Review of the possible role of Chlamydia pneumoniae infection in the pathogenesis of heart disease.
Keywords: clinical trials, antibiotic, treatment

Review of the possible role of Chlamydia pneumoniae infection in the pathogenesis of heart disease.
We are convinced that Chlamydia pneumoniae research has been unfavorably affected by the negative results of antibiotic treatment trials for the secondary prevention of late-stage coronary heart disease (CHD) (O'Connor et al., 2003; Cannon et al., 2005; Grayston et al., 2005). There is a widespread belief that the clinical trials showed that C. pneumoniae has no role in atherosclerotic disease. This flawed causal inference from these trials has contributed to slowing much-needed research on C. pneumoniae. It has also resulted in a nearly complete loss of momentum for research on the infection-based response to injury hypothesis as a key factor in the initiation and progression of CHD.
Box 1.
Chlamydia pneumoniae had been considered a possible cause of atherosclerosis. That antibiotics failed to prevent secondary coronary events in patients with established coronary artery disease has been erroneously interpreted as ruling out a causative role for C. pneumoniae. This misinterpretation has had a chilling effect on C. pneumoniae research.
Our concern has been confirmed by the sharp drop in published reports (PubMed citations) on C. pneumoniae since 2005. From 1999 to 2005, approximately 375 papers were published each year. There has been a 62% drop from that number with 143 being listed for 2013.
The antibiotic treatment trials were not etiologic studies. No inference regarding the role of C. pneumoniae in the cause of atherosclerosis can properly be made from the trials. A prior publication stated that the study design for these trials precluded proving or disproving a role for C. pneumoniae in the initiation or progression of atherosclerosis, and predicted an overreaction to either negative or positive results (Grayston 2000).
All subjects of these trials had established coronary artery disease with mostly advanced disease. Most had had a myocardial infarction (MI). The trials studied whether antibiotics could prevent secondary coronary events in patients who previously had had a coronary event. A coronary event was defined as an MI, a specified angina episode, surgical intervention or death.
While it was disappointing, it was not surprising that antibiotics given late in the course of atherosclerotic arterial disease did not prevent additional coronary events. The pathogenesis of MI has differences from the pathogenesis of atherosclerosis. In the animal model, antibiotic treatment was effective only when given early after C. pneumoniae inoculation. The animal model treatment studies were published after most of the clinical trials had been initiated.
Authors’ discussion of the lack of progress in C. pneumoniae research
The misinterpretation of the significance of the clinical trial results was already becoming known when the authors first began discussions that eventually led to this manuscript.
These discussions occurred at a remote mountainous ranch in Wyoming in 2007. The purpose of this ‘think tank’ was to discuss the state of C. pneumoniae research and to recommend areas for emphasis in future investigations. The stimulus for the retreat was our concern with the lack of progress in C. pneumoniae research.
A wide variety of research needing attention was discussed. During the second day of the discussions, the group was coalescing around the idea that while there were many individual technical and conceptual problems in C. pneumoniae research, the most important was the potential role of C. pneumoniae infection in atherosclerotic diseases.
The decisions to select this issue as the one for further study and to develop possible approaches for future research were influenced by the enormous importance of atherosclerotic diseases to human health. Atherosclerotic CHD is now the no. 1 killer throughout the industrialized world.
Two major topics
This manuscript will present two major topics: first, a description of the evidence for a potential etiologic association of C. pneumoniae and atherosclerosis, and second, a proposed method that could provide data for an etiologic association.
Infection has been proposed as a causal factor in heart disease for more than 100 years. In the last two decades, there has been increasing research on the role of infection in atherosclerotic cardiovascular disease. The accumulated data associating C. pneumoniae with atherosclerosis are particularly compelling.
C. pneumoniae in atherosclerosis
C. pneumoniae is unique among several microbes that have been associated with arterial disease in having been demonstrated with frequency in atherosclerotic lesions but not in normal arterial tissue (Kuo et al., 1995). The organism was found not only in coronary arteries but also in carotid (Jackson et al., 1997), aortic (Kuo et al., 1993), femoral and popliteal arteries (Kuo et al., 1997). These findings have been confirmed by a number of experienced investigative teams using multiple laboratory techniques, including PCR, microscopy with immunocytochemical stain, in situ DNA hybridization and isolation of the organism (Campbell and Kuo 2004; Watson and Alp 2008). In atheromas, C. pneumoniae is found within smooth muscle cells, macrophages and endothelial-derived foam cells (Kuo et al., 1993; Kuo and Campbell 2000).
In addition to the human studies, animal studies have contributed to the evidence for C. pneumoniae as an infectious cause of CHD. Chlamydia pneumoniae pulmonary infection has been shown to accelerate atherosclerotic disease in mice and rabbits prone to develop the disease due to genetic manipulation or high-fat diets (Muhlestein et al., 1998; Hu, Pierce and Zhong 1999; Moazed et al., 1999). In rabbits fed a regular diet, C. pneumoniae infection resulted in atherosclerotic changes (Fong et al., 1999). In contrast, although repeated infection did not induce atherosclerosis in normolipidemic mice, inflammatory changes were observed in the heart and aorta (Blessing et al., 2000). If a high-fat diet was initiated concurrently with infection in C57BL/6J mice, atherosclerotic lesion progress was accelerated (Blessing et al., 2002a). However, if infections preceded administration of high-fat diet, no augmentation of lesion development was observed. Taken together, these results suggest that in the mouse model C. pneumoniae infection is a co-risk factor with hyperlipidemia.
In human atherosclerotic lesions, although the organism has been cultured only a few times, the organism is frequently detected in mature atherosclerotic lesions by other methods, suggesting persistent infection. Similarly, following repeated pulmonary infection in mice, the organisms can be cultured from the aorta for 1–2 weeks post-infection, but can be detected by other methods in the aorta for 20 weeks post-infection, the endpoint of the experiment (Moazed et al., 1997).
In rabbit models of C. pneumoniae accelerated atherosclerosis, azithromycin prevented the accelerated intimal thickening. However, timing of antibiotic treatment was critical in blocking C. pneumoniae accelerated atherosclerosis. Specifically, initiation of treatment with either clarithromycin or azithromycin within a week after the first of three inoculations with C. pneumoniae was efficacious, while delayed treatment initiated 6 weeks after the first inoculation was not (Fong 2000). Studies done in apoE knockout mice using different treatment regimens of azithromycin (a dose after each of two inoculations or a 6-week course initiated after the third inoculation) did not demonstrate any reduction in C. pneumoniae accelerated atherosclerosis (Rothstein et al., 2001; Blessing et al., 2005). Importantly, following antibiotic treatment in both rabbit and mouse models, C. pneumoniae DNA or antigen was detected in the aorta, suggesting that infection was refractory to treatment (Muhlestein et al., 1998; Rothstein et al., 2001; Fong et al., 2002).
