Abstract
Background
Insulin-resistant subjects develop more severe and diffuse coronary artery atherosclerosis than insulin-sensitive controls but the mechanisms that mediate this atherosclerosis phenotype are unknown.
Research Objective
To determine the metabolic parameters that associate with the severity of coronary atherosclerosis in insulin resistant pigs fed a high fat/high NaCl diet.
Key Methods
The primary endpoint was severity of coronary atherosclerosis in adult pigs (Sus scrofa, n = 37) fed a high fat diet that also contained high NaCl (56% above recommended levels) for 1 year.
Principal Findings
Twenty pigs developed severe and diffuse distal coronary artery atherosclerosis (i.e., severe = intimal area as a percent medial area > 200% in at least 2 coronary artery cross sections and diffuse distal = intimal area as a percent medial area ≥ 150% over 3 sections separated by 2 cm in the distal half of the coronary artery). The other 17 pigs had substantially less coronary artery atherosclerosis. All 37 pigs had blood pressure in a range that would be considered hypertensive in humans and developed elevations in total and LDL and HDL cholesterol, weight gain, increased backfat, and increased insulin resistance (Bergman Si) without overt diabetes. Insulin resistance was not associated with atherosclerosis severity. Five additional pigs fed regular pig chow also developed increased insulin resistance but essentially no change in the other variables and little to no detectible coronary atherosclerosis. Most importantly, the 20 high fat/high NaCl diet -fed pigs with severe and diffuse distal coronary artery atherosclerosis had substantially greater increases (p< 0.05) in oxidized LDL (oxLDL) and fructosamine consistent with increased protein glycation.
Conclusion
In pigs fed a high fat/high NaCl diet, glycated proteins are induced in the absence of overt diabetes and this degree of increase is associated with the development of severe and diffuse distal coronary artery atherosclerosis.
Introduction
The increasing prevalence of insulin resistance and type 2 diabetes is likely to be attended by a substantial increase in cardiovascular disease (CVD).[1–3] Insulin resistance (IR) is defined as a decreased biological response to normal concentrations of serum insulin that over time leads to compensatory hyperinsulinemia.[2] Insulin resistant and diabetic humans often develop multiple and severe coronary atherosclerotic lesions that are diffuse in that they involve long arterial segments especially in the distal portions of the coronary arteries.[4–8] In addition, there is more extensive remodeling and fibrous caps are thicker.[5, 7] The resulting small caliber arteries are less amenable to angioplasty, stent placement, surgical reconstruction or bypass.[9] Even when coronary artery stent placement or bypass surgery is feasible, often disease progression outside of the stented segment of the coronary artery or bypass insertion site limits the duration of benefit in patients with IR and diabetes.[10] Remarkably, even the most aggressive medical treatment regimens do not lower the risk for CVD to the non-diabetic level.[9, 11–15] These findings strongly suggest that factors other than absolute glucose concentrations may be activating pathophysiological mechanisms that augment the development of atherosclerosis. Thus, there is a need for a relevant animal model of insulin resistance and/or type 2 diabetes that also exhibits severe and diffuse coronary and aortic atherosclerosis which are associated with identifiable biochemical abnormalities.
Our objective was to investigate whether the severity of atherosclerosis is associated not only with lipoprotein concentrations, weight, blood pressure, biomarkers of inflammation and IR in an animal model but also changes in parameters that measure protein glycation. Our experimental approach was to study normocholesterolemic pigs fed a high fat diet that also contained increased NaCl. Our choice of pigs was driven by the fact that, like humans, they develop coronary artery and aortic atherosclerosis and insulin resistance. In addition, pigs have been used in many studies to define the mechanisms that mediate increased atherosclerosis in diabetes.[16–18] Our results show that the experimental pigs developed varying degrees of coronary atherosclerosis that were grouped as either severe and diffuse distal or moderate. Atherosclerosis severity was not associated with increases in body weight, backfat, insulin or glucose levels, insulin resistance, blood pressure, or biomarkers of inflammation. Two other variables, however, that have been associated with increased oxidative protein glycation, oxLDL and fructosamine, were found to be substantially higher in animals with the severe and diffuse distal coronary atherosclerosis phenotype as compared to animals with moderate disease. Thus, although our data are associational and not mechanistic, this paradigm suggests that the increased protein glycation that occurs with feeding a high fat/high NaCl diet may enhance the severity of atherosclerosis development in insulin resistant, hypercholesterolemic pigs.
Materials and Methods
Experimental Pigs
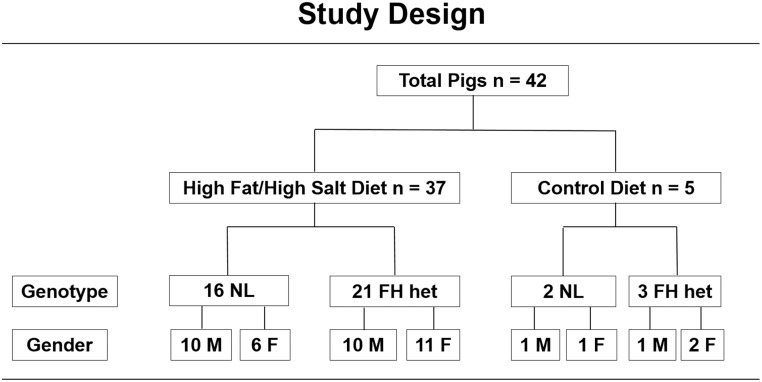
All pigs were produced and maintained in the same environmental conditions at the Francis Owen Blood Research Laboratory at the University of North Carolina at Chapel Hill. The background was Spotted Poland/China and Yorkshire crosses. Male and female pigs from the following two genotypes were used: (1) normocholesterolemic or (2) heterozygous familial hypercholesterolemic (FH) that have a recessive inheritance pattern.[19–21] Both genotypes of pigs are normocholesterolemic at baseline and only exhibit hypercholesterolemia when fed a high fat diet. In addition to being identified by normal cholesterol levels, FH heterozygotes are also identified from litters produced by known sires and dams with normal LDL receptor sequences or carrying the FH mutation, a missense mutation in a single base pair (C253➔ T253) of the LDL receptor that results in an arginine94 to cysteine94 mutation, located in the region that corresponds to exon 4 in the human ligand binding domain.[19–21] Pigs were maintained in agricultural style Hog Slats Inc buildings with ambient light and dark cycles, free access to water, with a temperature range of 55 to 85 degrees F. All pigs were checked daily for food consumption and general health issues. The UNC Division of Laboratory Animal Medicine provided regular veterinarian oversight. Forty-two pigs were entered into the year long study as they became available (Fig 1). Thirty-seven pigs were fed a high fat/high NaCL diet for one year: 16 normal (10 males, 6 females) and 21 heterozygous FH (10 males, 11 females). Five pigs were fed regular pig chow: 2 normal (1 male, 1 female) and 3 heterozygous FH (1 male, 2 females). The mean age at study entry was 3.2 ± 1.6 years.
Fig 1. Study Design.
The 42 pigs entered into this study are listed according to the diet they were fed, genotype (NL = normal, FH het = FH heterozygous) and gender.
Ethics Statement
All pigs were handled in strict accordance with the USDA regulations and the standards described in the 2010 Guide for the Care and Use of Laboratory Animals 8th edition (http://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-Use-of-Laboratory-Animals.pdf). All procedures and protocols were in accordance with institutional guidelines and approved by the Institutional Animal Care and Use Committee at the University of North Carolina in Chapel Hill (Animal Subject Assurance #A3410-01). When necessary, ketamine and acepromazine were given for sedation. See also PONE-D-15-01878R1 NC3Rs ARRIVE Guidelines Checklist Final in S1 File.
