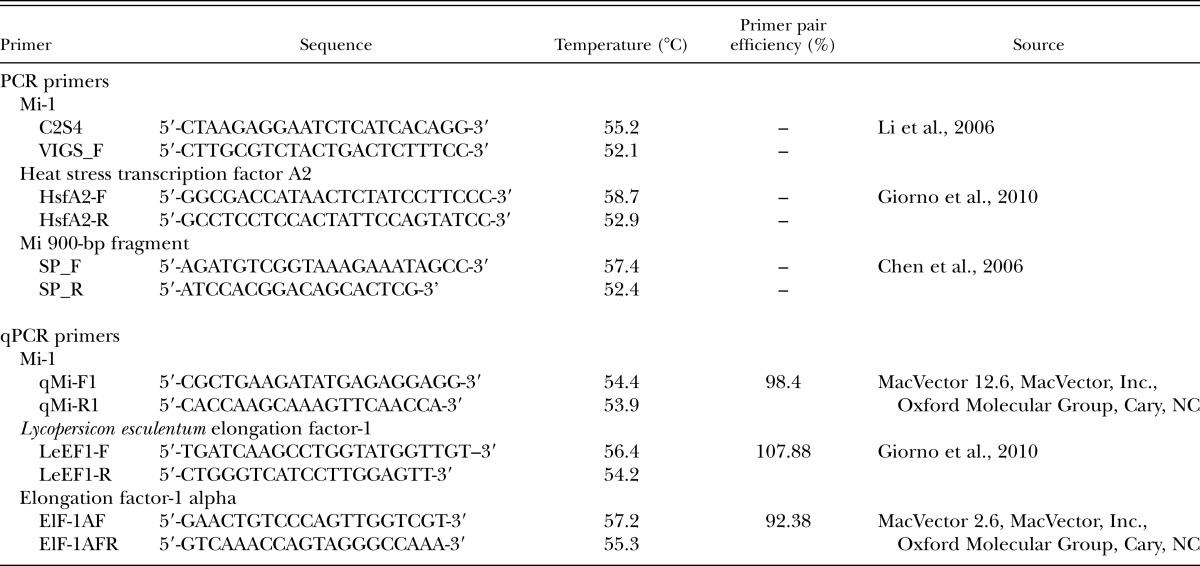
To initially verify the presence and absence of the Mi-1 gene in ‘Amelia’ and ‘Rutgers’, respectively, we collected root tissue from 3-wk-old plants of each cultivar and isolated DNA using a Zymo columns kit (Zymo Research Co., Irvine, CA), following manufacturer’s instructions. Mi-1 gene–specific primers (C2S4/VIGS_F; Table 1) and two sets of control gene primers (HsfA2 and SP), which should be present in both cultivars, were used to amplify DNA fragments with Invitrogen Platinum Taq DNA polymerase (Life Technologies, Grand Island, NY), following manufacturer’s instructions. Thermal cycling conditions were 94°C for 2 min, followed by 34 cycles of 94°C for 30 sec, 52°C for 30 sec, and 72°C for 5 min. Thermal cycling conditions for HsfA2 and SP control gene primers were similar, except annealing temperatures were set at 58°C for 30 sec and 57 for 1 min, respectively. PCR products were cloned into the pGEM-T Easy Vector System (Promega, Madison, WI) and sequenced for validation (University of Florida Interdisciplinary Center for Biotechnology Research, Gainesville, FL).
Table 1.
Primers used for 5′ and 3′ PCR and qPCR.
Mi-1 gene transcript analysis:
To assess whether changes in ‘Amelia’ resistance with heat treatment were related to transcriptional changes of the Mi-1 gene, the relative amount of Mi-1 mRNA in tomato cv. Amelia was estimated by real-time quantitative PCR (qPCR). Heat treated 3-wk-old plants (3 hr at 35°C) were inoculated with 500 M. incognita eggs 30 min after the heat treatment ended, and the roots were collected 21 hr later (at 1030 hr). RNA was isolated using the RNeasy mini kit (QIAGEN, Hilden, Germany), following the manufacturer’s instructions. Three primer pairs (qMi-1, qMi-2, and qMi-3; Table 1) were developed using the software MacVector 12.6 (MacVector, Inc., Oxford Molecular Group, Cary, NC). To test for primer pair efficiency, a fivefold dilution series of pure ‘Amelia’ DNA was used to generate a standard curve by plotting the Ct values obtained by qRT-PCR against the log [DNA] (Nicolaisen et al., 2009). The most efficient primer pair, qMi-1, was selected as well as housekeeping genes LeEF1 and Elf-1a to conduct q-PCR (Table 1). Thermal cycling conditions were 95°C for 2 min, followed by 40 cycles of 95°C for 5 sec and 60°C for 30 sec, then 95°C for 5 sec, and finally a melt curve of 65°C to 95°C with an increment of 0.5°C per 5 sec.
