Abstract
Background
The sensory nerve neuropeptide substance P (SP) regulates cardiac fibrosis in rodents under pressure overload conditions. Interestingly, SP induces transient increase expression of specific genes in isolated rat cardiac fibroblasts, without resultant changes in cell function. This suggests that SP ‘primes’ fibroblasts, but does not directly activate them. We investigated whether these unusual findings are specific to rodent fibroblasts or are translatable to a larger animal model more closely related to humans.
Methods
We compared the effects of SP on genes associated with extracellular matrix (ECM) regulation, cell-cell adhesion, cell-matrix adhesion and ECM in cardiac fibroblasts isolated from a non-human primate and Sprague-Dawley rats.
Results
We found that rodent and non-human primate cardiac fibroblasts showed similar ECM regulation and cell adhesion gene expression responses to SP. There were, however, large discrepancies in ECM genes which did not result in collagen or laminin synthesis in rat or non-human primate fibroblasts in response to SP.
Conclusions
This study further supports the notion that SP serves as a ‘primer’ for fibroblasts rather than initiating direct effects and suggests that rodent fibroblasts are a suitable model for studying gene and functional responses to SP in the absence of human or non-human primate fibroblasts.
Keywords: Cardiac fibroblast, Collagen, Non-human primate, Neuropeptide, Genes
Introduction
Recently, we demonstrated that the sensory nerve neuropeptide substance P (SP) plays an important role in cardiac fibrosis in response to hypertension [6]. Interestingly, our studies in isolated rat cardiac fibroblasts suggested that SP did not directly induce a pro-fibrotic phenotype in these cells since there was no detectable increase in collagen synthesis, no change to a myofibroblast phenotype, and no increase in cell migratory properties [6]. This was in keeping with Kumaran et al. [17] who previously reported that rat cardiac fibroblasts proliferated, but did not increase collagen synthesis in response to SP. However, despite this lack of functional change in our isolated cardiac fibroblast cultures, we did observe a transient increase in specific genes related to cell adhesion, extracellular matrix (ECM) regulation and ECM proteins in response to SP. Together, these findings suggest that SP does not induce abnormal ECM synthesis alone, but instead ‘primes’ cardiac fibroblasts by initiating proliferation and up-regulating pivotal genes in anticipation of subsequent pro-fibrotic stimuli. In support of this hypothesis, endothelin-1 (ET-1), a known pro-fibrotic stimuli [2,16], is regulated by SP in hypertensive rat hearts [6]. Since the aforementioned reports used rat cardiac fibroblasts, we wondered if this priming response without subsequent phenotype or functional changes was specific to the rodent or whether it was a conserved response of fibroblasts to SP that was also applicable to species more closely related to humans. We addressed this issue by exposing cardiac fibroblasts from an adult non-human primate cynomolgus monkey (Macaca fascicularis) to increasing concentrations of SP and comparing the mRNA response to the rat counterpart. We examined genes related to ECM regulation, cell-cell adhesion, cell-matrix adhesion and ECM. Additionally, we evaluated fibroblast functionality by determining synthesis of specific ECM proteins. We found non-human primate gene responses to SP to be similar to those of the rat, with the exception that these responses often occurred at lower SP concentrations in non-human primate cells. The results of this study demonstrate for the first time that rodent fibroblasts respond similarly to non-human primate fibroblasts justifying their use as a model system for studying gene and functional responses to SP.
Methods
Studies were performed using adult male Sprague-Dawley rats 8–10 weeks of age and one 16.6 year-old healthy, pathogen-free, female cynomolgus monkey (Macaca fascicularis). Rats (n=4) were housed under standard environmental conditions and maintained on commercial rat chow and tap water ad libitum. Rat studies conformed to the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were conducted according to a protocol approved by Institutional Animal Care and Use Committee at the Medical College of Wisconsin. The non-human primate underwent experimental necropsy to serve as a healthy control for another study. Physical exam prior to necropsy, including blood pressure and complete blood count and chemistries were normal. Procedures involving the non-human primate were approved by the Institutional Animal Care and Use Committee of Wake Forest University and conducted in accordance with federal, state, and institutional guidelines. The facilities and animal resources programs of Wake Forest University and Medical College of Wisconsin are fully accredited by the Association for Assessment and Accreditation of laboratory Animal Care. Following euthanasia achieved by overdose of pentobarbital, the hearts were removed and the left ventricle (LV) separated from the atria, right ventricle and great vessels for cardiac fibroblast isolations.
Cardiac Fibroblast Cell Cultures and Substance P Treatment
Cardiac fibroblasts from rats (n=4) and non-human primate (n=1) were isolated from LV tissue as previously described [19,21,22]. Briefly, the LV tissue was homogenised with 100 ng/μl of liberase TM (Roche) at 37°C for 15 minutes by a series of five digestions. The cells were pelleted by centrifugation at 800 rcf for 10 minutes and plated in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% FBS. Fibroblasts were purified by selective attachment to plastic culture ware. All fibroblasts were used after only one passage to minimise changes in phenotype associated with culture. Before treatment, the fibroblasts were plated at 1×10 cells/plate (n=4) and serum-starved in DMEM-F12 media for 24 hours. The cells were then treated in DMEM with 1.5% FBS with SP (Sigma Aldrich) at 0,100, 300 and 1000 nM concentrations for 24 hours.
