Abstract
Restoration of motor function following stroke involves reorganization of motor output through intact pathways, with compensatory brain activity likely variable by task. One class of motor tasks, those involved in self-care, is particularly important in stroke rehabilitation. Identifying the brain areas that are engaged in self-care and how they reorganize after stroke may enable development of more effective rehabilitation strategies. We piloted a paradigm for functional MRI assessment of self-care activity. In two groups, young adults and older adults, two self-care tasks (buttoning and zipping) produce activation similar to a bimanual tapping task, with bilateral activation of primary and secondary motor cortices, primary sensory cortex, and cerebellum. Quantitative differences include more activation of sensorimotor cortex and cerebellum in buttoning than bimanual tapping. Pilot subjects with stroke showed greater superior parietal activity across tasks than controls, potentially representing an increased need for sensorimotor integration to perform motor tasks.
Keywords: Functional MRI, Rehabilitation, Motor systems, Stroke, Aging
Introduction
An almost universal consequence of stroke is motor impairments resulting in self-care deficits (Bernspang et al. 1987). These deficits may occur directly as a result of reduced motor output through damage to the corticospinal tract or indirectly because of apraxia (Sunderland et al. 1999) or executive dysfunction. There is increasing focus on restoring self-care independence in stroke and acquired brain injury rehabilitation settings. That restoration is often based on teaching compensation for specific motor problems and using cues to supplement impaired motor sequencing (van Heugten et al. 1998). However, little is known regarding the brain substrates of these general approaches to rehabilitation. It is known that recovery from stroke produces changes in the pattern of movement-related activity in motor cortical areas (Weiller et al. 1992; Johansen-Berg et al. 2002). This neuronal plasticity appears to involve engagement of brain areas previously silent or minimally engaged during simple motor tasks, particularly contralesional primary motor cortex and secondary motor areas, e.g., premotor cortex and supplementary motor cortex (Schaechter and Perdue 2008).
Rehabilitation therapies are seldom based on physiological data, and there is little empirical evidence favoring one specific treatment over another (Pomeroy and Tallis 2000; Marsden and Greenwood 2005). Knowing how brain areas are engaged during recovery and rehabilitation would allow the physician to customize an individual patient’s treatment based on their specific pattern of damage, and to also better predict the outcome of treatment (Cramer et al. 2003). For example, one could avoid treatments known to depend on cortical areas that have been damaged in a particular individual. This will become a greater possibility as understanding of post-injury plasticity increases. As a necessary first step, we must understand how the intact brain accomplishes the kinds of motor tasks involved in rehabilitation.
These tasks are often self-care activities, such as dressing, grooming, and eating. Neurophysiological investigation of recovery of motor function after stroke, however, has typically included study of simple movements (Chollet et al. 1991; Weiller et al. 1992, 1993) but not more realistic tasks. The movements necessary for self-care are complex, involving bimanual manipulation of clothing objects such as shoelaces, buttons, and zippers, often under visual guidance. These movements require grasping with accurate force application and purposeful multijoint reaching movements. In contrast, the movements most often used in neurophysiological studies of motor function involve single or sequential finger tapping. While the network of brain areas involved in simple finger movements has been well characterized (Roland et al. 1982; Shibasaki et al. 1993), those supporting the more complex self-care movements remain unknown. The present study sought to find tasks that are appropriate to the MRI environment, are more ecologically valid than finger tapping, and that produce a circumscribed, interpretable pattern of sensorimotor brain activation.
We developed two self-care-related motor tasks that can be easily performed within the context of a functional neuroimaging experiment. Here, we investigated the functional activation related to those tasks, with two hypotheses: 1. The activation related to performance in normal subjects would be similar to commonly used finger-tapping task because the self-care tasks, while more complex, are overlearned; 2. Activation after recovery from stroke would reveal a more widespread pattern of activation due to task complexity and less practiced performance with current neural resources. These other areas would include non-primary motor cortices known to be involved in visuomotor processing (premotor areas, posterior parietal cortex), sequencing (SMA) and error correction (cerebellum.) We were also able to compare the brain activations of a pilot group of individuals with stroke to an age-matched control group and to compare this older group with a group of younger adult subjects, first used to validate the method. In this way we were able to ask whether aging and stroke affected the activation pattern related to performance of self-care movements.
Experimental procedures
Participants
Twelve participants in each of three groups were recruited for this study, which was carried out from 2002 to 2004. Those in the Adult group were between 21 and 50 years of age, and those in the Aged and Stroke (Pilot) group were over 50 years of age. No non-Stroke participant reported any history of stroke or other neurological impairment. A comprehensive neurological exam (Mental Status, Cranial Nerves, Motor, Sensory, Reflexes, and Coordination) was performed on each of the Aged participants, with no abnormal findings. For inclusion in the pilot study stroke survivors must have had a motor deficit immediately following stroke and experienced subsequent recovery to independence in self-care; one subject was severely hemiparetic (previously right-handed), but had recovered the ability to perform the requisite self-care tasks. None of the subjects had severe sensory deficits or neglect. The Wake Forest University Health Sciences Institutional Review Board approved all procedures, and subjects gave written informed consent and HIPAA acknowledgement prior to study participation.
Motor testing
Stroke subjects performed a battery of tests, including the Assessment of Motor and Process Skills (AMPS) (Fisher 1993), Motor Activity Log (MAL) (Uswatte et al. 2005) Trail-making Test (TMT) A and B, and the ABILHAND survey (Penta et al. 2001). The AMPS can be performed only by an occupational therapist that has been certified in this proprietary method, and such a therapist left the institution half-way through the pilot stroke group testing.