High Fat/High NaCl Diet and Regular Pig Chow
The high fat /high NaCl diet consisted of pig chow (5NP8 Wayne 15% Pig & Sow Pellets, Granville Milling, Granville NC) supplemented with 1% cholesterol, 20% beef tallow, and 0.75% cholate by weight.[22, 23] The NaCl content of the diets was measured (Eurofins Scientific Inc, Des Moines, IA) and 5NP8 provided 0.35% or 8 grams/day as recommended[24] and the high fat diet provided 0.55% or 12.5 grams/day, a 56% increase. The total calories in the high fat/high NaCl diet were distributed as: 43% fat, 12.5% protein, and 44.5% carbohydrate. The 5NP8 was fed to the 5 control pigs as the source of regular pig chow. All pigs were fed in the morning once per day in their pen to be able to monitor and confirm food consumption.
Sampling Protocol during Year Long Study
Weight, backfat, blood pressure, total and LDL and HDL cholesterol, oxLDL, fructosamine, aldosterone, triglycerides, Bergman Frequently Sampled Intravenous Glucose Tolerance Test (FSIVGTT), and serum and plasma inflammatory markers (TNF-alpha, IL-6, PAI-1, CRP) were obtained at baseline (BL), 3, 6, and 12 months of the year-long study. Serum glucose was measured monthly while the pig was fully conscious in addition to when it was sedated for the [25] FSIVGTT. Serum chemistry panels were obtained monthly to monitor general health status and processed by a commercial veterinary laboratory (Antech, Cary NC). At study termination all pigs were euthanized with an overdose of pentobarbital (6 grains/10 lbs. iv.) and tissue samples for morphometry were collected from all three coronary arteries and the abdominal aorta.
Bergman Frequently Sampled Intravenous Glucose Tolerance Test (FSIVGTT)
Each pig was sedated with ketamine 4–6 mg/kg intramuscular (IM) and acepromazine 0.1–0.3 mg/kg IM. Two intravenous catheters were placed, one for sampling and one for infusing glucose and insulin. A bolus of glucose (0.3 gm/kg IV) was administered as a 50% solution over ~5 to 10 min. Serum was prepared from blood samples obtained at -15, -10, -5, -1, 2, 3, 4, 5, 6, 8, 10, 14, and 19 minutes to measure insulin and glucose concentrations. At 20 minutes, an insulin bolus (0.03U/kg IV, Novolin R Human Insulin Regular, Novo Nordisk) was injected and blood samples for insulin and glucose concentrations were collected at 22, 25, 30, 40, 50, 70, 100, 140, and 180 minutes. The data were analyzed by the Bergman method to calculate an insulin sensitivity index (Si) using MINMOD Millennium version 6.02.[26] This method has been widely used to estimate insulin sensitivity in humans and pigs.[25, 27–32]
Measurement of Body Weight, Backfat, and Arterial Blood Pressure
Pigs were weighed on a FlexWeigh scale (model LPF-4824) with a digital readout (Velcon/FlexWeigh Model 5). Backfat has been used as an index of total body fat in pigs[33–38] and was measured by ultrasound over the last rib with the pig standing (Fukuda Denshi, model UF750XT). The intra-animal variability was < 7%. Arterial blood pressure was measured in the tail of conscious pigs (Veterinary Blood Pressure Monitor, model 9301V, CAS Medical Systems, Inc).[39–41] The results are the mean of 5 measurements taken over 5 min.
Total and LDL and HDL Cholesterol, Triglycerides, Insulin, and Glucose
Serum cholesterol was measured with the Cholesterol E kit (Wako, Richmond, VA) that utilizes an enzymatic colorimetric method for quantitative determination of total cholesterol in serum. LDL and HDL cholesterol were measured by colorimetric endpoint reactions using the Liquid Direct LDL and HDL Cholesterol Kits, respectively (Amresco, Solon, OH). Reactions were performed according to the manufacturer’s instructions modified for a 96 well plate and optical density was read using a Vmax kinetic microtiter plate reader (Molecular Devices). Triglycerides were measured by an enzymatic method using triglyceride reagent (Sigma Aldrich). Serum insulin was measured by a solid phase RIA (ICN, lower unit of detection is 2 μU/ml). The intraassay variability was 4% and the interassay variability was 6%. Serum glucose was measured with a 2300 STAT PLUS (YSI, Yellow Springs, Ohio).
Inflammatory Markers, oxLDL, Fructosamine, and Aldosterone
Pig specific ELISAs were used according to the manufacturer’s instructions to measure TNF-alpha (Biosource),[42] IL-6 (R&D Systems), PAI-1 (Molecular Innovations, Southfield, MI), [43] CRP (Pig CRP, Tri-Delta Diagnostics), and oxLDL (Mercodia, Uppsala, Sweden).[44–46] Fructosamine was measured in serum by a colorimetric endpoint reaction according to the manufacturer’s instructions (Raichem, San Diego, CA). Aldosterone was measured by enzyme immunoassay with a commercially available kit (Aldosterone EIA, Alpco Diagnostics, Salem, NH) using serum samples that were ether extracted, dried and reconstituted in sample buffer.
Morphometry of Coronary and Abdominal Aortic Atherosclerosis
Coronary artery and aortic atherosclerosis were measured as previously described.[23, 47] Digital, calibrated images of all coronary and aortic sections were made by scanning the histological sections using the Aperio ScanScope and these images were measured using Aperio ImageScope software. Coronary artery atherosclerosis histomorphometry measurements include medial area, intimal area, percent stenosis and intimal area as percent medial area.[23, 47] Abdominal aortic atherosclerosis measurements include histomorphometry (i.e., medial area, intimal area, and intimal area as percent medial area) as well as en face morphometry measurements of the total area, lesion area and percent surface area with raised lesions.[23, 47] Coronary and aortic atherosclerosis was measured by at least two blinded observers using the same microscope, computer, and software. The inter- and intra- observer variability was between 4 and 7%.
Definition of Severe and Diffuse Distal Coronary Artery Atherosclerosis
Severe coronary atherosclerosis was defined as mean intimal area as a percent of medial area of ≥ 200% in at least two coronary artery cross sections in the proximal or distal half. Diffuse distal coronary atherosclerosis was defined as a mean intimal area as a percent of medial area of ≥ 150% over at least three coronary artery cross sections separated by 2 cm in the distal half of the coronary artery.
Total Internal Elastic Lamina (IEL) Area as an Assessment of Coronary Artery Remodeling
The total area contained within the IEL has been used to assess coronary arteries for remodeling during atherogenesis in humans and pigs.[5, 48, 49] Thus, the total IEL area for all three coronary arteries from the three groups of pigs was measured as described,[23, 47] and the results were expressed in mm2 for each of the three coronary arteries from all pigs.
Percent of Coronary Artery Cross Sections with Fibrous Caps and Measurement of Fibrous Cap Thickness
The presence or absence of a fibrous cap was determined from visual inspection of all sections of each coronary artery from all pigs. The results are expressed as the percent of cross sections with fibrous caps. Maximal fibrous cap thickness was measured on the cross section with the greatest percent stenosis for a given coronary artery and is expressed in mm.
Biostatistical Analyses
For all groups, descriptive statistics are reported as means and SD for weight, backfat, blood pressure, triglycerides, total and LDL and HDL cholesterol, oxLDL, fructosamine, aldosterone, inflammatory markers, insulin, glucose, Bergman Si values, coronary and aortic atherosclerosis measurements. The coronary intimal area as a percent of medial area was used as the primary measure of atherosclerotic severity. The Wilcoxon rank sum statistic was used as the primary method for pairwise comparisons between severe and moderate atherosclerotic groups. Parametric repeated measures ANCOVA models with group, time, and baseline as explanatory factors were used as the primary method of finding association of logarithms of oxLDL, fructosamine, and aldosterone with atherosclerotic severity. In some of the models, atherosclerotic severity is dichotomous. Other models manage atherosclerotic severity as continuous, using a function of distal coronary arteries and intimal area as medial percentage. For blood pressure, total and LDL and HDL cholesterol, and triglycerides, Wilcoxon rank sum statistics for pairwise comparisons between groups and Wilcoxon signed ranks statistics for comparisons within groups were used with parametric repeated measures ANCOVA models in mutually supportive ways and with comparable results. The ANCOVA models additionally included gender and/or genotype as needed to account for their associations. A p-value less than 0.05 was the criterion for identifying a change within groups, a difference between groups, or an association of interest for future investigation.