Quantitative RT-PCR
Collected cells were lysed in PurZOL™. RNA was isolated according to the Aurum RNA isolation kit instructions (Bio-Rad). One μg/μl of total RNA was used for each cDNA reaction (iScript cDNA synthesis kit, Bio-Rad) and 1 μl of cDNA was used in each 20 μl RT-qPCR reaction (SsoAdvanced SYBR Green, Bio-Rad and Bio-Rad cycler). Each reaction was initiated with a 30 second denaturation step at 95°C followed by 39 cycles of a 10 second denature step at 95°C and a 30 second anneal/extension step at 60°C. The annealing temperature for each primer pair was calculated from the mean melting temperature of the forward and reverse primer. The rat and non-human primate primer sequences are presented in Table 1.
Table 1.
Primer sequences.
| Gene | Rat | Non-human primate |
|---|---|---|
|
MT-MMP1 (MMP-14) |
Forward sequence 5′-AAAGGGAACAAATACTGGAA-3′ | Forward sequence 5′- CCACCTACGGACCCAACATC-3′ |
| Reverse sequence 5′-ATGTAGTTAGGGGGATGGAA-3′ | Reverse sequence 5′- CAGAACCAGCGCTCCTTGAA | |
| MMP-2 | Forward sequence 5′-CAATACCTGAACCTT-3′ | Forward sequence 5′- ATGTTGTCTTGTGAGCGTGC-3′ |
| Reverse sequence 5′-CTGTATTGATCTGGTT-3′ | Reverse sequence 5′- AGGTATTGGCAACACTGCGG-3′ | |
| TIMP-2 | Forward sequence 5′-ATTTATCTACACGGCCCC-3′ | Forward sequence 5′- GGGCTGCGAGTGTAAGATCA-3′ |
| Reverse sequence 5′-CAAGAACCATCACTTCTCTTG-3′ | Reverse sequence 5′- AAGAAACTCCTGCTTGGGGG-3′ | |
| ICAM-1 | Forward sequence 5′-AGCATTTACCCCTCACCCAC-3′ | Forward sequence 5′- CAAACCTTTGACCTGCCAGC-3′ |
| Reverse sequence 5′-CATTTTCTCCCAGGCATTCTC-3′ | Reverse sequence 5′- CGAGAGGGAGTTGTTGCCAT-3′ | |
| CDH-2 | Forward sequence 5′-CACCCGGCTTAAGGGTGATT-3′ | Forward Sequence 5′- GGAAAAGTGGCAAGTGGCAG-3′ |
| Reverse sequence 5′-CGATCCTGTCTACGTCGGTG-3′, | Reverse sequence 5′- GTGGCTCCTTCACTGACTCC-3′ | |
| ITG- α5 | Forward sequence 5′-GAAGGGACGGAGTCAGTGTG-3′ | Forward sequence 5′- GGGTACCTGCTACCTCTCCA-3′ |
| Reverse sequence 5′-CTGGGTCATTCTGTGGGTCC-3′, | Reverse sequence 5′- GCTGAAATCTGAGCGGCAAG-3′ | |
| ITG- β1 | Forward sequence 5′-TTCAGACTTCCGCATTGGCT-3′ | Forward sequence 5′- GCCAAATGGGACACAGGTGA-3′ |
| Reverse sequence 5′-CCAATCAGCGACCCACAAAC-3′ | Reverse sequence 5′- TGCACAGGCGGTACTCATTT-3′ | |
| Collagen I | Forward sequence 5′-GGTTCTCCTGGCAAAGATGGACT-3′ | Forward sequence 5′- GTTTCTCCTTGGGGTCGGAG-3′ |
| Reverse sequence 5′-ACTGGTCATGCTCTCTCCAAACCA-3′, | Reverse sequence 5′- TGGTGGGATGTCTTCGTCTTG-3′ | |
| Collagen III | Forward sequence 5′-TCCTAACCAAGGCTGCAAGATGGA-3′ | Forward sequence 5′- AATCAGGTAGACCCGGACGA-3′ |
| Reverse sequence 5′-AGGCCAGCTGTACATCAAGGACAT-3′, | Reverse sequence 5′- TTCGTCCATCGAAGCCTCTG-3 | |
| Lama 2 | Forward sequence 5′-GCCACACGAGACCTGAAAGA-3′ | Forward sequence 5′- CAGATAGCGTCGCCAAAACG-3′ |
| Reverse sequence 5′-ACAAAACCAGGCTTGGGGAA-3′ | Reverse sequence 5′- AATGCAGTCACCTCCCGAAG-3′ | |
| Lama 4 | Forward sequence 5′-GCCACACGAGACCTGAAAGA-3′ | Forward sequence 5′- ACATTGAAGGGAGCTCAGCG-3′ |
| Reverse sequence 5′-ACAAAACCAGGCTTGGGGAA-3′ | Reverse sequence 5′- CATTGCATTTCTCGGCAGCA-3′ | |
| B-Actin | Forward sequence 5′-CGCCACCAGTTCGCCATGGAT-3′ | Forward sequence 5′-AGGAGAAGCTGTGCTACGTC-3′ |
| Reverse sequence 5′-TAGGGCGGCCCACGATGGAG-3′ | Reverse sequence 5′-ACTCCATGCCCAGGAAGGAA-3′ |
Hydroxyproline Assay
Hydroxyproline levels in the media were determined as a surrogate marker of collagen synthesis as we have published previously [19,21,22]. Briefly, 100 μl of cell culture media was incubated with 100 μl of 6 N HCL and hydrolysed at 107°C for 18 hours. The samples were dried by vacuum centrifuge and reconstituted with 500 μl of dH2O. The samples were then oxidised with 250 μl of chloramine T reagent and colour developed with 250 μl of Ehrlich’s reagent. Absorbance was read at 550 nm and hydroxyproline values determined from a hydroxyproline standard curve [7]. All the samples were run in duplicate with the average of the two replicates reported.
