Abstract
Backgrounds/Aims
Pancreaticoduodenctomy (PD) is associated with high rates of postoperative morbidity and mortality. Although many studies have shown that early postoperative enteral nutrition improves postoperative outcomes, limited clinical information is available on postoperative early oral feeding (EOF) after PD. The aim of this study was to evaluate the clinical feasibility, safety, and nutritional effects of EOF after PD.
Methods
Clinical outcomes were investigated in 131 patients who underwent PD between 2003 and 2013, including 81 whose oral feeding was commenced within 48 hours (EOF group) and 50 whose oral feeding was commenced after resumption of bowel movements (traditional oral feeding [TOF] group). Postoperative complications, energy intake, and length of stay (LOS) were reviewed.
Results
Demographic factors were similar in the two groups. The EOF group had a significantly shorter LOS (25.9±8.5 days vs. 32.3±16.3 days; p=0.01) than the TOF group. The rates of anastomotic leak (1.2% vs. 16%, p=0.00) and reoperation (3.7% vs. 20%, p=0.01) were significantly lower in the EOF group. In the clinically acute phase from postoperative day 1 to day 5, the mean daily calorie intake (847.0 kcal vs. 745.6 kcal; p=0.04) and mean daily protein intake (42.2 g vs. 31.9 g; p=0.00) in the EOF group were significantly higher than that in the TOF group.
Conclusions
Postoperative EOF is a clinically safe, feasible, and effective method of nutritional support after PD.
Keywords: Early oral feeding, Pancreaticoduodenectomy, Length of stay, Complication
INTRODUCTION
Pancreaticoduodenectomy (PD) is a technically demanding operation involving extensive surgical resection and reconstruction. Despite its high risk associated with surgical procedures, the operative mortality rate of PD has gradually decreased from 30% to 1-2% over the past 3 decades. Systematic reviews and meta-analyses of volume-outcome relationships in pancreatic surgeries have indicated that refinements in surgical technique and the regionalization of PD to high volume centers were keys to improve outcomes.1 Improvements in patient selection, intensive care, and nutrition play important roles in the reduction of mortality. Although malnutrition is a significant factor in postoperative outcomes, the optimal method for postoperative nutritional support after PD is currently unclear.
Traditional postoperative care after abdominal surgery includes the introduction of a nasogastric tube and starvation until bowel movement resumes. However, early oral feeding (EOF) omits the nasogastric tube placement or entails tube removal at 6-24 hours after surgery and starts oral intake of liquids and soft diet at will. Traditional management has shown no benefits compared to EOF.2,3,4
Many surgeons fear that postoperative EOF may cause mechanical injury at the anastomosis site or feeding intolerance in patients who have undergone PD. Few studies to date, however, have evaluated the efficacy of EOF after PD. Therefore, the objective of this study was to evaluate the clinical feasibility, safety, and nutritional effects of EOF after PD compared to TOF.
MATERIALS AND METHODS
This retrospective study using historical controls involved 131 patients, who underwent PD at Chonbuk National University Hospital from October 2003 to April 2013. Of these 131 patients, 30 had pancreatic cancer, 39 had ampulla of Vater cancer, 36 had distal bile duct cancer, 4 had duodenal cancer, 13 had intraductal papillary mucinous neoplasms, 3 had gallbladder cancer, 3 had trauma, 2 had chronic pancreatitis, and 1 had a pancreatic endocrine tumor. These patients were divided into EOF and traditional oral feeding [TOF] groups. The EOF group consisted of 81 patients who underwent PD since 2007 and started oral calorie intake within 48 hours after PD. All patients were encouraged to ingest tolerated amounts of water within 24 hours and a liquid diet within 48 hours after PD, with oral intake commenced carefully and adjusted according to patient tolerance. The TOF group consisted of 50 patients who underwent PD before 2007. Patients in the TOF group initially received total parenteral nutrition and started oral intake after bowel movement resumed.
We have performed a classic Whipple resection or pylorus-preserving pancreaticoduodenectomy (PPPD). Reconstruction was performed as a duct-to-mucosa pancreaticojejunostomy (end-to-end fashion), then a duct-to-mucosa choledochojejunostomy (end-to-side fashion), a retrocolicgastrojejunostomy (end-to-side fashion) in PD, or a retrocolicpylorojejunostomy in PPPD (end-to-side fashion), and a jejunojejunostomy (side-to-side fashion).
