Abstract
Gastrointestinal dysfunction remains a major cause of morbidity and mortality. Indeed, gastrointestinal (GI) motility in health and disease remains an area of productive research with over 1,400 published animal studies in just the last 5 years. Numerous techniques have been developed for quantifying smooth muscle activity of the stomach, small intestine, and colon. In vitro and ex vivo techniques offer powerful tools for mechanistic studies of GI function, but outside the context of the integrated systems inherent to an intact organism. Typically, measuring in vivo smooth muscle contractions of the stomach has involved an anesthetized preparation coupled with the introduction of a surgically placed pressure sensor, a static pressure load such as a mildly inflated balloon or by distending the stomach with fluid under barostatically-controlled feedback. Yet many of these approaches present unique disadvantages regarding both the interpretation of results as well as applicability for in vivo use in conscious experimental animal models. The use of dual element strain gages that have been affixed to the serosal surface of the GI tract has offered numerous experimental advantages, which may continue to outweigh the disadvantages. Since these gages are not commercially available, this video presentation provides a detailed, step-by-step guide to the fabrication of the current design of these gages. The strain gage described in this protocol is a design for recording gastric motility in rats. This design has been modified for recording smooth muscle activity along the entire GI tract and requires only subtle variation in the overall fabrication. Representative data from the entire GI tract are included as well as discussion of analysis methods, data interpretation and presentation.
Keywords: Bioengineering, Issue 91, gastrointestinal tract, gastric contractions, motility, in vivo recording, physiology, neuroscience, strain gage
Introduction
Experimental studies that record in vivo gastrointestinal (GI) motility across a number of experimental conditions remain a powerful tool for understanding the underlying normal and pathophysiological processes necessary for nutrient homeostasis. Traditionally, numerous experimental methodologies, some with similarities to those found in clinical practice 1, have been employed to directly quantify changes in GI contraction rate 2-5, intraluminal pressure 6, 7, or the GI transit of non-absorbable markers 8, 9 or stable isotopes 10-12. Each of these techniques has unique advantages and disadvantages, which have been addressed previously in the literature. For example, the utility of balloon manometry to quantify pressure changes has been questioned due to the inherent compliance of the balloon material while gastrointestinal recovery of nonabsorbable markers requires euthanizing the experimental animal for a single data point. Recently, the application and validation of a miniaturized arterial pressure catheter has been reported that offers a non-surgical method for monitoring gastric contractility in rats and mice 3. While an orogastrically placed pressure transducer effectively eliminates confounding variables on gastrointestinal function by avoiding invasive surgical procedures, such an approach is only suitable for anesthetized preparations. Furthermore, the lack of visual guidance does not permit consistent placement of the transducer within specific regions of the stomach. As such, this application is restricted to the stomach or colon since visualization, coupled with the relatively stiff transducer wire, within the duodenum or ileum is not an option.
Similarly, the biomagnetic alternate current biosusceptometry (ACB) technique has been validated for GI contraction analysis 4. While the ACB technique provides a noninvasive approach for measuring gastrointestinal contractions, ACB suffers from a similar limitation in that the use of ingested magnetic detection media does not permit precise recording of specific regions of the GI tract. This limitation can be overcome through the surgical implantation of magnetic markers. Nonetheless, the ACB technique necessitates that the animal be anesthetized for data collection.
Ultrasonomicrometry has been employed in some GI studies 13, 14 in order to take advantage of the small size, spatial, and temporal advantages of piezoelectric crystal transmitter/receivers. Waves of gastric smooth muscle contraction are not a high-frequency event and occur at a rate of approximately 3 - 5 cycles/min. Therefore, the temporal advantages of sonomicrometry may be unnecessary to justify the cost. Furthermore, while linear motion is accurately measured with sonomicrometry, limitations have been presented regarding accurate gastrointestinal data interpretation that may result from implanting an insufficient number of crystals 14.
Based upon the original designs of Bass and colleagues 2, 15 this visualized protocol more fully documents the step-by-step fabrication and experimental application of miniature, dual element strain gages that possess high sensitivity and flexibility for recording smooth muscle contractions along the entire GI tract. The dimensions of the strain gage elements are suitable for any rodent application since sensitivity and size of the finished strain gage are most dependent upon the silicone sheets encapsulating the elements. These strain gages are readily adapted for acute and chronic application in anesthetized and freely behaving laboratory animal models thereby providing a single technique for quantifying smooth muscle contractions.