Other biological effects consistent with a role of C. pneumoniae in atherosclerotic processes have been demonstrated in animal models including increased T-cell influx into the atherosclerotic lesion and earlier formation of complex lesions (Ezzahiri et al., 2002), enhanced endothelial dysfunction (Liuba et al., 2000, 2003), apoptosis of endothelial cells and degenerative changes associated with necrosis (Birck et al., 2013), and plaque destabilization as suggested by increased production of matrix metalloproteinases and reduced area of the fibrous cap (Ezzahiri et al., 2003) as well as increased intra-plaque hemorrhage in older mice (Campbell et al., 2010).
The human and animal studies have been used to demonstrate the role of C. pneumoniae infection in atherosclerosis. The question of how C. pneumoniae infection exacerbates atherosclerosis has been addressed in cell culture systems. In the presence of LDL, C. pneumoniae induces foam cell formation and simulates LDL oxidation, through chlamydial LPS and Hsp60, respectively (Kalayoglu and Byrne 1998; Kalayoglu et al., 1999a,b). Scavenger receptors mediate oxidized LDL (oxLDL) uptake (Ross 1993).
For endothelial cells, the lectin-like oxLDL receptor (LOX-1) is the major receptor for uptake of oxLDL (Sawamura et al., 1997; Kume et al., 1998). This scavenger receptor is also found on macrophages and smooth muscle cells (Moriwaki et al., 1998; Aoyama et al., 2000). Expression of LOX-1 is increased in hyperlipidemia and atherosclerotic lesions and activation of LOX-1 results in the up-regulation of pro-atherogenic factors (Kataoka et al., 1999; Chen et al., 2000; Li and Mehta 2000; Li et al., 2003; Zhu et al., 2005). Chlamydia pneumoniae has been shown to bind to the LOX-1 receptor, up-regulate LOX-1 expression, induce the expression of adhesion molecules and matrix metalloproteinases through LOX-1 activation, and promote uptake of ox-LDL (Yoshida et al., 2006; Campbell et al., 2012, 2013). Chlamydia pneumoniae infection of macrophages inhibits the expression of the cholesterol transporters ABCA1 and ABCG1 which play critical roles in cholesterol efflux and homeostasis (Liu et al., 2010; Korhonen et al., 2013; Zhao et al., 2014).
Chlamydia pneumoniae infection of vascular cells also induces the expression of pro-inflammatory cytokines, chemokines and growth factors, all of which could contribute to the chronic inflammatory processes of atherosclerosis (Kaukoranta-Tolvanen et al., 1996; Hu, Pierce and Zhong 1999; Gaydos 2000; Kothe et al., 2000; Netea et al., 2000, 2002; Summersgill et al., 2000; Coombes and Mahony 2001; Blessing et al., 2002b; Mamata et al., 2007; Eitel et al., 2012).
The findings of C. pneumoniae in atherosclerotic lesions provided the impetus for a number of investigators to attempt treatment of CHD with antibiotics known to be effective against C. pneumoniae. Results from three large randomized double blind studies of the effect of antibiotic treatment for secondary prevention of coronary events have been reported (O'Connor et al., 2003; Cannon et al., 2005; Grayston et al., 2005). Two of these studies had prolonged antibiotic treatment for one year (Cannon et al., 2005; Grayston et al., 2005). There were no differences in outcome between the groups given antibiotics or placebos. The overall results from a number of smaller trials were similarly negative (Andraws, Berger and Brown 2005). The significance of the failure of antibiotics to reduce coronary events in patients with established CHD has been discussed above.
Childhood atherosclerosis and C. pneumoniae
It is now well established that both atherosclerosis and C. pneumoniae infection are first seen in early childhood and that the prevalence of both increase with age.
A variety of studies in different populations using different techniques have shown that atherosclerosis begins in childhood. In autopsy studies in Japanese children, evidence of early atherosclerosis, fatty streaks, was found in 29% of aortas in those <1 year old and in 3.1% of coronary arteries of children 1–9 years old (Tanaka et al., 1988). In a US autopsy study of coronary arteries, the prevalence of fatty streaks in the coronary arteries increased with age from 50% at 2–15 years of age to 85% at 21–39 years of age. Raised fibrous-plaque lesions were seen in 8% of children 2–15 years of age and in 69% of adults 26–39 years of age (Berenson et al., 1998). Another study showed lipid-laden macrophages in the intima of the aorta and coronary arteries of young American children killed in motor accidents, with over 50% aged 10–14 years having some evidence of early atherosclerosis (Stary 1989). In the Pathobiological Determinants of Atherosclerosis in Youth study (McGill et al., 2000), in those 15–19 years old at time of death, raised fatty streaks were found in 20% of aortas and 10% of right coronary arteries. The prevalence increased to 40% of aortas and 30% of right coronary arteries showing the lesions by age 30–34.
Box 2.
Studies on the etiology of atherosclerosis must begin early in life:
Atherosclerotic lesions have been found in very young children and the prevalence increases with age.
Chlamydia pneumoniae infection first appears in early childhood and accelerates with school attendance.
In animals, C. pneumoniae pulmonary infection accelerates development of atherosclerotic disease.
Utilizing an intravascular ultrasound technique, it was found that 17% of otherwise healthy heart donors <20 years of age, 37% of those aged 20–29 years, 60% of those aged 30–39 years, 71% of those aged 40–49 years and 85% of those ≥50 years of age had evidence of coronary intimal thickening (Tuzcu et al., 2001).
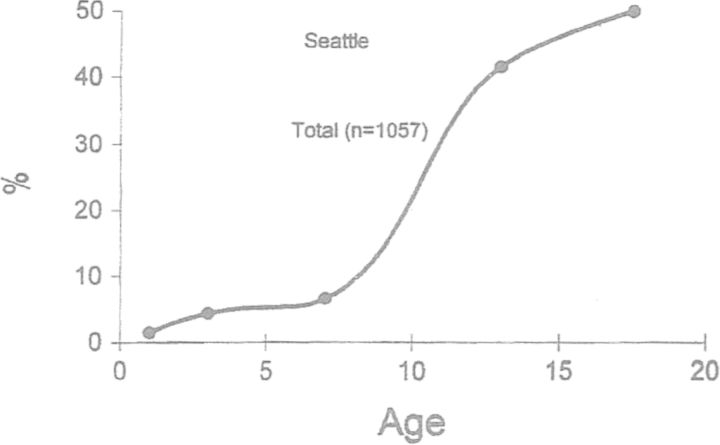
Seroepidemiologic studies with the microimmunofluorescence (MIF) test have provided information on C. pneumoniae infections in populations. Using several different serum banks, data on the prevalence and incidence of C. pneumoniae infections at different ages have been obtained (Aldous et al., 1992; Grayston 1994). The age-specific prevalence of C. pneumoniae-specific IgG antibody has shown a similar curve in several countries. Fig. 1 shows age prevalence in over 1000 Seattle residents below 20 years of age. The greatest increase in prevalence is seen from 5 to 15 years of age. While the prevalence rate is low in children under 5, many of the mild infections in these very young children may not produce long-lasting IgG antibody. This phenomenon has been demonstrated in very young children infected in the eye with C. trachomatis. Often two or three reinfections occur before antibody persists (Grayston et al., 1985).