Heat treatment experiments:
To compare differences between the level of M. incognita infection in plants at ambient temperatures to the level of infection following a single midday heat spike, subsets of ‘Amelia’ and ‘Rutgers’ plants were transferred to separate rooms: one designated as control and the other as heat treatment. The control greenhouse room was maintained at 25°C ± 2°C (0700–1800 hr; day): 21°C ± 1°C (1900–0600 hr; night), with 55% to 60% relative humidity. The heat-treatment room was maintained at the same conditions except for a single heat treatment day (1 d heat; 1 dH) of 35°C from 1200 to 1500 hr, preceded and followed by 1 hr of temperature transition (Fig. 1A). Each treatment was replicated with 10 plants of ‘Amelia’ and five of ‘Rutgers’. Plants were watered as needed with Hoagland’s solution maintained at the individual greenhouse room temperature. Soil temperature was monitored by StowAway TidbiT temperature data loggers (Onset Computer Corporation, Cape Cod, Bourne, MA) buried in pot substrate. One hour after the heat treatment, at which point the soil temperature had returned to 25°C ± 1°C, the plants were inoculated with M. incognita as described above (0 d post heat; 0 dPH). Plants were then maintained at control conditions, described above, for 14 d to allow for nematode development and galls production. This postinoculation period is likely insufficient for development of egg masses and secondary M. incognita infection.
To determine whether additional days of heat treatment would intensify the level of susceptibility, an additional set of plants were subjected to the heat treatment of 35°C between 1200 and 1500 hr for six consecutive days (6 d heat; 6 dH) before nematode inoculation (0 dPH). Furthermore, to determine whether the enhanced susceptibility following a single heat treatment persisted or decreased with time, plants were exposed to the heat treatment (1 dH), but then inoculated 6 d after the treatment (6 dPH). The control, 1 dH, and 6 dH treatments of this experiment were replicated, using eight plants each of ‘Amelia’ and ‘Rutgers’.
A separate experiment was conducted to better characterize changes in susceptibility. Plants were exposed to the heat treatment (1 dH), and then inoculated 2, 4, or 7 d after the treatment (2, 4, or 7 dPH). Again, the nematodes were allowed to develop for 14 d, and the number of galls were counted and compared to the gall production in other treatments.
Finally, the influence of previous M. incognita infection on secondary nematode infection was assessed. Plants were exposed to a single day of heat treatment (1 dH) and inoculated with M. incognita twice: on the same day as the heat treatment, and again 2, 4, 6, or 7 dPH (0 dPH + 2 dPH, 0 dPH + 4 dPH, or 0 dPH + 7 dPH).
Data collection and statistical analysis:
Roots of the inoculated tomato plants were stained with acid fuchsin 14 d postinoculation to quantify the number of nematode galls (Barker, 1985). Stained galls were counted using a light microscope.
Statistical analyses:
Quantitative PCR data was analyzed using the ΔΔCt method (Livak and Schmittgen, 2001). The ΔCt values were used to conduct an ANOVA to compare relative mRNA levels between tomato plants with or without heat treatment and with or without nematode inoculation.
A nonparametric bootstrap strategy was used to compare the number of galls (gall count) of any two treatments because the data were overdispersed and not normally distributed (Efron and Tibshirani, 1993). The difference between the mean gall counts of any two treatments was used as the test statistic. The null distribution of the difference was constructed by sampling with replacement from the mixed data from the two treatments. P-values were determined by comparing the observed difference to the constructed null distribution of the differences in mean gall counts. A Bonferroni correction was used in the case where multiple tests were conducted.
