Abstract
Background For the elderly, the association between waist circumference (WC) and mortality considering body mass index (BMI) remains unclear, and thereby also the evidence base for using these anthropometric measures in clinical practice. This meta-analysis examined the association between WC categories and (cause-specific) mortality within BMI categories. Furthermore, the association of continuous WC with lowest and increased mortality risks was examined.
Methods Age- and smoking-adjusted relative risks (RRs) of mortality associated with WC–BMI categories and continuous WC (including WC and WC2) were calculated by the investigators and pooled by means of random-effects models.
Results During a 5-year-follow-up of 32 678 men and 25 931 women, we ascertained 3318 and 1480 deaths, respectively. A large WC (men: ≥102 cm, women: ≥88 cm) was associated with increased all-cause mortality RRs for those in the ‘healthy’ weight {1.7 [95% confidence interval (CI): 1.2–2.2], 1.7 (95% CI: 1.3–2.3)}, overweight [1.1(95% CI: 1.0–1.3), 1.4 (95%: 1.1–1.7)] and obese [1.1 (95% CI: 1.0–1.3), 1.6 (95% CI: 1.3–1.9)] BMI category compared with the ‘healthy’ weight (20–24.9 kg/m2) and a small WC (<94 cm, men; <80 cm, women) category. Underweight was associated with highest all-cause mortality RRs in men [2.2 (95% CI: 1.8–2.8)] and women [2.3 (95% CI: 1.8–3.1]. We found a J-shaped association for continuous WC with all-cause, cardiovascular (CVD) and cancer, and a U-shaped association with respiratory disease mortality (P < 0.05). An all-cause (CVD) mortality RR of 2.0 was associated with a WC of 132 cm (123 cm) in men and 116 cm (105 cm) in women.
Conclusions Our results showed increased mortality risks for elderly people with an increased WC—even across BMI categories— and for those who were classified as ‘underweight’ using BMI. The results provide a solid basis for re-evaluation of WC cut-points in ageing populations.
Keywords: Waist circumference, body mass index, elderly, mortality
Introduction
The prevalence of overweight has increased for all age groups over the past decades in the Western world, including the elderly.1,2 For adults, overweight is known to be associated with many health problems and decreases in life expectancy,1,3 but for the elderly the association is less clear.4–7
In clinical practice, body mass index (BMI) and to a lesser extent waist circumference (WC) are widely used measures to assess an individual’s health risk. However, WC might be a better measure than BMI, given its relationship with harmful visceral adiposity.8 This might be particularly important for the elderly since they have more visceral adipose tissue than younger adults for a given WC.7,8 Several studies have examined the association between WC and mortality risks in elderly people, but findings are inconsistent.5,6,9–14
For WC, three categories (men: <94 cm, 94–101 cm and ≥102 cm, women: <80 cm, 80–87 cm and ≥88 cm)15 have been defined to indicate the increasing health risk with increasing WC.16,17 However, associations between these WC categories and mortality have not been studied extensively in the elderly. One study reported in never smoking men aged ≥55 years an elevated all-cause mortality risk in the upper two categories (94–101 cm and ≥102 cm) compared with the reference category (79–93 cm).18
Furthermore, since BMI is the most commonly used anthropometric measure, it is important to assess mortality risks associated with WC categories, within BMI categories. By studying combined categories, a more complete picture of risks becomes available and insight is gained on the magnitude of relative risks with increasing WC or with increasing BMI categories, keeping the other measurement the same. This has previously been studied, but not by stratifying for all combinations of WC and BMI categories, and in a smaller population of elderly.6,12
Given the unclear association between WC and mortality in the elderly, especially when also considering BMI, and the ageing of the population, more research in a large elderly population is needed. This would provide an evidence base for application of these anthropometric indicators. To our knowledge, only data from single cohort studies with limited generalizability have previously studied this association. Therefore, the aims of this meta-analysis, which included over 58 000 people aged 65–74 years, were 2-fold. The first aim was to examine the association between internationally defined WC categories and all-cause and cause-specific mortality risks, within standard BMI categories. The second aim was to examine the association of WC as a continuous variable with lowest and increased mortality risks.
Methods
Data sources and searches
Studies were identified by a PubMed search from 1984 until 1 November 2010, by examining the reference lists of identified reviews, and by suggestions from colleagues. The following search strategy was used: waist, or WC, or abdominal adiposity in the abstract, title or in the Medical Subject Heading (MeSH), and mortality in the abstract, title or mortality in MeSH, plus either prospective or cohort. This search resulted in 202 abstracts. Additionally, all investigators from a previous collaboration were contacted,19 and we searched on the website of the United States National Institute of Aging for eligible studies.
Study selection
Eligible studies were prospective cohort studies conducted in predominantly Caucasian populations. The studies had to include at least 400 people in the age range of 65–74 years at baseline, this ensured smaller studies were also included. WC, BMI and all-cause mortality had to be available. Additionally, it had to be possible to calculate hazard ratios [relative risks (RRs)] for a follow-up period of 5–8 years (preferably closest to 5 years). This follow-up range was chosen to ensure most subjects were still alive during follow-up, since life expectancy is about 80 years,20 and also to reduce heterogeneity between studies. Also, baseline conditions tend to change considerably over a longer follow-up period.
In Appendix 1 (available as Supplementary Data at IJE online), a flowchart of the identified studies is presented. We identified 100 studies as possibly eligible for inclusion in our meta-analysis. The investigators of these studies received an e-mail with an explanation of the purpose of the study, an invitation for participation and a request to ensure their study would meet the inclusion criteria. No financial support was offered to participate in this meta-analysis.
We could not find valid e-mail addresses for four investigators, thus 96 investigators were contacted by e-mail of whom 60 responded. Eighteen of these declined because the data did not fully meet the inclusion criteria. Fourteen investigators declined for financial reasons, due to lack of time or interest, or lost contact after initial response. Finally, 28 investigators responded from whom 29 cohort studies were included in the meta-analysis.
Data extraction
The investigators who agreed to participate were requested to perform Cox regression analyses to calculate RRs of mortality for WC as a categorical and continuous variable following a protocol with instruction. All analyses were stratified by sex.
For the combined WC–BMI categories, WC categories defined by Lean et al. and used in practice15–17 (i.e. <94, 94–101, ≥102 cm in men; <80, 80–87, ≥88 cm in women) and BMI categories underweight (<20 kg/m2), ‘healthy’ weight (20–24.9 kg/m2), overweight (25–29.9 kg/m2) and obese (≥30 kg/m2) were used. The investigators used a model to assess mortality risks for the 11 combined WC–BMI categories compared with the reference category (‘healthy weight’ and small waist) (Table 1). This model was adjusted for age and smoking status [current, former and never smokers (reference)].
Table 1.
Sex-specific combinations of WC and BMI categories used in the analyses
| WC categories (men/women) |
|||
|---|---|---|---|
| BMI categories (kg/m2) | Small waist (cm) | Medium waist (cm) | Large waist (cm) |
| Underweight <20 | <94/<80 | 94–101/80–87 | ≥102/≥88 |
| ‘Healthy’ weight 20–24.9 | <94/<80 (ref) | 94–101/80–87 | ≥102/≥88 |
| Overweight 25–29.9 | <94/<80 | 94–101/80–87 | ≥102/≥88 |
| Obese >30 | <94/<80 | 94–101/80–87 | ≥102/≥88 |
Since previous studies have shown a U-shaped relation between WC and mortality,10,11,21,22 the investigators used a model with WC as a continuous variable, including the linear and quadratic term of WC (WC and WC2). The models were first only adjusted for age and smoking status, and subsequently for BMI as well. All analyses were performed over a follow-up period of ∼5 years for all-cause mortality and, if available, for mortality from cardiovascular disease (CVD), cancer and respiratory disease (see Table 2 for definitions).