Procedure
Functional Magnetic Resonance Imaging (fMRI) was performed during a single 1-hour session while participants performed each of five motor tasks. The two self-care tasks were Buttoning and Zipping. During the Buttoning task, participants alternately fastened and unfastened the button attached to the cushion resting on their upper abdomen (Fig. 1). The cushion was made of pliable foam rubber with a denim cover. The button was placed so that it could easily be reached, requiring little arm movement. During the Zipping task, participants used their right hand to alternately move up and down the zipper attached to the cushion. There were also three comparison tasks involving sequentially tapping each finger on the cushion using the left, right, or both hands.
Fig. 1.
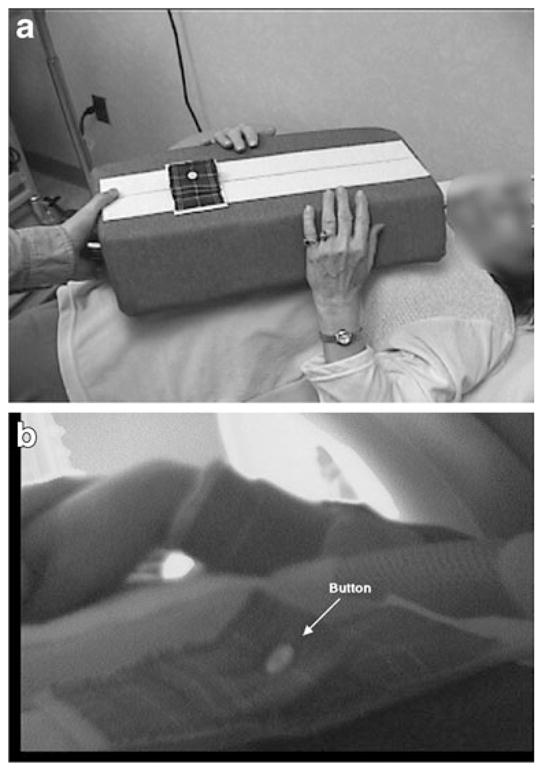
Set-up for tasks. a A participant is shown with the foam cushion and button in place, practicing the task outside the magnet. b The view through a head-coil mounted camera is shown with the participant in the magnet bore
At the beginning of the testing session, participants were shown how to perform each of the five tasks, and were allowed to practice each while lying down (to simulate the MRI environment). In the scanner, head movements were addressed in the following ways: a) participants were asked to keep their head as still as possible, b) their head was stabilized using cushions placed on either side, and c) a small piece of paper towel was placed on their forehead and taped at either end to the base of the head coil to provide cutaneous feedback.
Data acquisition and analysis
Each scanning session began with the collection of localizer and structural scans, followed by eight fMRI runs (one Right-hand tapping, one Left-hand tapping, and two each of Bimanual tapping, Buttoning, and Zipping; order determined by Latin-square). Each fMRI run lasted 3.5 min and consisted of alternating 30 s periods of repetitive, self-paced task performance and rest (each run began and ended with a rest period). A member of the research team stood beside the participant for the entire MRI session, instructing them prior to scan onset as to what task to perform, and when to begin and end the task (signaled by a light tap on the leg). Movements were videotaped using a custom-made MRI-compatible camera (Resonance Technology, Northridge, California) aimed at the task workspace, recorded on 8 mm digital videotape (Sony USA, New York NY), and analyzed offline for task timing.
Magnetic resonance imaging was performed using a GE Signa 1.5 Tesla Echo-Speed Horizon LX system, and consisted of a sagittal T1-weighted localizer, followed by a T1-weighted acquisition of the entire brain performed in the axial plane (24 cm FOV, 256×256 matrix, 3 mm slice thickness). This sequence was used during analysis both for anatomic overlays of the functional data, as well as for spatial normalization of the data sets to a standard atlas to permit comparisons to be made across individual participants. Functional imaging was performed in the axial plane using multislice gradient-echo echo planar imaging (EPI) with a field of view of 24 cm (frequency)×15 cm (phase), and an acquisition matrix of 64 mm×40 mm (28 slices, 5 mm thickness, no skip, TR=3,000, TE=40, flip angle=90 deg). This sequence delivered an effective voxel resolution of 3.75×3.75×5.00 mm, and allowed us to view changes in both superior, hand-related sensorimotor cortices and the cerebellum. There were 70 volumes acquired during each 3.5 min run.
The fMRI raw echo amplitudes were saved and transferred to a SUN Ultrasparc workstation (SUN Microsystems, Mountain View, CA) for off-line reconstruction using software developed in IDL (Research Systems Inc., Boulder Colorado). Correction for image distortion and alternate k-space line errors was performed on each image on the basis of data acquired during phase-encoded reference imaging (Alsop et al. 1995). Statistical parametric maps (SPMs) were generated using SPM99 (Friston et al. 1995) implemented in Matlab (The Mathworks Inc., Sherborn MA, USA), with an IDL interface. The T1-weighted images were normalized to a standard template in Montreal Neurological Institute (MNI) coordinate space within SPM99. The functional data sets were motion corrected (intra-run realignment) within SPM99 using the first image as the reference. The functional data sets were normalized to MNI space using image header information to determine the 16-parameter affine transform between the functional data sets and the T1-weighted images (Maldjian et al. 1997), in combination with the transform computed within SPM99 for the T1-weighted anatomic images to MNI space. The normalized data sets were resampled to 4×4×5 mm within MNI space using sync interpolation. A second realignment step (inter-run realignment) was then performed (within SPM99) between successive normalized runs within each participant, using the initial normalized run as the target. This was done to eliminate motion between the successive runs within each participant. The data sets were smoothed using an 8×8×10 mm full-width at half maximum Gaussian smoothing kernel, and SPMs were generated using the general linear model within SPM99. A 6 s time-shifted box-car waveform was used as the reference paradigm, and the ANCOVA model with global activity as a confound was employed for the statistical analysis. Temporal smoothing, detrending and high-pass filtering were performed as part of the SPM analysis. The SPM{t} were transformed to the unit normal distribution SPM{Z} and thresholded at p<0.05, corrected for multiple comparisons.