Postoperative outcomes including complications, energy intake, and length of stay (LOS) were analyzed. Postoperative complications included pneumonia, wound infection, intra-abdominal abscess, anastomotic leakage, delayed gastric emptying (DGE), upper gastrointestinal bleeding, postoperative bleeding, pleural effusion, small bowel obstruction, and reoperation. DGE was defined as nausea, vomiting, gastric distension or significant discomfort resulting in the discontinuation of oral feeding, as defined by the International Study Group of Pancreatic Surgery (ISGPS).5 The energy of postoperative nutrition allocated by the nutritional support team was initially calculated as kcal/day per patient and later expressed as percentage of necessary energy (25 kcal per kg body weight per day). The necessary amount of postoperative protein was set to be 1g per kg body weight per day.
Categorical variables were expressed as number (percentage) and compared using Fisher's exact tests and chi-square tests. Continuous variables were expressed as mean±SD and compared using Student's t tests. All statistical analyses were performed using SPSS 18.0 for Window (SPSS Inc., Chicago, Illinois, USA). A value of p<0.05 was considered statistically significant.
RESULTS
This study included 131 patients (87 men and 44 women, of mean age 61.1±10.6 years, range, 33-84 years). All patients showed satisfactory recovery after PD and were discharged from our department. The age, sex, weight, height and body mass index (BMI) of the EOF and TOF groups were similar. LOS (25.9±8.5 days vs. 32.3±16.3 days; p=0.01) were significantly shorter in the EOF than in the TOF group (Table 1).
Table 1.
Demographic and clinical characteristics of the EOF and TOF groups
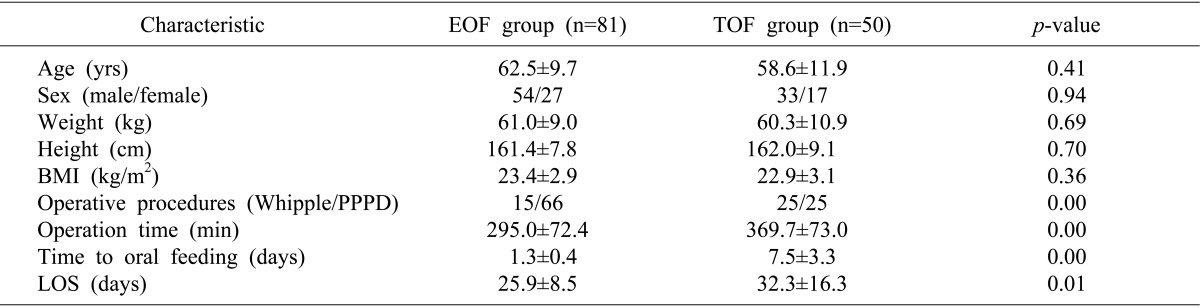
EOF, early oral feeding; TOF, traditional oral feeding; BMI, body mass index; PPPD, pylorus-preserving pancreaticoduodenctomy; LOS, length of stay.
A detailed description of postoperative complications is shown in Table 2. The rates of wound infection, intra-abdominal abscess, upper gastrointestinal bleeding, pleural effusion, pneumonia, postoperative bleeding and small bowel obstruction were similar in the two groups. However the rates of anastomotic leak (1.2% vs. 16%; p=0.00) and reoperation (3.7% vs. 20%; p=0.01) were significantly lower in the EOF than in the TOF group.
Table 2.
Postoperative complications in the EOF and TOF groups
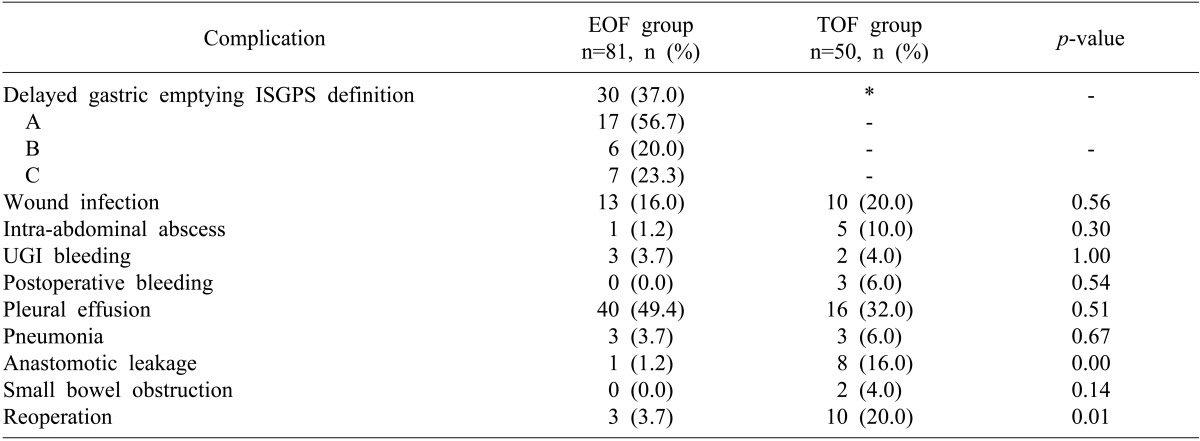
EOF, early oral feeding; TOF, traditional oral feeding; ISGPS, International Study Group of Pancreatic Surgery; UGI, upper gastrointestinal. *In the TOF group, the incidence of delayed gastric emptying could not be evaluated because all patients had to keep the nasogastric tube as traditional routine practice.