Laminin
Laminin protein levels were measured from the culture media using a commercially available ELISA kit (Abcam). This assay recognises the α, β, and γ sub-units of laminin. A human specific kit was used for non-human primate samples, whilst a rat specific kit was used for rat samples. All the samples were run in duplicate with the average of the two replicates reported.
Statistical Analysis
Statistical analysis of gene expression and secretion products of cardiac fibroblasts incubated with increasing concentrations of SP were performed using one-way ANOVA with Fisher’s least significant difference (LSD) posthoc test. Results are presented as mean ± SEM. Statistical significance was taken to be P<0.05. Analyses were performed using SPSS 11.5 software (Chicago, IL).
Results
Isolated Cardiac Fibroblast mRNA Responses to Substance P
Genes related to ECM regulation: Membrane type 1-matrix metalloproteinase (MT1-MMP) mRNA expression levels showed a significant concentration dependent up-regulation in rat fibroblasts at SP concentrations of 300 and 1000 nM (Figure 1A). In non-human primate fibroblasts, up-regulation of MT1-MMP occurred at 100 nM of SP. Non-human primate cells also showed greater basal expression of MT1-MMP. Matrix metalloproteinase (MMP)-2 mRNA was up-regulated in rat fibroblasts at 1000 nM of SP, but did not reach significance (Figure 1B). Levels of MMP-2 gene expression were undetectable in rat fibroblasts at 300 nM of SP. In nonhuman primate fibroblasts MMP-2 peak expression occurred at 300 nM. Non-human primate cardiac fibroblasts had higher basal expression of MMP-2. No significant changes in tissue inhibitor of metalloproteinases 2 (TIMP-2) mRNA were induced by SP in either rat or nonhuman primate fibroblasts, with similar levels of basal expression between the two species (Figure 1C).
Cell-cell adhesion genes: There was a significant concentration dependent increase intracellular adhesion molecule 1 (ICAM-1) mRNA in rat fibroblasts at 1000 nM of SP, while non-human primate fibroblast peak gene expression occurred at a lower concentration (300 nM) (Figure 2A). Cadherin 2 gene expression was up-regulated both in rat and non-human primate fibroblasts at 300 nM of SP (Figure 2B).
Cell-matrix adhesion genes: Among this category of genes, we examined integrin-α5 mRNA expression and found a non-significant up-regulation for this gene with increasing concentrations of SP in rat fibroblasts (300 and 1000 nM); in non-human primate fibroblasts, integrin-α5 mRNA expression was significantly elevated at 100 nM of SP (Figure 3A). We also examined integrin-β 1 mRNA expression, which was not significantly up-regulated in either rat or non-human primate fibroblasts (Figure 3B).
Extracellular matrix genes: Increasing concentrations of SP did not induce significant changes of collagen I α1 mRNA in rat fibroblasts compared to control; in nonhuman primate fibroblasts, SP induced a significant decrease in collagen I mRNA at 300 and 1000 nM (Figure 4A). Conversely, collagen III α1 mRNA, showed a significant increase at 1000 nM of SP in rat fibroblasts (Figure 4B), while non-human primate fibroblasts had a non-statistically significant decrease in collagen III mRNA at the low concentrations of SP. We also identified a non-significant increase in laminin 2 mRNA when rat fibroblasts were incubated with 300 and 1000 nM of SP (Figure 4C). Non-human primate fibroblasts showed a decrease of laminin 2 when incubated with 100 nM of SP. Similarly, laminin 4 mRNA expression was significantly up-regulated in rat fibroblasts at 1000 nM of SP and down-regulated in non-human primate fibroblasts at 300 and 1000 nM concentrations of SP (Figure 4D).
Figure 1.
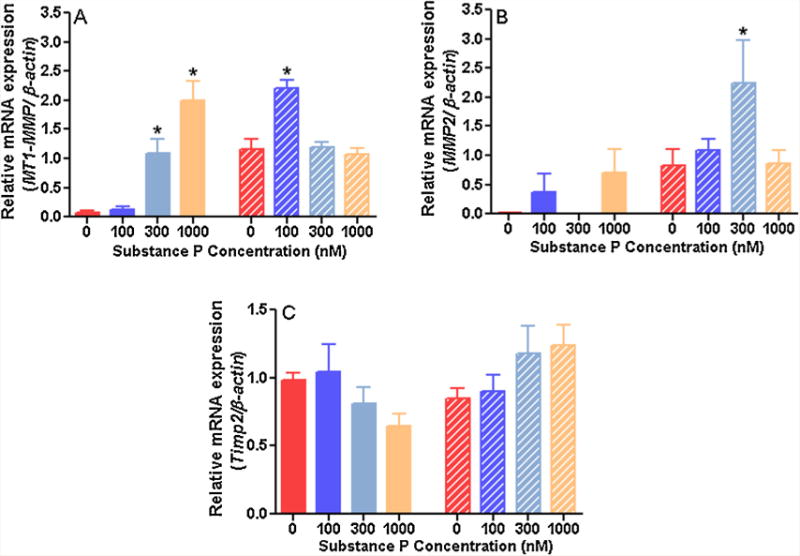
Relative expression of genes related to extracellular matrix regulation in rat (solid bars) and non-human primate (striped bars) cardiac fibroblasts incubated with increasing concentrations of substance P for 24 hours and normalised to β-actin (n=4). A) membrane type 1 matrix metalloproteinase (MT1-MMP); B) matrix metalloproteinase 2 (MMP-2); C) tissue inhibitor of metalloproteinase-2 (TIMP-2). All values are expressed as mean ± SEM. *p<0.05 vs. control group (0 nM of SP), one-way ANOVA with Fisher’s least significant difference (LDS) posthoc test.
