Abstract
Adolescent idiopathic scoliosis (AIS) and pectus excavatum (PE) are common pediatric musculoskeletal disorders. Little is known about the tissue of origin for either condition, or about their genetic bases. Common variants near GPR126/ADGRG6 (encoding the adhesion G protein-coupled receptor 126/adhesion G protein-coupled receptor G6, hereafter referred to as GPR126) were recently shown to be associated with AIS in humans. Here, we provide genetic evidence that loss of Gpr126 in osteochondroprogenitor cells alters cartilage biology and spinal column development. Microtomographic and x-ray studies revealed several hallmarks of AIS, including postnatal onset of scoliosis without malformations of vertebral units. The mutants also displayed a dorsal-ward deflection of the sternum akin to human PE. At the cellular level, these defects were accompanied by failure of midline fusion within the developing annulus fibrosis of the intervertebral discs and increased apoptosis of chondrocytes in the ribs and vertebrae. Molecularly, we found that loss of Gpr126 upregulated the expression of Gal3st4, a gene implicated in human PE, encoding Galactose-3-O-sulfotransferase 4. Together, these data uncover Gpr126 as a genetic cause for the pathogenesis of AIS and PE in a mouse model.
Introduction
Adolescent idiopathic scoliosis (AIS) and pectus excavatum (PE) are both common pediatric musculoskeletal disorders; however, little is known about the genetics and pathophysiology of either condition (1,2). Scoliosis is a complex rotational deformity of the spine, representing a common phenotypic manifestation associated with at least 126 known distinct human genetic disorders or disease loci and encompassing a range of neurological, connective tissue and musculature pathologies (OMIM: http://www.ncbi.nlm.nih.gov/omim). Interestingly, most cases of scoliosis occur without a defined cause and thus are considered idiopathic. While some forms of idiopathic scoliosis (IS) are observed in young children, the majority of IS cases manifest around adolescence termed adolescent idiopathic scoliosis or AIS. AIS presents in otherwise healthy children without any overt malformations of the vertebral bodies and is estimated to affect 3% of children worldwide (3). While the majority of AIS patients may only require intervention related to cosmesis, >10% are at risk for the progression of more severe forms of scoliosis (curvatures >40°) impairing normal pulmonary or ambulatory function (3). The etiology of AIS is hypothesized to entail disorders in the axial musculature, low bone density, neurological defects or even neuroendocrine disorders (4,5). On the other hand, PE is largely a congenital deformity of the anterior chest wall due the dorsal depression of the sternum that is observed in at least 27 distinct human genetic disorders or disease loci (OMIM). Non-syndromic PE is estimated to occur in 1:400 births and is thought to be due to defects in the sternum cartilage or overgrowth of the costal cartilage of the ribs (2). Most indications for PE correction are primarily for cosmetic reasons and associated socio-physiologic problems. However, most PE patients (95%) also display measurable deficiencies in cardiovascular and pulmonary function, which display marked improvement after surgical correction (2). Interestingly, PE and AIS have high concomitant incidence in humans (2,6,7); however, the molecular and cellular mechanisms underlying these axial skeletal disorders have not been elucidated in any vertebrate organism.
Large-scale genome wide association studies have implicated several loci as candidate genes for AIS and PE, including GPR126, CHL1, LBX1 and GAL3ST4 (8–11). For instance, several variants (rs6570507, rs7774095 and rs7755109) located within intronic regions of the GPR126 gene are linked to AIS in humans (8,12). Unfortunately, Gpr126-null mice die before weaning, highlighting the importance of Gpr126 for survival and precluding a direct test of this association (13). In zebrafish, Danio rerio, antisense oligonucleotide morpholino knockdown of gpr126 caused delayed ossification of the developing spine (8); however, mutants in gpr126 do not display scoliosis (14,15). Gpr126 is required in multiple tissues during development including in the endocardium for normal heart development in the mouse (16), in Schwann cells for myelination of peripheral axons in zebrafish and in mouse (13,14) and for inner ear development in zebrafish (15). Whereas Gpr126 is expressed in many cartilaginous tissues, including the vertebral body and the ribs of the mouse (8,17) (see also Supplementary Material, Fig. S1), its role in these tissues has not been determined in the mouse. Coincidentally, many connective tissue diseases in humans (e.g. Marfan syndrome) exhibit either PE or scoliosis, or both (2), suggesting a role of cartilage tissue in spine and sternum defects.
Here, we report a new mouse model of AIS and PE by genetically deleting Gpr126 in cartilage. This mouse model displays many of the hallmarks of AIS including postembryonic onset of spine curvatures with rotation and absence of malformations of vertebrae at birth. Moreover, we observe no defects in locomotion or overt behavioral abnormalities in these mutant mice. Loss of Gpr126 results in apoptosis in axial cartilage prior to onset of AIS and PE. We further discover that Gpr126 deficiency results in upregulation of Gal3st4, a gene previously linked to human PE. Our data confirm GPR126 as an AIS gene and implicate GPR126 as a candidate PE gene in humans. This novel mouse model is a useful tool for future exploration of the role of Gpr126 function in cartilage biology and for the discovery of novel therapies for AIS and PE.
Results
Loss of Gpr126 in cartilage tissues results in defects of the axial column
Gpr126 is broadly expressed in the mouse throughout embryonic and postnatal stages, including in vertebral bodies and rib cartilages (17,18) (Supplementary Material, Fig. S1). To examine Gpr126 expression in cartilage tissues in more detail, we performed in situ hybridization with an antisense probe for Gpr126 (17) on tissue serial sections from Col2Cre;Rosa26mTmG embryos. In these embryos, the Rosa26mTmG allele expresses membrane localized (m) Tomato fluorescent protein in all cells except those expressing Cre, where it expresses mGFP. The Col2Cre transgene targets all cartilaginous structures at E12.5 (19), likely acting in all osteochondroprogenitors that give rise to chondrocytes in the notochord, cartilages of the jaw and axial column as well as to osteoblasts (20). We observed strong Gpr126 RNA expression in GFP(+) cells located within Aggrecan-rich (Alcian Blue stained) cartilaginous tissues (Supplementary Material, Fig. S1). These results indicate that Gpr126 is expressed in cartilaginous tissues of the embryo that are effectively targeted by Col2Cre.
