Abstract
Objective
The purpose of this study was to evaluate the incidence and clinical significance of embolic events in patients undergoing endovascular femoropopliteal interventions with or without embolic protection devices (EPDs).
Methods
We reviewed the clinical data of 566 patients treated by 836 endovascular femoropopliteal interventions for lower extremity claudication (46%) or critical limb ischemia (54%) from 2002 to 2012. Outcomes were analyzed in 74 patients/ 87 interventions performed with EPDs (Spider Rx, Covidien, Plymouth, MN) and 513 patients/ 749 interventions performed without EPDs. TASC classification, run-off scores and embolic events were analyzed. End-points were morbidity, mortality, re-intervention, patency and major amputation rates.
Results
Both groups had similar demographics, indications, cardiovascular risk factors and run-off scores, but patients treated with EPDs had significantly (P<0.05) longer lesions (109±94 vs 85±76mm) and more often had occlusions (64% vs 30%) and TASC C/D lesions (56% vs 30%). Embolic events occurred in 35 of 836 interventions (4%), including 2 (2%) performed with EPD and 33 (4%) without EPD (P=0.35). Macroscopic debris was noted in 59 (68%) filter baskets. Embolic events were not associated with lesion length, TASC classification, run-off scores, treatment type or indication, but were independently associated with occlusion. Patients who had embolization required more re-interventions (20% vs 3%, P<.001) and major amputations at 30-days (11% vs 3%, P=0.02). There was no difference in hospital stay (2.4±4 vs 1.6±2 days, P=0.08), re-intervention (2% vs 4%) and major amputation (1% vs 4%) among patients treated with or without EPD, respectively. The two patients who developed embolization with EPDs had no clinical sequela and required no re-intervention. Most emboli were successfully treated by catheter aspiration or thrombolysis, but 8 patients (24%) treated without EPD required prolonged hospital stay, 7 (21%) had multiple re-interventions, 1 (3%) had unanticipated major amputation, and 1 (3%) died from hemorrhagic complications of thrombolysis. Median follow up was 20 months. At 2-years, primary patency and freedom for re-intervention was similar for TASC A/B and TASC C/D lesions treated with or without EPDs.
Conclusions
Rates of embolization are low in patients undergoing endovascular femoropopliteal interventions with (4%) or without (2%) EPD. Embolization is more frequent in patients with occlusions. While emboli in patients with EPD had no clinical sequel, those treated without EPD require multiple re-interventions in 21% or resulted in major amputation or death in 3%. Late outcomes were similar in patients treated with or without EPDs.
Introduction
Distal embolization is a well-known and feared complication of percutaneous interventions with potential devastating clinical sequelae.1–3 The use of embolic protection devices (EPDs) has been well accepted for carotid interventions and in select patients with coronary saphenous vein graft lesions.3–5 While EPDs have been designed and clinically tested for these procedures, its use during lower extremity revascularization has been criticized because of questionable significance of embolic events, increased cost and potential risk of complications such as vessel trauma or entrapment of the filter basket.6, 7
Distal embolization occurs in 1 to 20% of patients undergoing iliac, femoral and popliteal interventions.8–10 Clinical presentation is variable, ranging from asymptomatic emboli to major emboli with limb-threatening ischemia. Some patients who develop embolization may necessitate prolonged hospital stay, re-interventions to restore flow into the occluded artery, and the risk of limb loss has not been well described. The purpose of this study was to evaluate the incidence, predictive factors and clinical significance of embolic events in patients undergoing endovascular femoropopliteal interventions with or without EPDs.
Methods
The study was approved by the Institutional Review Board of the Mayo Clinic. We retrospectively reviewed the clinical data of consecutive patients treated for chronic lower extremity arterial insufficiency between 2002 and 2012. Indications for endovascular revascularization were claudication or critical limb ischemia. Patients with acute or acute on chronic symptoms were excluded from the study. Endovascular interventions consisted of angioplasty alone (PTA), angioplasty with primary or secondary stenting (PTAS) and percutaneous atherectomy. Patients who had hybrid femoral endarterectomy combined with endovascular femoropopliteal intervention were also analyzed.
Demographics, cardiovascular risk factors, medical treatment and indications for revascularization were reviewed. The severity of the infrainguinal arterial occlusive disease was graded using Rutherford categories 1 (mild claudication) to 6 (major tissue loss) according to the reporting standards of the Society for Vascular Surgery (SVS).11 Ankle-Brachial indices (ABIs) were obtained pre- and post-operatively. Conventional angiography was analyzed by an independent investigator to determine TASC II (Trans-Atlantic Inter-Society Consensus Document on Management of Peripheral Artery Disease) classification, run-off scores, technical success, and presence of embolic events.11, 12 Pre- and post-treatment completion angiography was used to identify emboli location, extent and resolution after treatment, if any. The presence of macroscopic debris on the filter basket was noted from procedural notes and/or photographs, when available. The amount of debris was classified as none, mild, moderate and severe, based on a subjective analysis by the treating physician. A basket full of debris was considered ‘severe’ and small or minimal debris was considered ‘mild’. Any amount of debris between these two categories was considered ‘moderate’.