Protocol
All procedures followed National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee at the Penn State Hershey College of Medicine. Rats were housed using common vivarium practices. Note: This protocol uses male Wistar rats ≥8 weeks of age and initially weighing 175 - 200 g.
1. Procedures for Fabrication of Strain Gage
Most tooling and components remain available from the original or successor companies and are summarized in Table 1.
- Preparation and Bonding of two single element strain gages
- Handle the strain gage elements (EA-06-031-350) carefully with clean Dumont #5 forceps. To limit unwanted movement of elements, use a small, clean, self-adhesive piece of paper with the adhesive side facing up to secure elements to the work surface without the risk of contamination or excessive adhesion.
- Bond two single strain gage elements back-to-back, to form a dual element. Clean the back of each element film with Isopropyl alcohol and allow drying by evaporation (drying with gauze often introduces fiber contaminants that are difficult to remove). Under stereomicroscope guidance (1 - 3x), and using a clean artist brush (10-0 camel hair), apply a thin film of epoxy-phenolic adhesive to the back of one element and immediately place the opposing back of the second element in contact and align the foil grids (Figure 1A).
- Place the bonded elements in a 50 - 60 °C oven O/N to fully cure the epoxy. NOTE: Do not clamp the bonded elements since excess epoxy may seep onto the elements and pressure may cause misalignment of the grids. The two-part epoxy has a usable refrigerated-life of only 6 weeks after mixing. Bond and cure a sufficient supply of elements at one time and store them in a clean, dust free, environment for later use.
- Sizing and wiring dual element strain gages
- Trim bonded dual elements to a final size of 3 x 3 mm with a #11 scalpel or single edge razor blade. Delay trimming the topmost portion of the dual elements at this time in order to have an area for safely handling the element (Figure 1A).
- Each element requires a four-conductor wire fabricated from three-conductor, bondable, Teflon insulated wire (P/N 336-FTE). Disassemble one 30 cm braided strand of three-conductor wire into three constituent wires.
- To make a four wire cable, pair one of the resulting single wires with the like-colored wire contained within a second 30 cm length of three conductor wire. In the following steps, these matching colored wires will be joined at the terminal end to form a common wire for the final strain gage (Figure 1B).
- Remove approximately 1mm of Teflon insulation from both ends of each wire with thermal wire strippers. Using activated rosin soldering flux and low temperature solder (melting point 183 ºC) tin the wire ends with a soldering pencil.
- For the next stage, a microsoldering tip is needed to form a more discrete solder joint to prevent heat damage to the film layer of the element (Figure 1C). To fabricate a smaller microsoldering tip, wrap a small piece of copper wire (~0.25 mm diameter) once around the standard soldering tip, ensuring that the copper wire extends beyond the length of the standard soldering tip.
- Flux just the solder pads on one side of the bonded dual element with a clean 10-0 brush and solder one single lead and one of the paired common leads to the solder pad (Figure 1D). Residual flux can be removed afterward with a clean brush dipped in resin solvent.
- Repeat the process on the opposite side, ensuring that the remaining common wire lead is soldered to the pad opposite the original common lead.
- Testing and epoxying dual element strain gages
- Solder gold socket connectors (E363/0) to the free ends of the wire leads. At this point, connect the strain gage to a recording amplifier (described below) to test the integrity of the dual element assembly.
- Measure the resistance with a good quality volt-ohm meter. Elements register a resistance of approximately 350 Ω. Resolder inadequate connections at this point with fresh solder.
- If the solder connections and the dual element assembly are deemed satisfactory, trim off any remaining element film.
- Insulate the solder joints on the element solder pads with a thin layer of two-part silicone-rubber epoxy resin (P/N E211). For best results, partly cure the resin for 20 - 30 min prior to application (Figure 1E).
- Encapsulating dual element strain gages in silicone
- Cut three pieces of 0.5 mm thick silicone sheet (P/N 20-20) to 15 mm 2 and clean the silicone with distilled water. Cut one piece of silicone sheet into a U-shape in order to accommodate the final dual element assembly without deforming the encapsulating silicone (Figure 1F).