A parametric bootstrap approach was used to compare the sum of mean gall counts of two treatments run separately to the mean gall counts of another treatment within the same experiment (e.g., the sum of mean gall counts from singly inoculated 0 and 2 dPH plants compared to the twice inoculated 0 dPH + 2 dPH plants). In this case, a nonparametric distribution of the gall counts was assumed. The null distribution was constructed using the singly inoculated treatments and assuming independence of their results, leading to the expectation that the expected mean gall counts of the twice inoculated treatments will be the sum of the two means of the singly inoculated treatments. That is, a previous inoculation at 0 dPH will not affect the infection rate of a second inoculation at 2 dPH, and hence, the resulting counts will match the sum of the counts when these two treatments were separate. The difference between the mean counts of the observed samples ([mean count of 0 dPH + mean count of 2 dPH] − [mean count of 0 dPH + 2 dPH]) were compared to those generated under the null distribution to determine the P-values.
Results
The presence of the Mi-1 gene–conferred resistance to ‘Amelia’:
Using Mi-1 gene–specific primers, we confirmed the presence of the Mi-1 gene in ‘Amelia’ and the absence in ‘Rutgers’ (Fig. 3). This was consistent with M. incognita resistance in ‘Amelia’, which only formed 4.1 ± 0.4 galls per plant and M. incognita susceptibility in ‘Rutgers’, which formed 132.0 ± 9.9 galls per plant when grown at 25°C (control, 0 dPH; Fig. 1B,C).
Fig. 3.
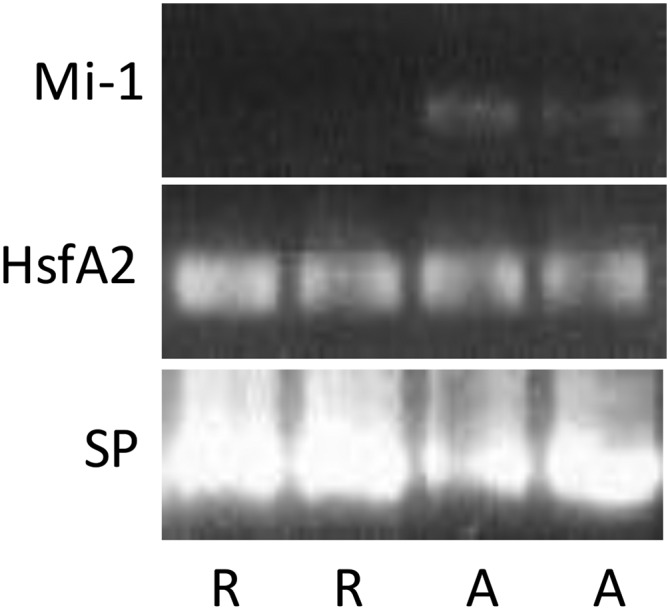
Polymerase chain reaction product indicating the presence of the Mi-1 gene in tomato cv. Amelia (A) and absence in cv. Rutgers (R), and the presence of housekeeping genes HsfA2 and SP in both cultivars.
Heat treatment increased gall number in ‘Amelia’, but resistance recovered with time. This recovery was unaffected by additional consecutive heat treatments or previous infection:
A single midday 3 hr heat spike of 35°C was sufficient to significantly reduce Mi-1 gene–conferred resistance in ‘Amelia’. The number of nematode galls formed in heat-treated plants was 3.4-fold higher than unheated ‘Amelia’ control plants (Bonferonni corrected P < 0.0001) (1 dH, 0 dPH; Fig. 1B). Results were similar in a second replicate of the experiment (Bonferonni-corrected P = 0.033) (1 dH, 0 dPH; Fig. 1C). There was no significant difference between the heat-treated ‘Rutgers’ and the control ‘Rutgers’ plants (Bonferonni-corrected P = 0.144 and P = 0.5124, respectively, shown in Fig. 1B,C).