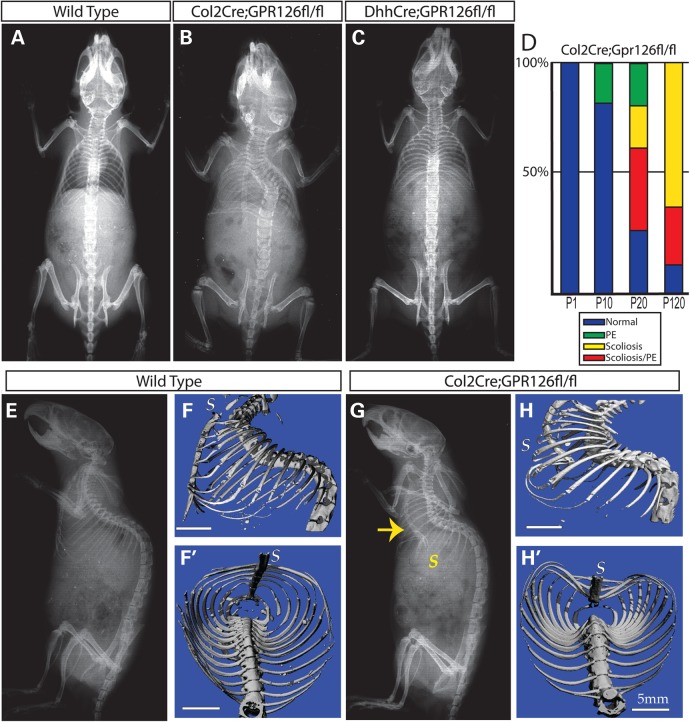
Given this expression pattern, we next sought to determine the role of Gpr126 in cartilage by deleting the gene with Col2Cre. Mice with the genotype of Col2Cre;Gpr126fl/fl were viable. However, X-ray analysis at postnatal (P) day 120 identified a significant fraction of Col2Cre;Gpr26fl/fl mice exhibiting thoracic scoliosis (Fig. 1A, B, D and Table 1). In contrast, scoliosis was not observed in DhhCre;Gpr126fl/fl animals, in which Gpr126 was deleted specifically in Schwann cell and Sertoli cell precursors (21,22) (Fig. 1C). In addition, we observed a significant fraction of the Col2Cre;Gpr26fl/fl mice with dorsal-ward PE-like deflections of the sternum (Fig. 1D–H). These data demonstrate that Col2Cre;Gpr126fl/fl mutant mice develop skeletal defects reminiscent of those in human AIS and PE patients.
Figure 1.
Conditional loss of Gpr126 in chondrocyte lineages results in scoliosis and PE. (A–C) Representative dorsal X-ray images of P120 (4-month-old) wild-type (A), Col2Cre;Gpr126fl/fl (B) or DhhCre;Gpr126fl/fl (C) mice. (D) Graphed phenotype prevalence in postnatal day in (P) 1, P10, P20 or P120 Col2Cre;Gpr126fl/fl mice. (E–H) Representative lateral X-ray (E and G) or μCT images (F and H) of P120 WT (E and G) and Col2Cre;Gpr126fl/fl (F and H) littermates. Yellow arrow denotes dorsal deflected sternum. (F′, H′) Coronal μCT images of WT (F′) and Col2Cre;Gpr126fl/fl (H′) littermates. S, sternum. Scale bars in F, F′, H, H′ are 5 mm.
Table 1.
Incidence of scoliosis and pectus excavatum in Col2Cre;Gpr126fl/fl mice
| Genotype | n | Weight (g) | Normal | Scoliosis | PE | PE/scoliosis |
|---|---|---|---|---|---|---|
| P1 | ||||||
| Wild type | 14 | 2.0 ± 0.5 | 14 | 0 | 0 | 0 |
| Col2Cre;Gpr126fl/fl | 8 | 2.1 ± 0.5 | 8 | 0 | 0 | 0 |
| P10 | ||||||
| Wild type | 8 | 5.1 ± 0.2 | 8 | 0 | 0 | 0 |
| Col2Cre;Gpr126fl/fl | 6 | 4.9 ± 0.5 | 5 | 0 | 1 | 0 |
| P20 | ||||||
| Wild type | 17 | 9.2 ± 1.2 | 17 | 0 | 0 | 0 |
| Col2Cre;Gpr126fl/fl | 11 | 7.9 ± 0.9 | 3 | 2 | 2 | 4 |
| P120 | ||||||
| Wild type | 11 | 23.8 ± 4.1 | 11 | 0 | 0 | 0 |
| Col2Cre;Gpr126fl/fl | 8 | 24.7 ± 2.8 | 1 | 5 | 0 | 2 |
We next evaluated the onset of the skeletal abnormalities in Col2Cre;Gpr26fl/fl-mutant mice (Fig. 1D and Table 1). They were mostly indistinguishable from their WT littermates from birth up to P10, although 1 out of 6 mutant mice displayed PE at P10. However, beginning at P20 and becoming more prevalent with age, a significant fraction of Col2Cre;Gpr26fl/fl mutant mice developed thoracic scoliosis. The severity of scoliosis was variable even among littermates, including both single and double curves and in some instances rotation of vertebral bodies (data not shown), akin to those seen in human AIS patients (23). A portion of scoliosis in humans is attributed to vertebral malformations including hemivertebrae and vertebral fusions (24); however, we did not observe vertebral malformations at any stage assayed. Hence, the lack of vertebral malformations, coupled with a postembryonic onset of pathology (P20), supports the categorization of Col2Cre;Gpr126fl/f-mutant mice as a model of human AIS. Interestingly, we observed that some mutant mice only displayed PE defects, whereas many mutants displayed co-occurrence of AIS and PE, particularly later in postnatal development (Fig. 1D and Table 1). By microtomography, we did not observe any significant changes in the trabecular bone of the vertebrae in Col2Cre;Gpr126fl/fl-mutant mice (Supplementary Material, Fig. S2). The mutant mice also did not show any overt defects in the craniofacial or appendicular skeleton (data not shown). As loss of Gpr126 function did not notably impair any of the bony structures, we conclude that Gpr126 is likely required in chondrocytes of the axial skeleton.