Figure 1.
Prevalence of C. pneumoniae MIF IgG antibody by age in Seattle children (Grayston 1994).
Sera from a family study in Seattle from 1963 to 1979 (Aldous et al., 1992) were used to determine incidence rates of C. pneumoniae infection based on 4-fold antibody titer rises. High annual rates of antibody rises were seen in the 5–9 year age group (9.2%) and the 10–14 year group (6.2%). These rates are consistent with the increase in prevalence rates from 5–14 years. The available clinical data suggest that many of the antibody conversions occurred in children who were asymptomatic. The symptomatic children usually had cough, a hallmark of C. pneumoniae disease. These incidence rates were confirmed by a study of sera collected longitudinally from children in Sweden. The reported annual MIF antibody conversion rates were 8.0% in 8 to 12 year olds and 5.9% in 12 to 16 year olds (Haidl, Sveger and Persson 1994).
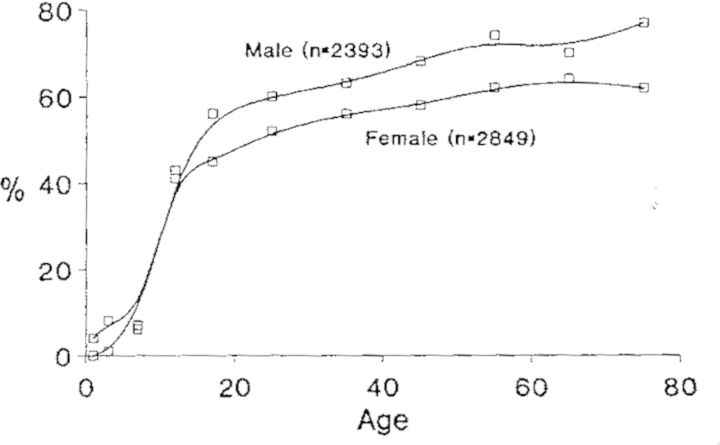
Chlamydia pneumoniae antibody prevalence rates continue to rise throughout adult life reaching 70–80% in the elderly. The rates are approximately the same in both sexes until age 15. Thereafter, the rates are higher in males, Fig. 2. The C. pneumoniae gender prevalence is different from other organisms causing respiratory infections where the prevalence rates are higher in women, presumably because they are in closer contact with children who are often the source of family infection. There is no known reason for C. pneumoniae to be more common in males but it is intriguing that atherosclerotic diseases are more common in males.
Figure 2.
Prevalence of MIF antibody to C. pneumoniae (TWAR) by age in among 5242 persons in Seattle. IgG titers ranged from 8 to 256 (Grayston 1992).
How to demonstrate C. pneumoniae etiology of CHD
We know of no simple ways to prove C. pneumoniae etiology of CHD and have proposed an expensive lengthy observational study supported by laboratory studies. Analogies could be the Framingham Heart Study (Dawber, Kannel and Lyell 1963) and the virus watch studies (Fox 1974). The plan calls for a large-scale natural history study to evaluate a causal link between C. pneumoniae and atherogenesis. This ambitious project is made feasible by recent advances in diagnostics for both early atherosclerosis and C. pneumoniae infection.
Family studies
The use of longitudinal evaluation of families to study the epidemiology of chronic infections, originally championed by Wade Hampton Frost (1880–1938), the first professor of Epidemiology in the United States at Johns Hopkins University. Frost's interests spanned the use of family-based data sets to study the epidemiology of both acute (e.g. influenza) and chronic (e.g. tuberculosis) infections, mainly in the 1920s and 1930s (Daniel 2009). Frost recognized that determinants of risk for individuals exposed to agents that cause chronic infections, as summarized by Fox (Fox 1974), are similar to those for pathogens that cause acute infections. The same three basic facts are required. These are (i) onset data for the first case (index case), (ii) roster of family members present and (iii) dates of successive cases. However, accurate assessment was found to be much more difficult for chronic infections since risk is not concentrated in the relatively brief period after exposure, but rather causal morbidity and mortality may occur at any subsequent time in the life of the infected individual. Frost found that two major values of family-based epidemiologic studies include developing reliable estimates for the risk of transmission of specific infectious agents, and the capacity to follow the natural history of specific pathogens. The latter proved to be especially valuable for chronic infections where most exposed individuals remained asymptomatic (Fox 1974). The so-called ‘virus watch’ method that was adopted by family-based epidemiologists who followed Frost generally assumes that most infected individuals will be subclinical and therefore the emphasis of sampling is on the occurrence of infection rather than the development of disease, especially acute disease.
The prototype for this sort of study is the polio virus watch investigation carried out by Fox and colleagues in Louisiana from 1953 to 1957, where it was established that subclinical infection far exceeded progression to paralytic disease (Fox et al., 1957; Gelfand et al., 1957). The polio virus watch study, which involved multiple sample collections for virus isolation in family members, antibody seroconversion measurements and verification of disease status in each household, was reproduced in a number of other family-based epidemiologic studies to gain insights into influenza, adenovirus, mycoplasma and tuberculosis infections (Fox 1974). The impact of the virus watch programs was extremely helpful in defining epidemiologic patterns for a number of acute and chronic viral and bacterial diseases. Similar, albeit far earlier, epidemiologic surveys were used over the course of 30–40 years to establish links between streptococcal pharyngitis, rheumatic fever and rheumatic heart disease, despite great initial resistance to seriously considering an infectious agent contributing to this very large public health problem in children (Benedek 2006), a condition now that is effectively avoided by prompt treatment of streptococcal pharyngitis (Guzmann-Cottrill et al., 2004).
The natural history of atherosclerotic cardiovascular disease has its genesis early in life. Carotid artery intima-media thickness in children and young adults, coupled with other risk factors (e.g. LDL cholesterol, elevated body mass index), predict atherosclerosis in adulthood (Juonala et al., 2010). Given the known involvement of infectious agents in chronic inflammatory events, and the existing associations between C. pneumoniae respiratory infections and atherosclerotic diseases, it should be possible to utilize the family-based epidemiologic methodologies to add to our knowledge on the role of C. pneumoniae infections in cardiovascular disease.