A second-level analysis was performed to generate group SPMs using a random-effects model within SPM99 with the individual contrast maps (Holmes and Friston 1998). The resulting maps were transformed to the unit normal distribution SPM{Z} and thresholded at p<0.05, corrected for multiple comparisons. Anatomic labels for significantly activated voxels were determined using the wfu_pickatlas (Maldjian et al. 2003). Only data from one run each (usually the second) of the Bimanual tapping, Buttoning, and Zipping tasks are presented here, as not all participants had two runs and we did not want to over represent an individual. The second level contrasts included: 1. Differences, within each group, between each ADL task and each of bimanual, right, and left tasks, with the hypothesis that the ADL tasks would be most similar to the bimanual task, with other differences based on the amount of use of each hand, 2. Differences within a task and between the Aged and the other two groups, with the hypothesis that the Aged group would have less focal activation than the Adult and the Stroke group would further have additional areas activated, with more increase in number of areas with task complexity.
Participants
There were 36 participants, 12 in each of the three groups, Adult (6 female, 29±7 year.), Aged (5 female, 61 ± 8 year), and Stroke (4 females; age 66.8 ± 7.76 years). The Stroke group was not statistically significantly different in age from the Aged group. One Aged participant was ambidextrous, but all other participants were right-handed. Besides these participants, four participants were excluded: 1. one Adult showed little motor activation in any task, 2. one Adult was left-handed and participated only for pilot purposes, 3. one Aged participant’s scans had reconstruction errors, and 4. one Aged participant was uncomfortable in the scanner and declined to participate further. Table 1 describes the demographic and medical characteristics of the stroke participants, which unfortunately is incomplete due to some loss of data and change in personnel. The lasting motor impairments among stroke patients varied from none to severe, but all Stroke participants were independent in their activities of daily living (ADL).
Table 1.
Subject characteristics, including rate of movement. The affected arm was not known for all stroke-affected subjects. For the functional measures, MAL, ABILHAND, higher is better. The AMPS and train-making (TM) have age-specific norms, but higher represents worse function. Task rate was taken from videotaped performance in the MRI, which was not always technically feasible. Tapping tasks are L (left), R (right), or Bi (bimanual), and ADL tasks are But (buttoning) and Zip (zipping)
| Subj. | Aff. arm | MAL (max 5) | ABIL-HAND (max 3) | AMPS motor (logits) | TMT A (sec) | TMT B (sec) | Task rate (#/30 s)
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L | R | Bi | But | Zip | |||||||
| 16 | R | 1.56 | 2.94 | 1.57 | 33 | 101 | 39 | 34 | 33 | 3.3 | 7.3 |
| 19 | R | 5 | 2.82 | 3.31 | 47 | 96 | 65 | 61 | 56 | 3.4 | 6.2 |
| 21 | R | 5 | 3.00 | 2.94 | 24 | 50 | 41 | 36 | 37 | 1.7 | 2.7 |
| 22 | L | 4.92 | 1.91 | 2.07 | 45 | 251 | 53 | 56 | 73 | 1.1 | 2.3 |
| 23 | R | 1.26 | 1.98 | – | 41 | 95 | – | – | – | – | – |
| 24 | R | 4.85 | 3.00 | 2.22 | 31 | 73 | 41 | 36 | 40 | 2.6 | 4.5 |
| 25 | L | 4.83 | 3.19 | – | 41 | 180 | 48 | 54 | 44 | – | 1.8 |
| 27 | R | 0.17 | 1.86 | 1.61 | 29 | 82 | 53 | – | 32 | 1.9 | 3.7 |
| 31 | ? | 4.1 | 1.74 | – | 29 | 59 | – | – | – | – | – |
| 32 | L | 4.38 | 2.57 | – | 41 | 66 | 41 | 54 | 40 | 2.6 | 4.7 |
| 38 | ? | 1.37 | 1.79 | – | 60 | 322 | – | – | – | – | – |
| 40 | R | 4.83 | 2.64 | – | 29 | 80 | 50 | 57 | 56 | 2.8 | 6.7 |
| mean | 3.52 | 2.49 | 2.38 | 37.00 | 118.92 | 47.89 | 48.50 | 45.67 | 2.43 | 4.43 | |
| stdev | 1.77 | 0.67 | 0.69 | 9.96 | 82.03 | 8.45 | 11.14 | 13.46 | 0.80 | 1.99 | |
Head movements
The realignment parameters generated by SPM during image realignment were used to estimate head movements in 6 dimensions (translation: x, y, and z; and rotation: pitch, roll, and yaw) for the Bimanual tapping, Buttoning, and Zipping tasks in 8 pilot participants (5 Adult and 3 Aged). The maximum values were generally within acceptable limits (Table 2), although movement in the z direction tended to be somewhat large during task performance. The Stroke group showed a similar range of head movements, although with more individual variation, with up to 1.5 mm and 1° of movement for a single realignment parameter.
Table 2.