Gpr126 is required for normal morphogenesis of the annulus fibrosis but not for the formation of the nucleus pulposus
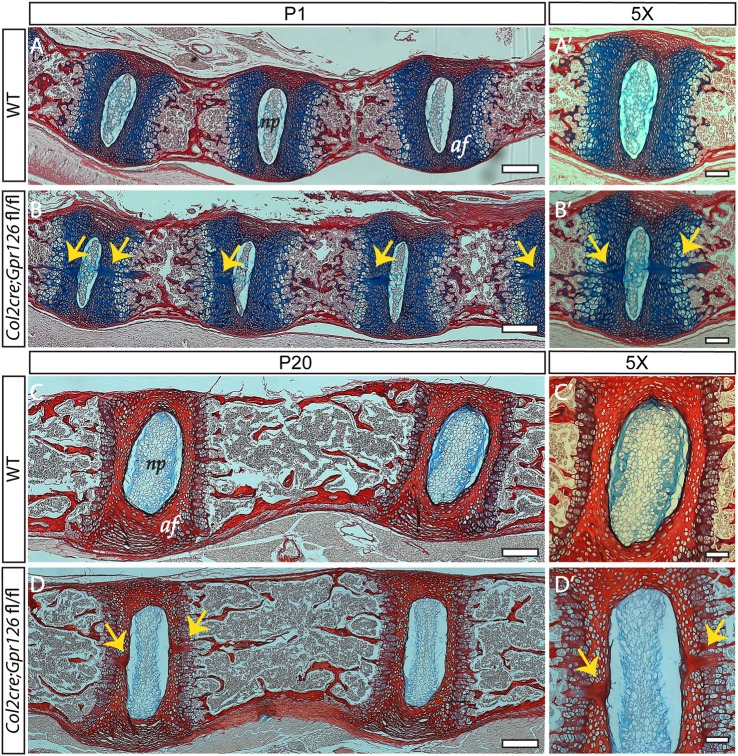
To determine the cellular etiology of AIS in Col2Cre;Gpr126fl/fl mutant mice, we evaluated histological sections of the spine and the sternum at P1, a time point prior to any phenotypic manifestation. Concurrent with formation of the annulus fibrosis, the notochord regresses in the region of the developing vertebral bodies, coupled with a transition of notochord to nucleus pulposus in the region of the developing intervertebral discs (25). In some cases, newborn Col2Cre;Gpr126fl/fl mice (P1) displayed multiple acellular clefts at the midline of the developing intervertebral bodies in the region of the annulus fibrosis (31.4%; n = 35; P = 0.01 Fisher's exact test); such clefts were only rarely observed in neonatal WT mice (5.9%; n = 34) (Fig. 2A–B′). In addition, these acellular clefts were also observed in Col2Cre;Gpr126fl/fl mice at P20 (12.5%; n = 16), a time point in which they were not observed in WT littermates (0.0%; n = 21) (Fig. 2C–D′). Thus, Gpr126 is required for the morphogenesis of the annulus fibrosis but not formation of the nucleus pulposus.
Figure 2.
Defective morphogenesis of the annulus fibrosis is observed in Col2Cre;Gpr126fl/fl-mutant mice. (A–D′) Sagittal sections of spinal columns from P1 (A–B′) or P20 (C–D′) Col2Cre;Gpr126fl/+ (WT) (A, A′, C, C′) or Col2Cre;Gpr126fl/fl (CKO) stained with Alcian Blue and Picrosirius Red (B, B′) or stained with Alcian Blue and Safranin O (D, D′). Yellow arrows denote acellular clefts. np, nucleus pulposus; af, annulus fibrosis. Scale bars in A–D are 200 microns and in A′–D′ are 100 microns.
Increased apoptosis in Gpr126 deficient cartilage suggests a common pathophysiology for AIS and PE
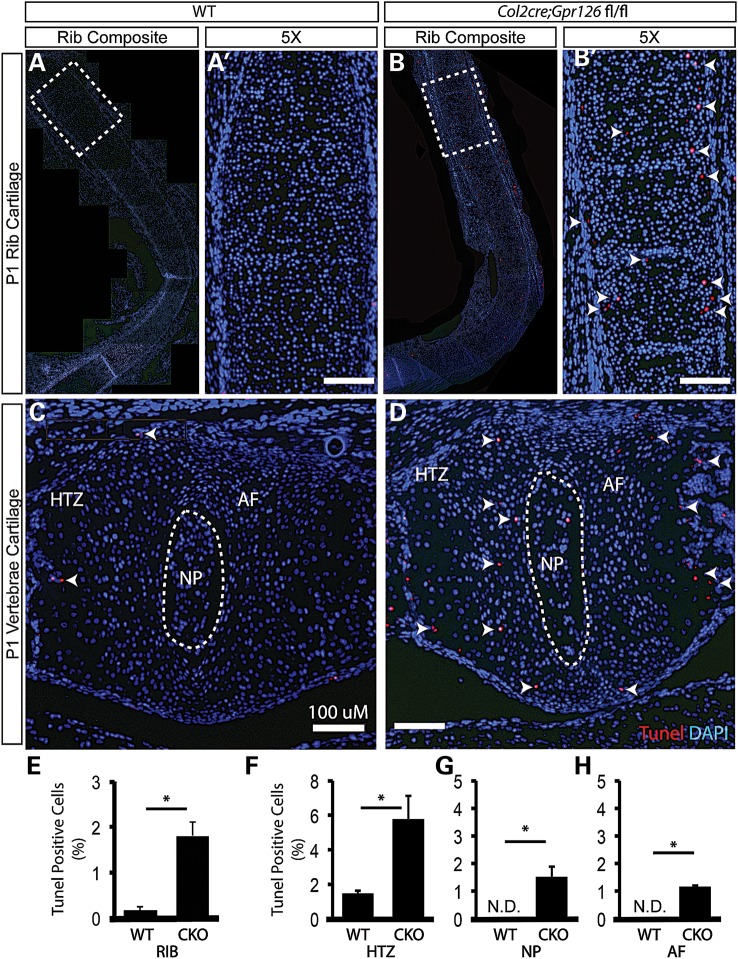
Increased apoptosis is thought to be a driving factor in cartilaginous disease such as osteoarthritis (26) and has been noted in intervertebral discs of human patients with AIS (27). The formation of PE in humans is postulated to occur by either biomechanical deficiencies or overgrowth of the sternocostal cartilages and/or costal cartilages of the ribs (2). We set out to address the role of cell survival or proliferation in Col2Cre;Gpr126fl/fl mice. In contrast to defects observed in the vertebral column (Fig. 2), we observed normal tissue architecture in the sternum and ribs of mutant mice at P1 (Supplementary Material, Fig. S3 and not shown). We did not observe noticeable changes in proliferation status in chondrocytes of the intervertebral regions (Supplementary Material, Fig. S4). Using Terminal deoxynucleotidyl transferase dUTP Nick End Labeling (TUNEL), we evaluated apoptosis in chondrocytes of the costal cartilage and vertebrae at P1, prior to onset of AIS and PE phenotypes. We detected increased apoptosis in mutant chondrocytes of both the costal cartilages of the ribs and of the vertebrae of Col2Cre;Gpr126fl/fl mutants (Fig. 3). In the intervertebral region, increased apoptosis was observed in all chondrocytes including within the hypertrophic zone, annulus fibrosis and within the nucleus pulposus (Fig. 3C, D and F–H). Apoptosis in cartilaginous elements of the axial skeleton prior to the manifestation of AIS and PE suggests a novel cellular etiology for both diseases.