Non-invasive measures of atherosclerosis
Basic cardiovascular research has led to a greater understanding of pathological processes underlying atherosclerosis, from initiation to progression of arterial plaques. Imaging techniques have also advanced beyond their use in merely defining anatomic features, and may now permit research efforts to probe cellular interactions, metabolic pathways and functional outcomes in the causal pathway of atherosclerosis and plaque rupture.
The thickness of the carotid artery intima measured by high-resolution ultrasonography may aid in detecting early vascular changes of atherosclerosis (Osika et al., 2007). The use of computed tomography (CT) has limited use in children because of the radiation exposure. Magnetic resonance imaging (MRI) is increasingly used to assess early atherosclerosis, since it allows characterization of cardiovascular structure, function and blood flow without exposure to ionizing radiation. It has been used to measure atheromatous plaques and characterize structure in the peripheral circulation. Both CT and MRI are currently being combined with positron emission tomography and fluorescent imaging to evaluate plaque biology in more detail (Sosnovik, Nahrendorf and Weissleder 2007). Arterial stiffness (atherosclerosis) can be assessed noninvasively by measuring a parameter known as the pulse wave velocity (PWV) between two major arteries located in the upper body (i.e. carotid or brachial artery) and the lower body (i.e. femoral or ankle). PWV reflects the time needed for the pulse wave to travel a given distance along the blood vessel. Stiffer arteries produce a higher PWV (Alpert and Collins 2007). PWV may have an advantage compared to other non-invasive techniques due to its simplicity of use and shorter time required for a measurement. A recent review (Camici et al., 2012) summarized the published literature reporting on advanced imaging techniques that have great potential for broad application in clinical practice. Studies will be needed to further prospectively evaluate these techniques. However, one can imagine that soon high-resolution methods will be compatible for use in longitudinal studies of atherosclerosis and underlying etiology.
Diagnosing C. pneumoniae infection
Although efforts have been made to standardize the detection of C. pneumoniae infection (Dowell et al., 2001), there is still lack of consensus on methods for differentiating acute versus chronic/persistent infection with C. pneumoniae. Nevertheless, past research efforts have provided sufficient information to choose and optimize diagnostic approaches suitable for monitoring C. pneumoniae infection for a family surveillance study. A list of approaches that can be considered for diagnosing C. pneumoniae airway infection is provided below.
PCR-based detection of C. pneumoniae-unique DNA sequences: this approach is both sensitive and specific (Al-Marzooq et al., 2011; Cho et al., 2012; Diaz and Winchell 2012). It can also be quantitative, which will allow infection burden to be correlated with clinical phenotypes.
Live C. pneumoniae organism isolation: although it is highly specific and can also provide materials for further characterization studies, the success rate is low lacking the required sensitivity as a routine screening tool (She et al., 2010). Improvement of isolation methods is needed.
Immunolabeling-based detection of C. pneumoniae antigen: besides the choice of target antigens and selection of antibodies, the assay platforms can also significantly affect both the detection specificity and sensitivity. Until they are improved, these assays will likely generate highly variable results and not be suitable for the proposed surveillance study.
Detection of C. pneumoniae-induced host responses including cytokines, antibodies and T cells: although C. pneumoniae is known to induce inflammatory cytokines via activating innate immunity receptor-mediated pathways, detection of these cytokines cannot be used for diagnosing C. pneumoniae infection due to the lack of specificity of the innate immunity receptors for recognizing C. pneumoniae. Only the host immune responses mediated by C. pneumoniae epitope-specific immune receptors can be used for indicating C. pneumoniae infection. However, due to the long half-life of immune molecules and memory lymphocytes, the immune response detection-based assays can only be used to indicate whether the subjects have ever been infected with C. pneumoniae. T-cell responses are more difficult to measure and also require more blood samples. Thus, T-cell responses are not suitable for routine screening for C. pneumoniae infection. However, C. pneumoniae epitope-specific T-cell responses can be measured for investigating the roles of T-cell responses in C. pneumoniae pathogenesis.
Next-generation sequencing (NGS) (van Dijk et al., 2014) can be used for identifying co-infection agents and profiling microbiome in the airway (Goodrich et al., 2014). NGS can be used to target either the variable regions of 16s RNA genes (Langille et al., 2013) or cover the entire microbial genomes (Song, Jarvie and Hattori 2013) for both identifying microbial species and quantitating the relative amounts of each species in a given sample.
For screening participating families: nasopharyngeal swabs and peripheral blood mononuclear cells (PBMC) can be used for monitoring acute C. pneumoniae infection by detecting C. pneumoniae DNA and for profiling the microbiota and co-infection agents by using NGS. Since the microbiota and co-infection agents in the airway likely affect the pathogenicity of C. pneumoniae, it is important to simultaneously monitor the microbial species co-existing in the nasopharyngeal and peripheral blood cells. At the same time, the plasma samples left from the above preparation of PBMC will be used for determining C. pneumoniae exposure status. Both the overall titers and antigen specificities of C. pneumoniae-specific antibodies will be determined. The latter can be carried out as described for mapping C. trachomatis antibody specificities (Wang et al., 2010). These measurements together will establish a baseline for each study subject family.
During follow-up, the same samples will be collected periodically (as determined for observing clinical phenotypes) for monitoring both C. pneumoniae infection and the microbiota and host antibody responses.
These laboratory-measured parameters will be correlated with clinical responses observed in the same study subjects/families.
Correlation of C. pneumoniae infection rate and burden with clinical diseases will allow identification of disease with C. pneumoniae infection.
Correlation of airway microbiota profiles and co-infection status with clinical diseases will allow us to map airway microbial species associated with clinical diseases or lack of diseases.
Correlation of anti-C. pneumoniae antibody titers and antigen-specificity profiles with clinical diseases will allow us to identify both pathogenic and protective antigens as has been done for C. trachomatis-infected women (Budrys et al., 2012).
Correlation between the above three parameters will allow investigation into the impact of the airway microbiota and co-infection on C. pneumoniae infection and pathogenicity, and on host responses to C. pneumoniae infection.
CONCLUSIONS
A family study could be designed that would provide information about atherosclerotic disease status among family members and the prevalence of C. pneumoniae in nasopharyngeal swabs and PBMC, as well as changing titers of host humoral and cellular responses to C. pneumoniae antigens. Advances in Chlamydia proteomics and genomics offer the promise of improved diagnostic and immunologic tests to better define infection status (primary, secondary, persistent) (Bunk et al., 2008). Clearly, new technologies involving highly sensitive and specific sequencing methods may be brought to bear on differential gene expression patterns associated with ongoing productive infection and C. pneumoniae persistence. These technologies associated with advances in vascular imaging may further help define those who suffer from infection-provoked vascular disease. Given the remarkable advances in non-invasive vascular imaging technologies, it would also be possible to obtain information on the initiation and progression of atherosclerotic lesions in large- and medium-sized arteries. Simultaneous longitudinal data on vessel health and infection status would provide insight into a possible etiologic association (Bunk et al., 2008). The plan would encourage evaluation of many other potential etiologic factors (e.g. obesity, hypercholesterolemia and other microbes).