Figure 2.
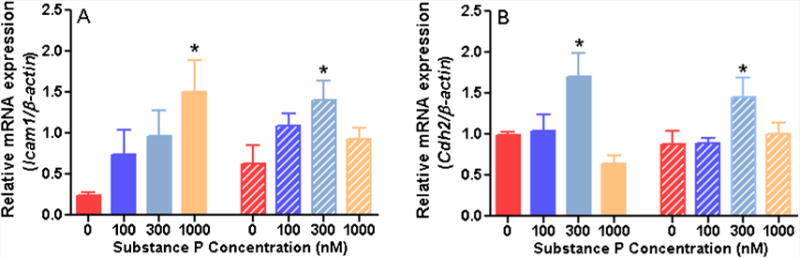
Relative expression of genes related to cell-cell adhesion in rat (solid bars) and non-human primate (striped bars) cardiac fibroblasts incubated with increasing concentrations of substance P for 24 hours and normalised to β-actin (n=4). A) Intercellular adhesion molecule 1 (ICAM1); B) Cadherin 2 (Cdh2). All values are expressed as mean ± SEM.*p<0.005 vs. control group (0 nM of SP), one-way ANOVA with Fisher’s least significant difference (LDS) posthoc test.
Figure 3.
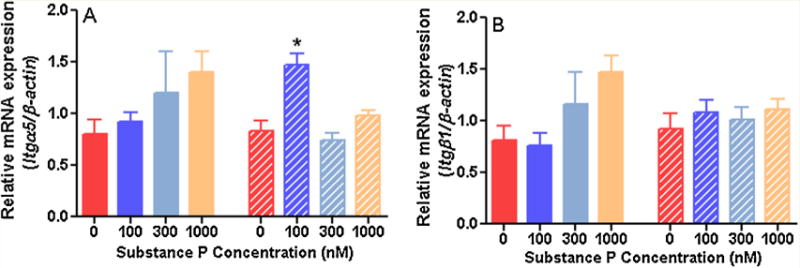
Relative expression of genes related to cell-matrix adhesion in of rat (solid bars) and non-human primate (striped bars) cardiac fibroblasts incubated with increasing concentrations of substance P for 24 hours and normalised to β-actin (n=4). A) Integrin-α 5 (Itgα5); B) Integrin-β1 (Itgβ1). All values are expressed as mean ± SEM. *p<0.005 vs. control group (0 nM of SP), one-way ANOVA with Fisher’s least significant difference (LDS) posthoc test.
Figure 4.
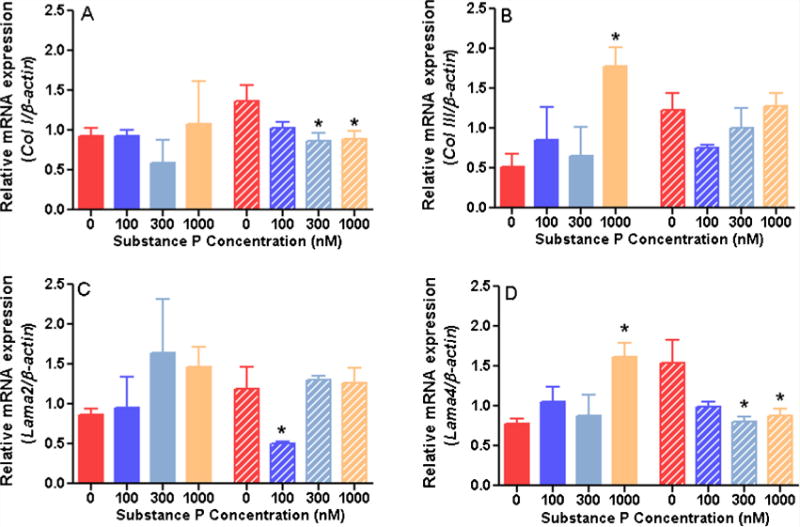
Relative expression of genes related to extracellular matrix proteins in of rat (solid bars) and non-human primate (striped bars) cardiac fibroblasts incubated with increasing concentrations of substance P for 24 hours and normalised to β-actin (n=). A) Collagen I (Col I); B) Collagen III (Col III); C) Laminin 2 (Lama2); D) Laminin 4 (Lama4). All values are expressed as mean ± SEM. *p<0.005 vs. control group (0 nM of SP), one-way ANOVA with Fisher’s least significant difference (LDS) posthoc test.