Technique of embolic protection
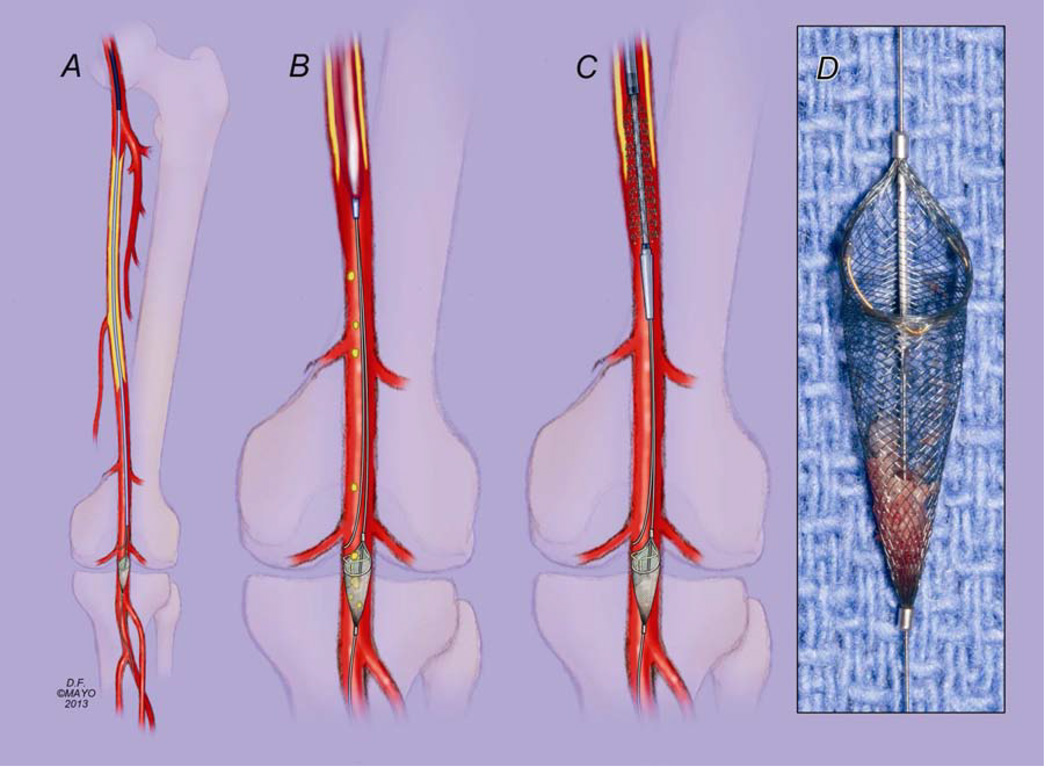
Percutaneous endovascular femoropopliteal interventions were performed under attended local anesthesia with monitored anesthesia care or general anesthesia. The decision to use EPD was left at the discretion of the treating physician. These were favored during atherectomy procedures and for recanalization of long-segment occlusions, particularly in those patients with single-vessel run-off. Most commonly contra-lateral trans-femoral access was used and a 5–7 Fr hydrophilic sheath was positioned in the common femoral artery (Fig 1). After systemic heparinization and diagnostic angiography, the femoropopliteal lesion was crossed using standard technique with angled or straight 0.035-inch Glidewire (Terumo, Somerset, NJ) and a catheter support. Reentrance devices were used selectively in patients with occlusions after reentrance was not successful using catheter and guide-wire technique. Once true lumen access was confirmed by hand injection using 0.035-inch catheter, a 0.014-inch Spider RX embolic protection device (Covidean, Plymouth MN) was advanced via the 0.035-inch catheter and deployed at or below the level of the knee joint. A 0.018-inch V18 guidewire (Boston Scientific, Natick, MA) was advanced via the 0.035-inch catheter to be used as a “buddy wire” to provide support for interventions done using 0.035-inch system. This facilitated advancement of 0.035-inch stents and retrieval of the filter basket (Fig 1). After successful endovascular revascularization, the EPD was retrieved, examined and baskets with debris were photographed for documentation. Completion angiography was obtained. Percutaneous closure was performed using a Perclose device (Abbott Vascular, Abbott Park, IL).
Figure 1.
Double-wire technique of superficial femoral artery intervention with embolic protection. Once true lumen access is confirmed with passage of a 0.035-inch catheter, a 0.014-inch Spider RX embolic protection device (Covidien, Plymouth MN) is loaded inside the 0.035-inch catheter, advanced and deployed at or below the level of the knee joint. A 0.018-inch V18 guidewire (Boston Scientific, Natick MA) is used as a “buddy wire” to provide support for interventions done using 0.035-inch system, and to facilitate retrieval of the filter basket while avoiding entrapment of the retrieval catheter in the struts of the stent (A). Balloon angioplasty is performed with the EPD in place (B), followed by deployment of a self-expanding stent (C). Moderate to severe macroscopic debris was captured in 45% of filter baskets (D).
Definitions and end-points
Primary end-point was presence of embolization, defined by angiographic evidence of occlusion or filling defect in a previously patent artery or branch distal to the treated segment. Secondary end-points were presence of macroscopic debris on the filter basket, mortality, morbidity, re-intervention, patency and major amputation rates. Early post-procedure events were defined as occurring within the first 30 days or within hospital stay if longer than 30 days. Re-interventions and major amputations were analyzed to determine its relationship to the embolic event. Technical success was defined as < 30% residual stenosis on completion angiography. Immediate post-embolization treatment, need for additional procedures and final resolution of the embolization were recorded. For patients treated with EPD, technical problems related to filter deployment or retrieval were noted. Patency rates and freedom from re-interventions were defined using the proposed reporting standards of the SVS.11
Statistical Analysis
Outcomes were analyzed in patients treated with or without EPDs (Spider Rx, Covidien, Plymouth, MN). Continuous variables were reported as mean and standard deviation, and categorical variables as frequency and percentages. Time dependent outcomes were estimated by the Kaplan-Meier method and differences were determined by Log Rank test. Univariate and multivariate logistic regression analysis were performed to identify predictors of embolization. Results were reported as percent or odds ratio (OR) with 95% confidence interval (95% CI). The Pearson χ2 or Fisher exact test was used for analysis of categorical variables. Differences between means were tested with two-sided t test, the Wilcoxon rank sum test, or the Mann-Whitney test. A value of P<.05 was used to determine statistical significance.
