Abstract
Background
This study aimed to investigate the endothelial function by reactive hyperemia index (RHI) in patients with depression, subjects recovering from depression, and subjects without a history of depression.
Material/Methods
Outpatients were recruited from a general hospital in China; 62 patients diagnosed with depression and the 17-item Hamilton Rating Scale for Depression (HAMD17) total scores ≥17 were enrolled as the depression group, 62 patients with a history of depression, discontinuation of antidepressants therapy at least 3 months ago, and HAMD17 ≤7 were recruited as remission group, and 62 subjects without a history of depression served as the control group (HAMD17 ≤7).
Results
The mean RHI was 1.93, 2.34, and 2.19 in depression, control, and remission groups, respectively, showing a significant difference among the 3 groups (P=0.0004). In addition, a marked difference in RHI was found between depression and control groups (P=0.0003) and between depression and remission groups (P=0.0270). However, there was no significant difference between remission and control groups (P=0.3363).
Conclusions
There is a relationship between depression and endothelial dysfunction in outpatients from a general hospital in China. The improvement of depression is synchronous with the improvement of endothelial function.
Keywords: Cross-Sectional Studies, Depression, Endothelial Cells, Remission Induction
Background
Increasing clinical epidemiologic studies revealed that some common chronic diseases (such as coronary heart disease [CHD], diabetes mellitus [DM], and cerebral infarction [CI]) are closely associated with depression, and they were reciprocally causative and had an interaction [1–4]. To the best of our knowledge, the mechanisms underlying the concomitant depression in patients with chronic diseases are usually ascribed to the disruption of the hypothalamic-pituitary-adrenal (HPA) axis and the involvement of varieties of proinflammatory cytokines. In the past decade, some investigators proposed the concept of vascular depression [5]. Depression may not only be a result of vascular diseases, but also a risk factor of vascular diseases. The relationship between depression and vascular endothelial dysfunction (a feature in the early stage of vascular diseases) is currently an important research area [6].
According to embryology, vascular cells compose a barrier between the vascular wall and deep tissues. Further studies revealed that endothelial cells also have endocrine characteristics and are able to regulate inflammatory reactions and coagulation. Endothelial dysfunction is mainly characterized by endothelium-dependent diastolic dysfunction, which is ascribed to the reduction in nitric oxide (NO) production. Currently, 2 methods are employed to detect endothelial function: intravascular injection of acetylcholine and pressurization with a cuff. In terms of intravascular injection of acetylcholine, when the endothelial function is normal, acetylcholine may induce the vascular dilation via NO pathway; however, when the endothelial dysfunction is present, vasoconstriction occurs after injection of acetylcholine [7,8]. Although this method is regarded as the criterion standard for evaluating endothelial function, it is not widely used in clinics because it is invasive, time-consuming, and expensive. When pressurization with a cuff is used to block the blood flow of the brachial artery, rapid blood flow after deflation may cause a high shear stress to the endothelial cells and induce them to release NO, resulting in vascular dilation. Then, the flow-mediated dilation is measured to evaluate the endothelial function [9,10]. This method may be influenced by many factors and highly dependent on the level of detection ability, which results in low repeatability and reliability. EndoPAT is a peripheral arterial tonometry in which the change in the finger arterial pulse volume is measured to evaluate the endothelial function by reactive hyperemia peripheral arterial tonometry index (RHI) [10,11]. It has no disadvantages related to the above mentioned methods and can be widely applied in clinical practices.
A recent meta analysis [12] showed that both severe and transient depression were related to endothelial dysfunction. However, whether the endothelial function becomes normal or is still abnormal during the following remission of depression or even complete recovery from depression is still unclear (that is endothelial function and depression status are independent characteristics or mutually dependent) [13–16]. This may be ascribed to the fact that the vascular endothelial dysfunction is influenced by a variety of factors such as lifestyle, concomitant diseases, and pharmacotherapy. Studies have been conducted to investigate the endothelial function in patients who have recovered from depression by undergoing anti-depression therapies [13,17]. However, these studies focused on the influence of antidepressant treatment on endothelial function. Few studies have been conducted to investigate the relationship between endothelial function and depression recovery after excluding the influence of antidepressants.