Results
The detailed results of the atherosclerosis data are presented first followed by the metabolic parameters that were monitored during the year long study to determine which ones were associated with the development of either the severe and diffuse distal versus moderate coronary atherosclerosis phenotype. Additional analyses that identified associations with p<0.05 for gender and genotype (i.e., normocholesterolemic and FH heterozygous) are listed.
Severity of Coronary Artery Atherosclerosis
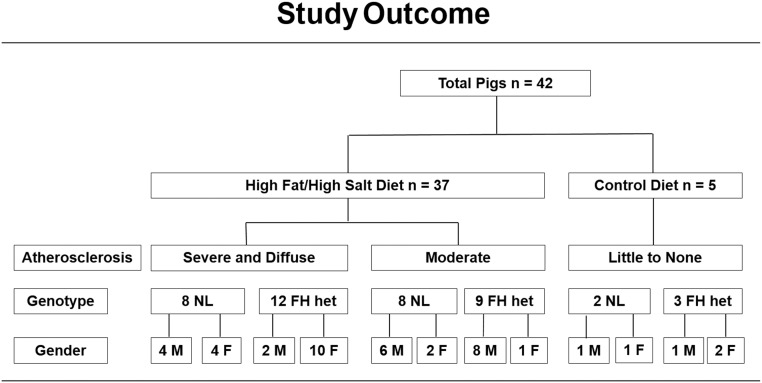
At the end of the study, 20 pigs (6 males and 14 females) fed the high fat/high NaCl diet were found to have met the criteria for both severe and diffuse distal coronary atherosclerosis (Figs 2–4). The other 17 pigs (14 males and 3 females) also fed the same high fat/high NaCl diet had substantially less or moderate coronary atherosclerosis. When the extent of coronary atherosclerosis was compared between the severe and moderate atherosclerosis groups for the proximal and distal halves of the coronary arteries, the severe group had much larger intimal area, percent stenosis, and intimal area as a percent medial area (Figs 3 and 4 and Table 1, p < 0.001). In a logistic regression model that controlled for gender, severe and diffuse coronary atherosclerosis was associated with female pigs (p = 0.003). Such a model that controlled for genotype instead of gender indicated no association of normal versus heterozygous FH genotype with severity of atherosclerosis (p = 0.963). The 5 pigs fed regular, low fat pig chow had little to no detectible coronary atherosclerosis (Table 1).
Fig 2. Study Outcome.
The 42 pigs are described as in Fig 1 and grouped by atherosclerosis phenotype that developed during the 12-month study.
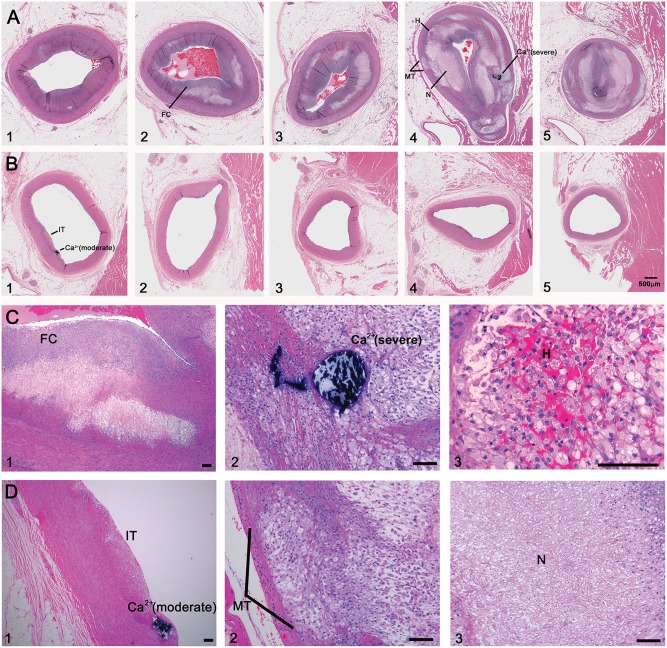
Fig 4. Coronary Artery Atherosclerosis in Insulin Resistant Pigs.
Panels A1 to A5: Representative serial sections from the proximal to distal left anterior descending coronary artery are shown from an IR pig with severe and diffuse distal coronary atherosclerosis. The mean intimal area as a percent of medial area for all three coronary arteries for this pig was 203.6% proximally and 338.5% distally. Panels B1 to B5: In contrast, a pig with moderate coronary atherosclerosis had a mean intimal area as a percent of medial area for all three coronary arteries of 11.3% proximally and 5.8% distally. Features of coronary atherosclerosis in rows A and B are shown at higher magnification in rows C and D, respectively: intimal thickening (IT) and calcification in a pig with moderate atherosclerosis (Ca2+ (moderate), Panels B1 and D1), calcification in a pig with severe atherosclerosis (Ca2+ (severe), Panels A4 and C2), fibrous cap (FC, Panel A2 and C1), hemorrhage into the plaque (H, Panels A4 and C3), medial thinning (MT, Panels A4 and D2), necrosis (N, Panels A4 and D3). (Hematoxylin and Eosin, mag bar = 500 μm is shown in B5 for rows A and B; mag bar = 10 μm in all panels in rows C and D).
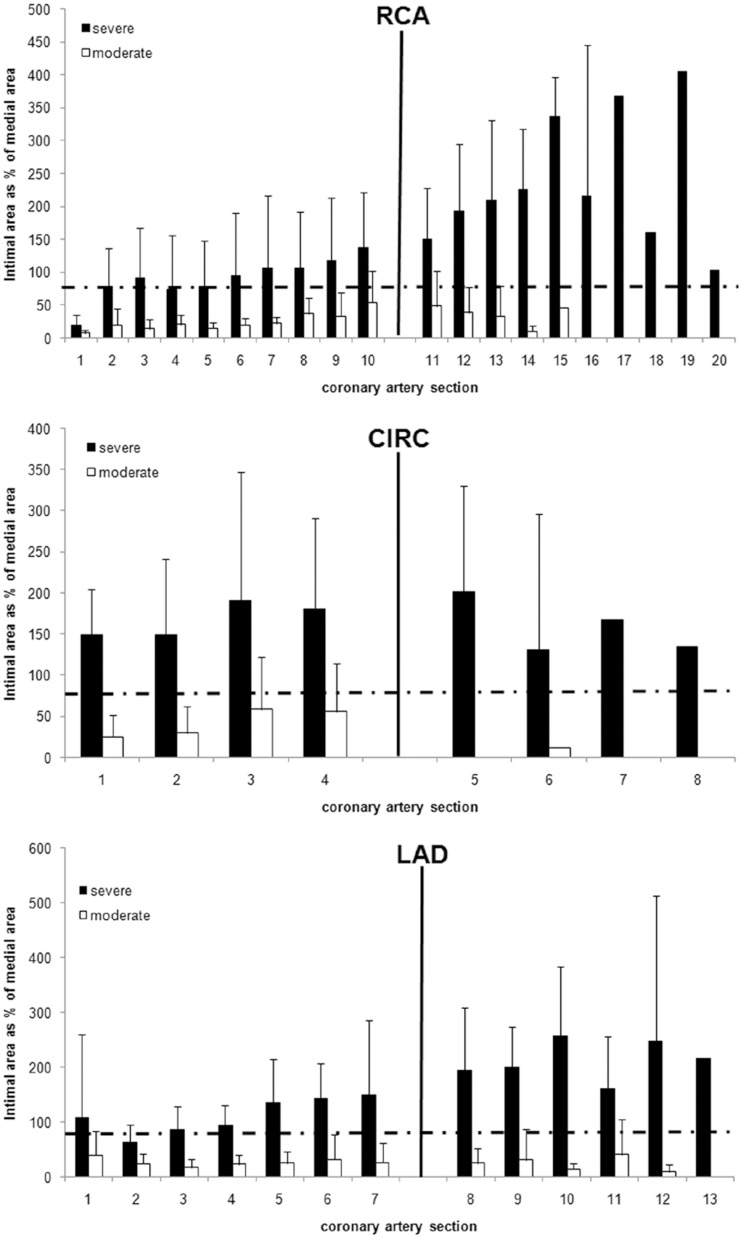
Fig 3. Intimal Area as % Medial Area from Serial Coronary Artery Sections.