For C. pneumoniae research, a number of applied and basic benefits would be derived from such a program. These include the opportunity to develop and implement new diagnostic tests, the creation of much needed collections of C. pneumoniae isolates for genomic studies, improved understanding of the relationship between immune responses and disease pathogenesis, mapping C. pneumoniae antigens associated with protective immunity vs. pathological responses, and identification of genetic determinants associated with infection susceptibility and cardiovascular health.
The recent recommendations of the American Academy of Pediatrics to test blood cholesterol and in some circumstances to undertake cholesterol lowering treatment of young children emphasize the early age for prevention of heart disease (Volanen et al., 2006). The proposed family study could include in its design studies that would add scientific rigor to the pediatric recommendations.
CHD is the leading cause of death in the industrialized world. Current patient management including life-style changes has reduced CHD deaths and improved quality of life for millions, but there is no cure for the disease. It is critical that the cause(s) of early atherosclerotic changes be determined, to provide an opportunity for prevention or cure. Both atherosclerosis and evidence of prior C. pneumoniae infection are found in most adults. If C. pneumoniae infection or other microbes are found to be closely associated with the initiation and development of CHD, a targeted antibiotic treatment or preferably a preventive vaccine could be tested. Given the magnitude of the problem, research to define the role of infection in heart disease should rightfully be a top-tier national health priority. Studies such as a Family Heart Watch Program, despite the high cost, should be undertaken.
Acknowledgments
This paper is dedicated to the memory of Walter Stamm, our friend and colleague. The authors thank Charles Knirsch and Lee Ann Campbell for assistance with this manuscript.
Footnotes
This article is expanded upon a similarly titled ‘abstract’ by the same authors published in the Proceedings of the Thirteenth International Symposium on Human Chlamydial Infections, held at Asilomar Conference Grounds, Pacific Grove, CA, June 22–27, 2014 (Publisher: International Chlamydia Symposium, San Francisco, CA 94110, USA; editors: Schachter, Byrne, Chernesky et al., 2014, pp. 507–513).
Deceased.
FUNDING
The retreat was supported in part by non-categorical research funds from the University of Washington.
Conflict of interest statement. None declared.
REFERENCES
- Al-Marzooq F, Imad MA, How SH, et al. Development of multiplex real-time PCR for the rapid detection of five bacterial causes of community acquired pneumonia. Trop Biomed. 2011;28:545–56. [PubMed] [Google Scholar]
- Aldous MB, Grayston JT, Wang SP, et al. Seroepidemiology of Chlamydia pneumoniae TWAR infection in Seattle families, 1966–1979. J Infect Dis. 1992;166:646–9. doi: 10.1093/infdis/166.3.646. [DOI] [PubMed] [Google Scholar]
- Alpert BS, Collins RT. Assessment of vascular function: pulse wave velocity. J Pediatr. 2007;150:219–20. doi: 10.1016/j.jpeds.2006.12.042. [DOI] [PubMed] [Google Scholar]
- Andraws R, Berger JS, Brown DL. Effects of antibiotic therapy on outcomes of patients with coronary artery disease: a meta-analysis of randomized controlled trials. JAMA. 2005;293:2641–7. doi: 10.1001/jama.293.21.2641. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Chen M, Fujiwara H, et al. LOX-1 mediates lysophosphatidylcholine-induced oxidized LDL uptake in smooth muscle cells. FEBS Lett. 2000;467:217–20. doi: 10.1016/s0014-5793(00)01154-6. [DOI] [PubMed] [Google Scholar]
- Benedek TG. The history of bacteriologic concepts of rheumatic fever and rheumatoid arthritis. Sem Arthritis Rheu. 2006;36:109–23. doi: 10.1016/j.semarthrit.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Berenson GS, Srinivasan SR, Bao W, et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. New Engl J Med. 1998;338:1650–6. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- Birck MM, Saraste A, Hyttel P, et al. Endothelial cell death and intimal foam cell accumulation in the coronary artery of infected hypercholesterolemic minipigs. J Cardiovasc Transl Res. 2013;6:579–87. doi: 10.1007/s12265-013-9463-2. [DOI] [PubMed] [Google Scholar]
- Blessing E, Campbell LA, Rosenfeld ME, et al. A 6 week course of azithromycin treatment has no beneficial effect on atherosclerotic lesion development in apolipoprotein E-deficient mice chronically infected with Chlamydia pneumoniae. J Antimicrob Chemoth. 2005;55:1037–40. doi: 10.1093/jac/dki128. [DOI] [PubMed] [Google Scholar]
- Blessing E, Campbell LA, Rosenfeld ME, et al. Chlamydia pneumoniae and hyperlipidemia are co-risk factors for atherosclerosis: infection prior to induction of hyperlipidemia does not accelerate development of atherosclerotic lesions in C57BL/6J mice. Infect Immun. 2002a;70:5332–4. doi: 10.1128/IAI.70.9.5332-5334.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing E, Kuo CC, Lin TM, et al. Foam cell formation inhibits growth of Chlamydia pneumoniae but does not attenuate Chlamydia pneumoniae-induced secretion of proinflammatory cytokines. Circulation. 2002b;105:1976–82. doi: 10.1161/01.cir.0000015062.41860.5b. [DOI] [PubMed] [Google Scholar]
- Blessing E, Lin TM, Campbell LA, et al. Chlamydia pneumoniae induces inflammatory changes in the heart and aorta of normocholesterolemic C57BL/6J mice. Infect Immun. 2000;68:4765–8. doi: 10.1128/iai.68.8.4765-4768.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budrys NM, Gong S, Rodgers AK, et al. Chlamydia trachomatis antigens recognized in women with tubal factor infertility, normal fertility, and acute infection. Obstet Gynecol. 2012;119:1009–16. doi: 10.1097/AOG.0b013e3182519326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunk S, Susnea I, Rupp J, et al. Immunoproteomic identification and serological responses to novel Chlamydia pneumoniae antigens that are associated with persistent C. pneumoniae infections. J Immunol. 2008;180:5490–8. doi: 10.4049/jimmunol.180.8.5490. [DOI] [PubMed] [Google Scholar]