Contrary to the expectation that additional days of heat might increase the susceptibility of ‘Amelia’ to M. incognita, plants exposed to six consecutive daily heat spikes (3 hr at 35°C) with nematode inoculation on the 6th day of heat (6 dH, 0 dPH) formed fourfold fewer galls than ‘Amelia’ exposed to a single heat spike (Bonferonni-corrected P = 0.0014; Fig. 1B). In a second replicate of the experiment, there was no significant difference between plants exposed to 1 or 6 dH (Bonferonni-corrected P = 0.6111) (6 dH, 0 dPH; Fig. 1C). Thus, Mi-1 resistance in ‘Amelia’ recovered with time, or at least, did not break down further, despite the additional days of heat treatment. Similarly, the number of galls that formed in ‘Amelia’ inoculated with nematodes 6 d after a single day of heat treatment (1 dH, 6 dPH) was not significantly different from ‘Amelia’ control plants (Bonferonni-corrected P = 0.9; Fig. 1B), nor from plants exposed to 6 consecutive days of heat spikes (Bonferonni-corrected P = 0.4614; Fig. 1B). Under the same treatment of 6 consecutive days of heat spikes (6 dH, 0 dPH), ‘Rutgers’ gall formation remained unaffected (Bonferonni-corrected P = 1.386 and P = 1.6644, respectively; Fig. 1B,C).
Furthermore, nematode infection did not appear to enhance M. incognita susceptibility nor reduce the ability of ‘Amelia’ to recover resistance with time (Fig. 2B,C). The average number of galls in ‘Amelia’ inoculated twice (0 dPH + 2 dPH) was not significantly different from the sum of those found in ‘Amelia’ inoculated solely on 0 or 2 dPH (Bonferonni-corrected P = 0.1122; Fig. 2B). Similarly, there was no significant difference when the second inoculation was 4 or 7 dPH (Bonferonni-corrected P = 1.0362, and 0.0802, respectively; Fig. 2B,C).
Heat treatment and nematode infection did not influence Mi-1 gene transcription:
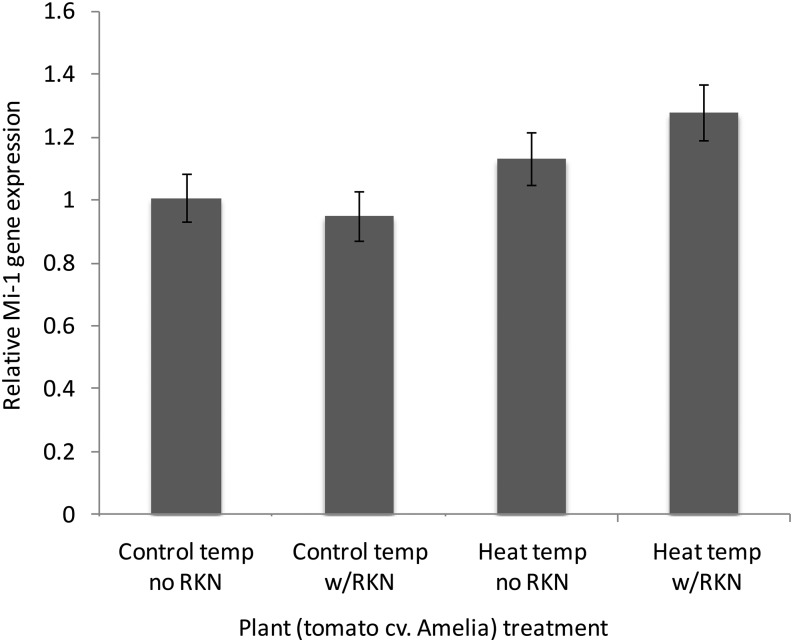
Although the presence of Mi-1 gene confers M. incognita resistance to ‘Amelia’, this resistance does not appear to be regulated at the transcriptional level. Transcript levels of Mi-1 gene in ‘Amelia’ plants 21 hr after exposure to heat (35°C), inoculation with M. incognita, or both, remained similar to transcript levels in ‘Amelia’ plants grown at 25°C without M. incognita inoculation (Fig. 4).
Fig. 4.
Effect of a single day of 3 hr heat at 35°C, followed by inoculation with 500 J2 Meloidogyne incognita, on the relative Mi-1 mRNA level in tomato cv. Amelia (N = 4 plants), using qMi-1 primers. Errors bars indicate ± standard error. Analysis of variance, F = 3.297, P = 0.0578.
Discussion
To date, Mi-1 is the only commercially available resistance gene for root-knot nematodes, one of the most damaging plant pathogens worldwide (Mantelin et al., 2013). Improved understanding of how and when heat exposure disrupts Mi-1 resistance is essential to developing strategies to reduce agro-economical losses associated with root-knot nematodes. Our research has shown that a single midday heat exposure of 35°C for 3 hr is sufficient to break Mi-1 resistance in S. lycopersicum cv. Amelia, leading to a significant increase in gall formation. However, this increase in susceptibility does not persist, and resistance is completely recovered 6 d after heat exposure. Recovery of Mi-1 gene resistance is not affected by additional days of heat or previous nematode infection.