Isolated Cardiac Fibroblast Functional Responses to Substance P
We evaluated the functional response of both rat and non-human primate cardiac fibroblasts by determining levels of the ECM components hydroxyproline and laminin. After 24 hours of incubation with increasing concentrations of SP, there was no increase in hydroxyproline synthesis by either rat or non-human primate cardiac fibroblasts, indicative of no change in collagen production (Figure 5A). Rat fibroblasts synthesised greater basal amounts of hydroxyproline compared to non-human primate cells. Conversely, laminin production was decreased by the highest concentration of SP in rat fibroblasts (1000 nM) and with 100 and 1000 nM of SP in non-human primate fibroblasts (Figure 5B). Rat fibroblasts produced greater basal amounts of laminin.
Figure 5.
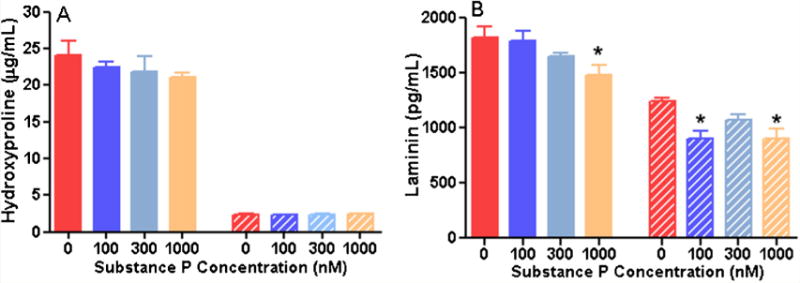
Evaluation of rat (solid bars) and non-human primate (stripe bars) cardiac fibroblast function as determined by secretion of A) hydroxyproline, B) laminin after 24 hours of incubation with increasing concentrations of substance P(n=4). All values are expressed as mean ± SEM. *p<0.005 vs. control group (0 nM of SP), one-way ANOVA with Fisher’s least significant difference (LDS) posthoc test.
Discussion
The results of our study address a critical question: are our previous findings in rodent cardiac fibroblasts, reflective of how human fibroblasts respond to SP? Previously, we reported that SP, a sensory nerve neuropeptide, regulates cardiac fibrosis in a rat model of hypertension [6]. Interestingly, SP did not have direct effects on cardiac fibroblasts to cause their conversion to a myofibroblast phenotype, or cause excess collagen synthesis. Rather, SP stimulated cardiac fibroblasts to transiently up-regulate genes related to ECM regulation, ECM proteins, and adhesion molecules, but without any functional changes. This led us to postulate that SP primes cardiac fibroblasts to be responsive to subsequent pro-fibrotic stimuli. This was supported by the additional finding that blockade of the neurokinin-1 receptor (NK-1R), the receptor for SP, prevented increased ET-1 production in the hypertensive heart; ET-1 is a known pro-fibrotic stimuli [2,16]. With NK-1R antagonists commercially available to treat nausea and vomiting in humans, it is important to determine whether our complicated findings in rat cardiac fibroblasts potentially translate to humans. We utilised cardiac fibroblasts from a non-human primate as an animal model more closely related to the human primate, to compare the effects of SP on cardiac fibroblast gene function, as well as ECM proteins. The major conclusion is that while there were some minor differences at the gene level, the overall effects of SP on rat and non-human primate cardiac fibroblasts at the gene and functional level were similar.
MMPs regulate ECM synthesis and degradation. MT1-MMP was up-regulated in cardiac fibroblasts from both species in our current study; however, this occurred at lower concentrations of SP in non-human primate fibroblasts, indicating a greater potency of SP on these cells. Conversely, the absolute response in rat cardiac fibroblasts was greater due to a lower baseline expression of MT1-MMP. While there was a trend for increased MMP-2 mRNA, no significant changes in expression were detected in rat fibroblasts; however, the intermediate concentration of SP (300 nM) did up-regulate MMP-2 gene expression in non-human primate fibroblasts. Both of these MMPs are important regulators of the ECM. MT1-MMP is increased in the LV of hypertensive patients [26], as well as pressure overloaded animals [35,36] and is important in the development of fibrosis due to its role in processing latent TGF binding protein-1, resulting in the release of active TGFβ[3,5,8,10,15,24,28]. MMP-2 is also important in fibrosis development; its extracellular activation by MT1-MMP stimulates collagen I synthesis in cardiac fibroblasts [11,13,31]. Alternatively, both these MMPs can also degrade the ECM, thus their imbalance causes ECM dysregulation. MMPs are inhibited by TIMPs, representing another layer of regulation of ECM homeostasis. SP did not alter TIMP-2 mRNA in either rat or non-human primate fibroblasts. Overall, there were distinct similarities in the way that these genes related to ECM regulation responded to SP in rat and non-human primate fibroblasts.