Results
Patient characteristics
A total of 566 patients were treated with 836 femoropopliteal endovascular interventions during the study period. Treatment of the contralateral limb was performed in 99 patients, whereas 171 had reinterventions in a previously treated limb. Of the 836 interventions, 74 underwent 87 interventions (10%) using EPDs, and 513 patients had 749 (90%) interventions performed without EPDs. There were 322 (57%) male and 244 (43%) female patients with a mean age of 72 ± 11 years (range, 28 to 105). Demographics, indications for revascularization and cardiovascular risk factors were similar in both groups, with the exception of more patients with hyperlipidemia in the EPD group (85% vs 73%; P < .02) and more patients with chronic kidney disease stage III to V in the non-EPD group (24% vs 10%; P < 0.01) (Table I). There were more patients in use of clopidogrel in the EPD group as compared to the non-EPD group (43% vs 23%; P < .001).
Table I.
Demographics, clinical presentation, cardiovascular risk factors and pre-admission medications of patients treated for chronic limb ischemia by percutaneous interventions with (EPD) or without embolic protection device (no EPD).
| All interventions n = 836 |
No EPD n = 749 |
EPD n = 87 |
P value | |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Demographics | ||||
| Age (mean ± SD) | 72 ± 11 | 72 ± 11 | 70 ± 10 | 0.06 |
| Female | 368 (44) | 337 (45) | 31 (36) | 0.1 |
| Clinical presentation | ||||
| Lower extremity claudication | 380 (46) | 332 (44) | 48 (55) | 0.07 |
| Critical limb ischemia | 456 (54) | 417 (56) | 39 (45) | 0.07 |
| Cardiovascular risk factors | ||||
| Hypertension | 714 (86) | 640 (86) | 74 (85) | 0.9 |
| Cigarette smoking | 577 (69) | 517 (69) | 60 (69) | 1 |
| Hyperlipidemia | 621 (74) | 547 (73) | 74 (85) | 0.02 |
| CAD | 436 (52) | 392 (52) | 44 (51) | 0.75 |
| CKD Stage >III | 187 (22) | 178 (24) | 9 (10) | 0.004 |
| Diabetes | 417 (50) | 381 (51) | 36 (41) | 0.09 |
| Baseline creatinine, mg/dL | 1.4 ± 1.3 | 1.5 ± 1.3 | 1.2 ± 1.2 | 0.08 |
| Preadmission medications | ||||
| Any antiplatelet therapy | 649 (78) | 583 (78) | 66 (76) | 0.7 |
| ASA | 589 (70) | 527 (70) | 62 (71) | 0.9 |
| Clopidogrel | 211 (25) | 174 (23) | 37 (43) | <.001 |
| Coumadin | 127 (15) | 109 (15) | 18 (21) | 0.13 |
| Statins | 540 (65) | 478 (64) | 62 (71) | 0.17 |
| Ezetimibe | 71 (8) | 63 (8) | 8 (9) | 0.8 |
| Nitrates | 158 (19) | 137 (18) | 21 (24) | 0.19 |
| Calcium channel blockers | 225 (27) | 202 (27) | 23 (26) | 0.92 |
| ACE inhibitor | 498 (60) | 441 (59) | 57 (66) | 0.23 |
| β-blockers | 543 (65) | 487 (65) | 56 (64) | 0.9 |
| Diuretics | 426 (51) | 378 (50) | 48 (55) | 0.67 |
SD, standard deviation; CAD, coronary artery disease; CKD, chronic kidney disease
TASC classification, extent of disease and run-off scores
Interventions were performed for native artery lesions in 604 cases (72%) or to treat femoropopliteal restenosis in 232 (28%). Patients in the EPD group had significantly more extensive disease as compared to those treated without distal protection. The EPD group had longer lesions (109 ± 94 vs 85 ± 76 mm; P<.03), and more often had interventions performed for occlusions (64% vs 30%; P<.001) and for TASC II C/D lesions (56% vs 30%; P<.001). Run-off scores were similar for both groups (4.4 ± 3 vs 4.7 ± 3; P=0.3), respectively (Table II).
Table II.
Angiographic and procedural characteristics of patients treated for chronic limb ischemia by percutaneous interventions with (EPD) or without embolic protection device (no EPD).
| All interventions n = 836 |
No EPD n = 749 |
EPD n = 87 |
P value | |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Lesion characteristics | ||||
| Length of stenosis (mm ± SD) | 88 ± 78 | 85 ± 76 | 109 ± 94 | 0.02 |
| Presence of occlusion | 272 (34) | 217 (30) | 55 (64) | <.001 |
| Length of occlusion (mm ± SD) | 119 ± 95 | 118 ± 89 | 121 ± 114 | 0.86 |
| SVS run-off score (mean ± SD) | 4.7 ± 2.7 | 4.7 ± 2.7 | 4.4 ± 2.6 | 0.27 |
| TASC II | ||||
| TASC A | 294 (35) | 274 (37) | 20 (23) | 0.02 |
| TASC B | 253 (30) | 235 (31) | 18 (21) | 0.05 |
| TASC C | 197 (24) | 166 (22) | 31 (36) | <.01 |
| TASC D | 72 (9) | 55 (7) | 17 (20) | <.001 |
| Treatment | ||||
| Balloon angioplasty | 822 (98) | 738 (90) | 84 (97) | 0.2 |
| Bare metal stenting | 367 (44) | 314 (42) | 53 (61) | <.001 |
| Self-expanding stent-grafts | 30 (4) | 21 (3) | 9 (10) | <.01 |
| Atherectomy | 38 (5) | 26 (4) | 12 (14) | <.001 |
| Hybrid procedure | 49 (6) | 43 (6) | 6 (7) | 0.8 |
| Vessel treated | ||||
| Femoral | 747 (89) | 664 (89) | 83 (95) | 0.06 |
| Popliteal | 387 (46) | 330 (44) | 57 (65) | <.001 |
| Femoral + popliteal | 312 (37) | 259 (35) | 53 (61) | <.0001 |
| Concomitant tibial | 133 (16) | 121 (16) | 12 (14) | 0.6 |
| Concomitant iliac | 84 (10) | 71 (10) | 13 (15) | 0.1 |
| Concomitant infra-renal aorta | 4 (0.5) | 1 (0.1) | 3 (3.4) | <.01 |
SD, standard deviation; TASC, Trans-Atlantic Inter-Society Consensus
Indications for EPDs
Selection of embolic protection evolved during the study, but was left at the discretion of the treating physician. Sixty-four (74%) interventions with EPD were performed by the senior author (GSO). In the first 17 cases, EPD was used primarily for recanalization of long occlusions in patients with single-vessel run-off or for atherectomy. Since 2010, >90% of the interventions performed by the senior author were done with EPD, including all patients undergoing recanalizations, and those who had single vessel run-off or required atherectomy.