We hypothesized the following: depression is closely related to endothelial dysfunction; the remission of depression symptoms is accompanied by the improvement of endothelial function; and that the endothelial function is worst in depression patients, best in non-depression patients, and moderate in remission patients.
Material and Methods
Subjects and setting
Patients were consecutively recruited from the Cardiology, Neurology, Endocrinology, and Clinical Psychology Clinics of Beijing Chaoyang Hospital from March 2013 to June 2014. These patients were aged 30–70 years, and had the ability to provide consent and cooperate with the questionnaire survey.
Subjects for the 3 groups were matched for age (±3y) and body mass index (±1 kg/m2) at a ratio of 1:1:1 and there were 62 subjects in each group. This cross-sectional study was approved by the Ethics Committee of Beijing Chaoyang Hospital. Informed consent was obtained from all subjects in this study.
Depression group: Depression was diagnosed according to the Chinese version of the Modified Structured Clinical Interview for DSM-IV diagnostic criteria for patient version [18] by 2 trained clinical psychiatrists. All patients were drug-naıve and in their first episode of illness (they experienced a depressive episode for the first time), and had 17-item Hamilton Rating Scale for Depression total scores (HAMD17) ≥17.
Remission group: Subjects had a self-reported history of physician-diagnosed depression, HAMD17 ≤7, and antidepressants had been discontinued for at least 3 months.
Control group: All healthy controls were interviewed with the Structured Clinical Interview for DSM-IV, non-patient version. They were free of depression, other psychiatric or neurological illnesses, history of head injury, and had HAMD17 ≤7.
Exclusion criteria were: severe cardiovascular disease (massive myocardial infarction and coronary artery bypass), acute diseases in internal medicine (such as acute nerve deafness), severe mental disorders (schizophrenia and bipolar disorder), and cognition impairment.
Demographic characteristics
Demographics characteristics, including gender, age (in years), and smoking history, were obtained by self-report.
Physical examination and medical history
Height was measured using a wall-mounted stadiometer and weight was assessed by using calibrated electronic scales. Body mass index (BMI) was calculated as kg/m2. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured on the left arm. Fasting glucose (FG), triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL) and low-density lipoprotein cholesterol (LDL), and self-reported history of physician-diagnosed diseases (such as CHD, DM and CI) were also recorded.
Depressive symptoms
Current depressive symptoms were determined with HAMD17 [19], which is the most common scale used for clinical evaluation of depression status. There are 17 items in this scale and each item is graded 0–4. HAMD17 ≥17 indicates severe depression, and HAMD17 ≤7 denotes no depressive symptoms. This scale has acceptable reliability and validity in medical samples.
Endothelial function
EndoPAT2000 (Itamar Medical, Caesarea, Israel) has been widely used to measure the endothelial function, which is a beat-to-beat plethysmographic recording of the finger arterial pulse-wave amplitude (PWA) with pneumatic probes [20,21]. A variety of clinical studies have confirmed that this technique is convenient and easy to handle and had good reliability and intraclass correlation coefficients (0.78) [20–27]. In addition, it also has high sensitivity and specificity. In detecting CHD, the sensitivity and specificity of this technique were as high as 80% and 85%, respectively [28].
Detection of endothelial function was done between 8 and 10 am. Patients were fasting and in supine position. A cuff was inflated to obstruct the blood flow of the brachial artery for 5 min, followed by deflation and subsequent vascular dilation. EndoPAT software (version 2.3.2) was employed to calculate the ratio of signal amplitude before flow obstruction to that after flow obstruction. Then, RHI was obtained. The higher the RHI was, the better the endothelial function.