Figure 3.
Col2Cre;Gpr126fl/fl-mutant cartilage displays increased apoptosis. Frontal sections of costal rib cartilage (A–B′) or sagittally sectioned intervertebral bodies (C and D) from P1 Col2Cre;Gpr126fl/+ (WT) (A, A′, C) or Col2Cre;Gpr126fl/fl (B, B′, D) stained with TUNEL to mark apoptotic cells (red nuclei; additionally marked by white arrowheads) and DAPI used to counterstain nuclei (blue nuclei). Dashed box highlights individual 5× image in A′, B′. (E–H) Quantification of the percentage of TUNEL-positive nuclei/total nuclei in WT and Col2Cre;Gpr126fl/fl (CKO) costal rib cartilages (E) and vertebral bodies (F–H). np, nucleus pulposus; af, annulus fibrosis; htz, hypertrophic zone. N.D., None detected. n = 5 stained sections each tissue/genotype, * P ≤ 0.05. Scale bars in A′, B′, C, D are 100 microns.
Gpr126 regulates transcription of Gal3st4 without affecting intracellular levels of cAMP in chondrocytes
Gpr126 function in Schwann cell is essential for peripheral myelination, in part through the elevation of intracellular cAMP levels (22). This signaling of Gpr126 through cAMP is also necessary for the transcriptional activation of genes important for myelination (28). Importantly, others and we reported that treatment with cAMP agonists rescued both Gpr126-dependent defects in peripheral nerve myelination in zebrafish and mice (14,22,28), as well as inner ear defects in gpr126-mutant zebrafish (15). While multiple cyclic AMP-hydrolyzing phosphodiesterase family genes are expressed in articulating cartilage tissues of the mouse (29), the selective inhibition of the phosphodiesterase-4 (PDE4) gene is known to provide chondroprotective effects for osteoarthritic cartilage (30). To test whether elevation of intracellular cAMP signaling could rescue the onset of AIS or PE defects in our mouse model, we injected newborn Col2Cre;Gpr126fl/fl and littermate controls daily with Rollipram, a known cAMP positive regulator via selective PDE4 inhibition (31). We utilized daily intraperitoneal injection of 5 mg/kg Rollipram starting at P1 until P20, a dosage shown to be beneficial in previous mouse models of Huntington's disease (32). However, we observed no significant reduction of either the incidence or severity of AIS or PE in Rollipram-treated Col2Cre;Gpr126fl/fl-mutant mice (Supplementary Material, Fig. S5A–C). To test the role of Gpr126 on intracellular levels of cAMP in chondrocytes, we measured cAMP concentrations in primary chondrocytes isolated from the costal cartilage of P5 mutant versus littermate control mice. Interestingly, we found that intracellular levels of cAMP were not significantly affected by loss of Gpr126 (Supplementary Material, Fig. S5D). These results indicate that Gpr126 is dispensable for maintaining basal cAMP levels in chondrocytes, in contrast to its role in Schwann cell precursors (22).
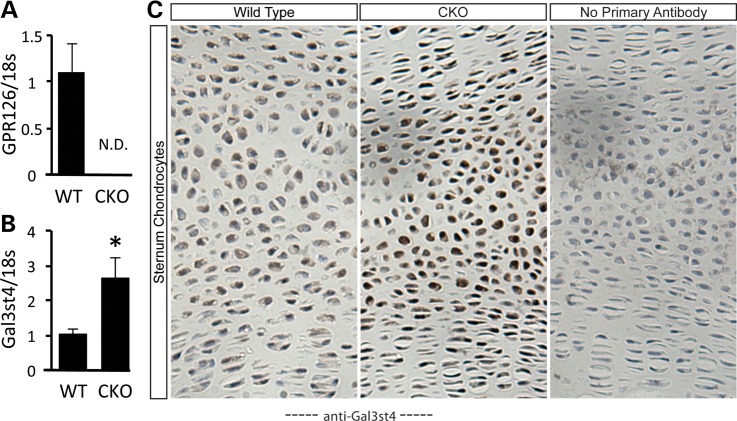
In the zebrafish larvae, Gpr126 controls the initiation of key transcription factors in Schwann cells (28). Therefore, we reasoned that misregulation of gene expression might be observed in Col2Cre;Gpr126fl/fl-mutant chondrocytes. Confirming efficient inactivation of Gpr126, its transcripts were undetectable in chondrocytes isolated from P5 Col2Cre;Gpr126fl/fl-mutant mice (Fig. 4A). We next assayed the mRNA levels of known markers of chondrocyte differentiation, as well as several candidate genes associated with AIS or PE in humans. No alterations in gene expression were observed for chondrocyte transcription factors (e.g. Sox5, Sox9 and Atf4) (Supplementary Material, Fig. S6A–C) or cartilage matrix components (e.g. Col2a1, Vcan and Fbn1) (Supplementary Material, Fig. S6D–F). Similarly, we did not detect any misregulation of Chl1 and Lbx1, both genes implicated in human AIS (9,10) (Supplementary Material, Fig. S6G and H). However, we did find that a gene previously implicated in human PE patients (11), Galactose-3-O-sulfotransferase 4 (Gal3st4), was significantly upregulated in Col2Cre;Gpr126fl/fl-mutant chondrocytes (Fig. 4B). Immunohistochemistry on sectioned sternum and ribs confirmed the elevated level of Gal3st4 protein in mutant chondrocytes (Fig. 4C). Taken together, these data show that Gpr126 regulates both chondrocyte survival and the expression of Gal3st4.