- Camici PG, Rimoldi OE, Gaemperli O, et al. Non-invasive anatomic and functional imaging of vascular inflammation and unstable plaque. Eur Heart J. 2012;33:1309–17. doi: 10.1093/eurheartj/ehs067. [DOI] [PubMed] [Google Scholar]
- Campbell LA, Kuo CC. Chlamydia pneumoniae–an infectious risk factor for atherosclerosis? Nat Rev Microbiol. 2004;2:23–32. doi: 10.1038/nrmicro796. [DOI] [PubMed] [Google Scholar]
- Campbell LA, Lee AW, Rosenfeld ME, et al. Chlamydia pneumoniae induces expression of pro-atherogenic factors through activation of the lectin-like oxidized LDL receptor-1. Pathog Dis. 2013 doi: 10.1111/2049-632X.12058. doi:10.1111/2049-632X.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LA, Puolakkainen M, Lee A, et al. Chlamydia pneumoniae binds to the lectin-like oxidized LDL receptor for infection of endothelial cells. Microbes Infect. 2012;14:43–9. doi: 10.1016/j.micinf.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LA, Yaraei K, Van Lenten B, et al. The acute phase reactant response to respiratory infection with Chlamydia pneumoniae: implications for the pathogenesis of atherosclerosis. Microbes Infect. 2010;12:598–606. doi: 10.1016/j.micinf.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon CP, Braunwald E, McCabe CH, et al. Antibiotic treatment of Chlamydia pneumoniae after acute coronary syndrome. New Engl J Med. 2005;352:1646–54. doi: 10.1056/NEJMoa043528. [DOI] [PubMed] [Google Scholar]
- Chen M, Kakutani M, Minami M, et al. Increased expression of lectin-like oxidized low density lipoprotein receptor-1 in initial atherosclerotic lesions of Watanabe heritable hyperlipidemic rabbits. Arterioscl Throm Vas Biol. 2000;20:1107–15. doi: 10.1161/01.atv.20.4.1107. [DOI] [PubMed] [Google Scholar]
- Cho MC, Kim H, An D, et al. Comparison of sputum and nasopharyngeal swab specimens for molecular diagnosis of Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella pneumophila. Ann Lab Med. 2012;32:133–8. doi: 10.3343/alm.2012.32.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes BK, Mahony JB. cDNA array analysis of altered gene expression in human endothelial cells in response to Chlamydia pneumoniae infection. Infect Immun. 2001;69:1420–7. doi: 10.1128/IAI.69.3.1420-1427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel TM. Wade Hampton Frost and the index case concept. Int J Tuberc Lung D. 2009;13:1345–6. [PubMed] [Google Scholar]
- Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann NY Acad Sci. 1963;107:539–56. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- Diaz MH, Winchell JM. Detection of Mycoplasma pneumoniae and Chlamydophila pneumoniae directly from respiratory clinical specimens using a rapid real-time polymerase chain reaction assay. Diag Micr Infec Dis. 2012;73:278–80. doi: 10.1016/j.diagmicrobio.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Dowell SF, Peeling RW, Boman J, et al. Standardizing Chlamydia pneumoniae assays: recommendations from the Centers for Disease Control and Prevention (USA) and the Laboratory Centre for Disease Control (Canada) Clin Infect Dis. 2001;33:492–503. doi: 10.1086/322632. [DOI] [PubMed] [Google Scholar]
- Eitel J, Meixenberger K, van Laak C, et al. Rac1 regulates the NLRP3 inflammasome which mediates IL-1beta production in Chlamydophila pneumoniae infected human mononuclear cells. PLoS One. 2012;7:e30379. doi: 10.1371/journal.pone.0030379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzahiri R, Stassen FR, Kurvers HA, et al. Chlamydia pneumoniae infection induces an unstable atherosclerotic plaque phenotype in LDL-receptor, ApoE double knockout mice. Eur J Vasc Endovasc. 2003;26:88–95. doi: 10.1053/ejvs.2002.1913. [DOI] [PubMed] [Google Scholar]
- Ezzahiri R, Nelissen-Vrancken HJ, Kurvers HA, et al. Chlamydophila pneumoniae (Chlamydia pneumoniae) accelerates the formation of complex atherosclerotic lesions in Apo E3-Leiden mice. Cardiovasc Res. 2002;56:269–76. doi: 10.1016/s0008-6363(02)00544-8. [DOI] [PubMed] [Google Scholar]
- Fong IW. Antibiotics effects in a rabbit model of Chlamydia pneumoniae-induced atherosclerosis. J Infect Dis. 2000;181:S514–8. doi: 10.1086/315607. Suppl 3. [DOI] [PubMed] [Google Scholar]
- Fong IW, Chiu B, Viira E, et al. De Novo induction of atherosclerosis by Chlamydia pneumoniae in a rabbit model. Infect Immun. 1999;67:6048–55. doi: 10.1128/iai.67.11.6048-6055.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong IW, Chiu B, Viira E, et al. Influence of clarithromycin on early atherosclerotic lesions after Chlamydia pneumoniae infection in a rabbit model. Antimicrob Agents Ch. 2002;46:2321–6. doi: 10.1128/AAC.46.8.2321-2326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JP. Family-based epidemiologic studies. The second Wade Hampton Frost Lecture. Am J Epidemiol. 1974;99:165–79. doi: 10.1093/oxfordjournals.aje.a121600. [DOI] [PubMed] [Google Scholar]
- Fox JP, Gelfand HM, Leblanc DR, et al. Studies on the development of natural immunity to poliomyelitis in Louisiana. I. Over-all plan, methods and observations as to patterns of seroimmunity in the study group. Am J Hyg. 1957;65:344–66. doi: 10.1093/oxfordjournals.aje.a119874. [DOI] [PubMed] [Google Scholar]
- Gaydos CA. Growth in vascular cells and cytokine production by Chlamydia pneumoniae. J Infect Dis. 2000;181:S473–8. doi: 10.1086/315612. Suppl 3. [DOI] [PubMed] [Google Scholar]
- Gelfand HM, Leblanc DR, Fox JP, et al. Studies on the development of natural immunity to poliomyelitis in Louisiana. II. Description and analysis of episodes of infection observed in study group households. Am J Hyg. 1957;65:367–85. doi: 10.1093/oxfordjournals.aje.a119876. [DOI] [PubMed] [Google Scholar]
- Goodrich JK, Di Rienzi SC, Poole AC, et al. Conducting a microbiome study. Cell. 2014;158:250–62. doi: 10.1016/j.cell.2014.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayston JT. Infections caused by C. pneumoniae strain TWAR. Clin Infect Dis. 1992;15:757–61. doi: 10.1093/clind/15.5.757. [DOI] [PubMed] [Google Scholar]
- Grayston JT. Chlamydia pneumoniae (TWAR) infections in children. Pediatr Infect Dis J. 1994;13:675–84. doi: 10.1097/00006454-199408000-00001. [DOI] [PubMed] [Google Scholar]