Table 2.
Characteristics of studies included in the meta-analysis of men and women (m/w) separately
| Study | Mean Age (years) | Mean WC (cm) | Mean BMI (kg/m2) | Never Smokers (%) | Year(s) of baseline | Mean Follow–up (years) | Self- reported (S) or Measured (M) BMI and WC | No. Available for Analyses | No. All-cause mortality | No. CVD mortality | No. Cancer mortality | No. Respiratory mortality | Definition of endpoint cause-specific mortalitya |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1913 Men Birth cohort27 | 67/NA | 96/NA | 25/NA | 23/NA | 1980–81 | 5.0/NA | M | 707/NA | 90/NA | 53/NA | 30/NA | 1/NA | A |
| Aerobics Center Longitudinal Study28 | 67/68 | 94/79 | 26/25 | 64/80 | 1979–2003 | 4.9/5.0 | M | 1780/437 | 87/9 | 37/3 | 36/5 | 0/0 | A,B |
| Australian National Heart Foundation Study29 | 67/67 | 95/83 | 27/26 | 27/63 | 1989–90 | 4.6/4.9 | M | 346/384 | 95/39 | 39/11 | 40/14 | 0/0 | B |
| British Regional Heart Study30 | 70/NA | 98/NA | 27/NA | 28/NA | 1998–2000 | 5.0/NA | M | 2204/NA | 282/NA | Unknown | Unknown | Unknown | NA |
| Catalonia study31 | 69/69 | 97/71 | 26/23 | 23/94 | 1994–95 | 5.0/4.8 | M | 207/228 | 21/5 | 6/0 | 8/2 | 5/0 | A,B |
| Cardiovascular Health Study6 | 70/69 | 98/93 | 27/27 | 29/52 | 1989–90 & 1992–93 | 4.7/4.9 | M | 1850/2287 | 208/134 | 97/54 | 71/55 | 15/9 | Adjudicated by committee of physicians |
| Cohort Study in Spain5 | 70/70 | 103/98 | 28/30 | 28/93 | 2000–01 | 4.7/4.8 | M | 675/1081 | 90/78 | Unknown | Unknown | Unknown | NA |
| Cohort of Swedish Men32 | 69/NA | 97/NA | 26/NA | 37/NA | 1997 | 5.1/NA | S | 9014/NA | 707/NA | 359/NA | 217/NA | 41/NA | A |
| 3C-Dijon Study33 | 70/70 | 95/83 | 26/26 | 29/80 | 1999–2001 | 5.8/5.9 | M | 979/1500 | 71/53 | Unknown | Unknown | Unknown | NA |
| Doetinchem | 68/68 | 101/95 | 27/28 | 15/56 | 1998–2002 | 4.8/4.9 | M | 236/224 | 19/7 | 5/3 | 8/2 | 0/0 | B |
| Cohort study34 | |||||||||||||
| North Carolina Established Populations for Epidemiologic Studies of the Elderly35 | 72/73 | 99/92 | 27/28 | 21/58 | 1992–93 | 4.1/4.6 | M | 233/295 | 78/50 | 26/24 | 31/12 | 7/4 | CVD: 390–459.9 (ICD-9); Cancer: 140 to 208.9 (ICD-9); Respiratory disease: 460–519.9 (ICD-9) |
| Finnish Twin Cohort36 | NA/70 | NA /90 | NA/28 | NA/87 | 1996–2001 | 4.9 | M | NA/404 | NA/18 | n.a /5 | NA/10 | NA/0 | A |
| Gubbio Population Study37 | 69/69 | 94/86 | 28/28 | 18/79 | 1988–92 | 4.7/4.9 | M | 327/398 | 41/16 | 16/6 | 18/8 | 0/0 | B |
| Health 2000 Health Examination Survey38 | 69/70 | 100/94 | 27/29 | 34/82 | 2000–01 | 6.2/6.7 | M | 358/474 | 81/61 | 41/17 | 24/28 | 5/3 | A |
| Harvard Alumni Health Study39 | 69/NA | 94/NA | 25 | 36/NA | 1988 | 4.8/NA | M | 4416/NA | 338/NA | 123/NA | 152/NA | 57/NA | ICD-7 |
| Hoorn study40 | 69/70 | 96/89 | 26/27 | 8/61 | 1989–90 | 4.7/4.8 | M | 345/439 | 49/37 | 13/13 | 22/14 | 1/1 | B |
| Invecchiare in Chianti Study41 | 70/70 | 96/91 | 27/28 | 26/77 | 1998–2000 | 4.2/4.3 | M | 261/289 | 17/9 | 8/1 | 7/6 | 0/0 | B |
| Longitudinal Aging Study Amsterdam42 | 70/70 | 99/96 | 26/28 | 7/53 | 1992–93 | 4.6/4.8 | M | 388/415 | 68/31 | 26/12 | 29/11 | 5/1 | A,B |
| MacArthur Successful Aging Study43 | 72/72 | 98/88 | 26/26 | 31/55 | 1988 | 5.9/6.4 | M | 303/349 | 79/48 | 19/16 | 29/13 | 10/3 | A,B |
| Melbourne Collaborative Cohort Study44 | 67/67 | 95/82 | 27/27 | 32/72 | 1990–94 | 5.6/5.7 | M | 3326/3919 | 305/174 | 101/47 | 146/100 | 15/8 | CVD:I00-I99 (ICD-10); 390–459 (ICD-9) Cancer: C00-C97 (ICD-10); 140–209 (ICD-9) Respiratory disease: J00-J99 (ICD-10); 460–519 (ICD-9) |
| Normative Aging Study45 | 68/NA | 99/NA | 28/NA | 30/NA | 1990–98 | 4.8/NA | M | 809/NANA | 64/NA | 11/NA | 38/NA | 8/NA | ICD-9: CVD: 410-414.9, 430–438.9; Cancer:140-208.9; Respiratory diseases: 460–519.9 |
| Prospective Investigation of the Vasculature in Uppsala Seniors46 | 70/70 | 95/87 | 27/27 | 90/88 | 2001–04 | 7/7 | M | 500/503 | 67/44 | Unknown | Unknown | Unknown | NA |
| Population Study of Women in Gothenburg47 | NA/71 | NA/85 | NA/26 | NA/59 | 1980–81, 1992–93, 2000–01 | NA/5.0 | M | NA/915 | NA/51 | NA/19 | NA/21 | Unknown, (between 1 and 6) | Deaths during 1980–91:Classified from death certificate; From 1991: A,B |
| Rotterdam study48 | 70/70 | 95/88 | 26/27 | 6/52 | 1989–92 | 4.7/4.9 | M | 1028/1301 | 125/87 | 45/35 | 53/34 | 2/1 | A |
| Third Scottish Multinational MONItoring of trends and determinants in CArdiovascular disease study49 | 6969 | 95/84 | 26/27 | 13/38 | 1992 | 4.5/4.6 | M | 200/212 | 46/30 | Unknown | Unknown | Unknown | NA |
| Survey in Europe on Nutrition and the Elderly: a Concerned Action50 | 72/73 | 97/89 | 26/27 | 20/84 | 1988–90 | 4.5/4.8 | M | 751/773 | 163/65 | 62/28 | 53/34 | 14/1 | B |
| Study of Health in Pomerania51 | 70/70 | 100/90 | 28/29 | 15/67 | 1997–2001 | 4.7/4.9 | M | 382/299 | 58/17 | 17/3 | 31/7 | 0/0 | Death certificates and internists |
| Swedish Mammography Cohort32 | NA/69 | NA/85 | NA/25 | NA/65 | 1997 | NA/5.2 | S | NA/8210 | NA/385 | NA/118 | NA/199 | NA/16 | A |
| Whitehall II study52 | 69/69 | 95/86 | 26/28 | 44/52 | 2002–04 | 5.5/5.6 | M | 1323/595 | 101 | 21/10 | 31/16 | 1/4 | A |
aEndpoints defined by the International Classification of Diseases (ICD)-10; CVD: I00–I99; Cancer: C00–97; Respiratory disease: J00–J99 are indicated with A. Endpoints defined by the ICD-9: CVD: 390–460; Cancer: 140–240; Respiratory disease: 460–520 are indicated with B. All exceptions are written out. NA: not available.