The mean ± SD for intimal area as % medial area of all sections from all three coronary arteries are shown by severe and diffuse (black), moderate (white), or control (grey) groups. Sections were taken at 1 cm intervals. The solid black vertical line indicates the division between the proximal and distal halves of each coronary artery. The horizontal black line at 200% indicates the criteria for severe coronary atherosclerosis. The hashed line at 150% indicates diffuse and distal coronary atherosclerosis when it occurs over 3 sections (i.e., 2 cm). Using these criteria, pigs in the severe atherosclerosis group also exhibited diffuse and distal coronary atherosclerosis in all three coronary arteries.
Table 1. Proximal and Distal Coronary Artery Atherosclerosis Morphometry.
| Proximal Coronary Arteries | Distal Coronary Arteries | |||||||
|---|---|---|---|---|---|---|---|---|
| Medial area (mm2) | Intimal area (mm2) | % Stenosis | Intimal area as % medial area | Medial area (mm2) | Intimal area (mm2) | % Stenosis | Intimal area as % medial area | |
| Severe and Diffuse Atherosclerosis (n = 20) | ||||||||
| Mean | 6.4 | 7.1 | 57.8 | 142.6 | 3.0 | 5.6 | 68.5 | 210.4 |
| ± SD | 1.5 | 3.2 | 18.6 | 69.6 | 1.1 | 2.4 | 21.2 | 90.7 |
| Moderate Atherosclerosis (n = 17) | ||||||||
| Mean | 6.4 | 1.8 | 24.3 | 30.4 | 2.9 | 1.6 | 33.3 | 52.0 |
| ± SD | 2.1 | 1.3 | 15.6 | 18.5 | 1.3 | 1.4 | 24.2 | 38.9 |
| Control (n = 5) | ||||||||
| Mean | 4.2 | 0.5 | 6.5 | 12.8 | 1.7 | 0.2 | 3.7 | 10.1 |
| ± SD | 0.9 | 0.1 | 1.5 | 2.9 | 0.3 | 0.1 | 1.3 | 4.5 |
| p* | ||||||||
| Severe vs Moderate | 0.775 | < 0.001 | < 0.001 | < 0.001 | 0.478 | < 0.001 | < 0.001 | < 0.001 |
| Severe vs Control | 0.002 | < 0.001 | < 0.001 | < 0.001 | 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Moderate vs Control | 0.031 | 0.011 | 0.006 | 0.039 | 0.071 | < 0.001 | < 0.001 | 0.006 |
* Wilcoxon rank sum statistic for differences between groups.
Coronary Artery Remodeling Assessed by Total IEL Area
The severe atherosclerosis group had substantially larger total IEL areas when compared to the moderate (p < 0.001) or control groups (p<0.035) for all three coronary arteries (Table 2). The data are consistent with a greater degree of positive remodeling.[5, 48, 49] Also, there was no evidence of pairwise vessel to vessel differences in the total IEL area when comparing the three coronary arteries within the three groups of pigs (p > 0.1) except for the right versus circumflex coronary artery comparison within the moderate atherosclerosis group (p = 0.044).
Table 2. Mean Coronary Artery Total IEL Area (mm2), Percent of Coronary Artery Cross Sections Containing Fibrous Caps (FC), and Fibrous Cap Thickness (mm) in All 3 Coronary Arteries.
| Right | Circumflex | Left Anterior Descending | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total IEL Area (mm2) | % with FC* | Fibrous Cap (mm) | Total IEL Area (mm2) | % with FC* | Fibrous Cap (mm) | Total IEL Area (mm2) | % with FC* | Fibrous Cap (mm) | |
| Severe and Diffuse Atherosclerosis (n = 20) | |||||||||
| Mean | 10.1 | 81.6 | 0.472 | 9.9 | 88.8 | 0.521 | 9.8 | 83.9 | 0.377 |
| ± SD | 2.1 | 16.4 | 0.184 | 3.1 | 24.2 | 0.277 | 2.1 | 17.0 | 0.139 |
| Moderate Atherosclerosis (n = 17) | |||||||||
| Mean | 6.2 | 26.2 | 0.405 | 5.0 | 41.3 | 0.482 | 6.0 | 32.2 | 0.346 |
| ± SD | 1.7 | 22.5 | 0.194 | 2.4 | 41.9 | 0.208 | 2.3 | 37.1 | 0.124 |
| Control (n = 5) | |||||||||
| Mean | 6.1 | 1.3 | 0.510 | 6.6 | 0 | N/A | 6.4 | 0 | N/A |
| ± SD | 1.1 | 3.0 | 0.000 | 1.7 | 0 | N/A | 0.6 | 0 | N/A |
| p † | |||||||||
| Severe vs Moderate | < 0.001 | < 0.001 | 0.161 | < 0.001 | < 0.001 | 0.914 | < 0.001 | < 0.001 | 0.470 |
| Severe vs Control | < 0.001 | < 0.001 | N/A | 0.035 | < 0.001 | N/A | < 0.001 | < 0.001 | N/A |
| Moderate vs Control | 1.00 | < 0.001 | N/A | 0.164 | < 0.001 | N/A | 0.820 | < 0.001 | N/A |
*percent of coronary artery sections containing fibrous caps (FC),
† Wilcoxon rank sum statistic for differences between groups,
N/A is not applicable,
Percent of Coronary Artery Cross Sections with Fibrous Caps and Fibrous Cap Thickness
The percent of coronary artery cross sections containing fibrous caps was much greater in the severe and diffuse atherosclerosis group for all three coronaries (Table 2, p < 0.001). There was no evidence among animals with detectible fibrous caps of differences between the mean fibrous cap thicknesses for the severe and moderate groups (p > 0.1). Only one of the five control pigs had a very small fibrous cap lesion and that lesion was located in the right coronary artery of that pig.
Abdominal Aortic Atherosclerosis
The 20 pigs that met the criteria for severe and diffuse distal coronary atherosclerosis had much more abdominal aortic atherosclerosis than the 17 pigs with moderate coronary atherosclerosis (Table A in S1 File for intimal area and intimal area as % medial area (p ≤ 0.004) and Table B in S1 File for en face measurement of % surface area with raised lesions, p ≤ 0.002). The 5 control pigs had raised lesions in the abdominal aorta (range was 2.9–28.6% of total surface area) that were much smaller than the other two groups (p ≤ 0.001). The intimal area as percent medial area was also much smaller in the control pigs (Table A in S1 File, p ≤ 0.04).
Change in Fasting Glucose, Insulin, and Insulin Sensitivity by Bergman FSIVGTT during the Year Long Study
Throughout the study, changes in fasting glucose values in conscious pigs in any group were relatively small (Table 3 shows quarterly glucose values only, monthly values not shown). In contrast, when the pigs were sedated for the Bergman FSIVGTT, the glucose values were higher, possibly due to the stress of the procedure or the sedatives used. Differences between the groups at each time point were relatively small as were change in any group over time (p ≥ 0.05, Table 3).[50, 51]
Table 3. Fasting Glucose Values.
| Fasting Glucose (mg/dl) conscious | Fasting Glucose (mg/dl) sedated | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 month | 6 month | 12 month | Baseline | 3 month | 6 month | 12 month | |
| Severe and Diffuse Atherosclerosis (n = 20) | ||||||||
| Mean | 73 | 78 | 75 | 77 | 112 | 125 | 132 | 136 |
| ± SD | 7 | 11 | 7 | 6 | 36 | 34 | 41 | 39 |
| p* | 0.054 | 0.163 | 0.050 | 0.091 | 0.092 | 0.062 | ||
| Moderate Atherosclerosis (n = 17) | ||||||||
| Mean | 75 | 73 | 76 | 75 | 114 | 113 | 114 | 121 |
| ± SD | 7 | 9 | 6 | 7 | 23 | 21 | 29 | 28 |
| p* | 0.377 | 0.875 | 0.704 | 0.882 | 0.924 | 0.162 | ||
| Control (n = 5) | ||||||||
| Mean | 76 | 71 | 69 | 72 | 137 | 110 | 117 | 130 |
| ± SD | 5 | 6 | 6 | 4 | 25 | 24 | 22 | 58 |
| p* | 0.248 | 0.121 | 0.516 | 0.067 | 0.251 | 0.821 | ||
| p † | ||||||||
| Severe vs Moderate | 0.263 | 0.136 | 0.807 | 0.476 | 0.661 | 0.254 | 0.156 | 0.247 |
| Severe vs Control | 0.235 | 0.116 | 0.067 | 0.154 | 0.086 | 0.371 | 0.530 | 0.265 |
| Moderate vs Control | 0.985 | 0.581 | 0.058 | 0.254 | 0.071 | 0.543 | 0.648 | 0.548 |
*Wilcoxon signed rank statistic for change from baseline within group.