The presence of the Mi-1 gene in ‘Amelia’ and absence in ‘Rutgers’ was consistent with resistance of ‘Amelia’ and susceptibility of ‘Rutgers’ to M. incognita race 1, as measured by number of galls formed. Similarly, other studies have found fewer M. incognita juveniles, egg masses, and galls in plants with the Mi-1 gene than in susceptible cultivars (Vos et al., 1998; Tzortzakakis et al., 1999; Jacquet et al., 2005; Lopez-Perez et al., 2006). The presence of a few galls in the control is not surprising: multiple studies, including ours, show that the Mi-1 gene confers resistance not immunity. Variation in Meloidogyne spp. reproduction on resistant tomato genotypes has been attributed to the plant’s genetic background, level of zygosity, and possibly an additional locus or other factor in the resistant genotype affecting resistance expression (Abdul-Baki et al., 1996; Jacquet et al., 2005; Verdejo-Lucas et al., 2013). These factors may also play a role in resistance stability and recovery, such that a different cultivar with the Mi-1 gene may respond differently to heat exposure compared to the heterozygous cv. Amelia. The lower number of galls in ‘Amelia’ relative to ‘Rutgers’ is expected because of high M. incognita susceptibility of ‘Rutgers’ (Ammati et al., 1986).
Our study is unique in that it used a short (3 hr) midday heat treatment, which is more comparable to field-like heat exposures during the day. Previous studies have found that resistance to M. incognita breaks down at ≥32°C; however, these studies employed heat treatments of ≥24 hr (Abawi and Barker, 1984; Zacheo et al., 1995; Devran et al., 2010). Zacheo et al. (1995) found that 2 d at 34°C was required to break resistance of cv. VFN8 to M. incognita. In addition to treatment timing, duration, and nematode exposure to heat, nematode species and plant cultivar differences may explain some of the conflicts with previous studies. For example, Verdejo-Lucas et al. (2013) found that Mi-1 resistance to M. arenaria and M. javanica remained intact in three of five cultivars exposed for 65 d to ambient soil temperatures as high as 34.1°C for ≥7.5 hr daily.
We found that 6 d with 3 hr of heat before nematode inoculation actually produced fewer galls than a single day of heat, showing that resistance returns over time or with a longer heat treatment. This suggests that Mi-1 resistance either acclimates to or recovers from exposure to high temperatures. Both of these hypotheses are also supported by a previous study, which found that 6 d of heat (constant 34°C) rendered seedlings susceptible for only 1 to 2 d after returning to control temperature (27°C) (Zacheo et al., 1995). Interestingly, the loss of susceptibility was accompanied by an increase in peroxidase activity, which is associated with the hypersensitive response, signifying the return of Mi-1 resistance (Zacheo et al., 1995). Contrary to the recovery hypothesis, resistance to M. incognita and M. arenaria was lost or reduced at 37°C for 35 d in six cultivars (Abdul-Baki et al., 1996). However, this lack of recovery may be the result of a lack of daily temperature fluctuation: temperatures were kept constant throughout that study. Araújo et al. (1982) found that plants exposed to differentially low nocturnal temperatures (19°C–25°C) and high diurnal temperatures (31°C) were better able to maintain resistance than plants exposed to constantly high temperatures. Daily temperature fluctuation may also explain the maintenance of resistance in the study by Verdejo-Lucas et al. (2013), who suggested that differences in the literature regarding resistance to root-knot nematodes and high temperature could be explained by the level of stress, where plants that experience daily fluctuations in soil temperature could recover Mi-1 resistance more easily than with longer heat treatments.
The reduction in gall number when ‘Amelia’ plants were inoculated 6 d after a single day of heat treatment suggests recovery of Mi-1 resistance, rather than acclimation to high temperature. Also, previously M. incognita–infected plants inoculated with a second aliquot of M. incognita did not show any additional susceptibility to this second infection. The increase in galls in these twice inoculated plants was consistent with the number of galls found in a plant inoculated only at the second time point (2, 4, or 7 dPH). Again, this suggests that the plant’s overall resistance does recover. It is useful to note that Dropkin (1969) found that once an individual M. incognita initiates development in the root, nematode growth will continue, even after a shift in temperature.