Statistical analysis
The demographics and clinical characteristics were compared with analysis of variance (ANOVA) or Kruskal-Wallis test for continuous variables and chi-square test for categorical variables. The linear regression model was used to analyze the effect of candidate covariates on RHI, and the candidate covariates included gender, age, BMI, SBP, DBP, FG, TC, HDL, LDL,TG, cardiovascular disease (yes/no), cerebrovascular disease (yes/no), and DM (yes/no). The affected factors on RHI were analyzed by mono-factor analysis. The general linear model was employed to compare RHI among the 3 groups after adjusting the covariates, and Bonferroni method was used for the correction of multiple comparisons among groups. A value of 2-tailed P<0.05 was considered statistically significant. Statistical analysis was performed with SAS 9.3.
Results
There were no significant differences in gender, age, BP, BMI, FG, LDL, HDL, TC, TG, or history of chronic diseases among the 3 groups (Table 1). Results showed that the demographics and clinical characteristics were comparable among three 3 groups and were matched for age and BMI.
Table 1.
Demographics and clinical characteristics of subjects in three groups.
| Depression | Remission | Non-depression | P | ||
|---|---|---|---|---|---|
| Gender | χ2=0.134 | 0.9352 | |||
| N (missing) | 62 (0) | 62 (0) | 62 (0) | ||
| Male/N (%) | 26 (41.94) | 25 (40.32) | 24 (38.71) | ||
| Female/N (%) | 36 (58.06) | 37 (59.68) | 38 (61.29) | ||
| Age | H=0.0163 | 0.9919 | |||
| X±SD | 54.18±9.69 | 54.15±9.63 | 54.00±9.65 | ||
| Median | 54.00 | 54.00 | 54.00 | ||
| Range | 33–70 | 33–70 | 33–70 | ||
| BMI | H=0.0451 | 0.9777 | |||
| X±SD | 25.09±2.91 | 25.09±2.79 | 25.14±2.84 | ||
| Median | 24.78 | 24.78 | 24.99 | ||
| Range | 19.05–31.28 | 19.05–30.41 | 19.05–30.41 | ||
| SBP | H=0.1044 | 0.9492 | |||
| X±SD | 132.02±11.4 | 131.77±9.46 | 132.58±11.26 | ||
| Median | 130.00 | 130.00 | 130.00 | ||
| Range | 100–160 | 110–160 | 120–165 | ||
| DBP | H=2.5992 | 0.2726 | |||
| X±SD | 78.56±10.1 | 76.29±8.54 | 75.95±8.43 | ||
| Median | 80 | 80 | 75 | ||
| Range | 60–105 | 60–95 | 60–100 | ||
| FG | H=4.6247 | 0.0990 | |||
| X±SD | 5.47±0.96 | 6.02±1.38 | 6.08±3.03 | ||
| Median | 5.33 | 5.77 | 5.49 | ||
| Range | 3.74–8.06 | 4.08–11.5 | 4.08–7.17 | ||
| TC | F=0.30 | 0.7443 | |||
| X±SD | 4.71±1.11 | 4.59±0.91 | 4.71±0.81 | ||
| Median | 4.75 | 4.65 | 4.73 | ||
| Range | 1.18–7.8 | 1.33–6.7 | 3.02–6.62 | ||
| HDL | H=0.7283 | 0.6948 | |||
| X±SD | 1.26±0.47 | 1.28±0.33 | 1.30±0.49 | ||
| Median | 1.23 | 1.25 | 1.25 | ||
| Range | 0.41–3.96 | 0.69–2.56 | 0.58–4.57 | ||
| LDL | H=1.6991 | 0.4276 | |||
| X±SD | 2.65±0.79 | 2.62±0.69 | 2.49±0.62 | ||