†Wilcoxon rank sum statistic for differences between groups.
Fasting insulin values measured during the Bergman FSIVGTT were higher at 3 (Table 4, p < 0.001), 6 (p < 0.001), and 12 (p < 0.001) months when compared to baseline values for the severe and diffuse coronary atherosclerosis group and at 12 months in the moderate group (p = 0.006). The control group showed little or no evidence of change (p > 0.05, Table 4).
Table 4. Fasting Insulin Levels and Bergman Si Values.
| Fasting Insulin (μU/ml)—sedated | Bergman Si | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 month | 6 month | 12 month | Baseline | 3 month | 6 month | 12 month | |
| Severe and Diffuse Atherosclerosis (n = 20) | ||||||||
| Mean | 11.8 | 20.4 | 27.2 | 27.2 | 4.1 | 3.9 | 3.7 | 3.6 |
| ± SD | 5.3 | 10.0 | 20.0 | 12.5 | 0.3 | 0.3 | 0.4 | 0.4 |
| p* | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||
| Moderate Atherosclerosis (n = 17) | ||||||||
| Mean | 13.0 | 17.3 | 16.7 | 18.8 | 4.0 | 3.7 | 3.7 | 3.5 |
| ± SD | 10.0 | 9.1 | 10.0 | 8.7 | 0.4 | 0.4 | 0.4 | 0.4 |
| p* | 0.150 | 0.203 | 0.006 | 0.005 | < 0.001 | < 0.001 | ||
| Control (n = 5) | ||||||||
| Mean | 10.4 | 16.7 | 19.0 | 16.6 | 4.0 | 4.0 | 3.9 | 3.7 |
| ± SD | 5.4 | 11.0 | 7.8 | 11.4 | 0.2 | 0.2 | 0.2 | 0.3 |
| p* | 0.253 | 0.067 | 0.378 | 1.000 | 0.033 | 0.058 | ||
| p † | ||||||||
| Severe vs Moderate | 0.775 | 0.266 | 0.127 | 0.056 | 0.103 | 0.312 | 0.282 | 0.581 |
| Severe vs Control | 0.530 | 0.408 | 0.818 | 0.088 | 0.310 | 0.205 | 0.179 | 0.584 |
| Moderate vs Control | 0.880 | 0.581 | 0.401 | 0.842 | 0.555 | 0.211 | 0.287 | 0.198 |
*Wilcoxon signed rank statistic for change from baseline within group.
†Wilcoxon rank sum statistic for differences between groups.
Analyses of fasting, 1 and 2 hour post prandial glucose and insulin levels in a subset of conscious pigs (n = 13 in the severe group, n = 6 in the moderate group, and n = 5 in the control group), showed similar trends (Tables F and G in S1 File).
The decreasing mean Si values in the severe and moderate atherosclerosis groups at 3, 6 and 12 months were consistent with increased insulin resistance (p <0.001 for the severe and diffuse atherosclerosis group, and p ≤ 0.005 for the moderate atherosclerosis group compared to baseline, respectively). Likewise, the control pigs exhibited decreases in insulin sensitivity at 6 months (p = 0.033) and nearly so at 12 months (p = 0.058). There was little or no evidence of differences in mean fasting glucose, insulin or Si values (Table 4) during the Bergman FSIVGTT between pairs of the three groups at any timepoint (Tables 3 and 4, p > 0.05).
Change in Serum Fructosamine Levels during the Year Long Study
The log transformed serum fructosamine levels were similar at baseline for all three groups (ANOVA, p = 0.06). Fructosamine increased substantially at 3, 6, and 12 months compared to baseline in the group that developed severe and diffuse atherosclerosis (p ≤ 0.017), but only at month 3 for the moderate group (Table 5, p < 0.001). There was no evidence of change in the control pigs. The serum fructosamine level was much higher at 3, 6, and 12 months in the severe and diffuse atherosclerosis group when compared to either the moderate atherosclerosis or control groups (p ≤ 0.031).
Table 5. Fasting Fructosamine Levels (μmol/L).
| Baseline | 3 month | 6 month | 12 month | |
|---|---|---|---|---|
| Severe and Diffuse Atherosclerosis (n = 20) | ||||
| Mean | 163 | 192 | 184 | 173 |
| ± SD | 18 | 26 | 17 | 21 |
| p* | < 0.001 | < 0.001 | 0.017 | |
| Moderate Atherosclerosis (n = 17) | ||||
| Mean | 148 | 171 | 159 | 150 |
| ± SD | 23 | 24 | 26 | 23 |
| p* | < 0.001 | 0.111 | 0.818 | |
| Control (n = 5) | ||||
| Mean | 150 | 155 | 154 | 149 |
| ± SD | 19 | 23 | 19 | 10 |
| p* | 0.221 | 0.058 | 0.961 | |
| p † | ||||
| Severe vs Moderate | 0.027 | 0.011 | <0.001 | 0.004 |
| Severe vs Control | 0.153 | 0.006 | 0.003 | 0.031 |
| Moderate vs Control | 0.880 | 0.283 | 0.928 | 0.842 |
*Wilcoxon signed rank statistic for change from baseline within group.
†Wilcoxon rank sum statistic for differences between groups.
Change in Weight, Backfat, and Blood Pressure during Year Long Study
The baseline, 3, 6, and 12 month weight and backfat values and blood pressure results are shown in Tables C and D in S1 File, respectively. Weight increased substantially over time compared to baseline in the moderate atherosclerosis group (p < 0.001) and in the severe group (p < 0.001). There was no evidence of differences between the severe and moderate groups for weight at study entry nor at any of the time points (p ≥ 0.4). Weight in the control group increased moderately over time (p <0.001 at month 12, Table C in S1 File). Backfat also increased substantially over time compared to baseline in the severe and diffuse atherosclerosis group (p ≤ 0.003) and in the moderate atherosclerosis group (p ≤ 0.005). There was no evidence of differences between the severe and moderate groups for backfat at study entry (p = 0.265) but there was ~ 1 cm more backfat at all three time points in the severe group (p ≤ 0.016). Back fat in the control group increased relatively minimally over time (p ≥ 0.070).
Blood pressure was in a range that would be considered mildly hypertensive in humans (142–160 / 93–108 mmHg) with little or no evidence of interpretable differences between the three groups (Table D in S1 File, p ≥ 0.10 for 19 of 24 cases). Blood pressure had little or no evidence for interpretable changes over time in any group (p≥ 0.10 for 15 of 18 cases).
Total, LDL, and HDL Cholesterol and Triglyceride Levels
The mean total cholesterol values ranged from 77 to 110 mg/dl at baseline, but it was lower for the group that developed moderate atherosclerosis than for both the group that developed severe and diffuse atherosclerosis and the control group (Table 6, p ≤ 0.004). A similar pattern was seen at baseline for LDL and HDL cholesterol (Tables 6 and 7) where the mean value for the moderate group was substantially lower than severe or control, but the control and severe were not substantially different. Fasting triglycerides values had little to no differences between the three groups at study entry (Table 7). For the pigs fed the high fat/high NaCl diet, fasting total, LDL, and HDL cholesterol increased substantially at 3, 6, and 12 months relative to baseline values within severe and moderate groups (p < 0.001), but evidence of change for triglycerides was only applicable to the severe group. There were substantial differences between the moderate and severe groups at 3, 6, and 12 months for total and LDL cholesterol and triglycerides (p < 0.02), but HDL had no interpretable differences. Control pigs showed no essentially no or minimal changes in these measurements over the 12 month study and had much lower total cholesterol, HDL, and LDL values when compared to the pigs being fed the high fat/high NaCl diet (p > 0.05 for all but one value).