- Coat the inner surfaces of the notch-free silicone sheets with clear silicone adhesive.
- Sandwich the dual element assembly within the notch and the aligned outer sheets, then gently press out any excess silicone, as well as air bubbles, from the center outward. Carefully clamp the encapsulated assembly between two blocks of metal bar stock for 24 hr to ensure uniform thickness and that no deformations occur.
- Allow the excess silicone to remain along the boundaries of the assembly and cure. This excess will be removed when the sheet silicone is trimmed to the desired final dimensions (commonly 6 mm x 8 mm; Figure 1G).
- Completion of wire connector and calibration
- Reinforce the solder joint of the gold socket connectors on the individual terminal wire leads with 3 mm (1/8 inch) shrink tubing and align within a plastic electrode pedestal (MS363, Figure 1H). Secure the electrode pedestal and wires with 0.125 and 0.25 inch diameter shrink tubing to prevent disconnection during the experiment (Figure 1I).
- Strain gage signals are processed through a high gain bridge amplifier (P/N AMP-01-SG). Connect the strain gage to the amplifier using a cable with a mated plug (363 - SL/6) to match the electrode pedestal. The threaded cap provides additional security to maintain uninterrupted signals during the experiment.
- Adjust the Bridge, Balance and Gain settings on the amplifier to a dedicated strain gage per manufacturer instructions. Affix the end of the strain gage where the wires exit horizontally to a rigid clamp and calibrate by placing a 1 g static load on the opposite end as originally described by Pascaud and colleagues 2, 16, 17.
2. Surgical Procedures for Acute Implantation of Strain Gage
- Animal Care and Preparation:
- Food deprive experimental animals the night before surgical implantation (water may be provided ad libitum).
- Deeply anesthetize the animal. Thiobutabarbital (100 – 150 mg/kg; i.p. for rats) is preferred for terminal (i.e., non-survival) strain gage implantation and experimentation due to sustained anesthetic effect and minimal alteration of gastric reflexes in the rat 10. Test for absence of paw pinch reflex to determine depth of anesthesia.
- Prepare the rat for aseptic surgery as dictated by the experimental design and approved IACUC guidelines including sterilizing surgical tools, shaving incision sites, applying vet eye ointment and disinfecting all surgical areas. NOTE: If the strain gage is being adapted for chronic use, sterilize the the strain gage only with ethylene oxide (gas) sterilization. The use of heat or chemical sterilization techniques may damage the strain gage.
- Tracheal intubation for terminal experiments:
- For long duration, terminal, experiments intubate the rat with a tracheal tube to maintain an open airway. Make a 1 - 2 cm midline incision on the ventral side of the neck from the inferior border of the mandible to the sternal notch.
- Separate the underlying strap muscles using blunt dissection at the midline to expose the trachea. Isolate the trachea from the underlying esophagus and place a loop of 3-0 ethilon suture between the trachea and esophagus to form a ligature.
- Open the trachea anteriorly by making a small incison in the membrane between two of the cartilaginous rings of the trachea just distal to the thyroid gland. Insert a small piece of polyethylene tubing (P/N PE-270), 5mm in length (and beveled at one end) into the trachea and secure it into place with the ligature.
- Put the strap muscles back in place and suture the overlying skin with 3-0 ethilon.
- Strain gage instrumentation to gastrointestinal surface:
- Thread the four corners of the strain gage with 4 - 5 cm lengths of 4-0, or smaller, sterile silk suture using a #14 taper point 3/8 circle needle prior to surgery. Silk suture provides a high level of flexibility and is less likely to damage the silicone encapsulating the strain gage element. NOTE: silk thread is acceptable for non-survival surgeries and for internal applications, where the wicking of bacteria across an epithelial barrier is not a risk. In applications requiring survival surgery, a prolene suture is necessary in order to reduce the risk of infection inherent in the braided cloth fibers of the silk suture.
- Perform a laparotomy by incising the abdominal skin along the midline. Section the rectus abdominus musculature along the connecting linea alba (avascular) to prevent bleeding. Then make a very superficial midline incision in the parietal peritoneum to avoid lacerating underlying abdominal viscera.
- Exteriorize the stomach with the aid of saline soaked cotton tipped applicators. Keep the stomach in position by carefully placing it on a saline soaked gauze pad at the caudal end of the abdominal incision.