Figure 4.
Increased Gal3st4 expression in Col2Cre;Gpr126fl/fl chondrocytes. (A and B) qPCR analysis of isolated costal rib cartilage-derived chondrocytes from Col2Cre;Gpr126fl/+ (WT) or Col2Cre;Gpr126fl/fl (CKO) mice. Gene expression is represented as fold change relative to 18S RNA. N = 3 samples per genotype. (C) Frontal sections of P1 sternum from WT or Col2Cre;Gpr126fl/fl mice stained with anti-Gal3st4 antibody (zoomed; 20X). N.D., none detected. *P ≤ 0.05.
Discussion
In this report, we demonstrate that GPR126, a gene implicated in human AIS (8,12), acts in axial cartilage to regulate normal spine and sternum development in the mouse. Mechanistically, Gpr126 appears to direct normal skeletal morphology by regulating morphogenesis of the annulus fibrosis, chondrocyte survival and the expression of Gal3st4 in cartilage tissues. The molecular details downstream of Gpr126 in axial cartilage tissues remain to be fully elucidated. Nevertheless, the current study expands not only our current knowledge of the cellular mechanisms underlying AIS and PE progression but also highlights the critical role of Gpr126 during postembryonic cartilage development. Finally, the Col2Cre;Gpr126fl/fl-mutant mouse provides a robust genetic model of AIS and PE to determine the pathophysiology of these common pediatric diseases.
While AIS has no known cause, neuropathic as well as osteopathic etiologies are hypothesized (3,33). Gpr126 is required for peripheral nerve development and myelination in both zebrafish and mice (13,22). In humans, scoliosis is associated with demyelinating peripheral neuropathies such as Charcot-Marie-Tooth disease (34). Here, we rule out a role for abnormal myelination of the peripheral nervous system as a primary etiology in AIS, as removal of Gpr126 in Schwann cell precursors using DhhCre resulted in no discernable scoliosis phenotype (Fig. 1C). On the other hand, removal of Gpr126 specifically in osteochondroprogenitor cells that give rise to both chondrocytes and osteoblasts (20) resulted in robust AIS and PE formation. Interestingly, AIS is associated with low bone mass in humans and bone mineral density is an important prognostic factor for curve progression (35–37). While Kuo and others reported that antisense oligonucleotide-mediated knockdown of gpr126 in larval zebrafish resulted in decreased ossification of the developing axial centra in adults (8), we did not observe either ossification defects or scoliosis in zebrafish with several gpr126-mutant alleles (our unpublished observations). Likewise, we observed no alterations in the overall shape, bone volume or mineral density of the vertebrae in Col2Cre;Gpr126fl/fl-mutant mice (Supplementary Material, Fig. S2), supporting the notion that decreased bone mineral density is not the cause of AIS in Col2Cre;Gpr126fl/fl-mutant mice. Instead, our data support a model wherein Gpr126 acts predominantly in chondrocytes/cartilage, whereas the role in bone is negligible.
The ablation of Gpr126 in Col2Cre-positive cells results in increased apoptosis and altered gene expression specifically in axial cartilage of the mouse. Interestingly, cartilage degradation during osteoarthritis is due to the progressive loss of the molecular and biomechanical properties of cartilage and is associated with increased apoptosis of chondrocytes (26). We speculate that the increase in apoptosis observed in the Col2Cre;Gpr126fl/fl-mutant cartilage also results in a gradual loss of the mechanical properties of cartilage during development which ultimately manifests as AIS and PE in the mouse. Based on our current observations, the formation of AIS or PE is stochastic at least until 4 months of age, wherein some mutant mice are phenotypically wild type (Fig. 1D). This suggests that the onset of pathology in the Col2Cre;Gpr126fl/fl-mutant mice occurs during a specific range of development, possibly during rapid growth prior to sexual maturity. Longitudinal studies on individual animals will be necessary to test this hypothesis.
Previous reports suggest that PDE4 inhibition may have chondroprotective effects in primary osteoarthritis patient-derived chondrocytes (29). Whereas we did not observe any suppression of AIS or PE phenotypes despite daily injection of Rollipram (a selective PDE4 inhibitor), it remains possible that other selective cAMP agonists could rescue the loss of Gpr126 signaling in chondrocytes. Our data support a model wherein Gpr126 signaling in the context of mouse chondrocytes may be inhibitory, acting to downregulate genes such as Gal3st4 during the development of cartilage tissues. Alternatively, it remains possible that Gpr126 functions independently of cAMP signaling in mouse axial cartilage. Future studies will be necessary to uncover the molecular effectors of Gpr126 in chondrocytes.
GPR126 is a member of the Adhesion GPCR class and thus postulated to facilitate both cell–cell and cell–matrix interactions (38). The N-terminal domains of adhesion GPCRs often interact with mediate contact with extracellular matrix (ECM) proteins (18), and Gpr126 can bind to ECM components Collagen type IV and Laminin-211 (39,40). It is likely that Gpr126 deletion in chondrocytes affects either adhesion to other chondrocytes, the notochord, invading osteoblasts and/or the cartilaginous matrix. Indeed, Collagen type IV was shown to activate human and zebrafish Gpr126 in heterologous systems (39,40). As the cartilaginous matrix is comprised of diverse collagen species, perhaps Gpr126 deficiency affects both intracellular signaling and matrix adhesion. Interestingly, the notochord sheath cells that surround both the notochord and its derivative the nucleus pulposus express Collagen Type IV at high levels (41). It is intriguing to speculate that GPR126 may function to coordinate communication, adhesion or morphogenesis of the vertebral chondrocytes and the regressing notochord during formation of the intervertebral discs. In support of this, we observed the presence of acellular clefts in the annulus fibrosis of Col2Cre;Gpr126fl/fl mice both at birth and postnatally (Fig. 2). These acellular regions are likely indicative of the failure of midline fusion of the cartilaginous anlagen during the transformation of the embryonic notochord into the nucleus pulposus (25). The presence of acellular regions or flaws in the vertebral body may affect the physical properties of the vertebrae both by changing the physical location and arrangement of the vertebral chondrocytes as well as by altering the composition of the ECM.