| Median | 2.58 | 2.62 | 2.55 | ||
| Range | 0.75–5.55 | 0.75–4.21 | 1.42–3.93 | ||
| TG | H=0.2367 | 0.8884 | |||
| X±SD | 1.77±0.90 | 1.73±0.94 | 1.72±1.01 | ||
| Median | 1.58 | 1.55 | 1.57 | ||
| Range | 0.42–4.33 | 0.42–4.62 | 0.43–6.76 | ||
| CHD | χ2=1,451 | 0.4841 | |||
| N (missing) | 62(0) | 61(1) | 62(0) | ||
| No/N (%) | 38(61.29) | 42(68.85) | 44(70.97) | ||
| Yes/N (%) | 24(38.71) | 19(31.15) | 18(29.03) | ||
| CI | χ2=1.787 | 0.4092 | |||
| N (missing) | 62(0) | 61(1) | 61(1) | ||
| No/N (%) | 46(74.19) | 39(63.93) | 45(72.58) | ||
| Yes/N (%) | 16(25.81) | 22(36.07) | 17(27.42) | ||
| DM | χ2=0.987 | 0.6105 | |||
| N (missing) | 61(1) | 61(1) | 62(0) | ||
| No/N (%) | 50(81.97) | 47(77.05) | 52(83.87) | ||
| Yes/N (%) | 11(18.03) | 14(22.95) | 10(16.13) |
BMI – body mass index (kg/m2); SBP – systolic blood pressure (mmHg); DBP – diastolic blood pressure (mmHg); FG – fasting glucose (mmol/L); TC – total cholesterol (mmol/L); HDL&LDL – high- and low-density lipoprotein cholesterol (mmol/L); TG – triglycerides (mmol/L); CHD – coronary heart disease [Y/N]; CI – cerebral infarction [Y/N]; DM – diabetes mellitus [Y/N]; HAMD17 – total scores of 17-item Hamilton Rating Scale for Depression.
The influence of 15 variables (e.g., gender, age, and clinical indicators of RHI) was analyzed by linear regression analysis (Table 2). The results showed that DBP, TG, CHD, and HAMD17 had significant effects on RHI (P<0.05).
Table 2.
Clinical variables showed association with RHI in univariate analysis.
| Variables | Parameter estimates | Standard error | t value | P value |
|---|---|---|---|---|
| Gender | 0.03924 | 0.08771 | 0.45 | 0.6551 |
| Age | −0.00407 | 0.00449 | −0.91 | 0.3656 |
| BMI | −0.01882 | 0.0152 | −1.24 | 0.2172 |
| SBP | −0.0055 | 0.00402 | −1.37 | 0.1727 |
| DBP | −0.01099 | 0.0047 | −2.34 | 0.0205 |
| FG | −0.02967 | 0.0214 | −1.39 | 0.1672 |
| TC | 0.0005933 | 0.04556 | 0.01 | 0.9896 |
| HDL | −0.10653 | 0.0994 | −1.07 | 0.2852 |
| LDL | 0.07075 | 0.06099 | 1.16 | 0.2475 |
| TG | −0.09247 | 0.04508 | −2.05 | 0.0417 |
| CHD | −0.4097 | 0.09083 | −4.51 | <0.0001 |
| CI | −0.0038 | 0.09739 | −0.04 | 0.9692 |
| DM | 0.03883 | 0.11379 | 0.34 | 0.7333 |
| HAMD17 | −0.0153 | 0.00268 | −5.72 | <0.0001 |
BMI – body mass index (kg/m2); SBP – systolic blood pressure (mmHg); DBP – diastolic blood pressure (mmHg); FG – fasting glucose (mmol/L); TC – total cholesterol (mmol/L); HDL&LDL – high- and low-density lipoprotein cholesterol (mmol/L); TG – triglycerides (mmol/L); CHD – coronary heart disease [Y/N]; CI – cerebral infarction [Y/N]; DM – diabetes mellitus [Y/N]; HAMD17 – total scores of 17-item Hamilton Rating Scale for Depression.