- Align the grid of the encapsulated strain gage in parallel with the circular smooth muscle fibers. Using the previously threaded sutures (step 2.3.1), attach the corners of the gage to the ventral serosal surface of the gastric corpus using a #14 taper point 3/8 circle needle. In order to minimize tissue damage and potential bleeding, do not use cutting-edged needles and do not perforate any superficial blood vessels on the surface of the stomach.
- Begin the suture pattern of the gage along the greater curvature of the stomach near the fundus/corpus boundary and proceed next along the fundus/corpus boundary toward the lesser curvature. The serosa underlying the strain gage should neither be slack nor overly stretched in order to obtain the best results.
- Carefully return the stomach to its anatomical position using saline-soaked cotton tipped applicators.
- In an acute model, exteriorize the strain gage leads at the caudal end of the midline incision before closure of the abdominal incision. Secure the free wires to the animal (e.g., hind foot) in order to provide strain relief during manipulation of the animal or terminal wire connector. Close the rectus abdominus muscles and the abdominal skin separately with 3-0 nylon suture. In a chronic model, secure the leads subcutaneously along the dorsal side of the rat and exteriorize them above the skull 18.
- After surgical instrumentation, place animals in a stereotaxic frame to support the head and elevate the upper torso. The latter step helps to reduce respiration artifact during recording. Monitor rectal temperature and maintain at 37±1 °C using a feedback-controlled heating pad.
- At the conclusion of terminal experiments utilizing thiobutabarbital anesthesia, the animal must be euthanized in a manner consistent with American Veterinary Medical Association (AVMA) Guidelines on Euthanasia.
- Gastric Motility recordings:
- Amplify the strain gage signal with any commercially available DC bridge amplifier.
- Record the DC output signals on a computer using the chart recorder function of any commercially available data acquisition system. NOTE: A hardcopy of the amplifier output can be generated through a polygraph chart recorder.
3. Representative Measurement of Gastric Contractions Following Brainstem Stimulation
- Exposure of brainstem and fourth ventricle
- After surgical instrumentation, and placement of the Thiobutabarbital-anesthetized animal in a stereotaxic frame, make a 1.5 - 2 cm midline skin incision from the occipital bone toward the base of the neck.
- Separate the connective tissue joining the bilateral muscle bellies of the underlying neck muscles along the midline (muscles from superficial to deep are levator auris longus cranial portion, levator auris longus caudal portion, and platysma cranial portion).
- Detach the levator auris longus from the occipital bone once the midline is clearly defined and exposed.
- Carefully expose the caudal region of the skull by using blunt dissection to detach platysma muscle from the underlying dura mater.
- Use a new 25 G needle to carefully detach the dura mater along the foramen magnum extending bilaterally to the occipital condyles.
- Use #5 Dumont forceps to remove the pia and arachnoid meninges overlying the fourth ventricle and expose the brainstem.
- Administering fourth ventricle thyrotropin releasing hormone or intravenous sodium nitroprusside
- Weigh and dissolve thyrotropin releasing hormone (TRH) in sterile saline to reach a final concentration of 50 µM TRH.
- Weigh and dissolve sodium nitroprusside (SNP) in sterile saline to reach a final concentration of 150 µM SNP.
- Using a 10 µl syringe, administer 2 µl of TRH (final dose equals 100 pmol) to the dorsal surface of the brainstem fourth ventricle to facilitate recording of gastric contractions.
- Using a sterile syringe and 27 G needle, administer 150 µmol/kg of SNP through the tail vein to facilitate recording of gastric relaxation.
Representative Results
Representative data from a Thiobutabarbital-anesthetized rat are shown in Figure 2. The top trace represents the gastric corpus contractions from the rat during the brainstem administration of thyrotropin releasing hormone (TRH, 100 pmol), a known motility-enhancing peptide 3, 19. It shows baseline contractions prior to the increase in phasic gastric smooth muscle activity. Note: Analysis of these peaks in gastric contractions follow the original formula devised by Ormsby and Bass 20
Motility Index= (N1x1) + (N2x2) + (N3x4) + (N4x8)
Based upon this formula, N equals the total number of peaks in a particular milligram range. Therefore, presuming that a 0 mg signal is indicative of no gastric motility, the grouping of peak-to-peak sinusoidal signals may be calculated as 25 - 50 mg, 60 - 100 mg, 110 - 200 mg and signals greater than 210 mg for N1 through N4, respectively. This formula is less sensitive to baseline tone fluctuations that naturally occur across several seconds or minutes. Such fluctuations would have to be subtracted in order to generate valid area using under the curve measurements 3.