Movement parameters: mean movement parameters derived by realignment of MRI volumes during functional scans. Coordinates are in mm, and rotations are in degrees
| SPM realignment parameter
|
||||||
|---|---|---|---|---|---|---|
| x | y | z | pitch | roll | yaw | |
| Bimanual finger tapping | ||||||
| Rest | 0.410 | 0.129 | 0.741 | 0.0102 | 0.0034 | 0.0076 |
| Task | 0.510 | 0.142 | 1.029 | 0.0135 | 0.0037 | 0.0088 |
| Buttoning | ||||||
| Rest | 0.401 | 0.224 | 0.822 | 0.0169 | 0.0085 | 0.0082 |
| Task | 0.478 | 0.260 | 1.050 | 0.0169 | 0.0113 | 0.0084 |
| Zipping | ||||||
| Rest | 0.465 | 0.178 | 0.895 | 0.0132 | 0.0040 | 0.0133 |
| Task | 0.436 | 0.379 | 1.116 | 0.0146 | 0.0040 | 0.0135 |
Results
Task-related activation
Task performance was confirmed by video monitoring and there was no deviation from the block design protocol. The location, magnitude, and anatomic label of each significantly activated voxel cluster in each task are listed for the three groups in Table 3. Each task produced a pattern of motor-related activity typical of dexterous hand movements, including cerebellum, sensorimotor cortex, and, in some cases, a distinct supplementary motor area (SMA) activation. Figures 2, 3 and 4 show areas of functional activation at the p<0.05 uncorrected level (a more liberal criterion than we chose for reporting specific voxels). The pattern of activation was qualitatively quite similar across all tasks.
Table 3.
Significantly activated main clusters (p<0.05 voxel level for FDR, corrected) for adult, aged, and stroke subjects in each task. L left side, R right side, M medial/midline, SMA supplementary motor area
| Region | Bimanual finger tapping task
|
Buttoning ADL task
|
Zipping ADL task
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Side | Talairach coordinates in MNI space
|
Z | Side | Talairach coordinates in MNI space
|
Z | Side | Talairach coordinates in MNI space
|
Z | |||||||
| x | y | z | x | y | z | x | y | z | |||||||
| Adult subjects (18–50 year) | |||||||||||||||
| Sensorimotor | L | −24 | −20 | 60 | 5.15 | L | −36 | −32 | 55 | 5.95 | L | −40 | −32 | 60 | 5.50 |
| Sensorimotor | R | 36 | −24 | 60 | 4.86 | R | 40 | −24 | 50 | 5.57 | R | 32 | −12 | 60 | 4.45 |
| Insula | L | −56 | 0 | 25 | 4.94 | R | 44 | 0 | 5 | 3.90 | |||||
| Cerebellum | R | 16 | −56 | −20 | 4.65 | M | 0 | −68 | −10 | 4.71 | R | 8 | −64 | −15 | 5.01 |
| Cerebellum | M/B | −4 | −64 | −20 | 5.14 | M | 4 | −56 | −10 | 5.21 | M/R | 8 | −64 | −15 | 5.01 |
| Cerebellum | L | −20 | −64 | −20 | 4.95 | L | −20 | −60 | −20 | 5.10 | LR | −20 | −68 | −20 | 4.04 |
| Aged subjects (>50 year) | |||||||||||||||
| Sensorimotor | R | 32 | −20 | 60 | 5.49 | R | 40 | −24 | 55 | 5.86 | R | 32 | −20 | 65 | 4.91 |
| Sensorimotor | R | 36 | −44 | 65 | 3.91 | ||||||||||
| Sensorimotor | L | −40 | −36 | 60 | 5.13 | L | −40 | −44 | 60 | 4.72 | L | −36 | −32 | 60 | 4.84 |
| SMA | M | 0 | −4 | 65 | 5.95 | M | 4 | −4 | 55 | 4.64 | M | −4 | −12 | 70 | 3.51 |
| Cerebellum | M | 0 | −68 | −15 | 4.87 | M/B | 0 | −60 | −15 | 4.99 | M/R | 12 | −56 | −20 | 3.97 |
| Cerebellum | R | 24 | −56 | −50 | 3.85 | ||||||||||
| PMAd | R | 52 | 8 | 35 | 4.00 | ||||||||||
| PMAv | R | 56 | 8 | 10 | 3.84 | ||||||||||
| Stroke subjects | |||||||||||||||
| SMA | M | 0 | −8 | 60 | 5.57 | M | 0 | −20 | 75 | 5.31 | M | −4 | −16 | 55 | 4.32 |
| Sensorimotor | R | 40 | −28 | 60 | 5.49 | R | 32 | −20 | 60 | 5.34 | R | 36 | −52 | 60 | 4.34 |
| Sensorimotor | L | −36 | −28 | 60 | 5.26 | L | −36 | −28 | 65 | 5.34 | L | −32 | −28 | 60 | 4.52 |
| Cerebellum | M | −4 | −64 | −15 | 4.82 | M | 0 | −68 | −15 | 5.27 | M | 0 | −68 | −15 | 4.20 |
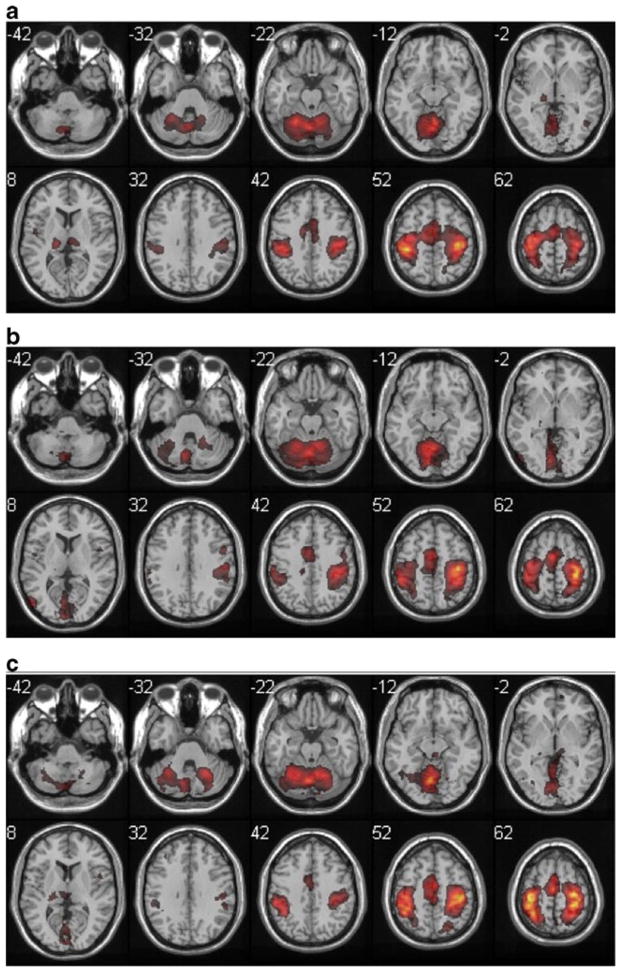
Fig. 2.