Additional analyses were performed for the models with WC as a categorical variable and WC as a continuous variable (with adjustment for BMI) for the following subgroups: subjects aged 65–69 years and 70–74 years; subjects aged 65–74 years; excluding mortality during the first 2 years of follow-up; excluding those with major chronic diseases (i.e. CVD, cancer and respiratory disease) at baseline; and only including never smokers.
The investigators were not asked to test the proportional hazard assumption for each requested analysis because it was considered too onerous. Nevertheless, the proportional hazard assumption was tested for each analysis in eight cohort studies and no violations were found [(global) test of Schoenfeld P > 0.05].
Descriptive statistics for each cohort (e.g. mean age, BMI and WC, number of subjects, total deaths, deaths from CVD, cancer and respiratory disease and percentage never smokers) were provided by the investigators.
Data synthesis and analysis
First, heterogeneity of the pooled RRs for the combined WC–BMI categories (received from the investigators) was tested by calculating the Cochran’s chi-square, its P-value and the I2 (percentage of variation across studies).23 Heterogeneity in the continuous analyses was tested by a chi-squared test from the random effects model.24 To account for any heterogeneity, a random-effects model was used for all models to pool the log RRs.
For the combined WC–BMI categories, the log RR for each WC–BMI category was pooled by a univariate meta-analysis.24
For the continuous analyses, we used a bivariate meta-analysis to pool the log RRs with the variance of each term and the covariance between terms.25 To assess the association between continuous WC and mortality, we tested if the regression coefficients for both terms were equal to 0. To plot a parabolic function between WC and mortality, the lowest risk was calculated by −EstimateWC/(2*EstimateWC2) which was the reference point (RR = 1.0) for the function. The RRs associated with the commonly used cut-points of 102 cm in men and 88 cm in women were reported. Also, the values of WC associated with a RR of 2.0 which we consider a clinically relevant increased mortality risk as supported by the National Cancer Institute.26
For the continuous analyses without and with adjustment for BMI, we tested the effect of BMI by means of a meta-regression analysis.24
Results
The 29 cohort studies included 32 678 men and 25 931 women aged 65–74 years of whom, respectively, 3318 and 1480 died. Table 2 shows the characteristics of the included cohorts by sex.
For the cohort studies where the cause of death was known (n = 24), the proportion of deaths assigned to CVD was 40.7% for men and 33.3% for women, the corresponding proportions for cancer were 38.7% and 45.1% and for respiratory diseases, 6.8% and 4.0%.
In general, there was no substantial heterogeneity in the analyses regarding the combined WC–BMI categories resulting in an I2 < 17.5% (P > 0.22, for the chi-squared test) (Appendix 4, Figure 4.1, 4.2, available as Supplementary Data at IJE online). Similarly, no substantial heterogeneity was found in the continuous analyses (P > 0.05 for the chi-squared test from the random-effects model (Appendix 4, Table 4.1, available as Supplementary Data at IJE online).
Associations between combined WC–BMI categories and mortality
For men and women, a large WC (≥102 cm, men, and ≥88 cm, women) was associated with increased all-cause mortality RRs for those in the ‘healthy’ weight, overweight and obese BMI category compared with those classified as ‘healthy’ weight (20–24.9 kg/m2) with a small WC (<94 cm, men and <80 cm, women) (Table 3). Overall, we observed a tendency for lower all-cause and CVD mortality risks in the overweight category compared with the ‘healthy’ weight category within WC categories for both men and women (men: Pall-cause = 0.02, PCVD = 0.03; women: Pall-cause = 0.18, PCVD = 0.36), although the RR for overweight men with a small WC in the association with CVD mortality was higher compared with ‘healthy’ weight men with a small waist (Table 3).
Table 3.
Relative risk (95% CI) of mortality from all causes, CVD and cancer per combined WC-BMI category in men and women aged 65–74 yearsa
| All-cause mortality |
CVD mortality |
Cancer mortality |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Small waist | Medium waist | Large waist | Small waist | Medium waist | Large waist | Small waist | Medium waist | Large waist | |||
| Men | Men | Men | |||||||||
| Underweight | 2.2 (1.8–2.8) | NA | NA | Underweight | 2.9 (2.0–4.2) | NA | NA | Underweight | 2.1 (1.5–3.0) | NA | NA |
| Healthy weight | 1.0 | 1.1 (1.0–1.3) | 1.7 (1.2–2.2) | Healthy weight | 1.0 | 1.4 (1.1–1.8) | 2.6 (1.6–4.1) | Healthy weight | 1.0 | 1.0 (0.7–1.4) | 1.7 (0.5–6.2) |
| Overweight | 0.9 (0.8–1.0) | 1.0 (0.9–1.1) | 1.1 (1.0–1.3) | Overweight | 1.5 (1.2–1.9) | 1.2 (1.0–1.5) | 1.4 (1.1–1.8) | Overweight | 0.8 (0.6–1.2) | 0.9 (0.8–1.1) | 1.3 (1.0–1.6) |
| Obese | NA | 1.2 (0.9–1.6) | 1.1 (1.0–1.3) | Obese | NA | 2.3 (1.5–3.7) | 1.7 (1.3–2.4) | Obese | NA | 0.8 (0.5–1.5) | 1.0 (0.8–1.3) |
| Women | Women | Women | |||||||||
| Underweight | 2.3 (1.8–3.1) | NA | NA | Underweight | 1.5 (0.8–2.8) | NA | NA | Underweight | 2.6 (1.5–4.4) | NA | NA |
| Healthy weight | 1.0 | 1.5 (1.2–1.8) | 1.7 (1.3–2.3) | Healthy weight | 1.0 | 1.2 (0.8–1.9) | 2.2 (1.3–3.8) | Healthy weight | 1.0 | 1.6 (1.1–2.3) | 1.5 (0.9–2.4) |
| Overweight | 1.0 (0.7–1.4) | 1.2 (0.9–1.5) | 1.4 (1.1–1.7) | Overweight | 1.1 (0.5–2.3) | 1.1 (0.7–1.7) | 1.2 (0.8–1.7) | Overweight | 1.2 (0.7–2.1) | 1.3 (0.9–1.9) | 1.5 (1.1–2.2) |
| Obese | NA | 1.6 (0.9–2.8) | 1.6 (1.3–1.9) | Obese | NA | 2.3 (0.9–5.7) | 1.5 (1.1–2.2) | Obese | NA | 1.3 (0.4–3.6) | 1.4 (0.9–2.2) |
aThe numbers of studies used in the analyses differ because some studies did not have sufficient cases in a category. When analysing all categories with the same number of studies which had information on all categories, the relative risks changed max. by 0.2.
