Abstract
Introduction
Chronic kidney disease may complicate diabetes, often manifesting with reduced glomerular filtration rate (GFR), albuminuria, or both. Although greater albuminuria and lower estimated GFR both predict adverse prognosis, whether a synergistic prognostic interaction occurs in patients with diabetes has not been defined in a large national cohort study.
Methods
We used 2000–2011 data from the National Kidney Foundation’s Kidney Early Evaluation Program (KEEP) for 42,761 participants with diabetes. Kaplan-Meier survival analysis and multivariable Cox regression were used to ascertain the association of estimated GFR, albumin-creatinine ratio (ACR), and their interaction on all-cause mortality and progression to end-stage renal disease (ESRD) at a median 4 years of follow-up.
Results
Of 42,761 participants with diabetes, 8,618 (20.2%) had estimated GFR <60 mL/min/1.732, 7,715 (18.0%) had ACR >30 mg/g, and 2,641 (6.2%) had both. The unadjusted incidence (per 1,000 person-years) of all-cause mortality increased from 3.1 (95% CI, 2.4–3.8) in participants with estimated GFR ≥105 mL/min/1.73 m2 and no albuminuria to 73.7 (95% CI, 54.9–92.5) in participants with estimated GFR <30 mL/min/1.73 m2 and macroalbuminuria (P <0.001). Progression to ESRD likewise increased from 0.2 (95% CI, 0–0.4) to 220.4 (95% CI, 177.2–263.6) per 1,000 person-years (P < 0.001). After adjustment for confounders, both estimated GFR and albuminuria were associated independently with mortality and progression to ESRD, with a strong synergistic interaction (P for interaction <0.001); estimated GFR <30 mL/min/1.73 m2 and macroalbuminuria together were associated with a 5-fold higher risk of mortality and a more than 1,000-fold higher risk of progression to ESRD (compared with patients with estimated GFR >60 mL/min/1.73 m2 and ACR <30 mg/g; P <0.001 for both outcomes).
Conclusions
In this large cohort of diabetic KEEP participants with more than 170,000 person-years of follow-up, both estimated GFR and albuminuria were associated independently with mortality and progression to ESRD, with a strong synergistic interaction.
INDEX WORDS: Albuminuria, chronic kidney disease, end-stage renal disease, diabetes mellitus, glomerular filtration rate, mortality, nonalbuminuric chronic kidney disease
Chronic kidney disease (CKD) is a major complication of diabetes mellitus manifested by albuminuria, decrease in estimated glomerular firate (eGFR), or both, and it occurs in up to 40% of patients with diabetes.1–3 Although diabetic nephropathy typically is characterized by albuminuria, the degree of albuminuria and eGFR decrease at the time of initial screening often varies widely in patients with diabetes. A substantial proportion of diabetic patients do not have albuminuria despite an abnormal eGFR. Studies have found that the absence of albuminuria in patients with diabetes ranges from 30%–40%4–7 and it was reported to be as high as 55% in one study.8 The long-term prognostic implications of albuminuria and eGFR in patients with diabetes have not been examined in a generalizable large national cohort followed up over time.
Furthermore, although both lower eGFR and greater albuminuria have been shown to independently predict poor prognosis,1–3,9–11 whether there is a synergistic prognostic interaction between these 2 factors in diabetic patients is largely unknown. While large population cohort studies (with a small proportion of patients with diabetes) have shown the absence of a synergistic interaction between eGFR and albuminuria, the prognostic effect of albuminuria and eGFR could be vulnerable to confounding by kidney disease type in mixed populations containing nondiabetic individuals. Thus, findings from these studies may not apply to patients with diabetes. Small studies of diabetic patients have suggested a lower risk of adverse outcomes in the absence of albuminuria;12,13 however, no study has examined a large national cohort of diabetic patients.
Addressing these knowledge gaps will aid clinicians in appropriately counseling and managing diabetic patients with CKD. Specifically, it will enable clinicians to appropriately risk stratify and aggressively treat these high-risk patients and inform and educate patients regarding their prognosis. Accordingly, we studied participants with diabetes in the National Kidney Foundation’s Kidney Early Evaluation Program (KEEP)14,15 to assess: (1) long-term prognosis by categories of albuminuria and eGFR status, and (2) whether a synergistic interaction exists between albuminuria and eGFR regarding impact on mortality and progression to end-stage renal disease (ESRD).
METHODS
KEEP Screening
KEEP is a national free community-based health screening program that targets populations at high risk of kidney disease. Enrollment has been described in detail previously.14–18 Eligible participants are 18 years or older with self-reported diabetes or hypertension or a first-degree relative with diabetes, hypertension, or kidney disease. Participants known to have undergone kidney transplant or who have ESRD and are receiving regular dialysis are excluded. All participants provide informed consent, then complete the screening questionnaire, which includes sociodemographic information, health history, risk factors, smoking status, and information for height, weight, and blood pressure. Plasma glucose and albumin-creatinine ratio (ACR) are measured. Blood samples are drawn from consenting participants and sent to a central laboratory.
Study Design and Study Population
In this observational cohort study, our study population consisted of KEEP participants enrolled in 2000–2011, with diabetes, and for whom eGFR and albuminuria measurements were available. A total of 150,972 participants were enrolled in KEEP, and 42,761 participants with diabetes were included in this analysis. Participants were interviewed with a standardized questionnaire. Self-reported demographic characteristics included age, race, and level of education. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement checklist of items required for reporting observational studies was considered in preparation of this report.19
Variable Definitions
Diabetes was defined as history of diabetes (self-report or retinopathy), use of diabetes medications, or newly diagnosed fasting glucose level ≥126 mg/dL, nonfasting glucose level ≥200 mg/dL, or hemoglobin A1c level ≥7%. Cardiovascular disease was defined as self-reported history of angina, heart attack, cardiac bypass surgery, coronary angioplasty, stroke, heart failure, abnormal heart rhythm, or coronary heart disease. Hypertension was defined as self-reported history of hypertension or use of antihypertensive medication. Blood pressure, height, and weight were measured by trained personnel and were categorized by the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) definitions as follows: normal, systolic blood pressure (SBP) <120 mm Hg and diastolic blood pressure (DBP) <80 mm Hg; prehypertension, SBP of 120–139 mm Hg or DBP of 80–89 mm Hg; stage 1, SBP of 140–159 mm Hg or DBP of 90–99 mm Hg; and stage 2, SBP ≥160 mm Hg or DBP ≥100 mm Hg. Body mass index (BMI) was calculated as weight (in kilograms) divided by height (in meters) squared. Hypercholesterolemia was defined as self-reported high cholesterol level, taking medication for high cholesterol level, or total cholesterol level >200 mg/dL or triglyceride level >150 mg/dL. Anemia was defined as hemoglobin level <13 g/dL in men and <12 g/dL in women. Family history of CKD was positive if the participant identified any family members who had kidney disease or received dialysis treatment.
Laboratory Data
Measurements of serum creatinine, eGFR, and albuminuria were performed at the time of screening. GFR was estimated using the CKD Epidemiology Collaboration (CKD-EPI) equation20 and categorized as ≥105, 90–<105, 75–<90, 60–<75, 45–<60, 30–<45, and <30 mL/min/1.73 m2. Albuminuria was defined from a spot urine ACR and categorized as no albuminuria (<30 mg/g), microalbuminuria (30–300 mg/g), or macroalbuminuria (>300 mg/g). Hemoglobin was measured from samples sent to a central laboratory.
Outcomes
All-cause mortality was the primary outcome of interest. All-cause mortality data were determined by linking KEEP participants to the Social Security Administration Death Master File as previously described.21 Progression to ESRD was a secondary outcome and was determined by linking the KEEP data with the US Renal Data System data. The last date of follow-up was December 31, 2011. Examination of KEEP data was approved by the Human Subjects Committee of the Minneapolis Medical Research Foundation (HSR 03-2262), Minneapolis, MN, and this protocol was approved by the Human Research Protection Office at Washington University (ID 201106346), St Louis, MO.