The second trace demonstrates a reduction in baseline gastric smooth muscle tone from the same animal in response to the nitric oxide donor, sodium nitroprusside (150 µmol/kg iv). Data representing an inhibition of gastric smooth muscle activity are readily analyzed by the reduction in signal voltage between baseline and maximal response. This voltage signal can then be used to derive the equivalent static load, in grams, if the strain gage was calibrated prior to the experiment. These representative data demonstrate the bidirectional capabilities of a dual element strain gage that has been properly attached to the gastric serosa.
The third trace represents basal smooth muscle contractions recorded by a subminiature strain gage sutured to the serosal surface of the duodenum of a fasted rat. The orientation of the strain gage elements were also in parallel with the circular muscle of the duodenum.
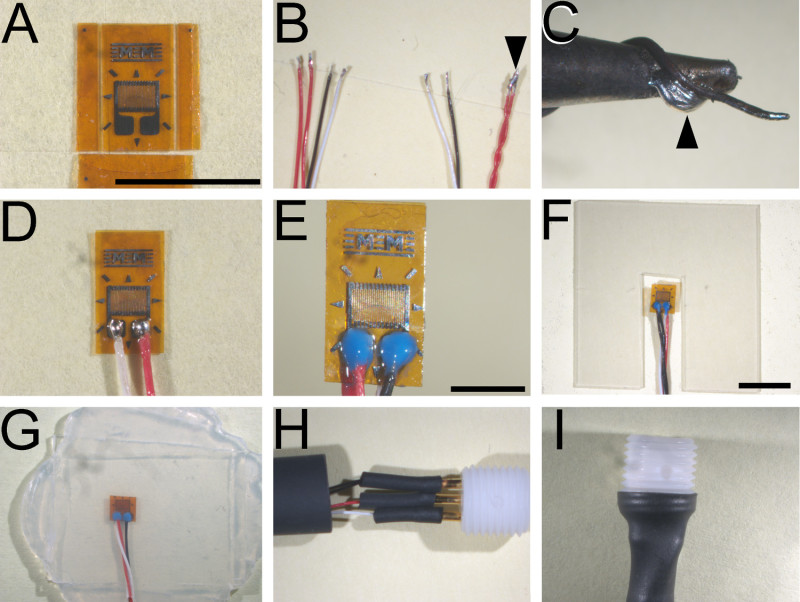 Figure 1. Principal stages of strain gage fabrication. (A) Dual bonded elements that have been trimmed on three of four sides to final dimensions. (B) Representative ends of wires configured for attachment to gage elements (left) and terminal connectors (right). Note that dual red leads are joined only at the terminal end (arrowhead). (C) Representative placement of a strand of copper wire in proximity to fine (1.5 mm) soldering tip. Maintaining fresh solder along this junction (arrowhead) ensures sufficient heat transfer through the micro tip to melt 63% Tin: 36.65% Lead: 0.35% Antimony solder. (D) Representative extent of solder joints between wire leads and solder pads on the gage element. (E) Properly potted solder joints. (F) Representative notch in the internal silicone laminate sheet to accommodate strain gage element without deforming completed element. (G) Bonded layers of silicone sheets (three in total) forming a completed strain gage prior to final sizing. (H) Wire connections to gold plated sockets are reinforced with layers of succeedingly larger diameter shrink tubing before insertion into electrode pedestal. (I) Final shrink wrap affixing of terminal connectors and electrode pedestal. Calibration bars: (A - D), 5 mm; (E), 2 mm; & (F - I), 5 mm. Please click here to view a larger version of this figure.