Despite the break in Mi-1 resistance, Mi-1 gene transcription was not affected by the 35°C heat spike. This suggests that heat-associated breakdown in Mi-1 resistance is affected by changes downstream of gene expression, such as protein conformational changes (Zhu et al., 2010).
From our results and previous studies, we suggest that soil temperatures ca. 35°C reduce the effectiveness of Mi-1 resistance in tomatoes. We also show that Mi-1 resistance can recover with time and that previous M. incognita infection does not make Mi-1 resistant plants more susceptible to further M. incognita infection. Hence, planting during the hottest season should be avoided, but occasional short-term high temperatures in the field will likely not greatly increase M. incognita gall formation. Understanding the effect of heat on Mi-1 resistance in tomatoes downstream of Mi-1 gene expression may offer insight into how this resistance works, as well as avenues for ameliorating the effect of heat stress.
Literature Cited
- Abawi GS, Barker KR. Effects of cultivar, soil temperature, and population levels of Meloidogyne incognita on root necrosis and Fusarium wilt of tomatoes. Phytopathology. 1984;74:433–438. [Google Scholar]
- Abdul-Baki AA, Haroon SA, Chitwood DJ. Temperature effects on resistance to Meloidogyne spp. in excised tomato roots. HortScience. 1996;31:147–149. [Google Scholar]
- Ammati M, Thomason IJ, McKinney HE. Retention of resistance to Meloidogyne incognita in Lycopersicon genotypes at high soil temperature. Journal of Nematology. 1986;18:491–495. [PMC free article] [PubMed] [Google Scholar]
- Anonymous 2012a. Food and agricultural commodities production/commodities by regions. FAOSTAT database. Rome, Italy: Food and Agriculture Organization of the United Nations.
- Anonymous 2012b. Tomato national and state statistics. Washington, DC: NASS United States Department of Agriculture.
- Araújo MT, Bassett MJ, Augustine JJ, Dickson DW. Effect of diurnal changes in soil temperatures on resistance to Meloidogyne incognita in tomato. Journal of Nematology. 1982;14:414–416. [PMC free article] [PubMed] [Google Scholar]
- Barker KR. 1985. Nematode extraction and bioassays. Pp. 19–35 in K. R. Barker, C. C. Carter, and J. N. Sasser, eds. An advanced treatise on Meloidogyne, vol. 2: Methodology. Raleigh, NC: North Carolina State University Graphics.
- Bendezu IF. Detection of the tomato M1 1.2 gene by PCR using non-organic DNA purification. Nematropica. 2004;34:23–30. [Google Scholar]
- Bleve-Zacheo T, Melillo MT, Castagnone-Sereno P. The contribution of biotechnology to root-knot nematode control in tomato plants. Pest Technology. 2007;1:1–16. [Google Scholar]
- Chen RG, Zhang LY, Zhang JH, Zhang W, Wang X, Ouyang B, Li HX, Ye ZB. Functional characterization of Mi, a root-knot nematode resistance gene from tomato (Lycopersicon esculentum L.) Journal of Integrative Plant Biology. 2006;48:1458–1465. [Google Scholar]
- Cooper WR, Jia L, Goggin L. Effects of jasmonate-induced defenses on root-knot nematode infection of resistant and susceptible tomato cultivars. Journal of Chemical Ecology. 2005;31:1953–1967. doi: 10.1007/s10886-005-6070-y. [DOI] [PubMed] [Google Scholar]
- Desaeger JA, Csinos AS. Root-knot nematode management in double-cropped plasticulture vegetables. Journal of Nematology. 2006;38:59–67. [PMC free article] [PubMed] [Google Scholar]
- Devran Z, Elekçioglu IH. The screening of F2 plants for the root-knot nematode resistance gene, Mi by PCR in tomato. Turkish Journal of Agriculture and Forestry. 2004;28:253–257. [Google Scholar]
- Devran Z, Sogut MA, Mutlu N. Response of tomato rootstocks with the Mi resistance gene to Meloidogyne incognita race 2 at different soil temperatures. Phytopathologia Mediterranea. 2010;49:11–17. [Google Scholar]
- Dropkin VH. Necrotic reaction of tomatoes and other hosts resistant to Meloidogyne: Reversal by temperature. Phytopathology. 1969;59:1632–1637. [Google Scholar]
- Efron B, Tibshirani RJ. 1993. An introduction to the bootstrap. Boca Raton, FL: Chapman and Hall/CRC.