Statistical Methods
Baseline characteristics were compared for KEEP participants across categories of eGFR and ACR using analysis of variance for continuous variables and χ2 test for categorical variables. We obtained locally weighted smoothing scatter plots (LOWESS22 plots) by performing locally weighted regression of mortality and progression to ESRD of eGFR for each ACR category: <30, 30–300, and >300 mg/g. Kaplan-Meier survival analysis was conducted to examine the unadjusted association of eGFR and ACR categories with outcomes of all-cause mortality and progression to ESRD. For the mortality analysis, censoring was performed at December 31, 2011; for the ESRD analysis, censoring was performed at December 31, 2011, and the date of death. Multivariable Cox proportional hazards regression was used to examine independent effects of eGFR and ACR categories on all-cause mortality and progression to ESRD in separate models. All models were adjusted for the following demographic and clinical characteristics: age; sex; race; insurance status; BMI; education status; risk factors such as family history of diabetes, hypertension, and chronic kidney disease; self-reported blood pressure categorized as normal, prehypertension, stage 1, or stage 2; hyperlipidemia; smoking status; laboratory values such as hemoglobin; and medications such as oral antidiabetic medications and insulin. Both eGFR and albuminuria categories were included as dummy variables in the model, with eGFR ≥105 mL/min/1.73 m2 and ACR <30 mg/g as the reference categories. A final model also included an eGFR (using 3 categories: ≥60, 30–59, and ≤30 mL/min/1.73 m2)–albuminuria interaction term in addition to the main effects to examine the combined effect of the 2 when adjusted for demographic and clinical factors. Relative risks are reported as hazard ratios (HRs) with 95% confidence intervals (CIs). The proportional hazards assumption for all Cox models was assessed by log–log plots and found to be true. All P values were 2 sided and P <0.05 was considered statistically significant. All analyses were conducted in SAS (version 9.1; SAS Institute Inc). LOWESS plots were generated in STATA (version 11.1, StataCorp LP).
RESULTS
Cohort Characteristics
A total of 150,972 participants were enrolled in KEEP in 2000–2011. Excluding participants who were already receiving dialysis (n = 287) left 150,685 participants, of whom 47,321 had diabetes. Excluding participants with missing values for albuminuria (n = 2,624), eGFR (n = 1,630), or both (n = 292) and those who newly developed ESRD after enrollment but before the screening questionnaire was administered (n = 14) yielded a final analytic cohort of 42,761.
Distribution of CKD
Of 42,761 participants with diabetes, 8,618 (20.2%) had eGFR <60 mL/min/1.72 m2 and 7,715 (18.0%) had ACR >30 mg/g (Tables 1 and 2). Participants with GFR <60 mL/min/1.72 m2 or ACR >30 mg/g were more likely to be older and male with more severe hypertension and poor glucose control and less likely to have a high school education (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics of the Cohort by eGFR and ACR Categories
| eGFR (mL/min/1.73 m2)
|
||||||||
|---|---|---|---|---|---|---|---|---|
| >105 | 90–<105 | 75–<90 | 60–<75 | 45–<60 | 30–<45 | <30 | P | |
| No. | 5,714 | 9,158 | 10,354 | 8,917 | 5,383 | 2,555 | 680 | |
| Age (y) | 43.9 ± 10.4 | 55.4 ± 10.0 | 61.0 ± 11.1 | 65.2 ± 10.4 | 69.1 ± 10.2 | 72.1 ± 9.9 | 69.8 ± 12.6 | <0.001 |
| Male sex | 26.1 | 33.5 | 35.3 | 35.7 | 34.3 | 32.0 | 38.7 | <0.001 |
| Race | <0.001 | |||||||
| White | 29.3 | 47.3 | 53.5 | 55.7 | 61.6 | 62.9 | 53.8 | |
| African American | 44.3 | 28.5 | 29.4 | 29.6 | 25.4 | 24.1 | 29.3 | |
| Native American | 5.2 | 4.5 | 3.0 | 3.1 | 3.0 | 3.3 | 3.2 | |
| Asian | 4.6 | 7.6 | 6.5 | 5.2 | 4.9 | 4.2 | 6.9 | |
| Other | 16.7 | 12.1 | 7.6 | 6.3 | 5.2 | 5.4 | 6.8 | |
| Health insurance | 64.8 | 73.2 | 82.9 | 87.5 | 89.4 | 92.3 | 88.7 | <0.001 |
| BMI (kg/m2) | 33.6 ± 8.0 | 32.4 ± 7.4 | 31.8 ± 6.9 | 31.6 ± 6.5 | 31.5 ± 6.7 | 31.5 ± 6.9 | 31.5 ± 6.7 | <0.001 |
| High school education | 81.1 | 82.0 | 83.7 | 83.3 | 80.3 | 78.6 | 77.7 | <0.001 |
| Family history | ||||||||
| Diabetes | 75.0 | 70.8 | 68.5 | 67.6 | 66.1 | 66.9 | 66.8 | <0.001 |
| Hypertension | 77.2 | 77.3 | 76.5 | 75.4 | 75.3 | 75.6 | 72.0 | 0.002 |
| Kidney disease | 22.7 | 17.7 | 17.7 | 16.1 | 16.7 | 18.8 | 21.1 | <0.001 |
| Self-reported hypertension | 53.0 | 65.9 | 70.6 | 77.7 | 84.7 | 90.1 | 93.8 | <0.001 |
| Systolic BP (mm Hg) | 131.0 ± 18.6 | 134.7 ± 19.0 | 136.8 ± 19.4 | 137.7 ± 19.4 | 138.1 ± 20.0 | 139.3 ± 21.4 | 141.1 ± 23.4 | <0.001 |
| Diastolic BP (mm Hg) | 81.0 ± 11.4 | 80.0 ± 11.1 | 79.4 ± 11.4 | 78.3 ± 11.3 | 76.3 ± 11.6 | 74.2 ± 12.3 | 74.3 ± 13.5 | <0.001 |
| Measured BPa | <0.001 | |||||||
| Normal | 19.9 | 15.5 | 13.6 | 12.5 | 13.1 | 13.9 | 16.3 | |
| Prehypertension | 43.8 | 43.1 | 41.2 | 41.7 | 40.2 | 37.3 | 32.4 | |
| Stage 1 | 25.7 | 29.3 | 30.8 | 30.7 | 31.3 | 31.9 | 28.1 | |
| Stage 2 | 10.7 | 12.1 | 14.3 | 15.1 | 15.4 | 16.9 | 23.2 | |
| Hypercholesterolemia | 56.9 | 66.4 | 68.9 | 72.1 | 73.7 | 72.0 | 70.5 | <0.001 |
| Smoking | <0.001 | |||||||
| Current | 15.2 | 11.7 | 8.5 | 6.9 | 5.5 | 4.1 | 5.6 | |
| Former | 22.7 | 30.1 | 33.4 | 34.8 | 35.9 | 36.4 | 37.7 | |
| Never | 62.1 | 58.1 | 58.1 | 58.3 | 58.6 | 59.5 | 56.7 | |
| Anemia | 15.4 | 9.9 | 11.3 | 15.3 | 24.2 | 40.4 | 65.5 | <0.001 |
| Hemoglobin (g/dL) | 13.5 ± 1.6 | 13.8 ± 1.4 | 13.7 ± 1.4 | 13.6 ± 1.4 | 13.3 ± 1.5 | 12.6 ± 1.5 | 11.8 ± 1.5 | <0.001 |
| Hemoglobin category | <0.001 | |||||||
| :S12.6 g/dL | 28.0 | 18.8 | 20.3 | 24.6 | 34.3 | 52.0 | 73.4 | |
| 12.7–13.6 g/dL | 27.0 | 26.3 | 26.9 | 26.8 | 26.2 | 24.0 | 14.3 | |
| 13.7–14.5 g/dL | 20.9 | 25.5 | 25.4 | 23.6 | 20.5 | 14.2 | 8.0 | |
| >14.5 g/dL | 24.1 | 29.5 | 27.4 | 25.0 | 18.9 | 9.8 | 4.4 | |
| Fasting glucose (mg/dL) | 144.6 ± 65.4 | 138.8 ± 66.1 | 136.1 ± 56.8 | 133.4 ± 54.7 | 134.2 ± 55.5 | 137.6 ± 59.3 | 140.2 ± 58.6 | <0.001 |
| Diabetes medication | <0.001 | |||||||
| Yes | 36.0 | 43.8 | 46.3 | 48.0 | 48.7 | 50.3 | 41.9 | |
| No | 34.2 | 31.0 | 30.2 | 28.0 | 27.5 | 25.3 | 27.8 | |
| Missing | 29.8 | 25.2 | 23.5 | 24.0 | 23.8 | 24.4 | 30.3 | |
| Insulin | ||||||||
| Yes | 13.2 | 12.5 | 12.7 | 13.8 | 17.8 | 24.0 | 30.7 | <0.001 |
| No | 57.0 | 62.4 | 63.7 | 62.5 | 58.5 | 52.8 | 39.4 | |
| Missing | 29.8 | 25.1 | 23.6 | 23.7 | 23.7 | 23.2 | 29.9 | |
| ACR category | <0.001 | |||||||
| <30 mg/g | 84.0 | 86.5 | 86.0 | 83.4 | 76.2 | 63.8 | 35.9 | |
| 30–300 mg/g | 14.8 | 12.4 | 12.5 | 14.7 | 20.2 | 28.1 | 35.4 | |
| >300 mg/g | 1.2 | 1.0 | 1.5 | 1.9 | 3.6 | 8.1 | 28.7 | |
Note: Unless otherwise indicated, values for continuous variables are given as mean ± standard deviation; values for categorical variables given as percentages.