Procedural characteristics
Balloon angioplasty was used in 822 (98%) interventions and primary or secondary stenting using self-expandable stents in 367 (44%). The mean number of stents was 1.8 (range, 1 to 8). Total treatment segment averaged 145 ± 107 mm (range, 10 to 510). Atherectomy was used in 38 interventions (5%) and self-expandable stent-grafts (Viabahn, WL Gore, Flagstaff, AZ) in 30 (4%). A hybrid approach with femoral endarterectomy, patch angioplasty or interposition graft was performed in 49 interventions (6%).
Patients with EPD were more often treated with bare metal self-expandable stents (61% vs 42%; P<.001), stent-grafts (10% vs 3%; P<.01) and atherectomy (14% vs 4%; P<.001), with similar number of hybrid procedures in both groups (7% vs 6%; P=0.8) (Table II). Total treatment length (198 ±120 mm vs 138 ± 103 mm; P<.0001) was longer in the EPD group as compared to patients treated without EPD, respectively. Technical success was achieved in 93% of the interventions, with no differences between the groups. The diameter of the EPD varied from 3 to 7 mm, and a 6 mm Spider™ Rx was used in 38% of cases. Two patients (2.3%) had decreased flow caused by the EPD. In one patient filter retrieval was difficult using the 0.014 wire, which prompted routine use of the double wire technique described in Figure 1.
Embolic events
Embolic events occurred in 35 interventions (4%), including two (2%) performed with EPD and 33 (4%) without EPD (P=0.35). Presence of occlusion was the only predictor for embolization by univariate analysis (Table III – online only). Use of bare metal stents, angioplasty or atherectomy was not associated with embolization. The location of emboli was at distal tibial vessels in 19 cases (54%), proximal tibial vessels in 11 (31%), below-knee popliteal artery or tibioperoneal trunk in three (9%) and SFA in two (6%). Of the 35 patients with embolization, three presented with subacute symptoms with duration of 2 to 6 weeks. A total of 23 embolizations were identified for TASC A/B lesions (4.2%), for an embolization rate of 2.6% with and 4.3% without EPD. For TASC C/D lesions, 12 embolic events were noted, for an embolization rate of 2.1% with and 5% without EPD.
Table III – online only.
Univariate analysis of predictors of embolization in patients treated for chronic limb ischemia by percutaneous interventions with (EPD) or without embolic protection device (no EPD).
| All interventions n = 836 |
Embolism n = 35 |
No embolism n = 801 |
P value | |
|---|---|---|---|---|
| % | ||||
| Pre-operative characteristics | ||||
| Use of EPD | 10 | 6 | 11 | 0.4 |
| Presence of occlusion | 34 | 51 | 33 | .04 |
| Previous stent occlusion | 3 | 9 | 3 | .08 |
| Previous by-pass occlusion | 1 | 0 | 1 | 0.5 |
| Length of occlusion (mm ± SD) | 119 ± 95 | 142 ± 94 | 117 ± 95 | 0.3 |
| Length of stenosis (mm ± SD) | 88 ± 78 | 69 ± 42 | 88 ± 79 | 0.2 |
| TASC II | 0.7 | |||
| A/B | 67 | 64 | 67 | |
| C/D | 33 | 36 | 33 | |
| Operative characteristics | ||||
| Angioplasty | 98 | 94 | 99 | .06 |
| Bare metal stent | 44 | 51 | 44 | 0.4 |
| Atherectomy | 5 | 9 | 4 | 0.2 |
| Length treated | 145 ± 107 | 134 ± 94 | 146 ± 107 | 0.6 |
EPD, embolic protection device; SD, standard deviation; TASC, Trans-Atlantic Intersociety Consensus.
Two patients had embolization with EPD. The exact mechanism of embolization could not be determined, but may represent failure of the EPD, including malposition, undersizing or dislodgement during retrieval; or formation of platelet or thrombus emboli beyond the filter device. Both patients had at moderate debris in the filter basket. In one patient, it is likely that embolization occurred during retrieval of the EPD. In the second patient the exact mechanism could not be determined, but complete resolution with t-PA indicates a thrombus emboli, which may have originated beyond the EPD due to stagnant flow or inadequate systemic heparinization.
In the 33 patients who had embolization after interventions without EPD, 25 were treated immediately with t-PA infusion in 16 and catheter aspiration in ten. Other adjunctive measures were intra-arterial nitroglycerin in two patients, angioplasty and stenting at the location of the embolization in two patients, and retrieval of the embolic material using an EPD or over-the-wire embolectomy one patient each. Of the patients who underwent immediate treatment, 12 had complete resolution, six had improvement and seven had no change. Among the 13 patients with persistent emboli, seven were started in continuous catheter-directed thrombolysis using t-PA. A total of 11 re-interventions for thrombolytic recheck or catheter-exchange were required among these seven patients. At the time of the last thrombolytic recheck, five had complete resolution, one had improvement, and one had no change. Eight patients (23%) with emboli underwent no additional treatment, including seven patients who had small emboli, which was not considered to be clinically significant and was associated with refilling via other collateral branches. Of the 35 patients who had emboli, complete resolution was noted in 18 (51%), partial resolution in seven (20%) and no resolution in three (9%) (Fig 2). None of the seven patients who had small emboli left untreated developed clinical sequela. Macroscopic debris was noted in in 59 (68%) filter baskets, 39 of which were described as moderate or severe amount of debris (45%, Fig. 1).