- Grayston JT. What is needed to prove that Chlamydia pneumoniae does, or does not, play an etiologic role in atherosclerosis? J Infect Dis. 2000;181:S585–6. doi: 10.1086/315595. Suppl 3. [DOI] [PubMed] [Google Scholar]
- Grayston JT, Kronmal RA, Jackson LA, et al. Azithromycin for the secondary prevention of coronary events. New Engl J Med. 2005;352:1637–45. doi: 10.1056/NEJMoa043526. [DOI] [PubMed] [Google Scholar]
- Grayston JT, Wang SP, Yeh LJ, et al. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985;7:717–25. doi: 10.1093/clinids/7.6.717. [DOI] [PubMed] [Google Scholar]
- Guzmann-Cottrill J, Jaggi P, Shulman S. Acute rheumatic fever: clinical aspects and insights into pathogenesis and prevention. Clin Appl Immunol Rev. 2004;4:263–76. [Google Scholar]
- Haidl S, Sveger T, Persson K. In: Longitudinal Pattern of Antibodies to Chlamydia Pneumoniae in Children. Orfila J, Byrne GI, Chernesky MA, et al., editors. Bologna, Italy: Societa Editrice Esculapio; 1994. pp. 189–92. [Google Scholar]
- Hu H, Pierce GN, Zhong G. The atherogenic effects of chlamydia are dependent on serum cholesterol and specific to Chlamydia pneumoniae. J Clin Invest. 1999;103:747–53. doi: 10.1172/JCI4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LA, Campbell LA, Kuo CC, et al. Isolation of Chlamydia pneumoniae from a carotid endarterectomy specimen. J Infect Dis. 1997;176:292–5. doi: 10.1086/517270. [DOI] [PubMed] [Google Scholar]
- Juonala M, Magnussen CG, Venn A, et al. Influence of age on associations between childhood risk factors and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study, the Childhood Determinants of Adult Health Study, the Bogalusa Heart Study, and the Muscatine Study for the International Childhood Cardiovascular Cohort (i3C) Consortium. Circulation. 2010;122:2514–20. doi: 10.1161/CIRCULATIONAHA.110.966465. [DOI] [PubMed] [Google Scholar]
- Kalayoglu MV, Byrne GI. Induction of macrophage foam cell formation by Chlamydia pneumoniae. J Infect Dis. 1998;177:725–9. doi: 10.1086/514241. [DOI] [PubMed] [Google Scholar]
- Kalayoglu MV, Hoerneman B, LaVerda D, et al. Cellular oxidation of low-density lipoprotein by Chlamydia pneumoniae. J Infect Dis. 1999a;180:780–90. doi: 10.1086/314931. [DOI] [PubMed] [Google Scholar]
- Kalayoglu MV, Miranpuri GS, Golenbock DT, et al. Characterization of low-density lipoprotein uptake by murine macrophages exposed to Chlamydia pneumoniae. Microbes Infect. 1999b;1:409–18. doi: 10.1016/s1286-4579(99)80044-6. [DOI] [PubMed] [Google Scholar]
- Kataoka H, Kume N, Miyamoto S, et al. Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation. 1999;99:3110–7. doi: 10.1161/01.cir.99.24.3110. [DOI] [PubMed] [Google Scholar]
- Kaukoranta-Tolvanen SS, Teppo AM, Laitinen K, et al. Growth of Chlamydia pneumoniae in cultured human peripheral blood mononuclear cells and induction of a cytokine response. Microb Pathogenesis. 1996;21:215–21. doi: 10.1006/mpat.1996.0056. [DOI] [PubMed] [Google Scholar]
- Korhonen JT, Olkkonen VM, Lahesmaa R, et al. ABC-cassette transporter 1 (ABCA1) expression in epithelial cells in Chlamydia pneumoniae infection. Microb Pathogenesis. 2013;61–62:57–61. doi: 10.1016/j.micpath.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Kothe H, Dalhoff K, Rupp J, et al. Hydroxymethylglutaryl coenzyme A reductase inhibitors modify the inflammatory response of human macrophages and endothelial cells infected with Chlamydia pneumoniae. Circulation. 2000;101:1760–3. doi: 10.1161/01.cir.101.15.1760. [DOI] [PubMed] [Google Scholar]
- Kume N, Murase T, Moriwaki H, et al. Inducible expression of lectin-like oxidized LDL receptor-1 in vascular endothelial cells. Circ Res. 1998;83:322–7. doi: 10.1161/01.res.83.3.322. [DOI] [PubMed] [Google Scholar]
- Kuo C, Campbell LA. Detection of Chlamydia pneumoniae in arterial tissues. J Infect Dis. 2000;181:S432–6. doi: 10.1086/315615. Suppl 3. [DOI] [PubMed] [Google Scholar]
- Kuo CC, Coulson AS, Campbell LA, et al. Detection of Chlamydia pneumoniae in atherosclerotic plaques in the walls of arteries of lower extremities from patients undergoing bypass operation for arterial obstruction. J Vasc Surg. 1997;26:29–31. doi: 10.1016/s0741-5214(97)70143-5. [DOI] [PubMed] [Google Scholar]
- Kuo CC, Gown AM, Benditt EP, et al. Detection of Chlamydia pneumoniae in aortic lesions of atherosclerosis by immunocytochemical stain. Arterioscl Throm. 1993;13:1501–4. doi: 10.1161/01.atv.13.10.1501. [DOI] [PubMed] [Google Scholar]
- Kuo CC, Grayston JT, Campbell LA, et al. Chlamydia pneumoniae (TWAR) in coronary arteries of young adults (15–34 years old) P Natl Acad Sci USA. 1995;92:6911–4. doi: 10.1073/pnas.92.15.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langille MG, Zaneveld J, Caporaso JG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814–21. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Liu L, Chen H, et al. LOX-1 mediates oxidized low-density lipoprotein-induced expression of matrix metalloproteinases in human coronary artery endothelial cells. Circulation. 2003;107:612–7. doi: 10.1161/01.cir.0000047276.52039.fb. [DOI] [PubMed] [Google Scholar]
- Li D, Mehta JL. Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation. 2000;101:2889–95. doi: 10.1161/01.cir.101.25.2889. [DOI] [PubMed] [Google Scholar]
- Liu W, He P, Cheng B, et al. Chlamydia pneumoniae disturbs cholesterol homeostasis in human THP-1 macrophages via JNK-PPARgamma dependent signal transduction pathways. Microbes Infect. 2010;12:1226–35. doi: 10.1016/j.micinf.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Liuba P, Karnani P, Pesonen E, et al. Endothelial dysfunction after repeated Chlamydia pneumoniae infection in apolipoprotein E-knockout mice. Circulation. 2000;102:1039–44. doi: 10.1161/01.cir.102.9.1039. [DOI] [PubMed] [Google Scholar]
- Liuba P, Pesonen E, Paakkari I, et al. Acute Chlamydia pneumoniae infection causes coronary endothelial dysfunction in pigs. Atherosclerosis. 2003;167:215–22. doi: 10.1016/s0021-9150(03)00019-4. [DOI] [PubMed] [Google Scholar]