NA: not available; if the number of studies was <5 then the RR for this category was not calculated, because of the low prevalence of these combinations.
The risks of all-cause, CVD and cancer mortality were (although not statistically tested) higher for those with a large WC compared with those having a medium WC, except within the obese category in the association with all-cause and CVD mortality, and for women within the ‘healthy’ weight category in the association with cancer mortality (Table 3).
Underweight was associated with highest all-cause mortality RRs in men {2.2 [95% confidence interval (CI): 1.8–2.8]} and women [2.3 (95% CI: 1.8–3.1)]. The RRs for cancer mortality were of the same magnitude. For CVD, an increased risk was found for men [RR = 2.9 (95% CI: 2.0–4.2)], but in women the RR was lower [RR = 1.5 (95% CI: 0.8–2.8)] (Table 3).
Associations between WC as a continuous variable and mortality
All-cause mortality
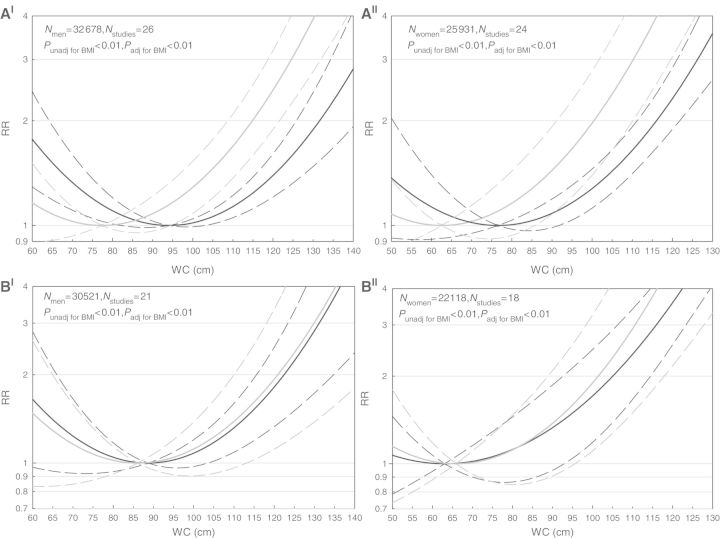
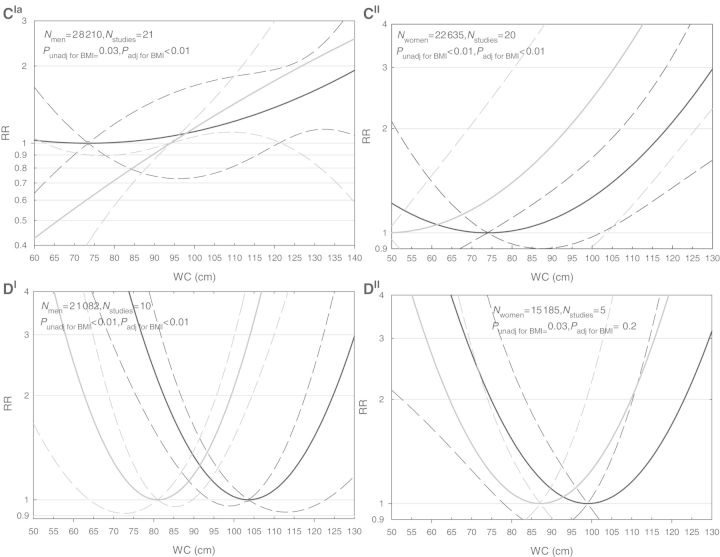
We observed a J-shaped association between WC and all-cause mortality adjusted for age and smoking status (P < 0.01) with the lowest risk at 94 cm and 77 cm for men and women, respectively (Figure 1A). The cut-points of 102 cm in men and 88 cm in women were associated with all-cause mortality RRs of 1.03 (95% CI: 1.00–1.07) and 1.06 (95% CI: 0.97–1.15), respectively. An RR of 2.0 was associated with a WC of 132 cm in men and 116 cm in women (Figure 1A).
Figure 1.
Relative risks of mortality from all causes (A), cardiovascular diseases (B), cancer (C) and respiratory disease (D) in men(I) and women(II) aged 65–74 years for WC as a continuous variable. All models were adjusted for age and smoking. In (A–D), solid lines indicate relative risks and dashed lines indicate 95% CIs. The black lines indicate the analyses unadjusted for BMI and the grey lines indicate the analyses with the adjustment for BMI. aIn this figure, for the analysis adjusted for BMI, a minimum of 94 cm was used, because there was no longer a parabolic association
Cause-specific mortality
Mortality from CVD, cancer and respiratory diseases were all associated with WC adjusted for age and smoking status in both men and women (P ≤ 0.03) (Figure 1B–D).
For CVD mortality, the lowest risk was at 89 cm and 63 cm for men and women, respectively. For men with a WC of 102 cm, the risk of CVD mortality was 1.11 (95% CI: 0.99–1.26) and for women with a WC of 88 cm this was 1.28 (95% CI: 0.92–1.77). An RR of 2.0 was associated with a WC of 123 cm in men and 105 cm in women (Figure 1B).
For cancer mortality, the lowest risk was at 73 cm and 74 cm for men and women, respectively. For men with a WC of 102 cm, the risk of cancer mortality was 1.13 (95% CI: 0.74–1.71) and for women with a WC of 88 cm this was 1.07 (95% CI: 0.90–1.27) (Figure 1C).
We observed a U-shaped relationship between WC and mortality from respiratory disease for both men and women. The lowest risk was at 104 cm for men and 99 cm for women. For men with a WC of 102 cm, the risk of mortality from respiratory diseases was 1.00 (95% CI: 0.98–1.03) and for women with a WC of 88 cm this was 1.15 (95% CI: 0.85–1.57) (Figure 1D).
Associations between WC as a continuous variable and mortality with adjustment for BMI
After adjusting for BMI, WC remained associated with mortality from all causes, CVD and cancer in both sexes, and with respiratory diseases in men but not in women. The curves for CVD mortality were similar to those that were not adjusted for BMI (Pmen = 0.99; Pwomen = 0.62), but the curves for mortality from all causes (Pmen < 0.01; Pwomen < 0.01) and respiratory diseases (Pmen < 0.01; Pwomen = 0.40) were shifted to the left for both sexes, and for cancer only in women (P = 0.15). Thus, the lowest risks were at lower values of WC, and the RRs associated with a similar WC were higher after adjusting for BMI compared with the analyses unadjusted for BMI (Figures 1A–D). The curve of cancer mortality in men became linear after adjustment for BMI (Figure 1C).
Additional analyses
We restricted our additional analyses to the four most relevant categories (i.e. underweight with a small WC, ‘healthy’ weight, overweight and obese combined with a large WC), because these categories gave the most consistent and strongest RRs in the main analyses.