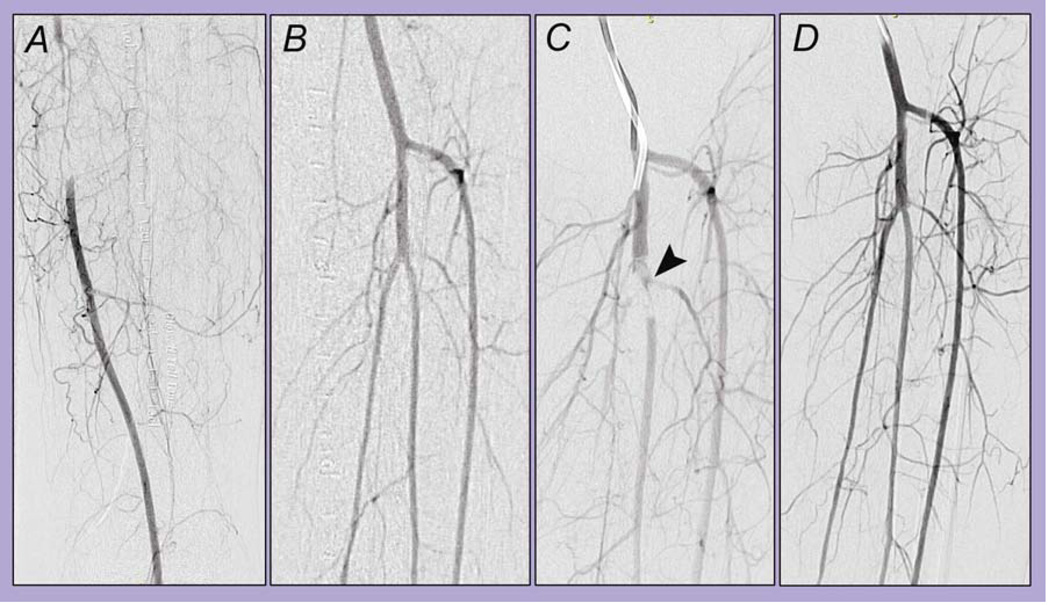
Figure 2.
Example of a case of distal embolization successfully treated with thrombolysis and catheter aspiration. Left lower extremity selective angiography demonstrates a segmental occlusion of the superficial femoral artery (SFA, A) and patent tibioperoneal trunk (B). After crossing of the SFA lesion, the angiogram reveals occlusion at the bifurcation of the tibioperoneal trunk, with compromise of flow to both arteries (C, arrowhead). After catheter aspiration of thrombus followed by continuous catheter-directed thrombolysis during eight hours, completion angiography demonstrates improved flow and patent tibioperoneal trunk (D).
Early outcomes
Patients with embolic events required more early re-interventions (20% vs 3%, P<.001) and early major amputations (11% vs 3%, P=.02) than those who did not have an embolization, with no difference in length of hospital stay (2.8 ± 6 vs 2.3 ± 4; P=0.5). There was no difference in hospital stay (2.4±4 vs 1.6±2 days, P=.08), re-intervention (2% vs 4%, P=0.4) and major amputation (1% vs 4%, P=0.3) among patients treated with or without EPD, respectively. None of the two patients who had small emboli with EPD developed clinical sequel or required re-intervention. In the group treated without EPD, eight (24%) patients required prolonged hospital stay, seven (21%) had re-interventions performed to restore lower extremity flow and one (3%) had major unanticipated amputation. This patient developed embolization of large calcific material to the tibioperoneal trunk and proximal anterior tibial artery, which was not felt to be amenable to endovascular maneuvers by the treating physician, resulting in acute ischemia and leading to below-knee amputation. One patient died from hemorrhagic complications of thrombolytic therapy used to treat an embolic event. The patient was an 84 years-old female with multiple co-morbidities, morbid obesity, end-stage renal disease on peritoneal dialysis and chronic atrial fibrillation, who developed puncture-related hemorrhage and multisystem organ failure during thrombolytic therapy. The patient expired after care was withdrawn per advanced directives and family request.