Group analysis of bimanual finger-tapping task-related activations, with color scale representing Z [1.5 to 18]. a adult, b aged, c stroke. Z coordinates in MNI space are shown next to each slice, with right hemisphere on the right for this and all other figures. Activity was very similar across the three groups, except for a more posterior extent of the frontoparietal cluster in the stroke group
Fig. 3.
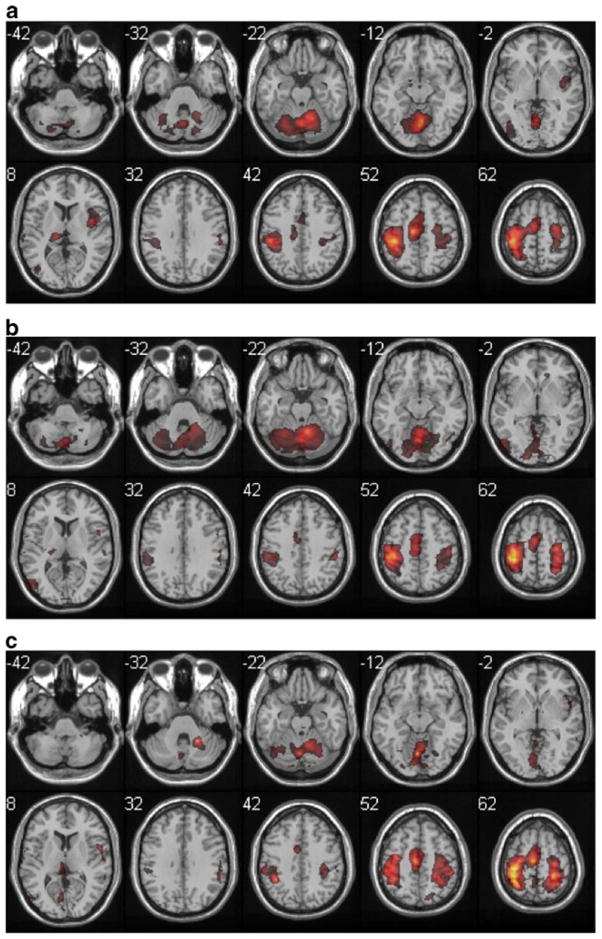
Group analysis of buttoning task-related activations, displayed as in Fig. 1, including the more posterior extent of the frontoparietal cluster
Fig. 4.
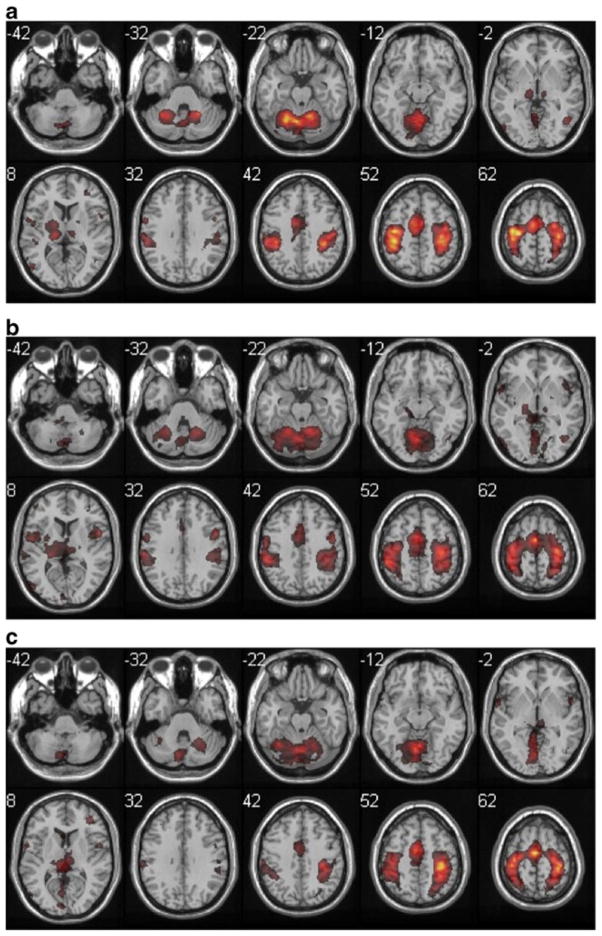
Group analysis of zipping task-related activations, displayed as in Fig. 1. Note the asymmetric activation (more left hemisphere activity) of sensorimotor cortex, consistent with the nature of the task. Activation was most symmetric in the stroke group
All participants performed the Zipping task with their right hand as prime mover, producing a strongly lateralized pattern of activity (right cerebellum, left cortex) in the Adult participants. The Aged participants showed a more bilateral pattern of activation in this task. In the Adult participants, the Bimanual tapping task produced a more anterior pattern of sensorimotor cortex activation (more primary motor cortex and dorsal premotor area) and the self-care tasks produced somewhat more posterior activation (more primary sensory cortex) both in the most activated voxel (Table 3) and the qualitative pattern of activation (Figs. 2, 3 and 4). In the Aged participants, sensorimotor cortex activity was more similar across the three tasks.