The associations between the WC–BMI categories and all-cause and CVD mortality did not differ by age group (Appendix 2, Table 2.1, 2.2, available as Supplementary Data at IJE online). Excluding the first 2 years of follow-up, or major chronic diseases at baseline, or only including never smokers did not change the interpretation of our findings (Appendix 3, Table 3.1, Figure 3.1, available as Supplementary Data at IJE online).
We found some differences between the main analyses and additional analyses. After excluding the first 2 years of follow-up, we observed an RR of 1.6 (95% CI: 0.8–3.2) for CVD mortality risk in women with a ‘healthy’ weight and a large WC, compared with an RR of 2.2 (95% CI: 1.3–3.8) including all subjects. However, the additional analyses confirmed that for those with a large WC being in the ‘healthy’ weight category is associated with a higher RR (1.6) than the overweight category [RR = 1.3; (95% CI: 0.8–2.0)]. Furthermore, the analyses for continuous WC showed a similar pattern for all-cause mortality (Appendix 3, Table 3.1, Figure 3.1, available as Supplementary Data at IJE online).
After exclusion of major chronic diseases at baseline, the RR for CVD mortality in underweight men was 2.5 (95% CI: 0.8–7.7) compared with an RR of 3.3 (95% CI: 1.5–7.3) including all men, but still this confirms that underweight is associated with CVD mortality with an RR of at least 2.0 (Appendix 3, Table 3.1, Figure 3.1, available as Supplementary Data at IJE online).
Results for never smokers were comparable to the total population, except for the CVD mortality risks in men with a large WC and overweight/obesity, which were higher among never smoking men (RR = 2.2) than for the total population [RR = 1.3 (overweight + large WC]; RR = 1.5 (obesity + large WC)]. In women, the patterns of the curves for the continuous analyses of WC were similar, but in men the steepness of the curves differed. As a consequence, in never smoking men, higher WC levels were accompanied by lower RRs for all-cause mortality compared with the RRs in all men (Appendix 3, Table 3.1, Figure 3.1, available as Supplementary Data at IJE online).
Discussion
This meta-analysis of 29 cohort studies, which included a total of 58 609 elderly people of whom 4798 died during 5 years of follow up, showed that both an increased WC and underweight (according to BMI) were associated with an increased risk of all-cause, CVD and cancer mortality risk.
Consistent with our study, others have reported stronger associations between WC (as a continuous variable) and mortality after adjustment for BMI.5,6,11,14,21,53 We also found that the RR of mortality in persons with a ‘healthy’ weight combined with a large waist was generally higher than for those with overweight and a large waist. These findings might be explained by body fat composition, in particular the proportion of hazardous visceral abdominal fat.54 In contrast to other studies, we also found strong associations with increased risks of mortality, particularly from all causes and CVD, but also from cancer, without adjustment for BMI.5,6,9,12,13 For respiratory diseases, a U-shaped association was observed between WC and mortality, whereas other studies reported an inverse association.9,12
Our results of the combined categories are difficult to compare with other studies as they have used different combined WC-BMI categories, reference categories, study groups or other outcome measures.6,12,55 However, these studies also found that underweight was associated with higher risks of coronary heart disease in adults,55 and all-cause and CVD mortality in the elderly.12
In our study, all analyses were conducted in a similar manner by the original investigators addressing the specific age-range of 65–74 years. This may be the reason that in general there appeared to be no substantial heterogeneity between studies. We included two cohort studies, one restricted to only men, the other only women, which excluded participants with cancer at baseline in the original data and used self-reported data of WC and BMI. However, excluding these studies from the analyses did not change our results meaningfully (data not shown).
Another strength of the included studies is that no overrepresentation of higher estimates of RR among studies with low precision (i.e. small studies) was detected in our data suggesting no substantial selection bias (Appendix 4, available as Supplementary Data at IJE online). We had a low response, only 28 out of 100 investigators participated but reasons for non-participation depended primarily on lack of time or financial sources. We included cohort studies according to their study characteristics rather than the published analyses. This meta-analysis was conducted according to a specific analysis protocol, requiring new analyses for each cohort; the exact information (required for this study) was not available in the literature already. Therefore, we do not think there is any participation bias in our study. Also, the additional analyses excluding the first 2 years, excluding major chronic diseases at baseline and including only never smokers did not affect our main conclusions.
To keep all analyses as similar as possible, we did not adjust for covariates, such as diet, physical activity and socio-economic status. These variables differ between studies in operationalization, and are often self-reported and thereby less accurate. Furthermore, two studies showed no major differences between the crude and adjusted risks (for these covariates) of mortality associated with WC.11,14 However, this might not have been the case if more precise measures were included. Sui and colleagues reported an association between abdominal obesity (≥102 cm, ≥88 cm) and all-cause mortality in adults ≥60 years [RR: 1.3 (95% CI: 1.0–1.6)], similar to our results, but this association attenuated after adjustment for cardiorespiratory fitness [RR: 1.0 (95% CI: 0.8–1.3)].56 This would imply that WC might not be independently associated with all-cause mortality and that cardiorespiratory fitness may be considered as an indicator instead. More research is needed to confirm these findings of Sui and colleagues, and to add evidence to underpin practical application. Finally, our analyses did not account for weight loss or weight gain prior to baseline, which both can be predictive of mortality risk,57 possibly due to underlying illnesses. However, the additional analysis when excluding major chronic diseases at baseline, did not affect the interpretation of our findings.
Another methodological issue is that the adjustment for BMI in the continuous analyses might have caused multicollinearity resulting in a less precise estimate with wide confidence intervals. However, in our analyses, the CIs were not substantially wider, which is supported by the lack of a near perfect correlation between BMI and WC (ρ < 0.95) and the variance inflation factor did not exceed 5.
In our study, underweight was associated with a high RR of mortality, which is commonly explained by underlying diseases or smoking. After excluding those with chronic diseases at baseline, or the first 2 years of follow-up, or including only never smokers this association persisted. This might be explained by the association of low BMI with malnutrition58 and sarcopenia59 which are in turn both associated with higher mortality risks.60,61 In addition, elderly people with underweight may have low-grade inflammation,62 and might be frailer.63 These mechanisms might contribute to the vulnerability for external hazards which can lead to death. More research into possible mechanisms is necessary to give more insight into the risk of mortality in underweight persons and give suitable recommendations for the treatment of the elderly.
Interestingly, we found lower all-cause and CVD mortality risks in the overweight category compared with the ‘healthy’ weight category within WC categories for both men and women, but only in men accompanied by a P < 0.05, probably because women had wider CIs. The lower risks within the overweight category are congruent with other studies which found that the lowest mortality risk was associated with overweight and an increased risk was in the ‘healthy’ weight category, indicating that the ‘healthy’ weight category might not be appropriate for the elderly.12,64–67 An explanation for this finding could be the age-related decline in height among the elderly which might induce a false increase in BMI.7 Furthermore, as mentioned above for underweight, these elderly persons with low BMI are prone to external hazards, whereas overweight might provide a metabolic buffer for diseases as previously reported in older people with chronic conditions.68 Therefore, the cut-point of 25 kg/m2 to indicate excess adiposity might not be appropriate for the elderly.