Late outcomes
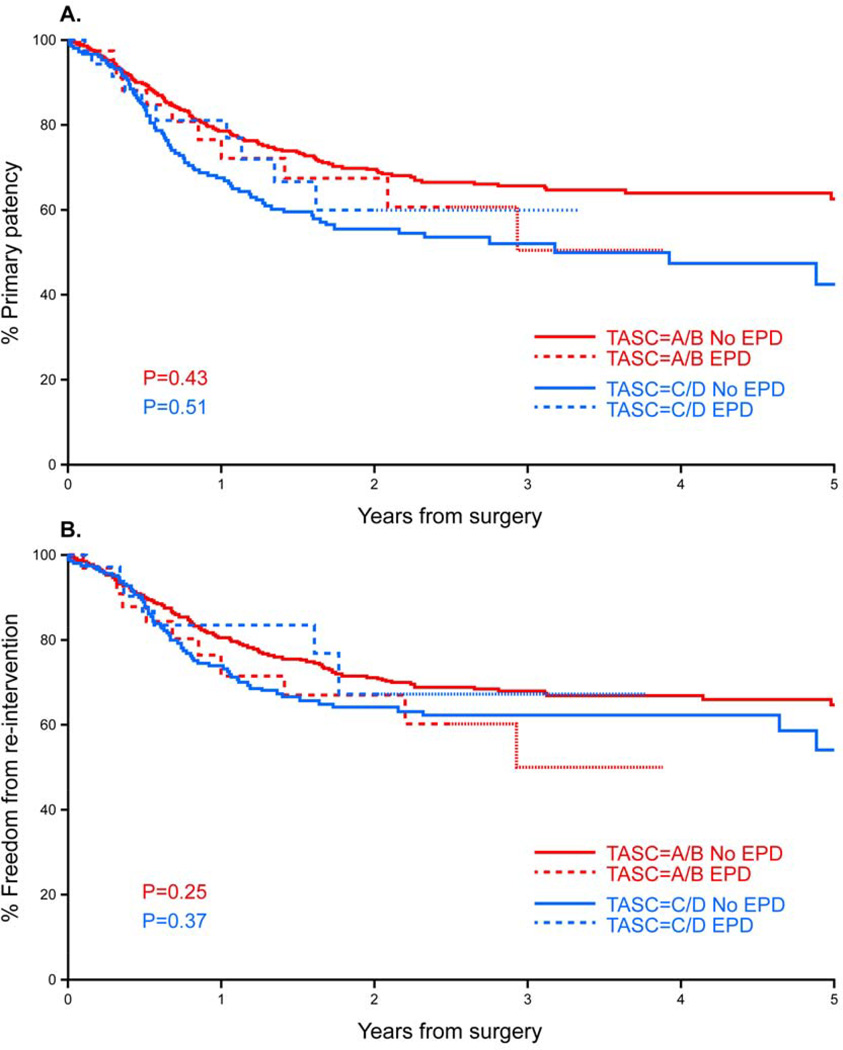
Mean follow-up was 14 months for the EPD group (range 0–43 months) and 21 months for patients treated without EPD (range 0–114 months). Late outcomes were analyzed separately in patients with TASC A/B and TASC C/D lesions. Primary patency in 2 years was 70% for TASC A/B lesions, with no difference among patients treated with (68%) or without EPD (70%; P=0.4). Freedom from re-intervention was also similar after two years, (68% for the EPD vs 72% for the non-EPD group; P=0.3). Primary patency at 2 years for TASC C/D lesions was 57%, and was significantly lower than patients with TASC A/B lesions (P<.01). There were no differences in primary patency rates at one year in patients treated with (81%) or without EPD (68%; P=0.5). Freedom from re-intervention was also similar after 18 months (83% for the EPD vs 67% for the non-EPD group; P=0.4). Comparing patients who had embolic events to those who did not, there were no differences in primary patency and freedom from re-intervention after one year for TASC A/B (86% vs 78% and 86% vs 80%) or TASC C/D lesions (67% vs 70% and 73% vs 76%), respectively (P=0.4) (Fig 3).
Figure 3.
Kaplan-Meier estimates of primary patency (A) and freedom from re-intervention (B) in patients treated for chronic limb ischemia by percutaneous interventions with (EPD) or without embolic protection device (no EPD).
Discussion
Endovascular interventions carry risk of embolization. Emboli in the carotid and coronary arteries can result in life-threatening complications or permanent disability.4, 13 For this reason, there has been little debate on the indications of EPDs when applied to these interventions. However, in the lower extremities, the clinical significance of distal embolization has not been well described. Although emboli may result in limb loss or require invasive treatment, most believe that its frequency is low and that these events carry little significance.8 This study describes a low frequency of emboli for interventions performed without embolic protection (4%), and even lower rates for those who had embolic protection (2%), despite more occlusions and longer lesions in the latter group. Although rates of emboli were similar, clinical consequences of emboli without EPD may be more severe.
The incidence of embolization during femoropopliteal interventions ranges from 2% to 100%.8, 9, 14 Prior reports have shown that clinical examination is a poor indicator of embolization. The wide variation in embolization rates in these reports is explained by differences in extent of disease, lesion type, treatment modality and diagnostic method.8, 9, 15, 16 Lam and associates employed continuous Doppler ultrasound monitoring in 60 patients treated by endovascular superficial femoral artery interventions. Embolic signals were noted in the popliteal artery in all interventions, and were significantly more frequent during stent deployment than during wire crossing or balloon angioplasty.8 In studies that evaluated presence of macroscopic debris in filter baskets, rates of embolization range from 55 to 100%.10, 15–19 We found that two thirds of the filter baskets had macroscopic debris, which was graded moderate to severe in 45%. This finding indicates that completion angiography provides limited assessment of emboli to smaller branches.
Angiographic evidence of embolization during lower extremity interventions ranges from 0% to 30%. Shrikkande and colleagues reported a large series of 2137 lesions treated in 1029 patients, with only 34 embolic events (1.6%).9 The rate of embolization was significantly higher (22%) among patients treated with atherectomy devices. Higher rates were also seen for patients with occlusions treated by recanalization, in-stent restenosis, and TASC C and D lesions. In that report, patency was restored in 32 of 34 cases, similar to our report. There were no differences in patency rates among patients who had or did not have embolic events. The authors concluded that selective use of EPDs should be considered in patients undergoing atherectomy, and in those treated for occlusions, TASC C and D lesions or in-stent restenosis.9
Current evidence on the use of EPDs for lower extremity interventions remains scarce. The PROTECT registry reported the results of a single-center prospective study of 40 patients who underwent endovascular treatment for infrainguinal occlusive disease with EPDs.16 Similar to our report, 55% of the filter baskets had macroscopic debris, with higher rates among patients treated by atherectomy (100%). There were no complications with filter retrieval, and one sidebranch embolization proximal to the filter was noted.16 A prospective, single-center study by Müller-Hülsbeck and associates evaluated the performance of the FilterWire EZ Embolic Protection System (Boston Scientific, Mountain View, CA) in 30 SFA interventions. There were no device-related complications, and macroscopic debris was found in 90% of filters. Microscopic analysis demonstrated a particle size ranging from 90 to 2000 µm, with the major components of emboli being platelets, erythrocytes, inflammatory cells, extracellular matrix and cholesterol.17 Spiliopoulos and associates have reported very low rates of embolization (0.57%) among 3147 percutaneous interventions. Their use of embolic protection in patients with subacute presentation of 3 to 6-months was suggested as a possible explanation for their low embolization rates.20 However, the study lacked information on the number of lesions that presented with subacute symptoms, number of patients treated by EPD and specific outcomes with or without EPD; reporting of these numbers is encouraged to support the authors’ opinion. Karnabitidis and colleagues demonstrated the use of the Spider FX device in 48 patients who underwent endovascular revascularization of infra-aortic lesions. There were three device failures, which included one side-branch occlusion, one distal embolization and one device-related vasospasm. A particle greater than 1 mm was detected in 70% of the baskets, with similar histologic characteristics as the aforementioned study. Increased lesion length, r eference vessel diameter, acute thrombosis and vessel occlusion were significantly associated with higher amounts of particles on the filter.15