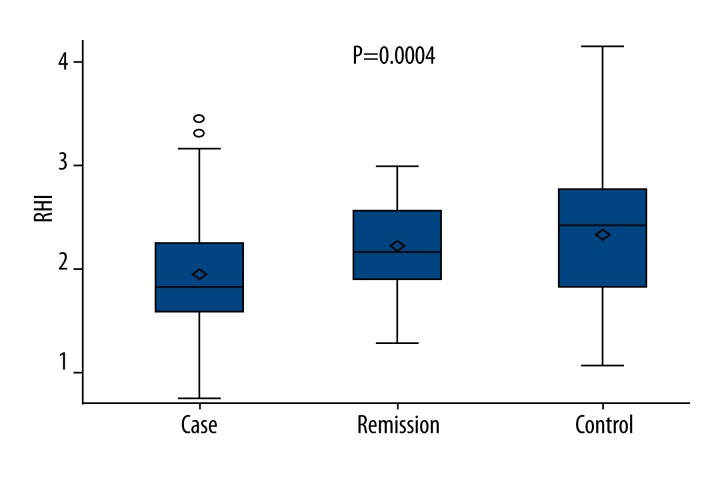
In terms of distribution balance of all covariates, a general linear model was used to compare RHI among the 3 groups without adjustments. The mean RHI was 1.93, 2.34, and 2.19 in the depression, control, and remission groups, respectively (Figure 1). The difference of RHI among the 3 groups was statistically significant (P=0.0004). Compared with the controls (2.337±0.697), RHI was significantly lower in the depression group (1.931±0.535; P=0.0003). RHI in the remission group (2.194±0.426) was markedly higher than in the depression group (P=0.027). However, there was no significant difference between remission and control groups (P=0.3363).
Figure 1.
RHI of patients in the 3 groups.
Discussion
After matching for age and BMI, patients in the 3 groups had comparable demographics and clinical characteristics. These patients were not receiving anti-depressants therapy when the RHI was measured. The results showed that depression was closely related to endothelial dysfunction, and endothelial function should be improved as symptoms of depression were relieved. The endothelial function in patients with remission from depression was comparable to that in non-depression patients.
Endothelial dysfunction is a critical step in the development of cardiovascular disease, such as hypertension, atherosclerosis, and thrombosis [29]. Several studies have demonstrated that endothelial function has relevance to blood pressure and lipids, which are cardiovascular risk factors. The relationship between depression symptoms and endothelial function is complicated. Some cross-sectional studies and retrospective studies [14,30–32] showed that depression in adolescence [14,20], climactereium [32,33] and senility, unipolar depression, bipolar depression [15], major depressive episodes, and minor depressive symptoms [6,20] were closely associated with endothelial dysfunction. Regarding biological mechanisms, depression may reduce synthesis of nitric oxide synthase [34] and create imbalance between dilation (by NO) and contraction (by ET-1) of blood vessels, which finally causes endothelial dysfunction. Depression directly influences the HPA axis and causes its overactivity to increase cortisol levels [35,36]. Cortisol may impair endothelial function. According to the behavioral mechanisms, depression may cause obesity [20], reduced exercises, and disturbed metabolism of glucose and lipid, which indirectly result in endothelial dysfunction. However, several studies [16,37] found a relationship between depression and CHD [16] and DM [37,38]. In the present study, patients were recruited from a general hospital in China, and results showed that DBP, TG, CHD, and HAMD total scores had significant effects on RHI, consistent with previous research [16].