Figure 1. Principal stages of strain gage fabrication. (A) Dual bonded elements that have been trimmed on three of four sides to final dimensions. (B) Representative ends of wires configured for attachment to gage elements (left) and terminal connectors (right). Note that dual red leads are joined only at the terminal end (arrowhead). (C) Representative placement of a strand of copper wire in proximity to fine (1.5 mm) soldering tip. Maintaining fresh solder along this junction (arrowhead) ensures sufficient heat transfer through the micro tip to melt 63% Tin: 36.65% Lead: 0.35% Antimony solder. (D) Representative extent of solder joints between wire leads and solder pads on the gage element. (E) Properly potted solder joints. (F) Representative notch in the internal silicone laminate sheet to accommodate strain gage element without deforming completed element. (G) Bonded layers of silicone sheets (three in total) forming a completed strain gage prior to final sizing. (H) Wire connections to gold plated sockets are reinforced with layers of succeedingly larger diameter shrink tubing before insertion into electrode pedestal. (I) Final shrink wrap affixing of terminal connectors and electrode pedestal. Calibration bars: (A - D), 5 mm; (E), 2 mm; & (F - I), 5 mm. Please click here to view a larger version of this figure.
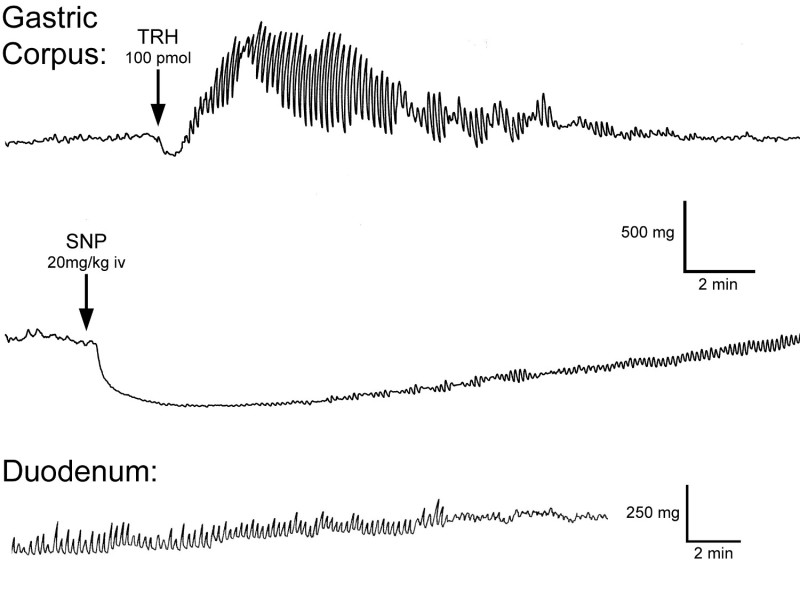 Figure 2. Representative motility traces generated with fabricated dual element strain gages. Recordings made from the anterior gastric corpus during an increase in gastric contractions (top trace) and during an inhibition of gastric contractions (middle trace) and duodenum (bottom trace) of fasted rats (200 - 250 g).
Figure 2. Representative motility traces generated with fabricated dual element strain gages. Recordings made from the anterior gastric corpus during an increase in gastric contractions (top trace) and during an inhibition of gastric contractions (middle trace) and duodenum (bottom trace) of fasted rats (200 - 250 g).
Discussion
The procedures presented here allow individual laboratories to fabricate sensitive miniature strain gages for biological applications including, but not limited to, gastrointestinal motility in small laboratory animals. Since the commercial manufacture of these strain gages has ceased, laboratories investigating gastrointestinal function are limited to other techniques which may not permit the full range of experimental applications that are available. This report provides an updated and more detailed description of previously described techniques 15. The text and accompanying video specifically address solutions to common pitfalls that we recognized during development and mastery of the fabrication process.
Each step, as described, presents techniques to successful fabrication. Careful attention to cleanly and securely soldering all connections as well as avoiding damage to the element with excessive heat from the soldering process are the most frequent challenges to success. The fine gage wire is prone to breaking if it is not properly reinforced with shrink tubing or silicone epoxy and will result in an absence of signal when the gage is gently flexed. A strain gage with a broken or disconnected wire in the vicinity of the gold connectors within the plastic terminal pedestal is the most common failure of a previously functional gage. Individual gages can be carefully disassembled by removing the shrink tubing in order to expose the broken wire. After resoldering the wire to the gold connector, the entire gage is reassembled with new shrink tubing.