Patients that present with subacute symptoms may have lesions that are at higher risk for embolic events. Shammas and colleagues reported increased risk of embolization during treatment of recent-onset (<6 months) occlusions. In that study, thrombus was identified by intravascular ultrasound in 94% of 17 patients. Embolization was identified in 18% of patients. The authors concluded that a thrombotic component is present in most patients with recent-onset occlusion, with a higher embolization risk for these lesions. Although we have not analyzed the duration of symptoms for all interventions in our series, the low number of patients with subacute presentation (9%) suggests that this accounts for a minority of all embolic events.21
In this study patients treated with EPDs had worse anatomical characteristics, including longer lesions and more occlusions, for which higher rates of embolization would be expected. Nonetheless, rates of embolization were lower (2% vs 4%) than what was observed among patients treated without EPDs, albeit this did not reach statistical significance. Most importantly, the two patients who had embolization with EPD had no clinical consequence and nor required re-intervention. Conversely, emboli in the group treated without EPD was associated with more re-interventions (21%), one unanticipated major amputation and one death. Although there was no significant relation between TASC classification and embolic events, the presence of occlusion was a risk factor as demonstrated in other studies.8, 9, 22 Our study did not show major complications directly related to the filter.
One question is whether the emboli have any impact on long-term results of revascularization. There were no differences in primary patency rates or freedom from re-intervention after stratification of patients according to TASC classification. Although presence of embolization was associated with more early re-interventions, these patients were not at higher risk of late re-interventions or loss of primary patency.
A few technical considerations deserve mention when using EPDs for lower extremity interventions. Often, these devices are selected in patients with difficult lesions, occlusions and dense calcifications. Because embolic protection devices are designed over a 0.014-inch platform, these devices often lack enough guide-wire support to advance 0.035 balloons and stents over difficult lesions. In addition, retrieval of the device can be problematic. In a patient treated by stenting, it may be difficult to advance the retrieval catheter over long stented segments over the 0.014-inch wire because the catheter may catch in the stent struts. We found that the use of a two-wire technique with the 0.014-inch Spider RX embolic protection device and a 0.018-inch “buddy wire” provides excellent support to advance 0.035-inch balloons, stents and to retrieve the device (Fig 1). The Spider Rx device has size range of 3 to 7mm, a working length of 320 cm, allowing use of long shaft balloons and stents via contra-lateral femoral approach.
This study has several limitations, notably the retrospective analysis of consecutive patients treated in a non-randomized fashion. Selection of EPDs was based on physician preference and did not follow a specific algorithm. There were differences between treatment groups, although ultimately these favored patients treated without EPD and were not significantly related to primary endpoints. It was not possible to determine if the technique used for recanalization was either subintimal or intra-luminal. We did not perform an analysis of symptoms duration before intervention and presence of subacute thrombotic occlusion; however, only one patient who had embolization had angiographic evidence of subacute thrombus, therefore it is unlikely that this would be a significant factor in our series. Most interventions performed with EPD were done by a single operator, and it is possible that technical aspects related to this individual affected outcomes. The power of the study was impaired by the small number of interventions performed with EPD, which likely affected the ability to detect small statistical differences between groups. There was no cost-related analysis; the use of EPDs is known to increase the cost of the procedure (by approximately US$ 1,000) but the impact of multiple re-interventions, early amputations and a death due to embolization is difficult to analyze. Finally, only one type of EPD was utilized in this investigation; it is unclear if the evidence found will be extended to other device designs in the same setting.
In summary, in this single-center, retrospective, non-randomized study, clinically significant embolic events were uncommon after endovascular femoropopliteal interventions performed with or without EPDs. Embolic events were associated with recanalization of chronic total occlusions. Although patients treated with EPDs had more advanced lesions, rates of embolization were lower in this group, albeit this did not reach statistical significance. Importantly, none of the patients who had emboli with EPD developed clinical sequel or required re-intervention, whereas one in four patients who developed embolization without EPD required escalating level of care. At the present time, these devices cannot be recommended for routine use, but should be considered in patients with occlusions and in those undergoing atherectomy, based on results of this study and other reports. Further analysis with a larger subset of patients, in a prospective, multi-center randomized setting is desirable to better understand the role of EPDs in lower extremity endovascular revascularizations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 2013 Annual Symposium of the Society for Clinical Vascular Surgery, Miami, Florida, March 2013