Table 6. Fasting Total and LDL Cholesterol.
| Total cholesterol (mg/dl) | LDL cholesterol (mg/dl) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 month | 6 month | 12 month | Baseline | 3 month | 6 month | 12 month | |
| Severe and Diffuse Atherosclerosis (n = 20) | ||||||||
| Mean | 103 | 583 | 481 | 413 | 57 | 226 | 186 | 157 |
| ± SD | 26 | 188 | 127 | 130 | 13 | 81 | 44 | 34 |
| p* | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Moderate Atherosclerosis (n = 17) | ||||||||
| Mean | 77 | 416 | 366 | 320 | 44 | 172 | 143 | 131 |
| ± SD | 20 | 107 | 163 | 74 | 14 | 38 | 50 | 27 |
| p* | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Control (n = 5) | ||||||||
| Mean | 110 | 113 | 119 | 106 | 57 | 64 | 66 | 61 |
| ± SD | 25 | 32 | 29 | 26 | 9 | 16 | 12 | 16 |
| p* | 0.922 | 0.132 | 0.465 | 0.133 | 0.027 | 0.335 | ||
| p † | ||||||||
| Severe vs Moderate | 0.002 | 0.003 | 0.021 | 0.015 | 0.005 | 0.017 | 0.009 | 0.016 |
| Severe vs Control | 0.564 | <0.001 | <0.001 | <0.001 | 0.809 | <0.001 | <0.001 | <0.001 |
| Moderate vs Control | 0.004 | <0.001 | 0.001 | <0.001 | 0.056 | <0.001 | 0.001 | <0.001 |
*Wilcoxon signed rank statistic for change from baseline within group.
†Wilcoxon rank sum statistic for differences between groups.
Table 7. Fasting HDL Cholesterol and Triglycerides.
| HDL cholesterol (mg/dl) | Triglycerides (mg/dl) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 month | 6 month | 12 month | Baseline | 3 month | 6 month | 12 month | |
| Severe and Diffuse Atherosclerosis (n = 20) | ||||||||
| Mean | 40 | 81 | 71 | 65 | 36 | 47 | 43 | 52 |
| ± SD | 8 | 29 | 13 | 15 | 29 | 28 | 25 | 39 |
| p* | <0.001 | <0.001 | <0.001 | 0.013 | 0.235 | 0.035 | ||
| Moderate Atherosclerosis (n = 17) | ||||||||
| Mean | 34 | 82 | 77 | 78 | 21 | 25 | 25 | 21 |
| ± SD | 11 | 28 | 28 | 19 | 6 | 12 | 13 | 11 |
| p* | <0.001 | <0.001 | <0.001 | 0.475 | 0.662 | 0.463 | ||
| Control (n = 5) | ||||||||
| Mean | 44 | 48 | 48 | 47 | 29 | 45 | 45 | 36 |
| ± SD | 6 | 6 | 8 | 7 | 12 | 21 | 30 | 15 |
| p* | 0.119 | 0.266 | 0.074 | 0.072 | 0.228 | 0.136 | ||
| p † | ||||||||
| Severe vs Moderate | 0.049 | 0.934 | 0.437 | 0.028 | 0.046 | 0.005 | 0.011 | 0.004 |
| Severe vs Control | 0.330 | 0.009 | <0.001 | 0.024 | 0.830 | 0.986 | 0.988 | 0.667 |
| Moderate vs Control | 0.025 | 0.006 | 0.048 | 0.001 | 0.182 | 0.032 | 0.240 | 0.020 |
*Wilcoxon signed rank statistic for change from baseline within group.
†Wilcoxon rank sum statistic for differences between groups.
Changes in Oxidized LDL
The mean oxLDL levels for the severe and diffuse atherosclerosis group at baseline, while similar to the moderate atherosclerosis group, was higher than the control group (Table 8, p = 0.015). The values increased substantially relative to the baseline at 3, 6, and 12 months in pigs fed the high fat/high NaCl diet (p ≤ 0.002) but not control pigs (p > 0.46). OxLDL was substantially higher in the severe atherosclerosis group at 3, 6 and 12 months compared to the moderate group and it was substantially lower for the control group (Table 8, p ≤ 0.002).
Table 8. Fasting oxLDL Levels (units/L).
| Baseline | 3 month | 6 month | 12 month | |
|---|---|---|---|---|
| Severe and Diffuse Atherosclerosis (n = 20) | ||||
| Mean | 20.4 | 47.9 | 42.6 | 39.8 |
| ± SD | 5.3 | 17.5 | 11.3 | 10.6 |
| p* | <0.001 | <0.001 | 0.002 | |
| Moderate Atherosclerosis (n = 17) | ||||
| Mean | 18.7 | 31.5 | 31.3 | 29.8 |
| ± SD | 4.8 | 7.1 | 8.2 | 7.0 |
| p* | <0.001 | <0.001 | <0.001 | |
| Control (n = 5) | ||||
| Mean | 13.6 | 12.6 | 14.1 | 13.5 |
| ± SD | 4.7 | 3.1 | 4.3 | 3.7 |
| p* | 0.465 | 0.465 | 0.465 | |
| p † | ||||
| Severe vs Moderate | 0.410 | <0.001 | <0.001 | 0.002 |
| Severe vs Control | 0.015 | <0.001 | <0.001 | <0.001 |
| Moderate vs Control | 0.088 | <0.001 | <0.001 | <0.001 |
*Wilcoxon signed rank statistic for change from baseline within group.
†Wilcoxon rank sum statistic for differences between groups.
Changes in Aldosterone
The mean aldosterone levels were similar for all three groups at baseline (p>0.9, Table E in S1 File). The values did not increase in the control group or group with moderate coronary atherosclerosis whereas in the group with severe disease there was a moderate increase above baseline values at 6 and 12 months (p ≤ 0.042). When controlling for gender, however, the changes in aldosterone were not associated with severe and diffuse atherosclerosis (p > 0.15). Serum potassium and sodium levels were assayed monthly and remained within the normal range (not shown).
Change in Inflammatory Markers during Year Long Study
The inflammatory markers (TNF-alpha, IL-6, PAI-1, CRP) did not differ between the moderate and severe groups in 9 pigs with severe and diffuse coronary atherosclerosis when compared to 8 pigs with moderate disease (data not shown). Since these values did not appear to be robust markers of IR or atherosclerosis in this model, the assays were not performed in the remaining pigs.
Adverse Events
During the year-long study, transient inappetance occurred in both the severe and diffuse (5/20) and moderate (6/17) groups but not the control group. In general, inappetance responded to feeding regular chow for a few days before restarting the high fat/high NaCL diet. If the pig had hemoccult positive stools, appropriate medications were given with the advice of the attending veterinarian (e.g., antacids and proton pump inhibitors). Two of the 6 pigs with inappetance in the moderate atherosclerosis group required blood transfusions for anemia due to gastrointestinal bleeding. In all cases, sampling was not interrupted by these events. One pig in the moderate atherosclerosis group was euthanized for persistent inappetance after 6 months on study. One pig in the severe atherosclerosis group died suddenly at age 10 months and, in addition to severe and diffuse coronary atherosclerosis, had a bladder infection at necropsy. Both of these animals are included in all analyses.