Of the 81 patients in the EOF group, 30 (37%) experienced DGE, with 21 requiring insertion of a nasogastric tube. Of these 21 patients, 9 (42.9%) showed resolution of DGE within one day and 19 (90.4%) within 5 days. Hospital stay was significantly longer in the 30 EOF patients with DGE than in the 51 without DGE (29.5±9.0 days vs. 23.8±7.5 days; p<0.05) (Table 3).
Table 3.
Characteristics of patients in the EOF group who did and did not experience postoperative delayed gastric emptying

EOF, early oral feeding; NG, nasogastric; POD, postoperative day; LOS, length of stay.
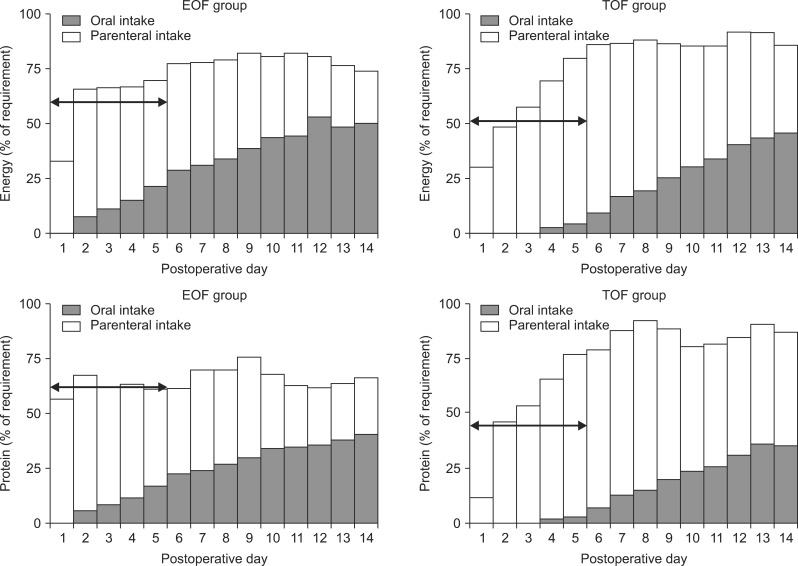
From postoperative days (POD) 1 to 14, patients in the EOF and TOF groups received 30% and 17%, respectively, of their energy goals through the enteral route and 41% and 51%, respectively, through the parenteral route. In addition, patients in the EOF and TOF groups received 23% and 14%, respectively, of their protein goals through the enteral route and 41% and 52%, respectively, through the parenteral route. The mean daily calorie intake (1018 vs. 972 kcal; p=0.30) and protein intake (44.3 vs. 44.4 g; p=0.98) from POD 1 to 14 were similar in the two groups. In the clinically acute phase, from POD 1 to 5, however, the mean daily calorie (847.0 kcal vs. 745.6 kcal; p=0.04) and protein (42.2 g vs. 31.9 g; p=0.00) intake were significantly greater in the EOF than in the TOF group. From POD 1 to 5, patients in these two groups also differed significantly in their mean percentages of total daily calorie intake (60.1% vs. 51.3%; p=0.02) and protein intake (62.1% vs. 45.9%; p=0.00) (Fig. 1).
Fig. 1.
Mean daily total calorie and protein intake in the EOF and TOF groups. Patients in the EOF and TOF groups received 30% and 17%, respectively, of their calorie goals through the enteral route and 41% and 51%, respectively, through the parenteral route from POD 1 to 14. Patients in the EOF and TOF groups received 23% and 14%, respectively, of their protein goals through the enteral route and 41% and 52%, respectively, through the parenteral route. The mean daily calorie (1018 kcal vs. 972 kcal; p=0.30) and protein (44.3 g vs. 44.4 g; p=0.98) intake from POD 1 to 14 were similar in the EOF and TOF groups. From POD 1 to 5, however, the mean daily calorie (847.0 kcal vs. 745.6 kcal; p=0.04) and protein (42.2 g vs. 31.9 g; p=0.00) intake was significantly higher in the EOF than in the TOF group, as was the mean percentage of total daily calorie (60.1% vs. 51.3%; p=0.02) and protein (62.1% vs. 45.9%; p=0.00) intake. Arrows indicate a percent of requirement of energy and protein from postoperative day 1 to 5. EOF: early oral feeding; TOF: traditional oral feeding; POD: postoperative day.