The ECM determines the physical characteristics and many of the biological properties of the chondrocytes embedded in the developing vertebrae. The major components of the ECM are fibrous proteins that provide tensile strength and elasticity (e.g. various collagens), adhesive glycoproteins (e.g. fibronectin and laminin) and proteoglycans (e.g. chondroitin/dermatan sulfate) that interact with other ECM components to provide a hydrated gel that resists compressive forces (42). In some cases, proteoglycans in the cartilage matrix are sulfonated, which provides a negative charge and is important for the normal hydration and assembly of the cartilage matrix. Proteoglycan sulfation is tightly regulated, both temporally and regionally, and these sulfation patterns are thought to provide heterogeneity for tissues throughout development. Importantly, loss-of-function of specific sulfotransferease genes affects a wide range of biological processes including morphogen signaling (43), axon guidance (44), somitogenesis and vascular assembly (45). GAL3ST4 catalyzes sulfonation of the C-3′ position of galactose residues in O-linked glycoproteins (46); recently, a mutation in GAL3ST4 (R11W) was identified in a familial study of PE in humans (11). While the exact role of Gal3st4 in mouse axial cartilage tissue is presently unclear, its increased expression may result in hypersulfation of proteoglycans in the ECM and change the physical properties of the developing axial cartilages. In this model, a reduction of the compressive strength of the vertebral bodies, sternum and rib cartilages could result in the pathology of AIS or PE. Future studies focused on the physical and mechanical properties of cartilage in Gpr126- and Gal3st4-deleted mouse models are warranted.
Regardless of the exact nature of GPR126 variants in humans, it is clear from our present study that Gpr126 functions in cartilage for normal stability of the spine and sternum. Importantly, many of the hallmarks of AIS are observed in this mutant model including postembryonic onset of the spine curvature with rotation, without underlying vertebral body defects, and no overt defects in locomotion and behavior. Thus, this mouse provides a novel genetic model of AIS. In addition, this work provides a novel genetic link for AIS and PE centered on Gpr126. Furthermore, our data suggest that both AIS and PE can have related pathophysiologies. To our knowledge, this represents the first genetic mouse model of both AIS and PE. These mice can be utilized as a preclinical model to define the relevant molecular targets and test novel therapeutic approaches. Future studies focused on the GPR126 locus for AIS or PE in humans and mechanistic studies of Gal3st4 in animal models are warranted in light of these data.
Materials and Methods
Mouse strains
Gpr126flox, Col2Cre, DhhCre and RosamTmG mouse strains are as previously described (19,21,22,47). The Animal Studies Committee at Washington University approved all mouse procedures.
Isolation of primary sternal chondrocytes
Rib cages were isolated from postnatal day (P) 5 mice. Muscle and bone was dissected away from the sternal and costal cartilage, which was then digested for 30 min in 2 mg/ml protease (Sigma P6911) at 37°C and washed two times in phosphate-buffered saline (PBS). This was followed by digestion with 3 mg/ml collagenase (Sigma C6885) for 1 h in DMEM at 37°C and 5% CO2 followed by three vigorous PBS washes to remove digested tissue. Cartilage was then further digested with 0.5 mg/ml collagenase overnight in DMEM at 37°C and 5% CO2. Cartilage was triturated with a 10-ml pipette tube and filtered through a 70-μm cell strainer and washed two times with PBS.
RNA isolation and qPCR
Total RNA was isolated from sternal chondrocytes at P5 using the RNAeasy kit with on-column DNase treatment (Qiagen). Reverse transcription was performed using 800 ng total RNA with the iScript cDNA synthesis kit (BioRad). Reactions were set up in technical and biological triplicates in a 96-well format on an ABI StepOne Plus, using SYBR green chemistry (SsoAdvanced, BioRad). The PCR conditions were 95°C for 3 min followed by 40 cycles of 95°C for 10 s and 60°C for 30 s. Gene expression was normalized to 18S rRNA, and relative expression was calculated using the 2−(ΔΔCt) method. Primers were used at 0.1 μm, and their sequences are listed in Supplementary Material, Table S1. PCR efficiency was optimized, and melting curve analyses of products were performed to ensure reaction specificity.
Analyses of mice
Radiographs of mouse skeletons were generated using a Faxitron x-ray system (Faxitron x-ray Corp) with 20-s exposure under 25 kV. Mice were euthanized with CO2 and immediately analyzed by X ray to minimize artifacts. Micro-computed tomography (μCT 40, Scanco Medical, AG) was used for three-dimensional reconstruction and quantification of bone parameters (threshold set at 200) from 100 slices underneath the growth plate. Histology was performed on spinal columns or sternums fixed in 10% buffered formalin overnight at room temperature followed by decalcification in 14% EDTA with change every 2 days for 2 weeks. After decalcification, samples were embedded in paraffin and sectioned at 6 μm of thickness. H&E, Saffrin-O and Picosirius Red/Alcian Blue staining were performed following standard protocols. TUNEL staining was performed as per manufacturers’ instructions using the In Situ Cell Death Detection Kit, TMR Red (Roche).
In situ hybridization and antibody staining
In situ hybridization was performed on 10-μm cryosectioned embryonic day (E)15.5 mouse embryos as previously described (48). Anti-GAL3ST4 antibody (ab116039 Abcam) staining was developed using DAB substrate (Roche).
Supplementary Material
Funding
This work supported by the National Institutes of Health (F32 AR060674 to C.K., R01 AR060456, R01 DK065789 to F.L., NIH NS079445 to K.R.M., F32AR063001 to R.S.G.); a Pilot and Feasibility from the Washington University Musculoskeletal Research Center (NIH P30 AR057235 to L.S.-K.) and small exploratory grants from the Scoliosis Research Society to R.S.G.
Supplementary Material
Acknowledgements
We thank C. Idleburg at the Washington University Musculoskeletal Histology and Morphometry Core for excellent histology support and C. Gurnett and J. Wallingford for critical comments on the manuscript.
Conflict of Interest statement. None declared.