Abbreviations: ACR, albumin-creatinine ratio; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate.
As defined by the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.
Table 2.
Baseline Demographic and Clinical Characteristics of the Cohort by ACR Categories
| ACR (mg/g)
|
||||
|---|---|---|---|---|
| <30 | 30–300 | >300 | P | |
| No. | 35,046 | 6,632 | 1,083 | |
| Age (y) | 59.9 ± 13.1 | 61.9 ± 13.8 | 62.0 ± 13.4 | <0.001 |
| Male sex | 32.3 | 38.9 | 39.0 | <0.001 |
| Race | <0.001 | |||
| White | 52.2 | 45.7 | 44.1 | |
| African American | 29.8 | 33.7 | 28.8 | |
| Native American | 3.3 | 4.6 | 8.7 | |
| Asian | 5.8 | 6.4 | 7.2 | |
| Other | 8.9 | 9.6 | 11.2 | |
| Health insurance | 81.2 | 79.5 | 75.1 | <0.001 |
| BMI (kg/m2) | 31.9 ± 7.0 | 32.6 ± 7.3 | 32.2 ± 7.3 | <0.001 |
| High school education | 83.0 | 78.1 | 75.4 | <0.001 |
| Family history | ||||
| Diabetes | 69.1 | 69.8 | 72.0 | 0.1 |
| Hypertension | 76.5 | 75.3 | 75.7 | 0.1 |
| Kidney disease | 17.6 | 19.6 | 21.1 | <0.001 |
| Self-reported hypertension | 70.2 | 79.2 | 86.8 | <0.001 |
| Systolic BP (mm Hg) | 134.6 ± 18.6 | 142.1 ± 21.9 | 151.2 ± 24.8 | <0.001 |
| Diastolic BP (mm Hg) | 78.3 ± 11.2 | 80.6 ± 12.8 | 82.2 ± 13.7 | <0.001 |
| Measured BPa | <0.001 | |||
| Normal | 15.6 | 10.6 | 6.0 | |
| Prehypertension | 43.7 | 33.3 | 25.0 | |
| Stage 1 | 29.2 | 32.9 | 32.0 | |
| Stage 2 | 11.5 | 23.1 | 37.0 | |
| Hypercholesterolemia | 68.1 | 68.9 | 70.5 | 0.2 |
| Smoking | <0.001 | |||
| Current | 8.7 | 10.9 | 10.3 | |
| Former | 31.5 | 34.7 | 36.5 | |
| Never | 59.8 | 54.4 | 53.3 | |
| Anemia | 14.8 | 22.9 | 36.3 | <0.001 |
| Hemoglobin (g/dL) | 13.6 ± 1.4 | 13.4 ± 1.7 | 12.9 ± 1.8 | <0.001 |
| Hemoglobin category | <0.001 | |||
| :S12.6 g/dL | 24.8 | 31.7 | 45.5 | |
| 12.7–13.6 g/dL | 27.0 | 23.3 | 20.9 | |
| 13.7–14.5 g/dL | 23.4 | 21.1 | 15.6 | |
| >14.5 g/dL | 24.7 | 23.9 | 18.0 | |
| Fasting glucose (mg/dL) | 132.2 ± 52.8 | 161.1 ± 81.9 | 164.0 ± 84.6 | <0.001 |
| Diabetes medication | <0.001 | |||
| Yes | 44.5 | 48.7 | 46.1 | |
| No | 31.1 | 24.2 | 22.8 | |
| Missing | 24.4 | 27.1 | 31.1 | |
| Insulin | <0.001 | |||
| Yes | 12.5 | 22.4 | 32.2 | |
| No | 63.2 | 51.0 | 36.7 | |
| Missing | 24.3 | 26.6 | 31.1 | |
| eGFR category | <0.001 | |||
| 2:105 mL/min/1.73 m2 | 13.7 | 12.7 | 6.3 | |
| 90–<105 mL/min/1.73 m2 | 22.6 | 17.2 | 8.8 | |
| 75–<90 mL/min/1.73 m2 | 25.4 | 19.5 | 14.5 | |
| 60–<75 mL/min/1.73 m2 | 21.2 | 19.8 | 15.6 | |
| 45–<60 mL/min/1.73 m2 | 11.7 | 16.4 | 17.6 | |
| 30–<45 mL/min/1.73 m2 | 4.7 | 10.8 | 19.2 | |
| <30 mL/min/1.73 m2 | 0.7 | 3.6 | 18.0 | |
Note: Unless otherwise indicated, values for continuous variables are given as mean ± standard deviation; values for categorical variables given as percentages.
Abbreviations: ACR, albumin-creatinine ratio; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate.
As defined by the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.
Prevalence of Albuminuria and Low eGFR
Diabetic KEEP participants had varying degrees of albuminuria and eGFR. Albuminuria was not uniformly present (Table 3). The prevalence of eGFR <60mL/min/1.73 m2 without albuminuria (ACR <30mg/g) was 14.0% (n = 5,977). Conversely, 11.9% (n = 5,074) of participants had albuminuria with eGFR >60 mL/min/1.73 m2. Both low eGFR and albuminuria (eGFR<60mL/min/1.73 m2 and ACR >30 mg/g) occurred in 2,641 (6.2%) participants. In the lowest eGFR category, <30 mL/min/1.73 m2, the absence of albuminuria was rare, occurring in only 244 (0.57%) participants.
Table 3.