- Gardner R, Panthee D. 2009a. Notice of release of NC 123S tomato breeding line. Raleigh, NC: North Carolina Agricultural Research Service (NCSU).
- Gardner R, Panthee D. 2009b. Notice of release of NC 111F2(98) tomato breeding line. Raleigh, NC: North Carolina Agricultural Research Service (NCSU).
- Gibeaut DM, Hulett J, Cramer GR, Seemann JR. Maximal biomass of Arabidopsis thaliana using a simple, low-maintenance hydroponic method and favorable environmental conditions. Plant Physiology. 1997;115:317–319. doi: 10.1104/pp.115.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorno F, Wolters-Arts M, Grillo S, Scharf K D, Vriezen W H, Mariani C. Developmental and heat stress-regulated expression of HsfA2 and small heat shock proteins in tomato anthers. Journal of Experimental Botany. 2010;61:453–462. doi: 10.1093/jxb/erp316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey RS, Barker KR. Comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Jacquet M, Bongiovanni M, Martinez M, Verschave P, Wajnberg E, Castagnone-Sereno P. Variation in resistance to the root-knot nematode Meloidogyne incognita in tomato genotypes bearing the Mi gene. Plant Pathology. 2005;54:93–99. [Google Scholar]
- Koenning SR, Overstreet C, Noling JW, Donald PA, Becker JO, Fortnum BA. Survey of crop losses in response to phytoparasitic nematodes in the United States for 1994. Journal of Nematology. 1999;31:587–618. [PMC free article] [PubMed] [Google Scholar]
- Lamberti F. 1979. Economic importance of Meloidogyne spp. in subtropical and Mediterranean climates. Pp. 341–357 in F. Lamberti and C. E. Taylor, eds. Root-knot nematodes (Meloidogyne species) systematics, biology and control. London, United Kingdom: Academic Press.
- Li Q, Xie Q, Smith-Becker J, Navarre D A, Kaloshian I. Mi-1-mediated aphid resistance involves salicylic acid and mitogen-activated protein kinase signaling cascades. Molecular Plant-Microbe Interactions. 2006;19:655–664. doi: 10.1094/MPMI-19-0655. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lopez-Perez JA, Le Strange M, Kaloshian I, Ploeg AT. Differential response of Mi gene-resistant tomato rootstocks to root-knot nematodes (Meloidogyne incognita) Crop Protection. 2006;25:382–388. [Google Scholar]
- Mantelin S, Bhattarai KK, Jhaveri TZ, Kaloshian I. Mi-1-mediated resistance to Meloidogyne incognita in tomato may not rely on ethylene but hormone perception through ETR3 participates in limiting nematode infection in a susceptible host. PLOS One. 2013;8:1–8. doi: 10.1371/journal.pone.0063281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melakeberhan H. Effects of temperature and nitrogen source on tomato genotypes response to Meloidogyne incognita infection. Fundamental and Applied Nematology. 1998;21:25–32. [Google Scholar]
- Milligan SB, Bodeau J, Yaghoobi J, Kaloshian I, Zabel P, Williamson VM. The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell. 1998;10:1307–1319. doi: 10.1105/tpc.10.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaisen M, Supronien S, Nielsen LK, Lazzaro I, Spliid NH, Justesen AF. Real-time PCR for quantification of eleven individual Fusarium species in cereals. Journal of Microbiological Methods. 2009;76:234–240. doi: 10.1016/j.mimet.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Oka Y, Koltai H, Bar-Eyal M, Mor M, Sharon E, Chet I, Spiegel Y. New strategies for the control of plant-parasitic nematodes. Pest Management Science. 2000;56:983–988. [Google Scholar]
- Ploeg AT. Effects of selected marigold varieties on root-knot nematodes and tomato and melon yields. Plant Disease. 2002;86:505–508. doi: 10.1094/PDIS.2002.86.5.505. [DOI] [PubMed] [Google Scholar]
- Reddy DDR. Analysis of crop losses in tomato due to Meloidogyne incognita. Indian journal of nematology. 1985;15:55–59. [Google Scholar]
- Riley DG, Joseph SV, Kelley WT, Olson S, Scott J. Host Plant Resistance to Tomato spotted wilt virus (Bunyaviridae: Tospovirus) in Tomato. HortScience. 2011;46:1626–1633. [Google Scholar]
- Roberts PA, Thomason IJ. Variability in reproduction of isolates of Meloidogyne incognita and Meloidogyne javanica on resistant tomato genotypes. Plant Disease. 1986;70:547–551. [Google Scholar]
- Rodriguez OJ. 2013. Exploitation of Solanum chilense and Solanum peruvianum in tomato breeding for resistance to Tomato yellow leaf curl disease. Valencia, Spain: Universitat Politecnica de Valencia.