Another important result in the present study was that the improvement of depression was accompanied by improvement of endothelial function. This may be ascribed to the following reasons: 1) endothelial dysfunction is dependent on depressive status. The improvement of depression may cause the cortisol secretion to become normal in the HPA axis. In the presence of stress, metyrapone, a drug able to inhibit the secretion of cortisol, is able to prevent endothelial dysfunction [36]. In our study, the endothelial function was measured on the basis of NO and was mainly characterized by vascular dilation. After anti-depressant therapy, the β receptor might become normal, leading to vascular dilation [15]. 2) Antidepressants may improve the endothelial function. Pizzi et al. [39,40] found the improvement of depression in CHD patients with depression following anti-depressant therapy was accompanied by the improvement of endothelial function. There is evidence showing that the endothelial dysfunction is still present in depression patients after anti-depressant therapy [13,15]. Among clinical studies, only the SADHART study [41] indicated that SSRIs might reduce the morbidity and mortality related to cardiovascular and cerebrovascular diseases. The evidence is still not enough to establish that successful anti-depressants therapy can reduce the incidence of cardiovascular events and/or increase the survival rate of these patients. Although the effect of SSRIs on nitric oxide synthase and NO production is ambiguous [40], anti-depressants as a confounding factor might obscure the relationship with endothelial function. In the present study, the influence of anti-depressants therapy on this relationship was excluded and results confirmed that the endothelial function recovered 3 months after discontinuation of anti-depressants therapy when clinical remission of depression was confirmed, and the endothelial function in remission patients was comparable to that of non-depression patients. Our findings are consistent with previously reported research [16] and demonstrate that the endothelial function after anti-depressants treatment could recover to the level of healthy controls.
EndoPAT2000 is able to identify atherosclerosis at an early stage and predict the adverse cardiovascular outcomes within 7 years [11]. This study for the first time employed EndoPAT for the non-invasive quantitative evaluation of vascular endothelial function in outpatients recruited from a general hospital. Our findings are consistent with results obtained with other methods for the detection of endothelial function. Thus, this technique is applicable in clinical studies on general screening and our results may be generalized.
This was a cross-sectional study that failed to evaluate the causative relationship between depression and endothelial function. This causative relationship needs to be confirmed in future prospective studies with larger sample sizes. Although paired comparisons were done among the 3 groups after balancing the clinical characteristics of subjects in these groups, which is a novelty of this study, the course of depression and details of anti-depressants therapy were not analyzed and evaluated. In future studies, investigators may focus on the relationship of changes in depression status with changes in endothelial function of a specific individual.
Conclusions
Taken together, depression in outpatients from a general hospital in China is closely related to endothelial dysfunction. The improvement of depression after anti-depression therapy is accompanied by improvement of endothelial function, which returns to a level comparable to that in healthy controls. Further studies with larger sample sizes are required to confirm these results.
Footnotes
Source of support: Departmental sources
References