Unadjusted Incidence of All-Cause Mortality and ESRD per 1,000 Person-Years
| ACR <30
|
ACR = 30–300
|
ACR >300
|
All
|
|||||
|---|---|---|---|---|---|---|---|---|
| No. | Incidence (95% CI) | No. | Incidence (95% CI) | No. | Incidence (95% CI) | No. | Incidence (95% CI) | |
| All-cause mortality | ||||||||
| eGFR 2:105 | 4,801 | 3.1 (2.4 to 3.8) | 845 | 8.9 (5.9 to 11.8) | 68 | 5.9 (−2.3 to 14.1) | 5,714 | 4 (3.2 to 4.8) |
| eGFR = 90–<105 | 7,925 | 5.6 (4.8 to 6.4) | 1,138 | 12.3 (9.2 to 15.3) | 95 | 24.3 (9.9 to 38.7) | 9,158 | 6.7 (5.9 to 7.5) |
| eGFR = 75–<90 | 8,907 | 8.5 (7.5 to 9.4) | 1,290 | 18.2 (14.7 to 21.8) | 157 | 29.4 (16.9 to 42) | 10,354 | 10.1 (9.1 to 11) |
| eGFR = 60–<75 | 7,436 | 11.1 (9.9 to 12.2) | 1,312 | 24.2 (20 to 28.3) | 169 | 41.6 (26.5 to 56.7) | 8,917 | 13.6 (12.4 to 14.8) |
| eGFR = 45–<60 | 4,103 | 20.7 (18.5 to 22.9) | 1,089 | 34.7 (29.2 to 40.2) | 191 | 62.2 (44.4 to 79.9) | 5,383 | 25 (22.9 to 27.1) |
| eGFR = 30–<45 | 1,630 | 27.7 (23.6 to 31.8) | 717 | 49 (40.6 to 57.5) | 208 | 87.1 (66.1 to 108.1) | 2,555 | 38 (34.2 to 41.9) |
| eGFR <30 | 244 | 47.3 (33.1 to 61.4) | 241 | 84.8 (65.4 to 104.3) | 195 | 73.7 (54.9 to 92.5) | 680 | 68.1 (58 to 78.2) |
| All | 35,046 | 10 (9.5 to 10.5) | 6,632 | 24.6 (22.8 to 26.5) | 1083 | 52 (45.4 to 58.7) | 42,761 | 13.3 (12.8 to 13.8) |
| Progression to ESRD | ||||||||
| eGFR 2:105 | 4,801 | 0.2 (0 to 0.4) | 845 | 2.3 (0.8 to 3.8) | 68 | 12.1 (0.2 to 23.9) | 5,714 | 0.7 (0.4 to 1) |
| eGFR = 90–<105 | 7,925 | 0.2 (0.1 to 0.4) | 1,138 | 1.8 (0.6 to 3) | 95 | 11.2 (1.4 to 21) | 9,158 | 0.6 (0.3 to 0.8) |
| eGFR = 75–<90 | 8,907 | 0.3 (0.1 to 0.5) | 1,290 | 1.1 (0.2 to 2) | 157 | 15.9 (6.5 to 25.3) | 10,354 | 0.6 (0.4 to 0.9) |
| eGFR = 60–<75 | 7,436 | 0.2 (0 to 0.3) | 1,312 | 3.3 (1.8 to 4.8) | 169 | 14.9 (5.7 to 24.2) | 8,917 | 0.9 (0.6 to 1.2) |
| eGFR = 45–<60 | 4,103 | 1 (0.5 to 1.4) | 1,089 | 4.3 (2.4 to 6.3) | 191 | 23.9 (12.5 to 35.2) | 5,383 | 2.4 (1.8 to 3.1) |
| eGFR = 30–<45 | 1,630 | 2.4 (1.2 to 3.6) | 717 | 15.9 (11.1 to 20.8) | 208 | 69.7 (49.3 to 90) | 2,555 | 10.5 (8.5 to 12.6) |
| eGFR <30 | 244 | 14.7 (6.7 to 22.7) | 241 | 125 (97.9 to 152) | 195 | 220.4 (177.2 to 263.6) | 680 | 97.8 (84.1 to 111.6) |
| All | 35,046 | 0.5 (0.4 to 0.6) | 6,632 | 6.7 (5.7 to 7.7) | 1,083 | 48.6 (41.7 to 55.5) | 42,761 | 2.5 (2.3 to 2.7) |
Abbreviations: ACR, albumin-creatinine ratio (given in mg/g); CI, confidence interval; eGFR, estimated glomerular figure rate (given in mL/min/1.73 m2), ESRD, end-stage renal disease.
Follow-up Period, Crude Rates for Death and ESRD
Median follow-up for all-cause mortality was 4.04 (interquartile range [IQR], 2.22–5.97) years, with a total of 179,746 person-years. Median follow-up for progression to ESRD was 4.18 (IQR, 2.20–5.96) years, with a total of 178,648 person-years. During follow-up, 2,390 participants died (5.59%) and 449 (1.05%) developed incident ESRD.
eGFR, Albuminuria, and Outcomes
Clear trends toward increasing death and higher rates of progression to ESRD occurred with lower eGFR and higher ACR. The unadjusted incidence (per 1,000 person-years) of all-cause mortality increased from 3.1 (95% CI, 2.4–3.8) in participants with eGFR ≥105 mL/min/1.73 m2 and no albuminuria to 73.7 (95% CI, 54.9–92.5) in participants with eGFR <30 mL/min/1.73 m2 and macroalbuminuria (P <0.001; Table 3). Progression to ESRD increased from 0.2 (95% CI, 0.1–0.3) per 1,000 person-years to 220.4 (95% CI, 177.2–263.6) per 1,000 person-years (P <0.001). Results of the LOWESS scatterplots are shown in Fig 1A and B. Probabilities of both death and progression to ESRD were higher in participants with lower eGFRs. At every eGFR value, the probabilities of death and progression to ESRD were higher with higher category of ACR. Figure 1B also shows that in participants with ACR >30 mg/g, a sharp inflection point occurred at eGFR of 40 mL/min/1.73 m2, showing a very high probability of progression to ESRD.
Figure 1.
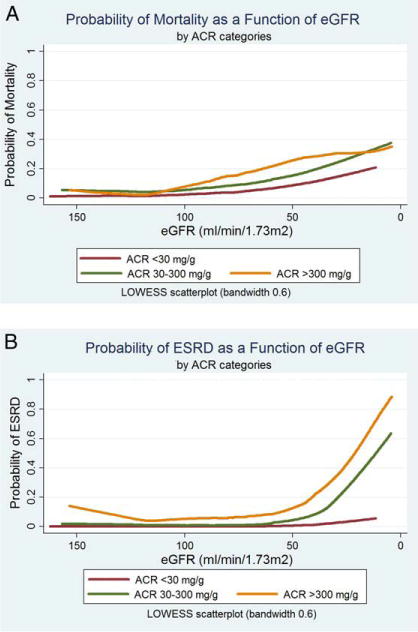
Probability of mortality and progression to end-stage renal disease (ESRD). LOWESS is a curve-fitting tech nique that provides locally weighted scatterplot smoothing. These graphs are produced by the following methodology: for each participant who lived or died (yi), a corresponding smoothed value of estimated glomerular filtration rate (eGFR; xi) was generated. The smoothed values of eGFR were obtained by running a regression of the dependent variable (mortality; yi) on the independent variable (eGFR; xi) and a few data near this point. The regression was weighted so the central point (xi; yi) was given the highest weight, and points farther away (based on the absolute distance |xj – xij|), less weight. The estimated regression line then was used to predict the smoothed value of eGFR. Because a separate weighted regression was performed for every point in the data, the procedure was repeated thou sands of times (exactly 42,761 times) to obtain the remaining smoothed values and the curves. Abbreviation: ACR, albumin-creatinine ratio.
Kaplan-Meier survival analysis estimated that 10-year survival probabilities were 95.2% for eGFR ≥105 mL/min/1.73 m2 and only 44.1% for eGFR<30 mL/min/1.73 m2 (Fig 2A). Survival probabilities were 87.9% for participants without albuminuria (ACR <30 mg/g) and 57.0% for those with macroalbuminuria (ACR >300 mg/g; Fig 2B). Similarly, regarding progression to ESRD, ESRD-free probability was 99.0% for eGFR ≤105mL/min/1.73 m2, but only 50.7% for eGFR <30 mL/min/1.73 m2 (Fig 3A). ESRD-free probability was 99.3% for participants without albuminuria (ACR <30 mg/g), but only 68.7% for those with macroalbuminuria (ACR >300 mg/g; Fig 3B). All log-rank test P values were <0.001 for these comparisons. These findings persisted and remained statistically significant after multivariable adjustment (Table 4). eGFR <30 mL/min/1.73 m2 was associated independently with mortality (HR, 1.74; 95% CI, 1.31–2.31; P <0.001). Albuminuria was a stronger independent predictor of mortality; the HR for participants with ACR >300 mg/g was 3.20 (95% CI, 2.73–3.74). Conversely, for the outcome of progression to ESRD, eGFR <30 mL/min/1.73 m2 was a stronger predictor (HR, 84.20; 95% CI, 46.57–152.22; P <0.001); the HR for albuminuria was 16.88 (95% CI, 12.20–23.36; P <0.001).