Since MMPs can initiate both ECM synthesis and degradation, we examined collagen I and III mRNA levels in fibroblasts treated with SP. SP had no effect on collagen I mRNA in rat fibroblasts, but did induce a small though significant down-regulation in non-human primate cells. Conversely, collagen III was up-regulated by SP in rat cells with no effect on non-human primate cells. These findings are consistent with our previously published findings for rat fibroblasts [6], and are similar to a previous report using rat tenocytes where collagen III and MMP-3 were up-regulated by SP [9]. Thus, we believe that the responses reported herein are likely reflective of general fibroblast responses to SP. Despite the divergent responses across species, the functional outcome was the same with neither rat nor non-human primate fibroblasts synthesising excess collagen (hydroxyproline) in response to SP. Similar to the collagen genes, there were divergent effects of SP on rat and non-human primate mRNA for the Lama2 and Lama4 isoforms of laminin. Lama2 was unchanged in rat fibroblasts, but decreased in non-human primate cells; Lama4 was increased in rat fibroblasts and decreased in non-human primate cells. Despite these species differences at the gene level, laminin synthesis was significantly decreased at the protein level in both rat and non-human primate fibroblasts. While this result fits with the gene expression pattern in the non-human primate fibroblasts, it does not follow gene expression in rat cells. Since the ELISA detected multiple laminin isoforms it is possible that other isoforms were down-regulated in the rat accounting for the overall decrease in laminin protein. These ECM genes represented the only group of genes that we examined that had large discrepancies between the rat and non-human primate, however, responses were the same at the protein level. Although there was no difference in hydroxyproline or laminin production between rat and non-human primate cardiac fibroblasts in culture despite differential gene expression patterns, it is still possible that the differences in ECM gene expression could be important under in vivo conditions.
Cell adhesion proteins are important modulators of numerous biological processes including tissue remodelling and inflammation [1]. We examined genes that encode the cell-cell adhesion proteins ICAM-1 and cadherin 2. ICAM-1 is a pro-inflammatory molecule found on the surface of a variety of cardiac cells types including cardiac fibroblasts [25], mediating their role in the recruitment of inflammatory cells [12,34]. In our study, SP induced up-regulation of ICAM-1 gene expression in both rat (1000 nM) and non-human primate fibroblasts (300 nM); non-human primate cells again were more sensitive to SP than rat cells. SP has been reported to induce an increase in ICAM-1 mRNA expression in human umbilical vein [23] and dermal microvascular endothelial cells [27]. Furthermore, the results of a study by Sapna et al. [29] demonstrated that rat cardiac fibroblasts also release soluble ICAM-1 after treatment with 1000 nM of SP for 24 h, the same concentration and incubation time at which we observed up-regulation of ICAM-1 mRNA. In that study however, SP had no effect on ICAM-1 mRNA. This discrepancy with our findings may be due to culture conditions; cardiac fibroblasts in that study were used for experimentation after two to three passages, whereas cells in our experiments were only passaged once. Cadherins are calcium dependent adhesion molecules that bind a cadherin of the same type on an adjacent cell [1]. We found that cadherin-2 mRNA was up-regulated by SP.
Integrins are fundamental components in the interaction between the extracellular matrix and cells. These membrane-bound matrix receptors consist of α and β chains that form heterodimers [20,30,32]. We evaluated the α 5 and β1 subunit gene expression which have been related to adverse remodelling induced by hypertension. We found that integrin-α5 mRNA expression appeared to show a concentration-dependent response to increasing concentrations of SP of rat cardiac fibroblasts, although this did not reach significance, while low concentrations of SP (100 nM) induced significant integrin-α5 gene up-regulation in non-human primate cardiac fibroblasts. SP did not have an effect on integrin– β1 expression in either rats or nonhuman primates, although there was a non-significant up-regulation of the β1 subunit at the highest concentrations of SP in rat cells (1000 nM). This is consistent with our previous study [6] where SP induced a significant increase of integrin–β1 and –α5 mRNA expression at 1000 nM of SP, however, in that study no effects on fibroblasts migration were found in response to SP, suggesting that despite integrin up-regulation at the gene level, there was no effect at the functional level. Whole animal studies have determined that the integrin α5 subunit is decreased and the β1 subunit is increased in cardiac fibroblasts of hypertensive animals [4]. Thus, it is plausible to speculate that SP is not capable of inducing functional integrin changes to fibroblasts directly; instead, a subsequent pro-fibrotic stimulus is necessary.
A limitation of this study is that we only measured mRNA levels of our targets of interest and did not investigate changes at the protein level with the exception of collagen (hydroxyproline) and laminin. Since there were no functional changes observed in this and our previous study [6] (i.e. myofibroblast conversion, migration), we believe that in all likelihood there are no changes at the protein level in response to SP. Even if there are changes, the data clearly demonstrates that there is no functional consequence of these changes. Additionally, SP induced changes in expression of specific genes at a lower concentration in non-human primate cells compared to rat fibroblasts. At this stage it is not clear why this occurs. One possibility is that non-human primates may have a greater abundance of NK-1R on cardiac fibroblasts. Alternatively, NK-1R from each species may differ to some extent in the intracellular signalling induced by their activation. A third possibility is that there may be differences in the level of the two isoforms of the NK-1R on rat and non-human primate cardiac fibroblasts. The full length NK-1R isoform is activated by lower concentrations of SP, whereas the truncated isoform requires higher concentrations. Very little is known about the function of the truncated isoform, or which isoform(s) are present on cardiac fibroblasts, however, species differences in the densities of the two isoforms may affect the concentrations of SP that elicits an effect.