Discussion
The study shows that 37 adult pigs fed a high fat/high NaCl diet exhibited a substantial increase in IR that was accompanied by weight gain, increased backfat, and elevations in total and LDL and HDL cholesterol levels. Those pigs that exhibited more severe as well as diffuse distal coronary artery and abdominal aortic atherosclerosis had substantially greater increases in fructosamine and oxLDL levels during the study compared to the pigs with moderate disease or the control pigs. Although all animals developed increased IR during the study as reflected in the decreasing Si values, the severity of IR was not associated with the severity of atherosclerosis. This finding suggests that this degree of IR did not discriminate between animals that develop severe and diffuse vs moderate atherosclerosis in this model. Since none of the IR pigs had diabetes, it appears that severe and diffuse atherosclerosis can develop in response to a high fat/high NaCl diet in the absence of overt hyperglycemia but this severe atherosclerosis phenotype is associated with increased oxLDL and fructosamine levels consistent with increased protein glycation. These findings are also reinforced by the fact that the normal chow-fed controls also developed IR but had no substantial change in cholesterol, oxLDL, or fructosamine levels and little to no atherosclerosis.
Insulin Resistance
The manner in which the hyperinsulinemic state or the loss of insulin sensitivity might lead to increased atherosclerosis remains poorly defined. Recently, several mechanisms that mediate insulin resistance in mice, pigs, and humans have been described.[52–58] It is not yet know if these mechanisms also mediate atherogenesis in addition to IR. Additionally the presence of IR is often associated with hypertension, lipoprotein abnormalities, and chronic kidney disease that further accentuate atherosclerosis risk.[59–64]
Although fully manifested diabetes mellitus in humans confers a greater risk for cardiovascular disease and other complications as compared to isolated IR without hyperglycemia, the presence of IR is a major independent risk factor with relative risk ratios between 2.2 and 2.7 when multifactorial linear regression analysis is utilized to assess risk.[2, 65, 66] Within this broad category of insulin resistance, there are several subgroups of patients with different metabolic abnormalities. Specifically some subjects have hyperlipidemia without overt diabetes, some have diabetes without hyperlipidemia, and both groups can have abnormalities in glucose dynamics and elevated levels of oxidized LDL as well as other glycated proteins such as HgbA1c and fructosamine.
Our IR pigs had elevated glucose levels during the Bergman FSIVGTT when sedated but not when fully conscious (Table 3). It is possible that the sedatives used in these pigs contributed to the glucose elevations (i.e., acepromazine and ketamine). Isoflurane has been shown to alter insulin sensitivity in pigs, possibly from direct drug effects as well as procedure-related stress.[50, 51] Importantly, since all Si measurements were done with sedation, the observed reduction in Si over 12 months is interpretable and was clearly progressive. Despite this increase in insulin resistance, however, there was no detectible association between the degree of changes in insulin sensitivity and the severity of atherosclerosis in the pigs in this study.
Cholesterol Levels and Atherosclerosis Severity
All pigs fed the high fat/high NaCl diet exhibited hypercholesterolemia but the levels were higher in pigs that developed severe and diffuse atherosclerosis. This difference in total and LDL cholesterol levels accounts for some, but not all, of the observed differences between groups according to atherosclerosis severity. In mixed models that used a continuous measure of coronary artery atherosclerosis severity, controlling for gender, baseline values, and variations in total cholesterol over time, there are still substantial strengths of association between coronary artery atherosclerosis severity and oxLDL (p = 0.035) and fructosamine (p = 0.022) that are independent of the changes in total or LDL cholesterol. Such models also indicated an association between the variation of total cholesterol over time with fructosamine (p< 0.001) and oxLDL (p< 0.001). Thus, while there are associations of cholesterol levels and atherosclerosis severity, the more noteworthy issue for cholesterol was the extent to which it fully or partly explained the associations of atherosclerosis severity with increased levels of fructosamine and oxLDL.
Fructosamine
Fructosamine is used as an index of mean blood glucose and is predictive of incident diabetes and microvascular complications in humans.[67] In pigs, fructosamine is also a reliable and important marker of mean blood glucose since pig red blood cells are relatively impermeable to glucose and thus hemoglobin A1c is not a reliable index of mean glucose concentrations in this species.[16, 68] Fructosamine is also a measure of protein glycation.[69] In our study, the fructosamine levels measured during the fasting state rose relative to baseline values in the group of animals that developed severe and diffuse coronary atherosclerosis but the levels were not elevated to a point that they would reflect meaningful and/or sustained hyperglycemia.
With regards to blood glucose levels, studies in humans have shown that stress-induced hyperglycemia correlates with subsequent development of diabetes and that the subjects who were predisposed to develop glucose intolerance during the FSIVGTT had higher fructosamine levels at baseline.[70] As mentioned above, the IR pigs appeared to exhibit stress-associated hyperglycemia during the Bergman FSIVGTT that may have accounted for some portion of the higher fructosamine levels. Fructosamine has also been shown to correlate with 2 hr postprandial glucose[71] and relatively short periods of hyperglycemia have been shown to increase protein glycation even if fasting glucose is not increased.[72] Presumably the increase in fructosamine in the IR pigs with severe and diffuse atherosclerosis reflects a modest increase in mean daily glucose levels, although periods of post prandial hyperglycemia of short duration followed by periods of normoglycemia as the sole cause cannot be excluded. Regardless of the cause, the degree of fructosamine increase was substantially greater in pigs with severe and diffuse atherosclerosis than the change that occurred in the group with moderate atherosclerosis or the control group. Therefore, it is possible that even a relatively modest change in fructosamine reflects a contribution of increased mean glucose values that would contribute to increased protein glycation and atherosclerosis development.
With regards to atherosclerosis, most reports have compared the degree of fructosamine change that occurs with impaired glucose tolerance to subjects with normal glucose tolerance and correlated this with a change in atherosclerotic risk. Studies in experimental animal models have reported increases in fructosamine over the range noted in our animals as being associated with biochemical changes that are believed to predispose to increase risk of atherosclerosis and some studies in humans with impaired glucose tolerance have demonstrated an increase in carotid intimal media thickening.[73] Although studies in which fructosamine changes alone, independent of changes in lipoproteins, predispose to increased coronary and aortic atherosclerosis have not been reported, there are studies wherein changes in advanced glycation end products or glycated albumin (another surrogate measurement of serum glycated protein concentrations) have been shown to predict atherosclerosis risk.[74–77] When measures of advanced glycation end products other than fructosamine or glycated albumin are utilized, there are also strong associations with the presence of advanced atherosclerosis.[78–80] Importantly, in a prospective study in adult humans without diabetes, higher HgbA1c was independently associated with progression of advanced coronary artery calcification.[81] Therefore, the combination of abnormal blood glucose dynamics that are not classified as overt diabetes and elevated concentrations of glycated proteins is associated with the development of advanced coronary atherosclerosis in humans and pigs.
Oxidized LDL (oxLDL)
Increases in oxidized LDL in humans, pigs, and rodents are associated with increased atherosclerosis and reduction in oxLDL is associated with reduced atherosclerosis.[82–87] The mechanism by which these changes in oxLDL might modulate atherosclerosis severity cannot be completely separated from the changes induced by protein glycation since exposure of LDL to high glucose concentrations results in abnormal glycation as well as oxidation.[88] In humans, increased glycemic index is also associated with increased oxLDL when total LDL remains unchanged.[82, 89–91] The more modest increase in postprandial glucoses in subjects with impaired glucose tolerance can also lead to elevated oxLDL[92–95] and treatment of those subjects lowers oxLDL.[96–99] Also, reductions in fat mass, dietary fat, and blood glucose have been shown to be associated with decreases in oxLDL.[100–103] A few studies have reported increases in oxLDL in nondiabetic subjects with impaired glucose tolerance[104–106] and one reported a positive correlation between oxLDL in normal subjects who also exhibited higher fructosamine levels.[104] Moreover, even small increases in postprandial glucose that are within the normal range can lead to increases in oxidized LDL[82, 107] and small reductions in postprandial glucose in patients with type 2 diabetes also lead to decreased oxidized LDL.[82, 107] Since oxidized LDL was increased in the pigs that developed severe and diffuse atherosclerosis, it is possible that increases solely in their postprandial glucoses were sufficient to contribute to the elevation in oxLDL levels.