Figure 2.
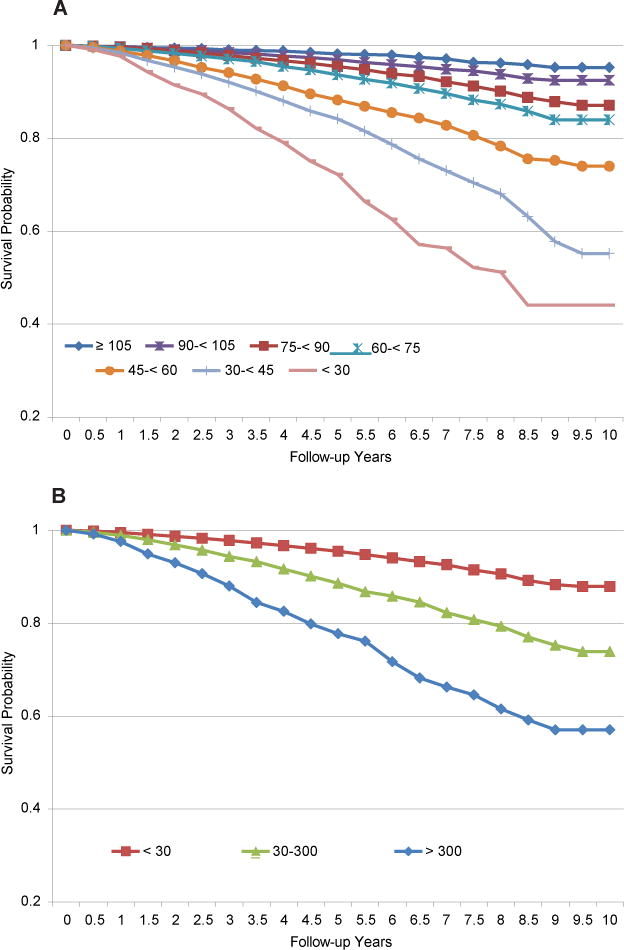
Kaplan-Meier estimates of long-term survival by (A) estimated glomerular rate (eGFR; mL/min/1.73 m2) and (B) albumin-creatinine ratio (ACR; mg/g). Abbreviation: ESRD, end-stage renal disease.
Figure 3.
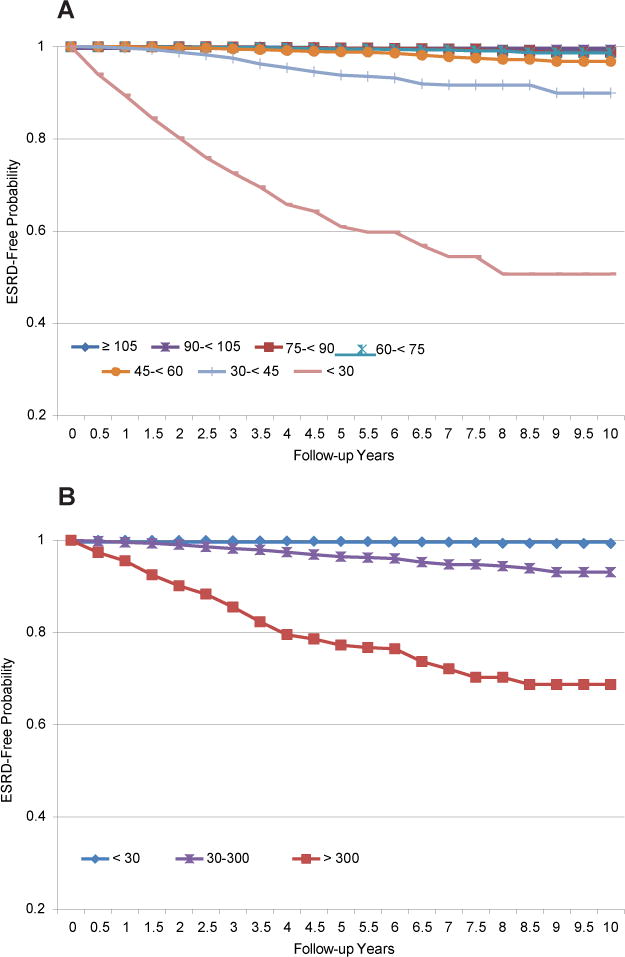
Kaplan-Meier estimates of long-term end-stage renal disease (ESRD) free probability by (A) estimated glomerular rate (eGFR; mL/min/1.73 m2) and (B) albumin-creatinine ratio (ACR; mg/g).
Table 4.
Independent Prognostic Effect of Reduced eGFR and albuminuria on All-Cause Mortality and Progression to ESRD
| All-Cause Mortality
|
Progression to ESRD
|
|||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| eGFR category | ||||
| 2:105 mL/min/1.73 m2 | 1.00 (reference) | 1.00 (reference) | ||
| 90–<105 mL/min/1.73 m2 | 0.84 (0.66–1.06) | 0.1 | 1.51 (0.77–2.93) | 0.2 |
| 75–<90 mL/min/1.73 m2 | 0.88 (0.70–1.11) | 0.3 | 1.83 (0.97–3.47) | 0.06 |
| 60–<75 mL/min/1.73 m2 | 0.92 (0.73–1.16) | 0.5 | 2.86 (1.54–5.33) | <0.001 |
| 45–<60 mL/min/1.73 m2 | 1.23 (0.97–1.56) | 0.08 | 5.93 (3.25–10.80) | <0.001 |
| 30–<45 mL/min/1.73 m2 | 1.40 (1.09–1.80) | 0.009 | 18.48 (10.27–33.22) | <0.001 |
| <30 mL/min/1.73 m2 | 1.74 (1.31–2.31) | <0.001 | 84.20 (46.57–152.22) | <0.001 |
| ACR category | ||||
| <30 mg/g | 1.00 (reference) | 1.00 (reference) | ||
| 30–300 mg/g | 1.79 (1.62–1.97) | <0.001 | 6.44 (4.81–8.61) | <0.001 |
| >300 mg/g | 3.16 (2.70–3.70) | <0.001 | 15.11 (10.90–20.95) | <0.001 |
Note: Both multivariable Cox proportional models adjusted for the following covariates: age, sex, race, insurance status, body mass index, education level, family history of diabetes, hypertension, chronic kidney disease, self-reported hypertension, measured blood pressure, hypercholesterolemia, smoking status, hemoglobin level, diabetes medications, and insulin use.
Abbreviations: ACR, albumin-creatinine ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HR, hazard ratio.
Synergistic Interaction between Albuminuria and eGFR on Outcomes
The unadjusted interaction between eGFR and albuminuria was highly significant for mortality and progression to ESRD (P <0.001 for both). In multivariable analyses, the interaction term of eGFR (using 3 categories: ≥60, 30–59, and ≤30 mL/min/1.73 m2) with albuminuria (3 categories) remained statistically significant (P <0.001 for outcomes of mortality and progression to ESRD). In this fully adjusted model, participants with eGFR <30 mL/min/1.73 m2 and macroalbuminuria were 5 times more likely to die than participants with normal kidney function and no albuminuria (Table 5). Albuminuria alone and the albuminuria-eGFR interaction were stronger predictors of long-term mortality than low eGFR (Wald χ2 statistic, 8.27 for eGFR, 18.07 for albuminuria, and 15.71 for the albuminuria-eGFR interaction).
Table 5.