Task comparison
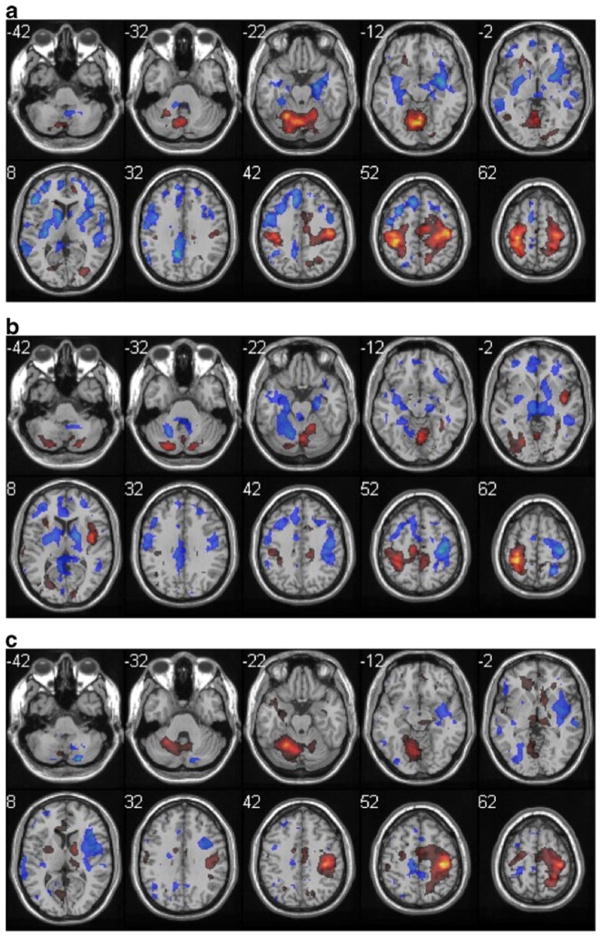
We used random effects analysis (paired t-test) to compare the pattern of activation between each of the tasks within each of the two normal groups (half of the comparisons are shown in Table 4, and three comparisons in Fig. 5). The Buttoning and Bimanual tapping tasks both produced greater left cerebellar and right cortical activation than the Zipping task. This is not surprising, given that the Zipping task was primarily performed with the right hand. The largest difference cluster was with the aged group, comparing Buttoning to Zipping, and was in the R sensorimotor cortex. Zipping had few voxels more activated in comparison to bimanual tapping in the normal groups, and none in the stroke one. Buttoning elicited more activity than tapping in the largest number of areas, with more right-sided motor and sensory activity in the Aged group.
Table 4.
Significant activations in each ADL condition and control conditions, by group. Only the main clusters from SPM are shown
| Region | Buttoning > bimanual
|
Zipping > bimanual
|
Buttoning > zipping
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ext. | Talairach coordinates in MNI space
|
Z | Ext. | Talairach coordinates in MNI space
|
Z | Ext. | Talairach coordinates in MNI space
|
Z | |||||||
| x | y | z | x | y | z | x | y | z | |||||||
| Adult subjects (18–50 year) | |||||||||||||||
| L SMC | 108 | −32 | −32 | 45 | 4.48 | 62 | −28 | −36 | 65 | 4.56 | |||||
| L cerebellum | 79 | −20 | −60 | −20 | 4.41 | 85 | −20 | −0 | −20 | 5.07 | |||||
| R SMC | 62 | 44 | −20 | 50 | 4.33 | 167 | 40 | −24 | 55 | 5.64 | |||||
| R insula | 16 | 40 | −4 | 15 | 3.90 | ||||||||||
| Aged subjects (>50 year) | |||||||||||||||
| R SMC | 42 | 44 | −20 | 50 | 4.76 | 305 | 44 | −20 | 55 | 5.80 | |||||
| L cerebellum | 15 | −4 | −68 | 10 | 4.35 | 37 | −16 | −64 | 20 | 4.42 | |||||
| R PMAd | 23 | 20 | −28 | 75 | 3.86 | ||||||||||
| L SMC | 13 | −28 | −36 | 65 | 3.54 | ||||||||||
| SMA | 21 | 4 | −4 | 60 | 3.98 | ||||||||||
| Stroke subjects | |||||||||||||||
| L SMC | 86 | −28 | −28 | 70 | 4.37 | ||||||||||
| R SMC | 64 | 24 | −36 | 60 | 4.24 | 79 | 32 | −16 | 60 | 4.81 | |||||
| L cerebellum | 50 | −24 | −52 | −20 | 4.13 | 48 | −8 | −64 | −20 | 3.96 | |||||
| R cerebellum | 18 | 16 | −64 | −40 | 3.79 | ||||||||||
Fig. 5.
Task differences in adult group, displayed as in Fig. 1, except that color scale represents negative Z scores from −7.5 to −1.5 in cold colors and positive differences from 1.5 to 14.5 in hot colors. a buttoning > bimanual. b zipping > bimanual. c buttoning > zipping
Group comparison
Random effects analyses (paired t-tests) were used to compare activations between the Adult and Aged groups and between the Aged and Stroke groups. In spite of the qualitative differences noted above, these analyses revealed no significantly differentially activated voxels between the Adult and Aged groups for any of the tasks. But comparison of Stroke to Aged revealed greater activation for Stroke in the superior parietal and secondary sensory areas, across all tasks (Fig. 6).
Fig. 6.