- Rosskopf EN, Chellemi DO, Kokalis-Burelle N, Church GT. Alternatives to methyl bromide: A Florida perspective. Plant Health Progress. 2005:1–25. [Google Scholar]
- Sasser JN, Carter CC, Hartman KM. 1984. Standardizations of host suitability studies and reporting of resistance to root-knot nematodes. Raleigh, NC: North Carolina State University Graphics.
- Srinivasan R, Riley D, Diffie S, Sparks A, Adkins S. Whitefly population dynamics and evaluation of whitefly-transmitted Tomato Yellow Leaf Curl Virus (TYLCV)-resistant tomato genotypes as whitefly and TYLCV reservoirs. Journal of Economic Entomology. 2012;105:1447–1456. doi: 10.1603/ec11402. [DOI] [PubMed] [Google Scholar]
- Trudgill DL. Resistance to and tolerance of plant parasitic nematodes in plants. Annual Review of Phytopathology. 1991;29:167–192. [Google Scholar]
- Tzortzakakis EA, Blok VC, Phillips MS, Trudgill DL. Variation in root-knot nematode (Meloidogyne spp.) in Crete in relation to control with resistant tomato and pepper. Nematology. 1999;1:499–506. [Google Scholar]
- Verdejo-Lucas S, Blanco M, Cortada L, Javier Sorribas F. Resistance of tomato rootstocks to Meloidogyne arenaria and Meloidogyne javanica under intermittent elevated soil temperatures above 28 °C. Crop Protection. 2013;46:57–62. [Google Scholar]
- Vos P, Simons G, Jesse T, Wijbrandi J, Heinen L, Hogers R, Frijters A, Groenendijk J, Diergaarde P, Reijans M, Fierens-Onstenk J, de Both M, Peleman J, Liharska T, Hontelez J, Zabeau M. The tomato Mi-1 gene confers resistance to both root-knot nematodes and potato aphids. Nature Biotechnology. 1998;16:1365–1369. doi: 10.1038/4350. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bao Z, Zhu Y, Hua J. Analysis of temperature modulation of plant defense against biotrophic microbes. Molecular Plant-Microbe Interactions. 2009;22:498–506. doi: 10.1094/MPMI-22-5-0498. [DOI] [PubMed] [Google Scholar]
- Williamson VM. Root-knot nematode resistance genes in tomato and their potential for future use. Annual Review of Phytopathology. 1998;36:277–293. doi: 10.1146/annurev.phyto.36.1.277. [DOI] [PubMed] [Google Scholar]
- Williamson VM, Hussey RS. Nematode pathogenesis and resistance in plants. Plant Cell. 1996;8:1735–1745. doi: 10.1105/tpc.8.10.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson VM, Kumar A. Nematode resistance in plants: The battle underground. Trends in Genetics. 2006;22:396–403. doi: 10.1016/j.tig.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Zacheo G, Blevezacheo T, Pacoda D, Orlando C, Durbin RD. The association between heat-induced susceptibility of tomato to Meloidogyne incognita and peroxidase activity. Physiological and Molecular Plant Pathology. 1995;46:491–507. [Google Scholar]
- Zhang L, Allen LH, Jr., Vaughan MM, Hauser BA, Boote KJ. Solar ultraviolet radiation exclusion increases soybean internode lengths and plant height. Agricultural and Forest Meteorology. 2014;184:170–178. [Google Scholar]
- Zhu Y, Qian WQ, Hua J. 2010. Temperature modulates plant defense responses through NB-LRR proteins. PLoS Pathogens 6:e1000844.