Synergistic Interaction of eGFR and Albuminuria: Results From Fully Adjusted Multivariable Cox Proportional Models
| ACR <30 | ACR = 30–300 | ACR >300 | |
|---|---|---|---|
| All-cause mortality | |||
| eGFR 2:105 | 1.00 (reference) | 2.67 (1.78–4.02) | 1.83 (0.45–7.49) |
| eGFR = 90–<105 | 0.89 (0.67–1.18) | 1.81 (1.27–2.57) | 3.93 (2.07–7.46) |
| eGFR = 75–<90 | 0.99 (0.76–1.31) | 1.69 (1.22–2.33) | 3.08 (1.87–5.06) |
| eGFR = 60–<75 | 0.98 (0.74–1.29) | 2.00 (1.47–2.73) | 3.46 (2.19–5.47) |
| eGFR = 45–<60 | 1.44 (1.09–1.92) | 2.16 (1.59–2.94) | 4.69 (3.16–6.96) |
| eGFR = 30–<45 | 1.60 (1.18–2.18) | 2.51 (1.82–3.46) | 5.67 (3.94–8.16) |
| eGFR <30 | 2.08 (1.38–3.15) | 4.20 (2.92–6.04) | 4.84 (3.33–7.04) |
| Progression to ESRD | |||
| eGFR 2:105 | 1.00 (reference) | 9.31 (2.79–31.01) | 56.97 (14.17–229.10) |
| eGFR = 90–<105 | 1.93 (0.56–6.62) | 12.33 (3.69–41.19) | 91.43 (24.22–345.18) |
| eGFR = 75–<90 | 3.20 (1.01–10.14) | 8.92 (2.49–31.94) | 100.10 (30.31–330.56) |
| eGFR = 60–<75 | 1.98 (0.52–7.45) | 32.84 (10.92–98.76) | 114.51 (35.14–373.19) |
| eGFR = 45–<60 | 13.03 (4.26–39.86) | 41.43 (13.70–125.23) | 177.68 (58.08–543.57) |
| eGFR = 30–<45 | 32.40 (10.44–100.58) | 155.79 (53.88–450.48) | 510.72 (176.77–1,475.60) |
| eGFR <30 | 161.35 (50.12–519.44) | 1,207.12 (423.99–3,436.68) | 1,530.91 (540.74–4,334.25) |
Note: Values shown are hazard ratio (95% confidence interval). Both multivariable Cox proportional models adjusted for the following covariates: age, sex, race, insurance status, body mass index, education level, family history of diabetes, hypertension, chronic kidney disease, self-reported hypertension, measured blood pressure, hypercholesterolemia, smoking status, hemoglobin level, diabetes medications, and insulin use.
Abbreviations: ACR, albumin-creatinine ratio (in mg/g); eGFR, estimated glomerular filtration rate (in mL/min/1.73 m2); ESRD, end-stage renal disease.
As a final note, participants with eGFR <30 mL/min/1.73 m2 and macroalbuminuria were at extremely high risk of developing ESRD during the median 4 years of follow-up (with HRs >1,000) compared with participants with normal kidney function and no albuminuria (Table 5).
DISCUSSION
In this large national cohort of screened participants with diabetes, we observed varying degrees of albuminuria and eGFR. Albuminuria was absent in 14% of participants with low eGFR. Conversely, 12% of participants had albuminuria only with no decrease in eGFR. Both decreased eGFR and albuminuria were independent predictors of mortality and progression to ESRD; however, albuminuria was a stronger independent predictor of mortality, whereas decreased eGFR was a stronger predictor of progression to ESRD. Additionally, we observed a highly significant interaction between low eGFR and greater degree of albuminuria, such that the presence of both factors amplified the risk of mortality and progression to ESRD beyond what would be expected by the simple combination of their independent effects. In particular, for the outcome of progression to ESRD, the HR for the combined occurrence of albuminuria and decreased eGFR was extremely high, implying that the combination of these 2 factors practically ensured progression to ESRD.
This study has several important implications. To our knowledge, it is the first large national inception cohort study in participants with diabetes to establish the independent prognostic impact of decreased eGFR and albuminuria on long-term mortality and progression to ESRD. Importantly, our study shows a strong synergistic interaction of these risk factors such that their adverse prognostic impact is amplified when both are present. This information can aid clinicians with risk stratification, facilitate appropriate counseling of patients regarding prognosis, and possibly influence the intensity of follow-up and medical management.
These data also emphasize the critical importance of albuminuria as a prognostic factor for progression to ESRD and mortality. Although both eGFR and albuminuria were associated with adverse events, albuminuria was a stronger predictor of mortality. More importantly, the synergistic interaction we observed between albuminuria and eGFR emphasizes the importance of efforts to prevent the onset of albuminuria, detect its presence through aggressive screening and surveillance, and once it is identified, closely monitor patients for further worsening of albuminuria and kidney function.
Compared with prior studies, we observed that albuminuria is a stronger predictor of mortality than low eGFR. In prior studies, this prognostic effect of albuminuria in nondiabetic patients was much more modest (only 1.5- to 2-fold higher risk of mortality across eGFR categories). In a population-level study (with only 5% diabetic patients) by Astor et al23 using NHANES (National Health and Nutrition Examination Survey) data, the risk of death during a median follow-up of 9 years doubled in participants with albuminuria versus those without albuminuria.23 Similarly, in a pooled meta-analysis24 from 21 general-population cohorts with a minority of diabetic patients, the long-term risk of death in the presence of albuminuria increased to twice as high as the risk without albuminuria.24 Our study, in contrast, demonstrates that in patients with diabetes, the prognostic impact of albuminuria is much greater.
Prior studies also tried to assess the interaction between albuminuria and eGFR.23–25 In a large pooled analysis from more than 21 population-level cohorts, the association between albuminuria and mortality remained constant and linear, independent of eGFR and conventional risk factors.24 A large-scale epidemiologic study from NHANES also demonstrated this constant risk relationship across all eGFRs.23 In contrast, our study shows a significant synergistic interaction between ACR and eGFR in a large diabetic population with long-term follow-up, such that the presence of both factors amplifies the risk of adverse events.
What are potential causes of decreased eGFR in the absence of albuminuria in participants with diabetes? Numerous factors could contribute, such as interstitial renal fibrosis, atherosclerosis of the renal arteries and arterioles, and possible cholesterol emboli, which may be underappreciated and may contribute to increasing nephron loss and tubulointerstitial changes.4–8 Pathology studies have identified atypical patterns of injury with renal structural changes, beyond the usual diabetic glomerulosclerosis, including tubulointerstitial changes and arteriolar hyalinosis with or without global glomerular sclerosis.26 Despite nephron loss and tubulointerstitial changes, the absence of albuminuria may be causally related to the favorable prognosis. Albuminuria is associated with the presence of endothelial markers of vascular damage, plasma von Willebrand factor, and thrombomodulin, which have significantly higher levels in diabetic patients with albuminuria than in patients without albuminuria, independent of eGFR.27–29 These results suggest that generalized endothelial damage in multiple vascular territories such as the heart or brain may occur in diabetic nephropathy during the microalbuminuric stage, which is not attributed to kidney damage or nephron loss per se.27–29 In the Framingham Offspring cohort, inflammatory biomarkers such as tumor necrosis factor a, interleukin 6, tumor necrosis factor receptor 2, intercellular adhesion molecule 1, and osteoprotegerin were associated independently with albuminuria and levels were elevated in patients with albuminuria.30 Inflammation is a major mediator of poor vascular outcomes and in conjunction with direct vascular damage, may explain why the absence of albuminuria is associated with favorable outcomes4–8 while its presence is related to adverse events.23,25,31–42
An elegant study from the Swedish National Diabetes Register,43 for which the aim was to identify clinical risk factors associated with the development of albuminuria and decreased kidney function in patients with type 2 diabetes, found that the development of albuminuria was associated independently with high BMI, elevated triglyceride level, low high-density lipoprotein cholesterol level, high SBP, high hemoglobin A1c level, smoking, and male sex (all P <0.001).43 Our study is consistent with this, and we also found that participants with albuminuria were more likely to have a high BMI and stage 2 hypertension and be current smokers. Thus, the risk factors associated with albuminuria are almost all modifiable. Future studies are needed to determine whether aggressive screening and timely interventions to prevent albuminuria could improve outcomes in this high-risk patient group.