In summary, we have found that non-human primate cardiac fibroblasts respond in a similar fashion at the gene level to rat cardiac fibroblasts. This was especially true for genes that encode proteins that regulate the ECM and cell adhesion. There were large discrepancies in the response to SP for genes that encode actual ECM proteins (i.e. collagen I, III, and laminin). However, this discrepancy did not have functional implications since SP had the same effect on ECM protein levels regardless of species. These findings in rat and non-human primate cardiac fibroblasts further support the concept that we previously put forth, that SP does not directly activate fibroblasts, but instead, primes the cell for response to subsequent pro-fibrotic stimuli by up-regulating important genes related to ECM production and cell adhesion. Further studies are necessary to uncover the pro-fibrotic stimuli that work synergistically with SP to induce the profibrotic phenotype that we observe in vivo. Several interesting additional observations from our studies are that although non-human primate cardiac fibroblasts had higher basal expression of several genes, non-human primate cardiac fibroblasts responded to lower concentrations of SP. If one extrapolates this finding to humans, it may mean that humans are more sensitive to the adverse effects of SP in disease. Increased levels of SP have been detected in heart failure patients [33], patients with angina pectoris [14], and in atherosclerotic lesions of human coronary arteries [18]. Overall, this study demonstrates that rat fibroblasts respond similarly to non-human primate cells, which makes rodent fibroblasts a suitable model to study fibroblast responses to SP in the absence of non-human primate cells.
Acknowledgments
Source of Funding
This work was supported by the National Heart, Lung and Blood Institute at the National Institutes of Health R00-HL093215 (S.P.L.) and T32-HL007792 training grant (H.M.D.).
Footnotes
Disclosure
G.C.M. received support as a Wake-Merck Cardiovascular Research Fellow supported by an education grant from Merck Pharmaceuticals.
References
- 1.Agarwal SK. Integrins and cadherins as therapeutic targets in fibrosis. Front Pharmacol. 2014;5:131. doi: 10.3389/fphar.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan A, Fenning A, Levick S, Hoey A, Brown L. Reversal of cardiac dysfunction by selective ET-A receptor antagonism. Br J Pharmacol. 2005;146(6):846–53. doi: 10.1038/sj.bjp.0706384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bobik A. Transforming growth factor-betas and vascular disorders. Arterioscler Thromb Vasc Biol. 2006;26(8):1712–20. doi: 10.1161/01.ATV.0000225287.20034.2c. [DOI] [PubMed] [Google Scholar]
- 4.Burgess ML, Terracio L, Hirozane T, Borg TK. Differential integrin expression by cardiac fibroblasts from hypertensive and exercise-trained rat hearts. Cardiovasc Pathol. 2002;11(2):78–87. doi: 10.1016/s1054-8807(01)00104-1. [DOI] [PubMed] [Google Scholar]
- 5.Dallas SL, Sivakumar P, Jones CJ, Chen Q, Peters DM, Mosher DF, et al. Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J Biol Chem. 2005;280(19):18871–80. doi: 10.1074/jbc.M410762200. [DOI] [PubMed] [Google Scholar]
- 6.Dehlin HM, Manteufel EJ, Monroe AL, Reimer MH, Jr, Levick SP. Substance P acting via the neurokinin-1 receptor regulates adverse myocardial remodeling in a rat model of hypertension. Int J Cardiol. 2013;168(5):4643–51. doi: 10.1016/j.ijcard.2013.07.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards CA, O’Brien WD., Jr Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980;104(2):161–7. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- 8.Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–93. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 9.Fong G, Backman LJ, Hart DA, Danielson P, McCormack B, Scott A. Substance P enhances collagen remodeling and MMP-3 expression by human tenocytes. J Orthop Res. 2013;31(1):91–8. doi: 10.1002/jor.22191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh AK. Factors involved in the regulation of type I collagen gene expression: implication in fibrosis. Exp Biol Med (Maywood) 2002;227(5):301–14. doi: 10.1177/153537020222700502. [DOI] [PubMed] [Google Scholar]
- 11.Goldsmith EC, Bradshaw AD, Spinale FG. Cellular mechanisms of tissue fibrosis. 2. Contributory pathways leading to myocardial fibrosis: moving beyond collagen expression. Am J Physiol Cell Physiol. 2013;304(5):C393–402. doi: 10.1152/ajpcell.00347.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graciano AL, Bryant DD, White DJ, Horton J, Bowles NE, Giroir BP. Targeted disruption of ICAM-1, P-selectin genes improves cardiac function and survival in TNF-alpha transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280(4):H1464–71. doi: 10.1152/ajpheart.2001.280.4.H1464. [DOI] [PubMed] [Google Scholar]
- 13.Guo C, Piacentini L. Type I collagen-induced MMP-2 activation coincides with up-regulation of membrane type 1-matrix metalloproteinase and TIMP-2 in cardiac fibroblasts. J Biol Chem. 2003;278(47):46699–708. doi: 10.1074/jbc.M307238200. [DOI] [PubMed] [Google Scholar]
- 14.Kambam JR, Merrill W, Parris W, Alhaddad R, Naukam R, Stewart J, et al. Substance P, acetylcholinesterase, and beta-endorphin levels in the plasma and pericardial fluid of patients with and without angina pectoris. J Lab Clin Med. 1990;116(5):707–10. [PubMed] [Google Scholar]