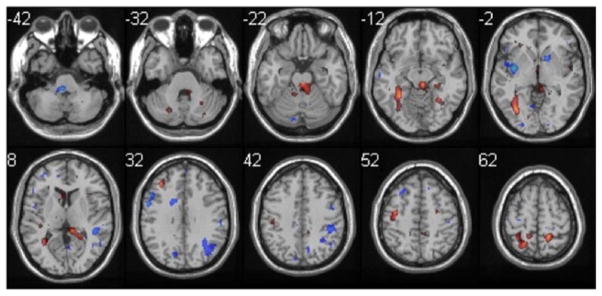
Difference image between stroke and aged in buttoning. Z scores −3.75 to −1.5, 1.5 to 3.51 are displayed as in Fig. 5. Note more activation in bilateral superior parietal cortex (BA 5) in stroke as compared to aged
Discussion
We show here that, in healthy participants, two dressing-related motor tasks—buttoning and zipping—produce circumscribed, interpretable patterns of activity that are similar to a bimanual finger-tapping task. This pattern of activation is similar in both younger and older age groups, and similar in stroke-affected individuals with only a few differences. While these dressing-related tasks are a more ecologically valid alternative to the often-used finger-tapping tasks for the study of recovery of self-care following stroke or other brain injury, the high degree of similarity between the brain activations regardless of task type suggests that the body of science on the simpler movements adequately probes the relevant brain motor systems.
We hypothesized first that self-care tasks would have similar activation to simple repetitive movements because they are overlearning and secondly that activation after recovery from stroke would be more divergent. Our first hypothesis was therefore confirmed, and may relate to the fact that the thousands of opportunities to practice these complex movements result in brain activity indistinguishable from simple repetitive movement. The second hypothesis was not confirmed, however. In fact, the same brain region, the superior parietal cortex, was activated more in stroke-affected individuals across tapping and dressing tasks.
While the only significant difference between buttoning and bimanual tapping was greater activation of the right primary sensory cortex, and then only in the Aged group, the overall pattern of activation was more robust in both sensorimotor cortices and the cerebellar vermis for the ADL-like task. Otherwise the task differences were expected, and reflected the relative hand contributions in each of the tasks. This lack of a larger difference between Zipping and other tasks is likely the result of the participants using their left hand to dynamically stabilize the workspace. While one hand is performing the more dexterous movement, the other needs to exert bimanually coordinated forces. In addition, consistent with studies of self-paced movements, there was frequent activation of supplementary motor area (SMA) (Cunnington et al. 2002). This activity is visible in Fig. 1, although it often fell below our (rather conservative) statistical threshold.
Age-related differences in brain activity were subtle. Others have found a more widespread pattern of brain activity related to simple bimanual tasks (Goble et al. 2010). Our data does not preclude this possibility, but our tasks were performed at a comfortable pace for each individual. This normalization of effort level may have prevented compensatory activation of additional brain regions, or their activation may have simply been below the threshold of detection. There is some support for this latter possibility in the qualitatively higher similarity and fewer significant voxel differences between bimanual tapping and self-care tasks in the Aged group as compared to the younger Adult group.
Stroke-related changes are interpretable only in light of the group heterogeneity but showed a consistent increase in activation of superior parietal cortex across all tasks. This area has been known to be part of the network for sensorimotor integration (Wise et al. 1997) and also to become more active in motor tasks after stroke e.g. (Gerloff et al. 2006). Here it may represent part of a common solution to the problem of coordinating movements with a partially damaged motor network, regardless of where that damage is.
While head movements generated by performing these tasks were generally within acceptable limits, it remains true that care should be taken to limit head movements, particularly in z translation and pitch, by instructing subjects as to the importance of keeping the head still, and by using head and trunk stabilization devices.
In conclusion, we have demonstrated that selected self-care tasks effectively elicit simple patterns of motor-related brain activity in younger, older, and relatively mildly stroke-affected adults. Superior parietal cortex was more activated in stroke patients across all tasks, as compared to normal subjects, suggesting a common role in recovery of motor function. It is possible that more disabling stroke will lead to a more significant recruitment of a broader range of motor areas for self-care tasks as compared to finger tapping, but this would require further study.
Acknowledgments
The authors acknowledge Robert Kraft, Paul Laurienti, Lumi Sawaki, and Todd Atwood for invaluable assistance. Dr. Wittenberg is now on Extended Educational Leave from his position as Staff Physician in the Geriatrics Research, Education and Clinical Center, VA Maryland Health Care System, and on sabbatical from his position as Associate Professor in Neurology at the University of Maryland, Baltimore, MD, U.S.A. He is currently a Senior Fellow at the Dept. of Kinesiology of KU Leuven, Belgium. Mr. Foster is a Principal Research Associate with Alnylam Pharmaceuticals in Cambridge, MA, USA, and Dr. Lovelace an Assistant Professor of Psychology at Shepherd University in Shepherdstown, WV, USA.
Grants This work was funded by a Scientist Development Grant #0230258N to G.F.W. from the American Heart Association. Continued analysis was supported by the Geriatrics Research, Education and Clinical Center of the VA Maryland Health Care System and was completed while the G.F.W. was a Senior Fellow at KU Leuven.
Footnotes
Disclosures The authors have nothing to disclose.