Our study should be interpreted in the context of several potential limitations. First, the median duration of follow-up was 4 years. Thus, longer term risks could not be assessed. Second, we used eGFR and albuminuria measurements obtained at baseline, without subsequent repeated testing. Third, because KEEP is a screening program, some data elements are subject to patient recall. Fourth, as with any observational study, unmeasured confounding may exist despite multivariate adjustments. Fifth, eGFR was estimated using the CKD-EPI equation.20 CKD classification among young blacks is very sensitive to the race coefficients and use of the CKD-EPI equation in KEEP, which has large racial heterogeneity, may possibly limit its generalizability to the US population.44 Nonetheless, in KEEP as in other populations, the CKD-EPI equation has been shown to more accurately reclassify people at lower risk of CKD and death into higher eGFR categories, resulting in more accurate risk prediction.45–48 A final note is that prior studies have shown variability in ACRs on repeated ACR testing, such that ~50% of tested adults will not have albuminuria on repeated testing.45–51 This could result in misclassification of the exposure variable, possibly biasing our findings toward the null. Despite this, we observed a strong independent relationship of albuminuria with adverse outcomes. Similarly, blood pressure measured at screening also could vary, possibly resulting in misclassification of hypertension as defined by the JNC 7.
In conclusion, both low eGFR and albuminuria are associated independently with higher rates of mortality and progression to ESRD in KEEP participants with diabetes. A significant synergistic interaction between lower eGFR and greater degree of albuminuria occurs in this group, such that the risk of mortality and progression to ESRD is amplified when both factors are present. Future studies are needed to determine whether early identification of modifiable risk factors for albuminuria and subsequent interventions to prevent its development can improve outcomes of patients with diabetes.
Acknowledgments
The KEEP Investigators are Peter A. McCullough, Adam T. Whaley-Connell, Andrew S. Bomback, Kerri Cavanaugh, Linda Fried, Claudine T. Jurkovitz, Mikhail Kosiborod, Samy McFarlane, Rajnish Mehrotra, Keith Norris, Rulan Savita Parekh, Carmen A. Peralta, Georges Saab, Stephen Seliger, Michael Shlipak, Lesley Inker, Manjula Kurella Tamura, John Wang; ex-officio Bryan Becker, Allan J. Collins, Nilka Ríos Burrows, Lynda A. Szczech, Joseph Vassalotti; advisory group, George Bakris, Wendy Brown; data coordinating center, Shu-Cheng Chen.
We thank the participants and staff who volunteered their time to make the KEEP screening a successful event; Chronic Disease Research Group colleague Nan Booth, MSW, MPH, ELS, for manuscript editing; and Monica R. Gannon, KEEP director, for regulatory assistance.
Support: KEEP is a program of the National Kidney Foundation Inc and is supported by Abbott, Amgen, LifeScan, Siemens, Genentech, GM Foundation, Nephroceuticals, and Pfizer. Dr Amin is supported by grant KM1CA156708, National Cancer Institute, National Institutes of Health (NIH), and grants UL1 TR000448, KL2 TR000450, and TL1 TR000449, Clinical and Translational Science Award, National Center for Advancing Translational Sciences (NCATS), NIH. Manuscript contents are solely the responsibility of the authors and do not necessarily represent the official view of NCATS or NIH. Dr Whaley-Connell is supported by the Department of Veterans Affairs Career Development Award-2, NIH grant R03AG040638, and the American Society of Nephrology-Association of Specialty Professors Development Grant in Geriatric Nephrology. Dr Kosiborod is supported by research grants from the American Heart Association, Medtronic Diabetes, Glumetrics, and Gilead.
Footnotes
Financial Disclosure: Dr Kosiborod is a consultant for Medtronic Diabetes, Genentech, Hoffman La Roche, Gilead, Glumetrics, Boehringer-Ingelheim, and Kowa Pharmaceuticals. The other authors declare that they have no other relevant financial interests.
References
- 1.Vora JP, Ibrahim HA, Bakris GL. Responding to the challenge of diabetic nephropathy: the historic evolution of detection, prevention and management. J Hum Hypertens. 2000;14(10–11):667–685. doi: 10.1038/sj.jhh.1001058. [DOI] [PubMed] [Google Scholar]
- 2.Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4(8):444–452. doi: 10.1038/ncpendmet0894. [DOI] [PubMed] [Google Scholar]
- 3.Soldatos G, Cooper ME. Diabetic nephropathy: important pathophysiologic mechanisms. Diabetes Res Clin Pract. 2008;82(suppl 1):S75–S79. doi: 10.1016/j.diabres.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 4.Penno G, Solini A, Bonora E, et al. Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J Hypertens. 2011;29(9):1802–1809. doi: 10.1097/HJH.0b013e3283495cd6. [DOI] [PubMed] [Google Scholar]
- 5.Macisaac RJ, Tsalamandris C, Panagiotopoulos S, Smith TJ, McNeil KJ, Jerums G. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care. 2004;27(1):195–200. doi: 10.2337/diacare.27.1.195. [DOI] [PubMed] [Google Scholar]
- 6.Macisaac RJ, Panagiotopoulos S, McNeil KJ, et al. Is nonalbuminuric renal insufficiency in type 2 diabetes related to an increase in intrarenal vascular disease? Diabetes Care. 2006;29(7):1560–1566. doi: 10.2337/dc05-1788. [DOI] [PubMed] [Google Scholar]
- 7.Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA. 2003;289(24):3273–3277. doi: 10.1001/jama.289.24.3273. [DOI] [PubMed] [Google Scholar]
- 8.Thomas MC, Macisaac RJ, Jerums G, et al. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (National Evaluation of the Frequency of Renal Impairment Co-existing With NIDDM [NEFRON] 11) Diabetes Care. 2009;32(8):1497–1502. doi: 10.2337/dc08-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson CA, Levey AS, Coresh J, Levin A, Lau J, Eknoyan G. Clinical practice guidelines for chronic kidney disease in adults: part II. Glomerular filtration rate, proteinuria, and other markers. Am Fam Physician. 2004;70(6):1091–1097. [PubMed] [Google Scholar]
- 10.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67(6):2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 12.Berhane AM, Weil EJ, Knowler WC, Nelson RG, Hanson RL. Albuminuria and estimated glomerular filtration rate as predictors of diabetic end-stage renal disease and death. Clin J Am Soc Nephrol. 2011;6(10):2444–2451. doi: 10.2215/CJN.00580111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rigalleau V, Lasseur C, Raffaitin C, et al. Normoalbuminuric renal-insufficient diabetic patients: a lower-risk group. Diabetes Care. 2007;30(8):2034–2039. doi: 10.2337/dc07-0140. [DOI] [PubMed] [Google Scholar]
- 14.Ohmit SE, Flack JM, Peters RM, Brown WW, Grimm R. Longitudinal study of the National Kidney Foundation’s (NKF) Kidney Early Evaluation Program (KEEP) J Am Soc Nephrol. 2003;14((7)(suppl 2)):S117–S121. doi: 10.1097/01.asn.0000070155.63971.b2. [DOI] [PubMed] [Google Scholar]
- 15.Brown WW, Peters RM, Ohmit SE, et al. Early detection of kidney disease in community settings: the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2003;42(1):22–35. doi: 10.1016/s0272-6386(03)00405-0. [DOI] [PubMed] [Google Scholar]