We found that a large waist (≥102 cm, men; ≥88 cm, women) was consistently associated with all-cause and CVD mortality within the ‘healthy’ weight, overweight and obese BMI category. This finding was supported by our continuous analyses which showed that an increased risk was associated with an increased WC either with or without adjustment for BMI. Furthermore, our results provide a solid basis for re-evaluation of currently defined cut-points for WC, which are based on adults aged 20–74 years.15 From our continuous analysis, we found no relevant elevated mortality risks between the value of the lowest risk and the standard WC cut-points of 102 cm for men and 88 cm for women. This suggests that cut-points for the elderly should be defined at higher WC values. For CVD mortality, a 2-fold increased risk was seen at WC levels of 123 cm for men and 105 cm for women, which can be considered as clinically relevant (almost) beyond discussion. However, we do not suggest that these levels should be the new WC cut-points. Thresholds to be used in (clinical) guidelines should be based on opinions and consensus about the relevance of increased risks—as found in epidemiological studies—which can differ. For example, Heim and colleagues69 suggested new WC cut-points of between 100 cm and 106 cm in men and 99 cm in women based on several health outcomes,69 which especially in women is indeed higher than the currently advocated cut-points.16,17 In addition, when defining cut-points to be used in clinical guidelines, the absolute prevalence rates need to be considered for practical reasons. We performed additional analyses in seven cohorts (data not shown in the article) to illustrate this issue, which revealed that the prevalence rates sharply increased between a WC level of 123 cm (1–2%) and 102 cm (12–48%) in men, with a similar pattern in women. So, a level of WC in between would include a large part of the population that is at risk and needs to be treated according to clinical guidelines.
Conclusion
In this elderly population, we found increased mortality risks associated with an increased WC—even across BMI categories—and also with being underweight according to BMI. Clinicians should be made aware of the usefulness of WC to measure adiposity in order to determine mortality risk in the elderly. This meta-analysis provides a solid basis for re-evaluation of WC cut-points in ageing populations.
Supplementary Data
Supplementary data are available at IJE online.
Funding
Funding was provided by an internal research budget of the National Institute of Public Health and the Environment, Bilthoven, The Netherlands.
Supplementary Material
Acknowledgements
We want to thank Rik P Bogers for his contribution to the design of the meta-analysis. Further, we want to thank Joyce van Yperen for analysing the data of the SENECA and Hoorn study. Bogers and van Yperen did not receive any compensation and confirmed their agreement. E.L.de.H. had full access to all of the data in the study (that were provided to her by the collaborating investigators) and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: W.J.E.B., L.C.P.G.M.d.G., Bogers and H.C.B. Acquisition of data: E.L.de.H. Statistical analysis: E.L.de.H., H.C.B. Interpretation of data: E.L.de.H., W.J.E.B., H.C.B., N.F., H.W., P.G.-C., S.W., M.C.Z., A.R., L.L., J.K.B., G.G.G., N.H., M.V., L.C.P.G.M.d.G. Drafting the article: E.L.d.H., W.J.E.B. Critical revision of the article: E.L.d.H., W.J.E.B., H.C.B., N.F., H.W., P.G.-C., S.W., M.C.Z., A.R., L.L., J.K.B., G.G.G., N.H., M.V., L.C.P.G.M.d.G. Study supervision: W.J.E.B.
WC elderly collaborators: All other investigators of the collaboration contributed by collecting data and calculating RRs. 1913 Men Birth Cohort: A. Rosengren, V. Sundh; Aerobics Center Longitudinal Study (ACLS): S. Blair, D.C. Lee, X. Sui; Australian National Heart Foundation Study (ANHFS): M. Woodward, T. Welborn, S. Dhaliwal; British Regional Heart Study (BRHS): G. Wannamethee; Catalonia Study: E. Roure, C. Castell; Cardiovascular Health Study (CHS): M.L. Biggs, Cohort of Swedish Men (COSM): A. Wolk, N. Orsini; 3C-Dijon Study: P. Ducimetiere; Doetinchem Cohort Study: M. Verschuren; Finnish Twin Cohort: J. Kaprio; Gubbio Population Study: A. Menotti; Harvard Alumni Health Study (HAHS): I.M. Lee, H. Sesso; Health 2000 Survey: P. Knekt, K. Sääksjärvi; Hoorn Study: J. Dekker, G. Nijpels, C. Stehouwer; Invecchiare in Chianti (InCHIANTI) Study: S. Bandinelli, A.M. Corsi, F. Lauretani; Longitudinal Aging Study Amsterdam (LASA): M. Visser, N. Heim; MacArthur Successful Aging Study: T. Seeman, S. Ishii; Melbourne Collaborative Cohort Study (MCCS): G. Giles, J. Bassett; Normative Aging Study (NAS): A. Spiro; North Carolina Established Populations for Epidemiologic Studies of the Elderly (EPESE): C. Phillips, D. Blazer; Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS): L. Lind; Rotterdam Study: M.C. Zillikens, A. Uitterlinden, A. Hofman, S. Walter, H. Tiemeier; Survey in Europe on Nutrition and the Elderly: a Concerned Action (SENECA): L. de Groot; Study of Health in Pomerania (SHIP): H. Wallaschofski, N. Friedrich, S. Baumeister; Cohort Study in Spain: P. Guallar-Castillón, F. Rodríguez-Artalejo; Study of Women in Gothenburg: L. Lissner, V. Sundh, I. Skoog; Swedish Mammography Cohort: A. Wolk, N. Orsini; Third Scottish MONICA Study: M. Woodward, H. Tunstall-Pedoe; Whitehall II Study: M. Shipley, M. Kivimäki.
Conflict of interest: None declared.
KEY MESSAGES.
WC as a measure for adiposity predicts (cause-specific) mortality risks for elderly persons, across BMI categories.
Our continuous analyses in 58 000 elderly persons aged 65–74 years provide a strong base for reconsidering the cut-points of WC.
A 2-fold increased risk of CVD mortality, within a period of approximately 5 years, was found at a WC of 123 cm in men and 105 cm in women.
Also underweight according to BMI is an important predictor for mortality risks.