References
- 1.Kastrup A, Nagele T, Groschel K, Schmidt F, Vogler E, Schulz J, et al. Incidence of new brain lesions after carotid stenting with and without cerebral protection. Stroke. 2006;37(9):2312–2316. doi: 10.1161/01.STR.0000236492.86303.85. [DOI] [PubMed] [Google Scholar]
- 2.Oderich GS, Tallarita T, Gloviczki P, Duncan AA, Kalra M, Misra S, et al. Mesenteric artery complications during angioplasty and stent placement for atherosclerotic chronic mesenteric ischemia. J Vasc Surg. 2012;55(4):1063–1071. doi: 10.1016/j.jvs.2011.10.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topol EJ, Yadav JS. Recognition of the importance of embolization in atherosclerotic vascular disease. Circulation. 2000;101(5):570–580. doi: 10.1161/01.cir.101.5.570. [DOI] [PubMed] [Google Scholar]
- 4.Baim DS, Wahr D, George B, Leon MB, Greenberg J, Cutlip DE, et al. Randomized trial of a distal embolic protection device during percutaneous intervention of saphenous vein aortocoronary bypass grafts. Circulation. 2002;105(11):1285–1290. [PubMed] [Google Scholar]
- 5.Gray WA, Hopkins LN, Yadav S, Davis T, Wholey M, Atkinson R, et al. Protected carotid stenting in high-surgical-risk patients: The ARCHeR results. Journal of Vascular Surgery. 2006;44(2):258–268. doi: 10.1016/j.jvs.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 6.Bates MC, Campbell JE. Pitfalls of embolic protection. Tech Vasc Interv Radiol. 2011;14(2):101–107. doi: 10.1053/j.tvir.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Cremonesi A, Manetti R, Setacci F, Setacci C, Castriota F. Protected carotid stenting - Clinical advantages and complications of embolic protection devices in 442 consecutive patients. Stroke. 2003;34(8):1936–1941. doi: 10.1161/01.STR.0000081000.23561.61. [DOI] [PubMed] [Google Scholar]
- 8.Lam RC, Shah S, Faries PL, McKinsey JF, Kent KC, Morrissey NJ. Incidence and clinical significance of distal embolization during percutaneous interventions involving the superficial femoral artery. J Vasc Surg. 2007;46(6):1155–1159. doi: 10.1016/j.jvs.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 9.Shrikhande GV, Khan SZ, Hussain HG, Dayal R, McKinsey JF, Morrissey N. Lesion types and device characteristics that predict distal embolization during percutaneous lower extremity interventions. J Vasc Surg. 2011;53(2):347–352. doi: 10.1016/j.jvs.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Shammas NW, Coiner D, Shammas GA, Christensen L, Dippel EJ, Jerin M. Distal embolic event protection using excimer laser ablation in peripheral vascular interventions: results of the DEEP EMBOLI registry. J Endovasc Ther. 2009;16(2):197–202. doi: 10.1583/08-2642.1. [DOI] [PubMed] [Google Scholar]
- 11.Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: Revised version. Journal of Vascular Surgery. 1997;26(3):517–538. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 12.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR, et al. Intersociety consensus for the management of peripheral arterial disease (TASC II) Journal of Vascular Surgery. 2007;45:S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 13.Kastrup A, Groschel K, Krapf H, Brehm BR, Dichgans J, Schulz JB. Early outcome of carotid angioplasty and stenting with and without cerebral protection devices - A systematic review of the literature. Stroke. 2003;34(3):813–819. doi: 10.1161/01.STR.0000058160.53040.5F. [DOI] [PubMed] [Google Scholar]
- 14.Shammas NW, Shammas GA, Dippel EJ, Jerin M, Shammas WJ. Predictors of distal embolization in peripheral percutaneous interventions: a report from a large peripheral vascular registry. J Invasive Cardiol. 2009;21(12):628–631. [PubMed] [Google Scholar]
- 15.Karnabatidis D, Katsanos K, Kagadis GC, Ravazoula P, Diamantopoulos A, Nikiforidis GC, et al. Distal embolism during percutaneous revascularization of infra-aortic arterial occlusive disease: an underestimated phenomenon. J Endovasc Ther. 2006;13(3):269–280. doi: 10.1583/05-1771.1. [DOI] [PubMed] [Google Scholar]
- 16.Shammas NW, Dippel EJ, Coiner D, Shammas GA, Jerin M, Kumar A. Preventing lower extremity distal embolization using embolic filter protection: results of the PROTECT registry. J Endovasc Ther. 2008;15(3):270–276. doi: 10.1583/08-2397.1. [DOI] [PubMed] [Google Scholar]
- 17.Muller-Hulsbeck S, Humme TH, Schafer JP, Charalambous N, Paulsen F, Heller M, et al. Final Results of the Protected Superficial Femoral Artery Trial Using the Filter Wire EZ System. Cardiovascular and Interventional Radiology. 2010;33(6):1120–1127. doi: 10.1007/s00270-010-9936-5. [DOI] [PubMed] [Google Scholar]
- 18.Suri R, Wholey MH, Postoak D, Hagino RT, Toursarkissian B. Distal embolic protection during femoropopliteal atherectomy. Catheter Cardiovasc Interv. 2006;67(3):417–422. doi: 10.1002/ccd.20634. [DOI] [PubMed] [Google Scholar]
- 19.Konig CW, Pusich B, Tepe G, Wendel HP, Hahn U, Schneider W, et al. Frequent embolization in peripheral angioplasty: detection with an embolism protection device (AngioGuard) and electron microscopy. Cardiovasc Intervent Radiol. 2003;26(4):334–339. doi: 10.1007/s00270-003-2656-3. [DOI] [PubMed] [Google Scholar]
- 20.Spiliopoulos S, Katsanos K, Fragkos G, Karnabatidis D, Siablis D. Treatment of infrainguinal thromboembolic complications during peripheral endovascular procedures with AngioJet rheolytic thrombectomy, intraoperative thrombolysis, and selective stenting. Journal of Vascular Surgery. 2012;56(5):1308–1316. doi: 10.1016/j.jvs.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 21.Shammas NW, Dippel EJ, Shammas G, Gayton L, Coiner D, Jerin M. Dethrombosis of the Lower Extremity Arteries Using the Power-Pulse Spray Technique in Patients with Recent Onset Thrombotic Occlusions: Results of the DETHROMBOSIS Registry. Journal of Endovascular Therapy. 2008;15(5):570–579. doi: 10.1583/08-2453.1. [DOI] [PubMed] [Google Scholar]
- 22.Siablis D. Atheroembolization and peripheral vascular interventions: the evidence is mounting. J Endovasc Ther. 2009;16(2):203–205. doi: 10.1583/08-2642C.1. [DOI] [PubMed] [Google Scholar]