- 1.Davidson KW. Depression and coronary heart disease. ISRN Cardiol. 2012;2012:743813. doi: 10.5402/2012/743813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egede LE, Ellis C. Diabetes and depression: global perspectives. Diabetes Res Clin Pract. 2010;87:302–12. doi: 10.1016/j.diabres.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363:2611–20. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egede LE. Major depression in individuals with chronic medical disorders: prevalence, correlates and association with health resource utilization, lost productivity and functional disability. Gen Hosp Psychiatry. 2007;29:409–16. doi: 10.1016/j.genhosppsych.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963–74. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper DC, Milic MS, Tafur JR, et al. Adverse impact of mood on flow-mediated dilation. Psychosom Med. 2010;72:122–27. doi: 10.1097/PSY.0b013e3181cdbfc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lockhart CJ, McVeigh GE, Cohn JN. Measuring endothelial function. Curr Diab Rep. 2006;6:267–73. doi: 10.1007/s11892-006-0059-y. [DOI] [PubMed] [Google Scholar]
- 8.Poitras VJ, Pyke KE. The impact of acute mental stress on vascular endothelial function: evidence, mechanisms and importance. Int J Psychophysiol. 2013;88:124–35. doi: 10.1016/j.ijpsycho.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Rozanski A, Qureshi E, Bauman M, et al. Peripheral arterial responses to treadmill exercise among healthy subjects and atherosclerotic patients. Circulation. 2001;103:2084–89. doi: 10.1161/01.cir.103.16.2084. [DOI] [PubMed] [Google Scholar]
- 10.Kuvin JT, Mammen A, Mooney P, et al. Assessment of peripheral vascular endothelial function in the ambulatory setting. Vasc Med. 2007;12:13–16. doi: 10.1177/1358863X06076227. [DOI] [PubMed] [Google Scholar]
- 11.Goswami N, Gorur P, Pilsl U, et al. Effect of orthostasis on endothelial function: a gender comparative study. PLoS One. 2013;8:e71655. doi: 10.1371/journal.pone.0071655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper DC, Tomfohr LM, Milic MS, et al. Depressed mood and flow-mediated dilation: a systematic review and meta-analysis. Psychosom Med. 2011;73:360–69. doi: 10.1097/PSY.0b013e31821db79a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broadley AJ, Korszun A, Jones CJ, Frenneaux MP. Arterial endothelial function is impaired in treated depression. Heart. 2002;88:521–23. doi: 10.1136/heart.88.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajagopalan S, Brook R, Rubenfire M, et al. Abnormal brachial artery flow-mediated vasodilation in young adults with major depression. Am J Cardiol. 2001;88:196–68. a7. doi: 10.1016/s0002-9149(01)01623-x. [DOI] [PubMed] [Google Scholar]
- 15.Rybakowski JK, Wykretowicz A, Heymann-Szlachcinska A, Wysocki H. Impairment of endothelial function in unipolar and bipolar depression. Biol Psychiatry. 2006;60:889–91. doi: 10.1016/j.biopsych.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Sherwood A, Hinderliter AL, Watkins LL, et al. Impaired endothelial function in coronary heart disease patients with depressive symptomatology. J Am Coll Cardiol. 2005;46:656–59. doi: 10.1016/j.jacc.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 17.Finkel MS, Laghrissi-Thode F, Pollock BG, Rong J. Paroxetine is a novel nitric oxide synthase inhibitor. Psychopharmacol Bull. 1996;32:653–58. [PubMed] [Google Scholar]
- 18.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, D.C: American Psychiatric Press, Inc; 1996. [Google Scholar]
- 19.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomfohr LM, Martin TM, Miller GE. Symptoms of depression and impaired endothelial function in healthy adolescent women. J Behav Med. 2008;31:137–43. doi: 10.1007/s10865-007-9141-4. [DOI] [PubMed] [Google Scholar]
- 21.Tomfohr LM, Murphy ML, Miller GE, Puterman E. Multiwave associations between depressive symptoms and endothelial function in adolescent and young adult females. Psychosom Med. 2011;73:456–61. doi: 10.1097/PSY.0b013e3182228644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonetti PO, Barsness GW, Keelan PC, et al. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41:1761–68. doi: 10.1016/s0735-1097(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 23.Itzhaki S, Lavie L, Pillar G, et al. Endothelial dysfunction in obstructive sleep apnea measured by peripheral arterial tone response in the finger to reactive hyperemia. Sleep. 2005;28:594–600. doi: 10.1093/sleep/28.5.594. [DOI] [PubMed] [Google Scholar]
- 24.Mahmud FH, Earing MG, Lee RA, et al. Altered endothelial function in asymptomatic male adolescents with type 1 diabetes. Congenit Heart Dis. 2006;1:98–103. doi: 10.1111/j.1747-0803.2006.00015.x. [DOI] [PubMed] [Google Scholar]