- 16.McCullough PA, Steigerwalt S, Tolia K, et al. Cardiovascular disease in chronic kidney disease: data from the Kidney Early Evaluation Program (KEEP) Curr Diabetes Rep. 2011;11(1):47–55. doi: 10.1007/s11892-010-0162-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCullough PA, Whaley-Connell A, Brown WW, et al. Cardiovascular risk modification in participants with coronary disease screened by the Kidney Early Evaluation Program. Intern Med J. 2010;40(12):833–841. doi: 10.1111/j.1445-5994.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- 18.Whaley-Connell A, Bomback AS, McFarlane SI, et al. Diabetic cardiovascular disease predicts chronic kidney disease awareness in the Kidney Early Evaluation Program. Cardiorenal Med. 2011;1(1):45–52. doi: 10.1159/000322862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens LA, Li S, Kurella TM, et al. Comparison of the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study equations: risk factors for and complications of CKD and mortality in the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2011;57((3)(suppl 2)):S9–S16. doi: 10.1053/j.ajkd.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74(368):829–836. [Google Scholar]
- 23.Astor BC, Hallan SI, Miller ER, III, Yeung E, Coresh J. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol. 2008;167(10):1226–1234. doi: 10.1093/aje/kwn033. [DOI] [PubMed] [Google Scholar]
- 24.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79(12):1331–1340. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fioretto P, Mauer M, Brocco E, et al. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia. 1996;39(12):1569–1576. doi: 10.1007/s001250050616. [DOI] [PubMed] [Google Scholar]
- 27.Gaede P, Vedel P, Parving HH, Pedersen O. Elevated levels of plasma von Willebrand factor and the risk of macro- and microvascular disease in type 2 diabetic patients with microalbuminuria. Nephrol Dial Transplant. 2001;16(10):2028–2033. doi: 10.1093/ndt/16.10.2028. [DOI] [PubMed] [Google Scholar]
- 28.Vischer UM, Emeis JJ, Bilo HJ, et al. von Willebrand factor (vWf) as a plasma marker of endothelial activation in diabetes: improved reliability with parallel determination of the vWf propeptide (vWf:AgII) Thromb Haemost. 1998;80(6):1002–1007. [PubMed] [Google Scholar]
- 29.Hirano T, Ookubo K, Kashiwazaki K, Tajima H, Yoshino G, Adachi M. Vascular endothelial markers, von Willebrand factor and thrombomodulin index, are specifically elevated in type 2 diabetic patients with nephropathy: comparison of primary renal disease. Clin Chim Acta. 2000;299(1–2):65–75. doi: 10.1016/s0009-8981(00)00274-6. [DOI] [PubMed] [Google Scholar]
- 30.Upadhyay A, Larson MG, Guo CY, et al. Inflammation, kidney function and albuminuria in the Framingham Offspring cohort. Nephrol Dial Transplant. 2011;26(3):920–926. doi: 10.1093/ndt/gfq471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bello AK, Hemmelgarn B, Lloyd A, et al. Associations among estimated glomerular filtration rate, proteinuria, and adverse cardiovascular outcomes. Clin J Am Soc Nephrol. 2011;6(6):1418–1426. doi: 10.2215/CJN.09741110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tonelli M, Klarenbach SW, Lloyd AM, et al. Higher estimated glomerular filtration rates may be associated with increased risk of adverse outcomes, especially with concomitant proteinuria. Kidney Int. 2011;80(12):1306–1314. doi: 10.1038/ki.2011.280. [DOI] [PubMed] [Google Scholar]
- 33.Gansevoort RT, Nauta FL, Bakker SJ. Albuminuria: all you need to predict outcomes in chronic kidney disease? Curr Opin Nephrol Hypertens. 2010;19(6):513–518. doi: 10.1097/MNH.0b013e32833e4ce1. [DOI] [PubMed] [Google Scholar]
- 34.Weiner DE, Tabatabai S, Tighiouart H, et al. Cardiovascular outcomes and all-cause mortality: exploring the interaction between CKD and cardiovascular disease. Am J Kidney Dis. 2006;48(3):392–401. doi: 10.1053/j.ajkd.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 35.Arnlov J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112(7):969–975. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 36.Solomon SD, Lin J, Solomon CG, et al. Influence of albuminuria on cardiovascular risk in patients with stable coronary artery disease. Circulation. 2007;116(23):2687–2693. doi: 10.1161/CIRCULATIONAHA.107.723270. [DOI] [PubMed] [Google Scholar]
- 37.Berhane AM, Weil EJ, Knowler WC, Nelson RG, Hanson RL. Albuminuria and estimated glomerular filtration rate as predictors of diabetic end-stage renal disease and death. Clin J Am Soc Nephrol. 2011;6(10):2444–2451. doi: 10.2215/CJN.00580111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foster MC, Hwang SJ, Larson MG, et al. Cross-classification of microalbuminuria and reduced glomerular filtration rate: associations between cardiovascular disease risk factors and clinical outcomes. Arch Intern Med. 2007;167(13):1386–1392. doi: 10.1001/archinte.167.13.1386. [DOI] [PubMed] [Google Scholar]
- 39.Cerasola G, Mule G, Cottone S, Nardi E, Cusimano P. Hypertension, microalbuminuria and renal dysfunction: the Renal Dysfunction in Hypertension (REDHY) Study. J Nephrol. 2008;21(3):368–373. [PubMed] [Google Scholar]
- 40.Bouchi R, Babazono T, Yoshida N, et al. Association of albuminuria and reduced estimated glomerular filtration rate with incident stroke and coronary artery disease in patients with type 2 diabetes. Hypertens Res. 2010;33(12):1298–1304. doi: 10.1038/hr.2010.170. [DOI] [PubMed] [Google Scholar]
- 41.Ford I, Bezlyak V, Stott DJ, et al. Reduced glomerular filtration rate and its association with clinical outcome in older patients at risk of vascular events: secondary analysis. PLoS Med. 2009;6(1):e16. doi: 10.1371/journal.pmed.1000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–1352. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 43.Afghahi H, Cederholm J, Eliasson B, et al. Risk factors for the development of albuminuria and renal impairment in type 2 diabetes—the Swedish National Diabetes Register (NDR) Nephrol Dial Transplant. 2011;26(4):1236–1243. doi: 10.1093/ndt/gfq535. [DOI] [PubMed] [Google Scholar]
- 44.Peralta CA, Lin F, Shlipak MG, et al. Race differences in prevalence of chronic kidney disease among young adults using creatinine-based glomerular filtration rate-estimating equations. Nephrol Dial Transplant. 2010;25(12):3934–3939. doi: 10.1093/ndt/gfq299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McFarlane SI, McCullough PA, Sowers JR, et al. Comparison of the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study equations: prevalence of and risk factors for diabetes mellitus in CKD in the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2011;57((3)(suppl 2)):S24–S31. doi: 10.1053/j.ajkd.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pugliese G, Solini A, Bonora E, et al. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation provides a better definition of cardiovascular burden associated with CKD than the Modification of Diet in Renal Disease (MDRD) Study formula in subjects with type 2 diabetes. Atherosclerosis. 2011;218(1):194–199. doi: 10.1016/j.atherosclerosis.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 47.Stevens LA, Li S, Kurella TM, et al. Comparison of the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study equations: risk factors for and complications of CKD and mortality in the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2011;57((3)(suppl 2)):S9–S16. doi: 10.1053/j.ajkd.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD Study equation for estimated glomerular filtration rate. JAMA. 2012;307(18):1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newman DJ, Mattock MB, Dawnay AB, et al. Systematic review on urine albumin testing for early detection of diabetic complications. Health Technol Assess. 2005;9(30):iii–163. doi: 10.3310/hta9300. [DOI] [PubMed] [Google Scholar]
- 50.Watts GF, Taub NA, Mazurkiewicz J, Shaw KM. An examination of the covariability of subclinical albuminuria in insulin-dependent diabetes mellitus: implications for monitoring microalbuminuria. Diabetes Res Clin Pract. 1993;21(2–3):177–185. doi: 10.1016/0168-8227(93)90067-f. [DOI] [PubMed] [Google Scholar]
- 51.Watts GF, Kubal C, Chinn S. Long-term variation of urinary albumin excretion in insulin-dependent diabetes mellitus: some practical recommendations for monitoring microalbuminuria. Diabetes Res Clin Pract. 1990;9(2):169–177. doi: 10.1016/0168-8227(90)90109-7. [DOI] [PubMed] [Google Scholar]