With a bit of practice and careful attention to fabricating strain gages of uniform dimensions, affixing strain gages relative to clear landmarks (e.g., greater gastric curvature, fundus/corpus boundary), and avoiding damage to the vasculature, novice users will rapidly develop the ability to achieve consistent results.
Encapsulating the dual element in three layers of silicone creates a durable and flexible, yet highly sensitive strain gage that will last over repeated use with proper care. The high sensitivity of an unencapsulated strain gage is minimally affected by any resistance that is imparted by the silicon laminate. Thinner silicone sheets (P/N 20-05) are recommended in order to modify the gage for intestinal applications or for fabricating smaller gages for mice and discrete gut regions such as sphincters and esophagus. Extra caution is required since thinner gages have diminished resistance to tearing of the silicone sheet during implantation.
Surgical difficulties with the use of these gages often result from excessive manipulation of the visceral organs or misalignment of the gage during implantation. The former likely initiates neural and inflammatory processes that directly lead to impaired GI motility, 9, 21 though both pitfalls are easily remedied by refinement of surgical technique. This may include altering the length and starting point of the midline incision into the abdomen as well as minimizing the manipulation of the viscera during exteriorization and replacement of the stomach.
The validity and fidelity of these strain gages have been discussed previously 2, 15. We, and others, routinely measure gastric smooth muscle activity in acute, anesthetized preparations 16, 22. With adequate instrumentation, a single investigator can instrument and acquire data from up to four animals in a single day. Additionally, implantation of multiple gages within the same animal allows one to measure the relationship between adjacent, or distant, regions of the gastrointestinal tract.
In summary, the fabrication of these subminiature strain gages allows for a wider range of studies utilizing a common array of implantation techniques, instrumentation and data analysis. Among applications across the entire gastrointestinal tract, these gages allow for cross comparison of data collected from A) acute and/or chronic experimental designs; B) multiple (simultaneous) recording sites from within a single animal; and C) a wider range of experimental interventions.
Disclosures
The authors declare that they have no competing financial interests. The suppliers listed in this manuscript are provided for reference only.
Acknowledgments
Research funding was received through the National Institute of Neurological Disorders and Stroke (NS049177 and NS087834). The authors wish to acknowledge the intellectual contributions of the late Dr. Paul Bass and his colleagues to the original design of the strain gages; and Carol Tollefsrud for the fabrication and marketing of the strain gages until the cessation of production in 2010 as well as for her insightful correspondence.
References
- Szarka LA, Camilleri M. Methods for measurement of gastric motility. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296(3):G461–G475. doi: 10.1152/ajpgi.90467.2008. [DOI] [PubMed] [Google Scholar]
- Pascaud XB FAU, Genton MJ, Bass P. A miniature transducer for recording intestinal motility in unrestrained chronic rats. Am. J. Physiol. Endocrinol. Metab. Gastrointest. Physiol. 1978;4(5):532–538. doi: 10.1152/ajpendo.1978.235.5.E532. [DOI] [PubMed] [Google Scholar]
- Gourcerol G, Adelson DW, Million M, Wang L, Tache Y. Modulation of gastric motility by brain-gut peptides using a novel non-invasive miniaturized pressure transducer method in anesthetized rodents. Peptides. 2011;32(4):737–746. doi: 10.1016/j.peptides.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Américo MF, et al. Validation of ACB in vitro and in vivo as a biomagnetic method for measuring stomach contraction. Neurogastroenterol. Motil. 2010;22(12):1340–1374. doi: 10.1111/j.1365-2982.2010.01582.x. [DOI] [PubMed] [Google Scholar]
- Fujitsuka N, Asakawa A, Amitani H, Fujimiya M, Inui A. Ghrelin and Gastrointestinal Movement. Academic Press; 2012. Chapter Eighteen - Ghrelin and Gastrointestinal Movement; pp. 289–301. [DOI] [PubMed] [Google Scholar]