- 15.Koli K, Saharinen J, Hyytiainen M, Penttinen C, Keski-Oja J. Latency, activation, and binding proteins of TGF-beta. Microsc Res Tech. 2001;52(4):354–62. doi: 10.1002/1097-0029(20010215)52:4<354::AID-JEMT1020>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 16.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2013;1(4):549–74. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumaran C, Shivakumar K. Calcium- and superoxide anion-mediated mitogenic action of substance P on cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2002;282(5):H1855–62. doi: 10.1152/ajpheart.00747.2001. [DOI] [PubMed] [Google Scholar]
- 18.Laine P, Naukkarinen A, Heikkila L, Penttila A, Kovanen PT. Adventitial mast cells connect with sensory nerve fibers in atherosclerotic coronary arteries. Circulation. 2000;101(14):1665–9. doi: 10.1161/01.cir.101.14.1665. [DOI] [PubMed] [Google Scholar]
- 19.Levick SP, McLarty JL, Murray DB, Freeman RM, Carver WE, Brower GL. Cardiac mast cells mediate left ventricular fibrosis in the hypertensive rat heart. Hypertension. 2009;53(6):1041–7. doi: 10.1161/HYPERTENSIONAHA.108.123158. [DOI] [PubMed] [Google Scholar]
- 20.Manso AM, Kang SM, Ross RS. Integrins, focal adhesions, and cardiac fibroblasts. J Investig Med. 2009;57(8):856–60. doi: 10.231/JIM.0b013e3181c5e61f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLarty JL, Meléndez GC, Brower GL, Janicki JS, Levick SP. Tryptase/Protease-activated receptor 2 interactions induce selective mitogen-activated protein kinase signaling and collagen synthesis by cardiac fibroblasts. Hypertension. 2011;58(2):264–70. doi: 10.1161/HYPERTENSIONAHA.111.169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meléndez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension. 2010;56(2):225–31. doi: 10.1161/HYPERTENSIONAHA.109.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakagawa N, Sano H, Iwamoto I. Substance P induces the expression of intercellular adhesion molecule-1 on vascular endothelial cells and enhances neutrophil transendothelial migration. Peptides. 1995;16(4):721–5. doi: 10.1016/0196-9781(95)00037-k. [DOI] [PubMed] [Google Scholar]
- 24.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33(3):407–11. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 25.Niessen HW, Krijnen PA, Visser CA, Meijer CJ, Hack CE. Intercellular adhesion molecule-1 in the heart. Ann N Y Acad Sci. 2002;973:573–85. doi: 10.1111/j.1749-6632.2002.tb04703.x. [DOI] [PubMed] [Google Scholar]
- 26.Polyakova V, Hein S, Kostin S, Ziegelhoeffer T, Schaper J. Matrix metalloproteinases and their tissue inhibitors in pressure-overloaded human myocardium during heart failure progression. J Am Coll Cardiol. 2004;44(8):1609–18. doi: 10.1016/j.jacc.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 27.Quinlan KL, Song IS, Bunnett NW, Letran E, Steinhoff M, Harten B, et al. Neuropeptide regulation of human dermal microvascular endothelial cell ICAM-1 expression and function. Am J Physiol. 1998;275(6 Pt 1):C1580–9. doi: 10.1152/ajpcell.1998.275.6.C1580. [DOI] [PubMed] [Google Scholar]
- 28.Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem. 2005;280(9):7409–12. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- 29.Sapna S, Shivakumar K. Substance P enhances soluble ICAM-1 release from adult rat cardiac fibroblasts by a p42/44. Cell Biol Int. 2007;31(8):856–9. doi: 10.1016/j.cellbi.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 30.Sarrazy V, Koehler A, Chow M, Zimina E, Li CX, Kato H, et al. Integrins alphavbeta5 and alphavbeta3 promote latent TGF-beta1 activation by human cardiac fibroblast contraction. Cardiovasc Res. 2014;102(3):407–17. doi: 10.1093/cvr/cvu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spinale FG, Janicki JS, Zile MR. Membrane-associated matrix proteolysis and heart failure. Circ Res. 2013;112(1):195–208. doi: 10.1161/CIRCRESAHA.112.266882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart JA, Jr, Gardner JD, Brower GL, Janicki JS. Temporal changes in integrin-mediated cardiomyocyte adhesion secondary to chronic cardiac volume overload in rats. Am J Physiol Heart Circ Physiol. 2014;306(1):H101–8. doi: 10.1152/ajpheart.00541.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valdemarsson S, Edvinsson L, Ekman R, Hedner P, Sjoholm A. Increased plasma level of substance P in patients with severe congestive heart failure treated with ACE inhibitors. J Intern Med. 1991;230(4):325–31. doi: 10.1111/j.1365-2796.1991.tb00452.x. [DOI] [PubMed] [Google Scholar]
- 34.Xia Y, Lee K, Li N, Corbett D, Mendoza L, Frangogiannis NG. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol. 2009;131(4):471–81. doi: 10.1007/s00418-008-0541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yarbrough WM, Mukherjee R, Stroud RE, Rivers WT, Oelsen JM, Dixon JA, et al. Progressive induction of left ventricular pressure overload in a large animal model elicits myocardial remodeling and a unique matrix signature. J Thorac Cardiovasc Surg. 2012;143(1):215–23. doi: 10.1016/j.jtcvs.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zile MR, Baicu CF, Stroud RE, Van LA, Arroyo J, Mukherjee R, et al. Pressure overload-dependent membrane type 1-matrix metalloproteinase induction: relationship to LV remodeling and fibrosis. Am J Physiol Heart Circ Physiol. 2012;302(7):H1429–37. doi: 10.1152/ajpheart.00580.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