References
- 1.Branca F, Nikogosian H, Lobstein T. The Challenge of Obesity in the WHO European Region and the Strategies for Response. Denmark: World Health Organization; 2007. [Google Scholar]
- 2.Eiben G, Dey DK, Rothenberg E, et al. Obesity in 70-year-old Swedes: secular changes over 30 years. Int J Obes. 2005;29:810–17. doi: 10.1038/sj.ijo.0802940. [DOI] [PubMed] [Google Scholar]
- 3.Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138:24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 4.Grabowski DC, Ellis JE. High body mass index does not predict mortality in older people: analysis of the Longitudinal Study of Aging. J Am Geriatr Soc. 2001;49:968–79. doi: 10.1046/j.1532-5415.2001.49189.x. [DOI] [PubMed] [Google Scholar]
- 5.Guallar-Castillon P, Balboa-Castillo T, Lopez-Garcia E, et al. BMI, waist circumference, and mortality according to health status in the older adult population of Spain. Obesity. 2009;17:2232–38. doi: 10.1038/oby.2009.115. [DOI] [PubMed] [Google Scholar]
- 6.Janssen I, Katzmarzyk PT, Ross R. Body mass index is inversely related to mortality in older people after adjustment for waist circumference. J Am Geriatr Soc. 2005;53:2112–18. doi: 10.1111/j.1532-5415.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 7.Zamboni M, Mazzali G, Zoico E, et al. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes. 2005;29:1011–29. doi: 10.1038/sj.ijo.0803005. [DOI] [PubMed] [Google Scholar]
- 8.Lemieux S, Prud'homme D, Bouchard C, Tremblay A, Despres JP. A single threshold value of waist girth identifies normal-weight and overweight subjects with excess visceral adipose tissue. Am J Clin Nutr. 1996;64:685–93. doi: 10.1093/ajcn/64.5.685. [DOI] [PubMed] [Google Scholar]
- 9.Baik I, Ascherio A, Rimm EB, et al. Adiposity and mortality in men. Am J Epidemiol. 2000;152:264–71. doi: 10.1093/aje/152.3.264. [DOI] [PubMed] [Google Scholar]
- 10.Dolan CM, Kraemer H, Browner W, Ensrud K, Kelsey JL. Associations between body composition, anthropometry, and mortality in women aged 65 years and older. Am J Public Health. 2007;97:913–18. doi: 10.2105/AJPH.2005.084178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–20. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 12.Price GM, Uauy R, Breeze E, Bulpitt CJ, Fletcher AE. Weight, shape, and mortality risk in older persons: elevated waist-hip ratio, not high body mass index, is associated with a greater risk of death. Am J Clin Nutr. 2006;84:449–60. doi: 10.1093/ajcn/84.1.449. [DOI] [PubMed] [Google Scholar]
- 13.Reis JP, Macera CA, Araneta MR, Lindsay SP, Marshall SJ, Wingard DL. Comparison of overall obesity and body fat distribution in predicting risk of mortality. Obesity. 2009;17:1232–39. doi: 10.1038/oby.2008.664. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117:1658–67. doi: 10.1161/CIRCULATIONAHA.107.739714. [DOI] [PubMed] [Google Scholar]
- 15.Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. 1995;311:158–61. doi: 10.1136/bmj.311.6998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obesity: Preventing and Managing the Global Epidemic. Report of a WHO consultation. Geneva: World Health Organization: 2000. [PubMed] [Google Scholar]
- 17.Clinical Practice Guidelines for the Management of Overweight and Obesity in Adults. Canberra: National Health & Medical Research Council; 2003. [Google Scholar]
- 18.Visscher TL, Seidell JC, Molarius A, van der Kuip D, Hofman A, Witteman JC. A comparison of body mass index, waist-hip ratio and waist circumference as predictors of all-cause mortality among the elderly: the Rotterdam study. Int J Obes Relat Metab Disord. 2001;25:1730–35. doi: 10.1038/sj.ijo.0801787. [DOI] [PubMed] [Google Scholar]
- 19.Bogers RP, Bemelmans WJE, Hoogenveen RT, et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med. 2007;167:1720–28. doi: 10.1001/archinte.167.16.1720. [DOI] [PubMed] [Google Scholar]
- 20.World Population Prospects, The 2006 Revision, Highlights. New York: United Nations; 2007. [Google Scholar]
- 21.Bigaard J, Tjonneland A, Thomsen BL, Overvad K, Heitmann BL, Sorensen TI. Waist circumference, BMI, smoking, and mortality in middle-aged men and women. Obes Res. 2003;11:895–903. doi: 10.1038/oby.2003.123. [DOI] [PubMed] [Google Scholar]
- 22.Folsom AR, Kushi LH, Anderson KE, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women's Health Study. Arch Intern Med. 2000;160:2117–28. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002;21:589–624. doi: 10.1002/sim.1040. [DOI] [PubMed] [Google Scholar]
- 25.Riley RD, Abrams KR, Lambert PC, Sutton AJ, Thompson JR. An evaluation of bivariate random-effects meta-analysis for the joint synthesis of two correlated outcomes. Stat Med. 2007;26:78–97. doi: 10.1002/sim.2524. [DOI] [PubMed] [Google Scholar]
- 26.National Cancer Institute, US National Institute of Health. Epidemiology in a Nutshell. 2002 http://benchmarks.cancer.gov/2002/07/epidemiology-in-a-nutshell/ (20 December 2011, date last accessed) [Google Scholar]
- 27.Larsson B, Svardsudd K, Welin L, Wilhelmsen L, Bjorntorp P, Tibblin G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed) 1984;288:1401–04. doi: 10.1136/bmj.288.6428.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CD, Sui X, Blair SN. Combined effects of cardiorespiratory fitness, not smoking, and normal waist girth on morbidity and mortality in men. Arch Intern Med. 2009;169:2096–101. doi: 10.1001/archinternmed.2009.414. [DOI] [PubMed] [Google Scholar]
- 29.Dhaliwal SS, Welborn TA. Central obesity and multivariable cardiovascular risk as assessed by the Framingham prediction scores. Am J Cardiol. 2009;103:1403–07. doi: 10.1016/j.amjcard.2008.12.048. [DOI] [PubMed] [Google Scholar]
- 30.Wannamethee SG, Shaper AG, Lennon L, Whincup PH. Decreased muscle mass and increased central adiposity are independently related to mortality in older men. Am J Clin Nutr. 2007;86:1339–46. doi: 10.1093/ajcn/86.5.1339. [DOI] [PubMed] [Google Scholar]
- 31.Castell C, Tresserras R, Serra J, Goday A, Lloveras G, Salleras L. Prevalence of diabetes in Catalonia (Spain): an oral glucose tolerance test-based population study. Diabetes Res Clin Pract. 1999;43:33–40. doi: 10.1016/s0168-8227(98)00125-9. [DOI] [PubMed] [Google Scholar]
- 32.Levitan EB, Yang AZ, Wolk A, Mittleman MA. Adiposity and incidence of heart failure hospitalization and mortality: a population-based prospective study. Circ Heart Fail. 2009;2:202–08. doi: 10.1161/CIRCHEARTFAILURE.108.794099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahamat A, Richard F, Arveiler D, et al. Body mass index, hypertension and 5-year coronary heart disease incidence in middle aged men: the PRIME study. J Hypertens. 2003;21:519–24. doi: 10.1097/00004872-200303000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Verschuren WM, Blokstra A, Picavet HS, Smit HA. Cohort profile: the Doetinchem Cohort Study. Int J Epidemiol. 2008;37:1236–41. doi: 10.1093/ije/dym292. [DOI] [PubMed] [Google Scholar]
- 35.Cornoni-Huntley J, Ostfeld AM, Taylor JO, et al. Established populations for epidemiologic studies of the elderly: study design and methodology. Aging. 1993;5:27–37. doi: 10.1007/BF03324123. [DOI] [PubMed] [Google Scholar]