- 25.Nohria A, Gerhard-Herman M, Creager MA, et al. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol (1985) 2006;101:545–48. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- 26.Yinon D, Lowenstein L, Suraya S, et al. Pre-eclampsia is associated with sleep-disordered breathing and endothelial dysfunction. Eur Respir J. 2006;27:328–33. doi: 10.1183/09031936.06.00010905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selamet Tierney ES, Newburger JW, Gauvreau K, et al. Endothelial pulse amplitude testing: feasibility and reproducibility in adolescents. J Pediatr. 2009;154:901–5. doi: 10.1016/j.jpeds.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 28.Bonetti PO, Pumper GM, Higano ST, et al. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–41. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 29.Zheng Z, Zeng Y, Huang H, Xu F. MicroRNA-132 may play a role in coexistence of depression and cardiovascular disease: a hypothesis. Med Sci Monit. 2013;19:438–43. doi: 10.12659/MSM.883935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavoie KL, Pelletier R, Arsenault A, et al. Association between clinical depression and endothelial function measured by forearm hyperemic reactivity. Psychosom Med. 2010;72:20–26. doi: 10.1097/PSY.0b013e3181c2d6b8. [DOI] [PubMed] [Google Scholar]
- 31.Greenstein AS, Paranthaman R, Burns A, et al. Cerebrovascular damage in late-life depression is associated with structural and functional abnormalities of subcutaneous small arteries. Hypertension. 2010;56:734–40. doi: 10.1161/HYPERTENSIONAHA.110.152801. [DOI] [PubMed] [Google Scholar]
- 32.Harris KF, Matthews KA, Sutton-Tyrrell K, Kuller LH. Associations between psychological traits and endothelial function in postmenopausal women. Psychosom Med. 2003;65:402–9. doi: 10.1097/01.psy.0000035720.08842.9f. [DOI] [PubMed] [Google Scholar]
- 33.Wagner JA, Tennen H, Mansoor GA, Abbott G. History of major depressive disorder and endothelial function in postmenopausal women. Psychosom Med. 2006;68:80–86. doi: 10.1097/01.psy.0000195868.68122.9e. [DOI] [PubMed] [Google Scholar]
- 34.Chrapko WE, Jurasz P, Radomski MW, et al. Decreased platelet nitric oxide synthase activity and plasma nitric oxide metabolites in major depressive disorder. Biol Psychiatry. 2004;56:129–34. doi: 10.1016/j.biopsych.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Osika W, Montgomery SM, Dangardt F, et al. Anger, depression and anxiety associated with endothelial function in childhood and adolescence. Arch Dis Child. 2011;96:38–43. doi: 10.1136/adc.2008.152777. [DOI] [PubMed] [Google Scholar]
- 36.Broadley AJ, Korszun A, Abdelaal E, et al. Metyrapone improves endothelial dysfunction in patients with treated depression. J Am Coll Cardiol. 2006;48:170–75. doi: 10.1016/j.jacc.2005.12.078. [DOI] [PubMed] [Google Scholar]
- 37.Wagner J, Tennen H, Mansoor G, Abbott G. Endothelial dysfunction and history of recurrent depression in postmenopausal women with Type 2 diabetes: a case-control study. J Diabetes Complications. 2009;23:18–24. doi: 10.1016/j.jdiacomp.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Kheirabadi GR, Toghani F, Kousha M, et al. Is there any association of anxiety-depressive symptoms with vascular endothelial function or systemic inflammation? J Res Med Sci. 2013;18:979–83. [PMC free article] [PubMed] [Google Scholar]
- 39.Pizzi C, Mancini S, Angeloni L, et al. Effects of selective serotonin reuptake inhibitor therapy on endothelial function and inflammatory markers in patients with coronary heart disease. Clin Pharmacol Ther. 2009;86:527–32. doi: 10.1038/clpt.2009.121. [DOI] [PubMed] [Google Scholar]
- 40.van Zyl LT, Lesperance F, Frasure-Smith N, et al. Platelet and endothelial activity in comorbid major depression and coronary artery disease patients treated with citalopram: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy Trial (CREATE) biomarker sub-study. J Thromb Thrombolysis. 2009;27:48–56. doi: 10.1007/s11239-007-0189-3. [DOI] [PubMed] [Google Scholar]
- 41.Swenson JR, O’Connor CM, Barton D, et al. Influence of depression and effect of treatment with sertraline on quality of life after hospitalization for acute coronary syndrome. Am J Cardiol. 2003;92:1271–76. doi: 10.1016/j.amjcard.2003.08.006. [DOI] [PubMed] [Google Scholar]