DISCUSSION
In the past, patients who underwent major gastrointestinal surgery having an intestinal anastomosis were not fed orally, primarily to stabilize the intestinal anastomosis to avoid possible mechanical pressure induced by food passage. However, it remains unclear whether delayed postoperative oral feeding would enhance recovery after surgery, especially since a period of starvation following gastrointestinal surgery showed no benefits to patients.6 In addition, experimental and clinical studies have suggested that early postoperative enteral feeding could enhance wound healing and anastomotic integrity while reducing septic complications.7,8 Little was known, however, about the efficacy of EOF after PD.
Early postoperative enteral feeding has been shown to be superior to parenteral nutrition in supporting function of the immune system, reducing infection rates, and maintaining gut integrity.4,6,8,9 Early postoperative enteral feeding has been associated with increased rates of nausea, vomiting, diarrhea, abdominal distension, excess gas production, feeding tube related complications, DGE, and patient intolerance to feeding.10 Therefore, full nutritional support only through enteral feeding is quite difficult.
It has been reported that total parenteral nutrition was not as effective as standard means of postoperative nutritional support for patients undergoing major pancreatic resection for malignancy.9 In fact, total parenteral nutritional support for patients following major pancreatic resection significantly increased complication rates, with most of these complications associated with infections. Clinical studies have suggested that the combination of enteral and parenteral nutrition is better than either one alone after pancreatic surgery. For example, a comparison of enteral feeding with the combination of enteral and parenteral nutrition revealed that the dropout rate in the enteral group (62.5%) was significantly higher than that in the combined group (11.1%) due to gastrointestinal side effects.10
To facilitate recovery and nutritional support after PD, we discontinued preoperative nasogastric tube insertion and introduced EOF in 2007. The combination of EOF with side-to-side jejunojejunostomy is especially advantageous to decrease DGE by maintaining the myoelectric activity of the gut and protecting the gastric mucosa from alkaline reflux.11 EOF has been found to stimulate enterocyte growth, improve mucosal barrier functions, decrease bacterial translocation, and stimulate small bowel peristalsis within hours after laparotomy.12,13,14,15,16 EOF can provide natural and adequate nutrition, therefore avoiding the need for prolongedparenteral nutritionwhich can lead to the intestinal membrane to atrophy and higher hospital expenses.
EOF within 24 hours has been shown to be safe in patients following major upper gastrointestinal surgery such as gastrectomy and Whipple's procedures.4 The first large randomized trial to question the existing routine of nil by mouth in patients undergoing upper gastrointestinal surgery found that nil by mouth was unnecessary in most patients. A prospective trial has found that early postoperative enteral feeding may contribute to decreased rates of postoperative morbidity and mortality by improving protein metabolism.17 Our analysis of postoperative complication rates after PD also demonstrated that, compared to TOF, EOF had significant positive effects, including lower rates of anastomotic leakage and reoperation.
The median LOS after PD in the Republic of Korea, as determined by reviewing Korean health insurance claims, was 26.9 days in 2013. This LOS, and the median LOS of 25.9 days in the present study, is somewhat longer than those reported in Western countries. This could be due to the unique social insurance system in Korea, in which the cost of hospitalization is kept relatively low. Therefore, patients are inclined to stay longer than the optimum LOS. The average LOS in the EOF group was six days less than in the TOF group, which was both clinically and economically important. Reduction in hospital stay might indicate a more rapid return of gastrointestinal function and a decreased rate of postoperative complications.
EOF can supply targeted amounts of calories and protein better than TOF during early postoperative stages. We found that protein and energy intake in the EOF group was lower than that of a previous study.10 The intake data might be improved by more intensive and effective care.
This study has several limitations, including the relatively small number of patients and its retrospective design. Therefore, a prospective trial comparing EOF with TOF after PD in a large number of patients in the future is needed.
In conclusion, postoperative EOF is a clinically safe, feasible, and effective method of nutritional support in patients who have undergone PD. Moreover, compared to the traditional management, EOF after PD significantly decreased LOS without increasing complication rates.