References
- 1.Kouwenhoven J.W., Castelein R.M. (2008) The pathogenesis of adolescent idiopathic scoliosis: review of the literature. Spine, 33, 2898–2908. [DOI] [PubMed] [Google Scholar]
- 2.Brochhausen C., Turial S., Muller F.K., Schmitt V.H., Coerdt W., Wihlm J.M., Schier F., Kirkpatrick C.J. (2012) Pectus excavatum: history, hypotheses and treatment options. Interact. Cardiovasc. Thoracic Surg., 14, 801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wise C.A., Gao X., Shoemaker S., Gordon D., Herring J.A. (2008) Understanding genetic factors in idiopathic scoliosis, a complex disease of childhood. Curr. Genom., 9, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinstein S.L. (1989) Adolescent idiopathic scoliosis: prevalence and natural history. Instr. Course Lect., 38, 115–128. [PubMed] [Google Scholar]
- 5.Ahn U.M., Ahn N.U., Nallamshetty L., Buchowski J.M., Rose P.S., Miller N.H., Kostuik J.P., Sponseller P.D. (2002) The etiology of adolescent idiopathic scoliosis. Am. J. Orthop. (Belle. Mead. NJ), 31, 387–395. [PubMed] [Google Scholar]
- 6.Hong J.Y., Suh S.W., Park H.J., Kim Y.H., Park J.H., Park S.Y. (2011) Correlations of adolescent idiopathic scoliosis and pectus excavatum. J. Pediatr. Orthop., 31, 870–874. [DOI] [PubMed] [Google Scholar]
- 7.Gurnett C.A., Alaee F., Bowcock A., Kruse L., Lenke L.G., Bridwell K.H., Kuklo T., Luhmann S.J., Dobbs M.B. (2009) Genetic linkage localizes an adolescent idiopathic scoliosis and pectus excavatum gene to chromosome 18 q. Spine, 34, E94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kou I., Takahashi Y., Johnson T.A., Takahashi A., Guo L., Dai J., Qiu X., Sharma S., Takimoto A., Ogura Y., et al. (2013) Genetic variants in GPR126 are associated with adolescent idiopathic scoliosis. Nat. Genet., 45, 676–679. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S., Gao X., Londono D., Devroy S.E., Mauldin K.N., Frankel J.T., Brandon J.M., Zhang D., Li Q.Z., Dobbs M.B., et al. (2011) Genome-wide association studies of adolescent idiopathic scoliosis suggest candidate susceptibility genes. Hum. Mol. Genet., 20, 1456–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi Y., Kou I., Takahashi A., Johnson T.A., Kono K., Kawakami N., Uno K., Ito M., Minami S., Yanagida H., et al. (2011) A genome-wide association study identifies common variants near LBX1 associated with adolescent idiopathic scoliosis. Nat. Genet., 43, 1237–1240. [DOI] [PubMed] [Google Scholar]
- 11.Wu S., Sun X., Zhu W., Huang Y., Mou L., Liu M., Li X., Li F., Li X., Zhang Y., et al. (2012) Evidence for GAL3ST4 mutation as the potential cause of pectus excavatum. Cell Res., 22, 1712–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J.F., Yang G.H., Pan X.H., Zhang S.J., Zhao C., Qiu B.S., Gu H.F., Hong J.F., Cao L., Chen Y., et al. (2015) Association of GPR126 gene polymorphism with adolescent idiopathic scoliosis in Chinese populations. Genomics, 105, 101–107. [DOI] [PubMed] [Google Scholar]
- 13.Monk K.R., Oshima K., Jors S., Heller S., Talbot W.S. (2011) Gpr126 is essential for peripheral nerve development and myelination in mammals. Development, 138, 2673–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monk K.R., Naylor S.G., Glenn T.D., Mercurio S., Perlin J.R., Dominguez C., Moens C.B., Talbot W.S. (2009) A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science, 325, 1402–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng F.S., Abbas L., Baxendale S., Holdsworth C.J., Swanson A.G., Slanchev K., Hammerschmidt M., Topczewski J., Whitfield T.T. (2013) Semicircular canal morphogenesis in the zebrafish inner ear requires the function of gpr126 (lauscher), an adhesion class G protein-coupled receptor gene. Development, 140, 4362–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waller-Evans H., Promel S., Langenhan T., Dixon J., Zahn D., Colledge W.H., Doran J., Carlton M.B., Davies B., Aparicio S.A., et al. (2010) The orphan adhesion-GPCR GPR126 is required for embryonic development in the mouse. PLoS One, 5, e14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patra C., van Amerongen M.J., Ghosh S., Ricciardi F., Sajjad A., Novoyatleva T., Mogha A., Monk K.R., Muhlfeld C., Engel F.B. (2013) Organ-specific function of adhesion G protein-coupled receptor GPR126 is domain-dependent. Proc. Natl Acad. Sci. USA, 110, 16898–16903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patra C., Monk K.R., Engel F.B. (2014) The multiple signaling modalities of adhesion G protein-coupled receptor GPR126 in development. Recept. Clin. Investig., 1, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long F., Zhang X.M., Karp S., Yang Y., McMahon A.P. (2001) Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development, 128, 5099–5108. [DOI] [PubMed] [Google Scholar]
- 20.Wang W., Nyman J.S., Ono K., Stevenson D.A., Yang X., Elefteriou F. (2011) Mice lacking Nf1 in osteochondroprogenitor cells display skeletal dysplasia similar to patients with neurofibromatosis type I. Hum. Mol. Genet., 20, 3910–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaegle M., Ghazvini M., Mandemakers W., Piirsoo M., Driegen S., Levavasseur F., Raghoenath S., Grosveld F., Meijer D. (2003) The POU proteins Brn-2 and Oct-6 share important functions in Schwann cell development. Genes Dev., 17, 1380–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mogha A., Benesh A.E., Patra C., Engel F.B., Schoneberg T., Liebscher I., Monk K.R. (2013) Gpr126 functions in Schwann cells to control differentiation and myelination via G-protein activation. J. Neurosci., 33, 17976–17985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenke L.G., Betz R.R., Harms J., Bridwell K.H., Clements D.H., Lowe T.G., Blanke K. (2001) Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J. Bone Joint Surg. Am., 83-A, 1169–1181. [PubMed] [Google Scholar]