Contributor Information
George F. Wittenberg, Program in Rehabilitation, Department of Neurology, Neuroscience Program & Department of Physiology and Pharmacology, Wake Forest University School of Medicine, Winston-Salem, NC, USA
Christopher T. Lovelace, Program in Rehabilitation, Department of Neurology, Neuroscience Program & Department of Physiology and Pharmacology, Wake Forest University School of Medicine, Winston-Salem, NC, USA. Department of Psychology, Shepherd University, Shepherdstown, WV, USA
Donald J. Foster, Program in Rehabilitation, Department of Neurology, Neuroscience Program & Department of Physiology and Pharmacology, Wake Forest University School of Medicine, Winston-Salem, NC, USA
Joseph A. Maldjian, Radiologic Sciences, Wake Forest University School of Medicine, Winston-Salem, NC, USA
References
- Alsop DC, Hatabu H, Bonnet M, Listerud J, Gefter W. Multi-slice, breathhold imaging of the lung with submillisecond echo times. Magnetic Resonance in Medicine. 1995;33:678–682. doi: 10.1002/mrm.1910330513. [DOI] [PubMed] [Google Scholar]
- Bernspang B, Asplund K, Eriksson S, Fugl-Meyer AR. Motor and perceptual impairments in acute stroke patients: effects on self-care ability. Stroke. 1987;18:1081–1086. doi: 10.1161/01.str.18.6.1081. [DOI] [PubMed] [Google Scholar]
- Chollet F, Di Piero V, Wise RJS, Brooks DJ, Dolan RJ, Frackowiak RSJ. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Annals of Neurology. 1991;29:63–71. doi: 10.1002/ana.410290112. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Benson RR, Burra VC, Himes D, Crafton KR, Janowsky JS, Brown JA, Lutsep HL. Mapping individual brains to guide restorative therapy after stroke: rationale and pilot studies. Neurological Research. 2003;25:811–814. doi: 10.1179/016164103771953899. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. NeuroImage. 2002;15:373–385. doi: 10.1006/nimg.2001.0976. [DOI] [PubMed] [Google Scholar]
- Fisher AG. The assessment of IADL motor skills: an application of many-faceted Rasch analysis. American Journal of Occupational Therapy. 1993;47:319–329. doi: 10.5014/ajot.47.4.319. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Heather JD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, Waldvogel D, Wittenberg GF, Ishii K, Cohen LG, Hallett M. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129:791–808. doi: 10.1093/brain/awh713. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Van Impe A, De Vos J, Wenderoth N, Swinnen SP. The neural control of bimanual movements in the elderly: brain regions exhibiting age-related increases in activity, frequency-induced neural modulation, and task-specific compensatory recruitment. Human Brain Mapping. 2010;31:1281–1295. doi: 10.1002/hbm.20943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Friston K. Generalisability, random effects and population inference. NeuroImage. 1998;7:S754. [Google Scholar]
- Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125:2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Schulder M, Liu WC, Mun IK, Hirschorn D, Murthy R, Carmel P, Kalnin A. Intraoperative functional MRI using a real-time neurosurgical navigation system. Journal of Computer Assisted Tomography. 1997;21:910–912. doi: 10.1097/00004728-199711000-00013. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Marsden J, Greenwood R. Physiotherapy after stroke: define, divide and conquer. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76:465–466. doi: 10.1136/jnnp.2004.053827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penta M, Tesio L, Arnould C, Zancan A, Thonnard JL. The ABILHAND questionnaire as a measure of manual ability in chronic stroke patients: Rasch-based validation and relationship to upper limb impairment. Stroke. 2001;32:1627–1634. doi: 10.1161/01.str.32.7.1627. [DOI] [PubMed] [Google Scholar]
- Pomeroy VM, Tallis RC. Need to focus research in stroke rehabilitation. Lancet. 2000;355:836–837. doi: 10.1016/s0140-6736(99)08143-x. [DOI] [PubMed] [Google Scholar]
- Roland PE, Meyer E, Shibasaki T, Yamamoto YL, Thompson CJ. Regional cerebral blood flow changes in cortex and basal ganglia during voluntary movements in normal human volunteers. Journal of Neurophysiology. 1982;48:467–480. doi: 10.1152/jn.1982.48.2.467. [DOI] [PubMed] [Google Scholar]
- Schaechter JD, Perdue KL. Enhanced cortical activation in the contralesional hemisphere of chronic stroke patients in response to motor skill challenge. Cerebral Cortex. 2008;18:638–647. doi: 10.1093/cercor/bhm096. [DOI] [PubMed] [Google Scholar]
- Shibasaki H, Sadato N, Lyshkow H, Yonekura Y, Honda M, Nagamine T, Suwazono S, Magata Y, Ikeda A, Miyazaki M, et al. Both primary motor cortex and supplementary motor area play an important role in complex finger movement. Brain. 1993;116:1387–1398. doi: 10.1093/brain/116.6.1387. [DOI] [PubMed] [Google Scholar]
- Sunderland A, Bowers MP, Sluman SM, Wilcock DJ, Ardron ME. Impaired dexterity of the ipsilateral hand after stroke and the relationship to cognitive deficit. Stroke. 1999;30:949–955. doi: 10.1161/01.str.30.5.949. [DOI] [PubMed] [Google Scholar]
- Uswatte G, Taub E, Morris D, Vignolo M, McCulloch K. Reliability and validity of the upper-extremity motor activity log-14 for measuring real-world arm use. Stroke. 2005;36:2493–2496. doi: 10.1161/01.STR.0000185928.90848.2e. [DOI] [PubMed] [Google Scholar]
- van Heugten CM, Dekker J, Deelman BG, van Dijk AJ, Stehmann-Saris JC, Kinebanian A. Outcome of strategy training in stroke patients with apraxia: a phase II study. Clinical Rehabilitation. 1998;12:294–303. doi: 10.1191/026921598674468328. [DOI] [PubMed] [Google Scholar]
- Weiller C, Chollet F, Friston KJ, Ramsay SC, Frackowiak RSJ. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Annals of Neurology. 1992;31:463–472. doi: 10.1002/ana.410310502. [DOI] [PubMed] [Google Scholar]
- Weiller C, Ramsay SC, Wise RJS, Friston KJ, Frackowiak RSJ. Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Annals of Neurology. 1993;33:181–189. doi: 10.1002/ana.410330208. [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annual Review of Neuroscience. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]