- Monroe MJ, Hornby PJ, Partosoedarso ER. Central vagal stimulation evokes gastric volume changes in mice: a novel technique using a miniaturized barostat. Neurogastroenterol. Motil. 2004;16(1):5–11. doi: 10.1046/j.1365-2982.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- Herman MA, et al. Characterization of noradrenergic transmission at the dorsal motor nucleus of the vagus involved in reflex control of fundus tone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294(3):720–729. doi: 10.1152/ajpregu.00630.2007. [DOI] [PubMed] [Google Scholar]
- Gondim FA, et al. Complete cervical or thoracic spinal cord transections delay gastric emptying and gastrointestinal transit of liquid in awake rats. Spinal Cord. 1999;37(11):793–799. doi: 10.1038/sj.sc.3100923. [DOI] [PubMed] [Google Scholar]
- Van Bree SHW, et al. Systemic inflammation with enhanced brain activation contributes to more severe delay in postoperative ileus. Neurogastroenterol. Motil. 2013;25(8):540–549. doi: 10.1111/nmo.12157. [DOI] [PubMed] [Google Scholar]
- Qualls-Creekmore E, Tong M, Holmes GM. Gastric emptying of enterally administered liquid meal in conscious rats and during sustained anaesthesia. Neurogastroenterol. Motil. 2010;22(2):181–185. doi: 10.1111/j.1365-2982.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualls-Creekmore E, Tong M, Holmes GM. Time-course of recovery of gastric emptying and motility in rats with experimental spinal cord injury. Neurogastroenterol. Motil. 2010;22(1):62. doi: 10.1111/j.1365-2982.2009.01347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KM, et al. Determination of gastric emptying in nonobese diabetic mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293(5):G1039–G1045. doi: 10.1152/ajpgi.00317.2007. [DOI] [PubMed] [Google Scholar]
- Adelson DW, Million M, Kanamoto K, Palanca T, Tache Y. Coordinated gastric and sphincter motility evoked by intravenous CCK-8 as monitored by ultrasonomicrometry in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286(2):G321–G332. doi: 10.1152/ajpgi.00057.2003. [DOI] [PubMed] [Google Scholar]
- Xue L, et al. Effect of modulation of serotonergic, cholinergic, and nitrergic pathways on murine fundic size and compliance measured by ultrasonomicrometry. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;290(1):G74–G82. doi: 10.1152/ajpgi.00244.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass P, Wiley JN. Contractile force transducer for recording muscle activity in unanesthetized animals. J. Appl. Physiol. 1972;32(4):567–570. doi: 10.1152/jappl.1972.32.4.567. [DOI] [PubMed] [Google Scholar]
- Holmes GM, Browning KN, Tong M, Qualls-Creekmore E, Travagli RA. Vagally mediated effects of glucagon-like peptide 1: in vitro and in vivo gastric actions. J. Physiol. 2009;587(19):4749–4759. doi: 10.1113/jphysiol.2009.175067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M, Qualls-Creekmore E, Browning KN, Travagli RA, Holmes GM. Experimental spinal cord injury in rats diminishes vagally-mediated gastric responses to cholecystokinin-8s. Neurogastroenterol. Motil. 2011;23(2):e69–e79. doi: 10.1111/j.1365-2982.2010.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyano Y, et al. The role of the vagus nerve in the migrating motor complex and ghrelin- and motilin-induced gastric contraction in suncus. PLoS ONE. 2013;8(5):e64777. doi: 10.1371/journal.pone.0064777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GM, Rogers RC, Bresnahan JC, Beattie MS. Thyrotropin-releasing hormone (TRH) and CNS regulation of anorectal motility in the rat. J Auton. Nerv. Syst. 1995;56:8–14. doi: 10.1016/0165-1838(95)00051-8. [DOI] [PubMed] [Google Scholar]
- Ormsbee HS, Bass P. Gastroduodenal motor gradients in the dog after pyloroplasty. Am. J. Physiol. 1976;230:389–397. doi: 10.1152/ajplegacy.1976.230.2.389. [DOI] [PubMed] [Google Scholar]
- Fukuda H, et al. Impaired gastric motor activity after abdominal surgery in rats. Neurogastroenterol. Motil. 2005;17(2):245–250. doi: 10.1111/j.1365-2982.2004.00602.x. [DOI] [PubMed] [Google Scholar]
- Browning KN, Babic T, Holmes GM, Swartz E, Travagli RA. A critical re-evaluation of the specificity of action of perivagal capsaicin. J. Physiol. 2013;591(6):1563–1580. doi: 10.1113/jphysiol.2012.246827. [DOI] [PMC free article] [PubMed] [Google Scholar]