- 24.Giampietro P.F., Dunwoodie S.L., Kusumi K., Pourquie O., Tassy O., Offiah A.C., Cornier A.S., Alman B.A., Blank R.D., Raggio C.L., et al. (2008) Molecular diagnosis of vertebral segmentation disorders in humans. Expert Opin. Med. Diagn., 2, 1107–1121. [DOI] [PubMed] [Google Scholar]
- 25.Smith L.J., Nerurkar N.L., Choi K.S., Harfe B.D., Elliott D.M. (2011) Degeneration and regeneration of the intervertebral disc: lessons from development. Dis. Model Mech., 4, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldring M.B. (2000) The role of the chondrocyte in osteoarthritis. Arthritis Rheum., 43, 1916–1926. [DOI] [PubMed] [Google Scholar]
- 27.Sitte I., Kathrein A., Pfaller K., Pedross F., Klosterhuber M., Lindtner R.A., Zenner J., Ferraris L., Meier O., Koller H. (2013) Morphological differences in adolescent idiopathic scoliosis: a histological and ultrastructural investigation. Spine, 38, 1672–1680. [DOI] [PubMed] [Google Scholar]
- 28.Glenn T.D., Talbot W.S. (2013) Analysis of Gpr126 function defines distinct mechanisms controlling the initiation and maturation of myelin. Development, 140, 3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tenor H., Hedbom E., Hauselmann H.J., Schudt C., Hatzelmann A. (2002) Phosphodiesterase isoenzyme families in human osteoarthritis chondrocytes--functional importance of phosphodiesterase 4. Br. J. Pharmacol., 135, 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowen M.E., Ayturk U.M., Kurek K.C., Yang W., Warman M.L. (2014) SHP2 regulates chondrocyte terminal differentiation, growth plate architecture and skeletal cell fates. PLoS Genet., 10, e1004364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barad M., Bourtchouladze R., Winder D.G., Golan H., Kandel E. (1998) Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc. Natl Acad. Sci. USA, 95, 15020–15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeMarch Z., Giampa C., Patassini S., Bernardi G., Fusco F.R. (2008) Beneficial effects of rolipram in the R6/2 mouse model of Huntington's disease. Neurobiol. Dis., 30, 375–387. [DOI] [PubMed] [Google Scholar]
- 33.Gorman K.F., Julien C., Moreau A. (2012) The genetic epidemiology of idiopathic scoliosis. Eur. Spine J., 21, 1905–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter G.T., Abresch R.T., Fowler W.M., Jr, Johnson E.R., Kilmer D.D., McDonald C.M. (1995) Profiles of neuromuscular diseases. Hereditary motor and sensory neuropathy, types I and II. Am. J. Phys. Med. Rehabil., 74, S140–e14149. [DOI] [PubMed] [Google Scholar]
- 35.Li X.F., Li H., Liu Z.D., Dai L.Y. (2008) Low bone mineral status in adolescent idiopathic scoliosis. Eur. Spine J., 17, 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pourabbas Tahvildari B., Erfani M.A., Nouraei H., Sadeghian M. (2014) Evaluation of bone mineral status in adolescent idiopathic scoliosis. Clin. Orthop. Surg., 6, 180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hung V.W., Qin L., Cheung C.S., Lam T.P., Ng B.K., Tse Y.K., Guo X., Lee K.M., Cheng J.C. (2005) Osteopenia: a new prognostic factor of curve progression in adolescent idiopathic scoliosis. J. Bone Joint Surg. Am., 87, 2709–2716. [DOI] [PubMed] [Google Scholar]
- 38.Langenhan T., Aust G., Hamann J. (2013) Sticky signaling--adhesion class G protein-coupled receptors take the stage. Sci. Signal., 6, re3. [DOI] [PubMed] [Google Scholar]
- 39.Petersen S.C., Luo R., Liebscher I., Giera S., Jeong S.J., Mogha A., Ghidinelli M., Feltri M.L., Schoneberg T., Piao X., et al. (2015) The adhesion GPCR GPR126 has distinct, domain-dependent functions in Schwann cell development mediated by interaction with laminin-211. Neuron, 85, 755–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paavola K.J., Sidik H., Zuchero J.B., Eckart M., Talbot W.S. (2014) Type IV collagen is an activating ligand for the adhesion G protein-coupled receptor GPR126. Sci. Signal., 7, ra76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayes A.J., Benjamin M., Ralphs J.R. (2001) Extracellular matrix in development of the intervertebral disc. Matrix Biol., 20, 107–121. [DOI] [PubMed] [Google Scholar]
- 42.Grodzinsky A.J., Levenston M.E., Jin M., Frank E.H. (2000) Cartilage tissue remodeling in response to mechanical forces. Ann. Rev. Biomed. Eng., 2, 691–713. [DOI] [PubMed] [Google Scholar]
- 43.Lin X., Buff E.M., Perrimon N., Michelson A.M. (1999) Heparan sulfate proteoglycans are essential for FGF receptor signaling during Drosophila embryonic development. Development, 126, 3715–3723. [DOI] [PubMed] [Google Scholar]
- 44.Bulow H.E., Hobert O. (2004) Differential sulfations and epimerization define heparan sulfate specificity in nervous system development. Neuron, 41, 723–736. [DOI] [PubMed] [Google Scholar]
- 45.Chen E., Stringer S.E., Rusch M.A., Selleck S.B., Ekker S.C. (2005) A unique role for 6-O sulfation modification in zebrafish vascular development. Dev. Biol., 284, 364–376. [DOI] [PubMed] [Google Scholar]
- 46.Seko A., Hara-Kuge S., Yamashita K. (2001) Molecular cloning and characterization of a novel human galactose 3-O-sulfotransferase that transfers sulfate to gal beta 1-->3galNAc residue in O-glycans. J. Biol. Chem., 276, 25697–25704. [DOI] [PubMed] [Google Scholar]
- 47.Muzumdar M.D., Tasic B., Miyamichi K., Li L., Luo L. (2007) A global double-fluorescent Cre reporter mouse. Genesis, 45, 593–605. [DOI] [PubMed] [Google Scholar]
- 48.Karner C.M., Chirumamilla R., Aoki S., Igarashi P., Wallingford J.B., Carroll T.J. (2009) Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat. Genet., 41, 793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.