Footnotes
This study was supported by a grant (0620220-1) from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea.
References
- 1.Gooiker GA, van Gijn W, Wouters MW, Post PN, van de Velde CJ, Tollenaar RA Signalling Committee Cancer of the Dutch Cancer Society. Systematic review and meta-analysis of the volume-outcome relationship in pancreatic surgery. Br J Surg. 2011;98:485–494. doi: 10.1002/bjs.7413. [DOI] [PubMed] [Google Scholar]
- 2.Okabayashi T, Kobayashi M, Nishimori I, Sugimoto T, Akimori T, Namikawa T, et al. Benefits of early postoperative jejunal feeding in patients undergoing duodenohemipancreatectomy. World J Gastroenterol. 2006;12:89–93. doi: 10.3748/wjg.v12.i1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akbarshahi H, Andersson B, Nordén M, Andersson R. Perioperative nutrition in elective gastrointestinal surgery--potential for improvement? Dig Surg. 2008;25:165–174. doi: 10.1159/000136478. [DOI] [PubMed] [Google Scholar]
- 4.Lassen K, Kjaeve J, Fetveit T, Tranø G, Sigurdsson HK, Horn A, et al. Allowing normal food at will after major upper gastrointestinal surgery does not increase morbidity: a randomized multicenter trial. Ann Surg. 2008;247:721–729. doi: 10.1097/SLA.0b013e31815cca68. [DOI] [PubMed] [Google Scholar]
- 5.Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Lewis SJ, Andersen HK, Thomas S. Early enteral nutrition within 24 h of intestinal surgery versus later commencement of feeding: a systematic review and meta-analysis. J Gastrointest Surg. 2009;13:569–575. doi: 10.1007/s11605-008-0592-x. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder D, Gillanders L, Mahr K, Hill GL. Effects of immediate postoperative enteral nutrition on body composition, muscle function, and wound healing. JPEN J Parenter Enteral Nutr. 1991;15:376–383. doi: 10.1177/0148607191015004376. [DOI] [PubMed] [Google Scholar]
- 8.Moore FA, Feliciano DV, Andrassy RJ, McArdle AH, Booth FV, Morgenstein-Wagner TB, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992;216:172–183. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brennan MF, Pisters PW, Posner M, Quesada O, Shike M. A prospective randomized trial of total parenteral nutrition after major pancreatic resection for malignancy. Ann Surg. 1994;220:436–441. doi: 10.1097/00000658-199410000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagata S, Fukuzawa K, Iwashita Y, Kabashima A, Kinoshita T, Wakasugi K, et al. Comparison of enteral nutrition with combined enteral and parenteral nutrition in post-pancreaticoduodenectomy patients: a pilot study. Nutr J. 2009;8:24. doi: 10.1186/1475-2891-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wayne MG, Jorge IA, Cooperman AM. Alternative reconstruction after pancreaticoduodenectomy. World J Surg Oncol. 2008;6:9. doi: 10.1186/1477-7819-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander JW. Bacterial translocation during enteral and parenteral nutrition. Proc Nutr Soc. 1998;57:389–393. doi: 10.1079/pns19980056. [DOI] [PubMed] [Google Scholar]
- 13.Fukuzawa J, Terashima H, Ohkohchi N. Early postoperative oral feeding accelerates upper gastrointestinal anastomotic healing in the rat model. World J Surg. 2007;31:1234–1239. doi: 10.1007/s00268-007-9003-9. [DOI] [PubMed] [Google Scholar]
- 14.Nachlas MM, Younis MT, Roda CP, Wityk JJ. Gastrointestinal motility studies as a guide to postoperative management. Ann Surg. 1972;175:510–522. doi: 10.1097/00000658-197204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deitch EA, Winterton J, Li M, Berg R. The gut as a portal of entry for bacteremia. Role of protein malnutrition. Ann Surg. 1987;205:681–692. doi: 10.1097/00000658-198706000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang W, Kudsk KA. Is there evidence that the gut contributes to mucosal immunity in humans? JPEN J Parenter Enteral Nutr. 2007;31:246–258. doi: 10.1177/0148607107031003246. [DOI] [PubMed] [Google Scholar]
- 17.Hochwald SN, Harrison LE, Heslin MJ, Burt ME, Brennan MF. Early postoperative enteral feeding improves whole body protein kinetics in upper gastrointestinal cancer patients. Am J Surg. 1997;174:325–330. doi: 10.1016/s0002-9610(97)00095-0. [DOI] [PubMed] [Google Scholar]