- 36.Kaprio J, Kujala UM, Koskenvuo M, Sarna S. Physical activity and other risk factors in male twin-pairs discordant for coronary heart disease. Atherosclerosis. 2000;150:193–200. doi: 10.1016/s0021-9150(99)00368-8. [DOI] [PubMed] [Google Scholar]
- 37.Menotti A, Lanti M, Puddu PE, et al. First risk functions for prediction of coronary and cardiovascular disease incidence in the Gubbio Population Study. Ital Heart J. 2000;1:394–99. [PubMed] [Google Scholar]
- 38.Knekt P, Reunanen A, Jarvinen R, Seppanen R, Heliovaara M, Aromaa A. Antioxidant vitamin intake and coronary mortality in a longitudinal population study. Am J Epidemiol. 1994;139:1180–89. doi: 10.1093/oxfordjournals.aje.a116964. [DOI] [PubMed] [Google Scholar]
- 39.Lee IM, Paffenbarger RS., Jr Change in body weight and longevity. JAMA. 1992;268:2045–49. [PubMed] [Google Scholar]
- 40.Wedick NM, Snijder MB, Dekker JM, et al. Prospective investigation of metabolic characteristics in relation to weight gain in older adults: the Hoorn Study. Obesity. 2009;17:1609–14. doi: 10.1038/oby.2008.666. [DOI] [PubMed] [Google Scholar]
- 41.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–25. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 42.Huisman M, Poppelaars J, van der Horst M, et al. Cohort Profile: The Longitudinal Aging Study Amsterdam. Int J Epidemiol. 2011;40:868–76. doi: 10.1093/ije/dyq219. [DOI] [PubMed] [Google Scholar]
- 43.Srikanthan P, Seeman TE, Karlamangla AS. Waist-hip-ratio as a predictor of all-cause mortality in high-functioning older adults. Ann Epidemiol. 2009;19:724–31. doi: 10.1016/j.annepidem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giles GG, English DR. The Melbourne Collaborative Cohort Study. IARC Sci Publ. 2002;156:69–70. [PubMed] [Google Scholar]
- 45.Bell B, Rose CL, Damon A. The Veterans Administration longitudinal study of healthy aging. Gerontologist. 1966;6:179–84. doi: 10.1093/geront/6.4.179. [DOI] [PubMed] [Google Scholar]
- 46.Ahlstrom T, Hagstrom E, Larsson A, Rudberg C, Lind L, Hellman P. Correlation between plasma calcium, parathyroid hormone (PTH) and the metabolic syndrome (MetS) in a community-based cohort of men and women. Clin Endocrinol. 2009;71:673–78. doi: 10.1111/j.1365-2265.2009.03558.x. [DOI] [PubMed] [Google Scholar]
- 47.Lissner L, Bjorkelund C, Heitmann BL, Seidell JC, Bengtsson C. Larger hip circumference independently predicts health and longevity in a Swedish female cohort. Obes Res. 2001;9:644–46. doi: 10.1038/oby.2001.85. [DOI] [PubMed] [Google Scholar]
- 48.Hofman A, Breteler MM, van Duijn CM, et al. The Rotterdam Study: objectives and design update. Eur J Epidemiol. 2007;22:819–29. doi: 10.1007/s10654-007-9199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tunstall-Pedoe H, Woodward M, Tavendale R, A'Brook R, McCluskey MK. Comparison of the prediction by 27 different factors of coronary heart disease and death in men and women of the Scottish Heart Health Study: cohort study. BMJ. 1997;315:722–29. doi: 10.1136/bmj.315.7110.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van 't Hof MA, Hautvast JG, Schroll M, Vlachonikolis IG. Design, methods and participation. Euronut SENECA investigators. Eur J Clin Nutr. 1991;45(Suppl 3):5–22. [PubMed] [Google Scholar]
- 51.Volzke H, Alte D, Schmidt CO, et al. Cohort Profile: The study of health in Pomerania. Int J Epidemiol. 2011;40:294–307. doi: 10.1093/ije/dyp394. [DOI] [PubMed] [Google Scholar]
- 52.Fransson EI, Batty GD, Tabak AG, et al. Association between change in body composition and change in inflammatory markers: an 11-year follow-up in the Whitehall II Study. J Clin Endocrinol Metab. 2010;95:5370–74. doi: 10.1210/jc.2010-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacobs EJ, Newton CC, Wang Y, et al. Waist Circumference and all-cause mortality in a large US cohort. Arch Intern Med. 2010;170:1293–301. doi: 10.1001/archinternmed.2010.201. [DOI] [PubMed] [Google Scholar]
- 54.Janssen I, Heymsfield SB, Allison DB, Kotler DP, Ross R. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr. 2002;75:683–88. doi: 10.1093/ajcn/75.4.683. [DOI] [PubMed] [Google Scholar]
- 55.Wormser D, Kaptoge S, Di Angelantonio E, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–95. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sui X, LaMonte MJ, Laditka JN, et al. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007;298:2507–16. doi: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bamia C, Halkjaer J, Lagiou P, et al. Weight change in later life and risk of death amongst the elderly: the European Prospective Investigation into Cancer and Nutrition-Elderly Network on Ageing and Health study. J Intern Med. 2010;268:133–44. doi: 10.1111/j.1365-2796.2010.02219.x. [DOI] [PubMed] [Google Scholar]
- 58.Venzin RM, Kamber N, Keller WC, Suter PM, Reinhart WH. How important is malnutrition? A prospective study in internal medicine. Eur J Clin Nutr. 2009;63:430–36. doi: 10.1038/sj.ejcn.1602948. [DOI] [PubMed] [Google Scholar]
- 59.Szulc P, Duboeuf F, Marchand F, Delmas PD. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: the MINOS study. Am J Clin Nutr. 2004;80:496–503. doi: 10.1093/ajcn/80.2.496. [DOI] [PubMed] [Google Scholar]
- 60.Saletti A, Johansson L, Yifter-Lindgren E, Wissing U, Osterberg K, Cederholm T. Nutritional status and a 3-year follow-up in elderly receiving support at home. Gerontology. 2005;51:192–98. doi: 10.1159/000083993. [DOI] [PubMed] [Google Scholar]
- 61.Roubenoff R. Sarcopenia and its implications for the elderly. Eur J Clin Nutr. 2000;54(Suppl. 3):S40–47. doi: 10.1038/sj.ejcn.1601024. [DOI] [PubMed] [Google Scholar]
- 62.Nakajima K, Yamaoka H, Morita K, et al. Elderly people with low body weight may have subtle low-grade inflammation. Obesity. 2009;17:803–08. doi: 10.1038/oby.2008.596. [DOI] [PubMed] [Google Scholar]
- 63.Hubbard RE, Lang IA, Llewellyn DJ, Rockwood K. Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci. 2010;65:377–81. doi: 10.1093/gerona/glp186. [DOI] [PubMed] [Google Scholar]
- 64.Allison DB, Gallagher D, Heo M, Pi-Sunyer FX, Heymsfield SB. Body mass index and all-cause mortality among people age 70 and over: the Longitudinal Study of Aging. Int J Obes Relat Metab Disord. 1997;21:424–31. doi: 10.1038/sj.ijo.0800423. [DOI] [PubMed] [Google Scholar]
- 65.Flicker L, McCaul KA, Hankey GJ, et al. Body mass index and survival in men and women aged 70 to 75. J Am Geriatr Soc. 2010;58:234–41. doi: 10.1111/j.1532-5415.2009.02677.x. [DOI] [PubMed] [Google Scholar]
- 66.Janssen I, Mark AE. Elevated body mass index and mortality risk in the elderly. Obes Rev. 2007;8:41–59. doi: 10.1111/j.1467-789X.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- 67.De Hollander EL, Van Zutphen M, Bogers RP, Bemelmans WJE, De Groot LCPGM. The impact of body mass index in old age on cause-specific mortality. J Nutr Health Aging. 2012;16:100–06. doi: 10.1007/s12603-011-0077-6. [DOI] [PubMed] [Google Scholar]
- 68.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–78. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 69.Heim N, Snijder MB, Heymans MW, Deeg DJ, Seidell JC, Visser M. Optimal cutoff values for high-risk waist circumference in older adults based on related health outcomes. Am J Epidemiol. 2011;174:479–89. doi: 10.1093/aje/kwr093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.