- McGill HC. McMahan CA, Jr, Zieske AW, et al. Associations of coronary heart disease risk factors with the intermediate lesion of atherosclerosis in youth. The Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscl Throm Vas. 2000;20:1998–2004. doi: 10.1161/01.atv.20.8.1998. [DOI] [PubMed] [Google Scholar]
- Mamata Y, Hakki A, Newton C, et al. Differential effects of Chlamydia pneumoniae infection on cytokine levels in human T lymphocyte- and monocyte-derived cell cultures. Int J Med Microbiol. 2007;297:109–15. doi: 10.1016/j.ijmm.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Moazed TC, Campbell LA, Rosenfeld ME, et al. Chlamydia pneumoniae infection accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. J Infect Dis. 1999;180:238–41. doi: 10.1086/314855. [DOI] [PubMed] [Google Scholar]
- Moazed TC, Kuo C, Grayston JT, et al. Murine models of Chlamydia pneumoniae infection and atherosclerosis. J Infect Dis. 1997;175:883–90. doi: 10.1086/513986. [DOI] [PubMed] [Google Scholar]
- Moriwaki H, Kume N, Kataoka H, et al. Expression of lectin-like oxidized low density lipoprotein receptor-1 in human and murine macrophages: upregulated expression by TNF-alpha. FEBS Lett. 1998;440:29–32. doi: 10.1016/s0014-5793(98)01414-8. [DOI] [PubMed] [Google Scholar]
- Muhlestein JB, Anderson JL, Hammond EH, et al. Infection with Chlamydia pneumoniae accelerates the development of atherosclerosis and treatment with azithromycin prevents it in a rabbit model. Circulation. 1998;97:633–6. doi: 10.1161/01.cir.97.7.633. [DOI] [PubMed] [Google Scholar]
- Netea MG, Kullberg BJ, Galama JM, et al. Non-LPS components of Chlamydia pneumoniae stimulate cytokine production through Toll-like receptor 2-dependent pathways. Eur J Immunol. 2002;32:1188–95. doi: 10.1002/1521-4141(200204)32:4<1188::AID-IMMU1188>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Netea MG, Selzman CH, Kullberg BJ, et al. Acellular components of Chlamydia pneumoniae stimulate cytokine production in human blood mononuclear cells. Eur J Immunol. 2000;30:541–9. doi: 10.1002/1521-4141(200002)30:2<541::AID-IMMU541>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- O'Connor CM, Dunne MW, Pfeffer MA, et al. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459–66. doi: 10.1001/jama.290.11.1459. [DOI] [PubMed] [Google Scholar]
- Osika W, Dangardt F, Gronros J, et al. Increasing peripheral artery intima thickness from childhood to seniority. Arterioscl Throm Vas. 2007;27:671–6. doi: 10.1161/01.ATV.0000256468.95403.6f. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Rothstein NM, Quinn TC, Madico G, et al. Effect of azithromycin on murine arteriosclerosis exacerbated by Chlamydia pneumoniae. J Infect Dis. 2001;183:232–8. doi: 10.1086/317941. [DOI] [PubMed] [Google Scholar]
- Sawamura T, Kume N, Aoyama T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–7. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- She RC, Thurber A, Hymas WC, et al. Limited utility of culture for Mycoplasma pneumoniae and Chlamydophila pneumoniae for diagnosis of respiratory tract infections. J Clin Microbiol. 2010;48:3380–2. doi: 10.1128/JCM.00321-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Jarvie T, Hattori M. Our second genome-human metagenome: how next-generation sequencer changes our life through microbiology. Adv Microb Physiol. 2013;62:119–44. doi: 10.1016/B978-0-12-410515-7.00003-2. [DOI] [PubMed] [Google Scholar]
- Sosnovik DE, Nahrendorf M, Weissleder R. Molecular magnetic resonance imaging in cardiovascular medicine. Circulation. 2007;115:2076–86. doi: 10.1161/CIRCULATIONAHA.106.658930. [DOI] [PubMed] [Google Scholar]
- Stary HC. Evolution and progression of atherosclerotic lesions in coronary arteries of children and young adults. Arteriosclerosis. 1989;9:I19–32. [PubMed] [Google Scholar]
- Summersgill JT, Molestina RE, Miller RD, et al. Interactions of Chlamydia pneumoniae with human endothelial cells. J Infect Dis. 2000;181:S479–82. doi: 10.1086/315620. Suppl 3. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Masuda J, Imamura T, et al. A nation-wide study of atherosclerosis in infants, children and young adults in Japan. Atherosclerosis. 1988;72:143–56. doi: 10.1016/0021-9150(88)90075-5. [DOI] [PubMed] [Google Scholar]
- Tuzcu EM, Kapadia SR, Tutar E, et al. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation. 2001;103:2705–10. doi: 10.1161/01.cir.103.22.2705. [DOI] [PubMed] [Google Scholar]
- van Dijk EL, Auger H, Jaszczyszyn Y, et al. Ten years of next-generation sequencing technology. Trends Genet. 2014;30:418–26. doi: 10.1016/j.tig.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Volanen I, Jarvisalo MJ, Vainionpaa R, et al. Increased aortic intima-media thickness in 11-year-old healthy children with persistent Chlamydia pneumoniae seropositivity. Arterioscl Throm Vas. 2006;26:649–55. doi: 10.1161/01.ATV.0000202664.76816.bb. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang Y, Lu C, et al. A genome-wide profiling of the humoral immune response to Chlamydia trachomatis infection reveals vaccine candidate antigens expressed in humans. J Immunol. 2010;185:1670–80. doi: 10.4049/jimmunol.1001240. [DOI] [PubMed] [Google Scholar]
- Watson C, Alp NJ. Role of Chlamydia pneumoniae in atherosclerosis. Clin Sci. 2008;114:509–31. doi: 10.1042/CS20070298. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Koide N, Mori I, et al. Chlamydia pneumoniae infection enhances lectin-like oxidized low-density lipoprotein receptor (LOX-1) expression on human endothelial cells. FEMS Microbiol Lett. 2006;260:17–22. doi: 10.1111/j.1574-6968.2006.00286.x. [DOI] [PubMed] [Google Scholar]
- Zhao GJ, Mo ZC, Tang SL, et al. Chlamydia pneumoniae negatively regulates ABCA1 expression via TLR2-Nuclear factor-kappa B and miR-33 pathways in THP-1 macrophage-derived foam cells. Atherosclerosis. 2014;235:519–25. doi: 10.1016/j.atherosclerosis.2014.05.943. [DOI] [PubMed] [Google Scholar]
- Zhu H, Xia M, Hou M, et al. Ox-LDL plays dual effect in modulating expression of inflammatory molecules through LOX-1 pathway in human umbilical vein endothelial cells. Front Biosci. 2005;10:2585–94. doi: 10.2741/1722. [DOI] [PubMed] [Google Scholar]