Atherosclerosis Morphology in Insulin Resistance
Coronary artery atherosclerosis in the IR pigs showed fibrous cap formation, foam cells, medial thinning, plaque hemorrhage, necrosis, and calcification (Fig 4). All of these characteristics have been described in atherosclerotic plaques from IR and diabetic humans.[5, 7] Similar histological findings have been described in pigs given streptozotocin and fed a high fat diet.[16] An important characteristic is the presence of distal coronary atherosclerosis which has been a consistent finding in asymptomatic IR and diabetic patients screened by electron beam computed tomography and magnetic resonance imaging,[4, 8] clinically symptomatic IR patients by quantitative angiography[6] and in type 2 diabetic patients who died suddenly.[5] Thick fibrous caps (>64 μm) are more common in coronary atherosclerotic plaques of humans with type 2 diabetes who died suddenly.[5] In our study, the fibrous caps were more abundant in pigs with severe atherosclerosis than those with moderate atherosclerosis (Table 2).
Clinical studies have reported an increase in coronary artery remodeling in diabetic victims of sudden death.[5] The mean total IEL areas for serial sections in all 3 coronary arteries from IR pigs with severe atherosclerosis was larger than those with moderate atherosclerosis (Table 2). This finding is consistent with positive remodeling.[5, 48, 49] Thus, the coronary atherosclerotic plaques in the IR pigs with severe and diffuse disease also exhibited many of the phenotypic characteristics that are found in IR humans.
Gender
Severe and diffuse atherosclerosis occurred more commonly in IR female pigs fed the high fat/high NaCl diet. One study conducted in nondiabetic female minipigs designed to develop metabolic syndrome found they became more insulin resistant and developed more atherogenic lipoprotein changes compared to males.[108] In insulin resistant humans, incident cardiovascular disease is more prevalent in women from many different ethnic groups including Caucasians, Latinos, American Indians, and several European populations.[109] Additionally increased levels of glycated proteins predicted mortality from coronary heart disease in non-diabetic women but not non-diabetic men.[74] The reason for this difference between genders is unknown. It is speculated that the difference may in part be explained by a higher burden of all risk factors for cardiovascular disease and a greater effect of high blood pressure and atherogenic dyslipidemia in women when compared to men.[110]
Mechanism of glycation induced changes in atherosclerosis severity
Although our study was not designed to identify or measure the mechanism by which changes in oxLDL or glycated proteins would induce atherogenesis, the cell surface receptor RAGE is thought to be a key mediator.[111] RAGE is induced in blood vessels under these conditions and the soluble form of RAGE that is secreted is increased in patients with severe atherosclerosis and abnormal protein glycation. The mechanism by which these combinations of metabolic changes induce atherosclerosis has been proposed to be activation of macrophages that in turn further oxidize LDL. An additional proposed mechanism is the inhibition of autophagy. Specifically glycated albumin results in autophagy dysfunction in pancreatic beta cells leading to enhanced pancreatic cell death.[112] A reduction in the rate of autophagy induced by glycated proteins has also been implicated in pericyte dropout and the subsequent development of diabetic retinopathy.[113] Finally inhibition of autophagy has been shown to be important for mediating changes in endothelial cells that have been associated with atherosclerosis.[114] The results of our study support the hypothesis that elevations in glycated proteins in IR pigs could lead to more severe and diffuse atherosclerosis at least in part through inhibition of autophagy.
Study Limitations
We chose to measure parameters that have been associated with increased atherosclerosis and insulin resistance in humans: blood pressure, body weight, body fat, hyperlipidemia, inflammatory markers, glycated proteins including fructosamine and oxLDL, and aldosterone. This study design addressed the extent to which each of these parameters, as measured at 4 time points, were associated with the severity of atherosclerosis that developed in pigs with comparable IR. The grouping of pigs by atherosclerosis severity was only possible after feeding the high fat diet for one year. As the investigators did not know which pigs had severe atherosclerosis until the end of the study, one can regard the investigators as being blinded. Likewise, evaluating these parameters for association with atherosclerosis severity could only be done after identifying the groups according to specific criteria that were defined a priori. Although possibly introducing some bias, this grouping is comparable to a ranking that classified somewhat more than half as severe and the others as moderate. We have additionally addressed this possibility by analyzing changes over time with appropriate and validated statistical methods.[115] We may have inadvertently omitted other parameters that could be associated with atherosclerosis severity that could also be analyzed in a similar fashion. We did not measure glycated proteins in tissues so we do not know the extent to which such measurements would or would not have correlated with the fructosamine and oxLDL levels measured in the circulation. The other limitation of our study is that it is purely hypothesis generating, and not hypothesis testing, as many pairwise comparisons were made.
Conclusion
Our goal was to develop a useful animal model of insulin resistance that also develops coronary artery and aortic atherosclerosis. Several investigators have used pigs to study the impact of diabetes on atherogenesis, most of whom have used chemical induction of insulin-deficiency.[16, 18, 116, 117] In contrast, we used pigs that developed increased insulin resistance in association with weight gain, increased backfat, increased total and LDL and HDL cholesterol levels, and elevated blood pressure. Our model has some features of the metabolic syndrome and about half of the animals developed more severe and diffuse atherosclerosis. The metabolic variables that are associated with atherosclerosis severity are increases in fructosamine and oxidized LDL. Our study had a robust number of pigs and it would be reasonable expect to see a similar effect size in future studies. This animal model should be useful for identifying and studying mechanisms of disease that are suggested by these associations and for testing new therapeutic approaches designed to reduce or prevent the development of severe and diffuse atherosclerosis in insulin resistant subjects. Our data provide a rational basis for determining the number of animals required for such a mechanistic-based study to detect significant differences between treatment groups.
Supporting Information
(DOCX)
Acknowledgments
The authors wish to thank Kent Passingham and the staff at the Francis Owen Blood Research Laboratory for outstanding care of these animals.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Heart, Lung, and Blood institute of the National Institutes of Health (HL069364, DRC, TCN) and the North Carolina Biotechnology Center (NCBC MRG1101, TCN and DRC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7. [DOI] [PubMed] [Google Scholar]
- 2. Kohen-Avramoglu R, Theriault A, Adeli K. Emergence of the metabolic syndrome in childhood: an epidemiological overview and mechanistic link to dyslipidemia. Clin Biochem. 2003;36:413–20. [DOI] [PubMed] [Google Scholar]
- 3. Pinhas-Hamiel O, Zeitler P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet. 2007;369:1823–31. [DOI] [PubMed] [Google Scholar]
- 4. Meigs JB, Larson MG, D'Agostino RB, Levy D, Clouse ME, Nathan DM, et al. Coronary artery calcification in type 2 diabetes and insulin resistance: the Framingham offspring study. Diabetes care. 2002;25:1313–9. [DOI] [PubMed] [Google Scholar]
- 5. Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, et al. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:1266–71. [DOI] [PubMed] [Google Scholar]
- 6. Graner M, Syvanne M, Kahri J, Nieminen MS, Taskinen MR. Insulin resistance as predictor of the angiographic severity and extent of coronary artery disease. Ann Med. 2007;39:137–44. [DOI] [PubMed] [Google Scholar]
- 7. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:1262–75. [DOI] [PubMed] [Google Scholar]
- 8. Weckbach S, Findeisen HM, Schoenberg SO, Kramer H, Stark R, Clevert DA, et al. Systemic Cardiovascular Complications in Patients With Long-Standing Diabetes Mellitus: Comprehensive Assessment With Whole-Body Magnetic Resonance Imaging/Magnetic Resonance Angiography. Invest Radiol. 2009;44. [DOI] [PubMed] [Google Scholar]
- 9. Flaherty JD, Davidson CJ. Diabetes and coronary revascularization. JAMA. 2005;293:1501–8. [DOI] [PubMed] [Google Scholar]
- 10. Zhang H, Yuan X, Osnabrugge RL, Meng D, Gao H, Zhang S, et al. Influence of diabetes mellitus on long-term clinical and economic outcomes after coronary artery bypass grafting. Ann Thorac Surg. 2014;97:2073–9. 10.1016/j.athoracsur.2014.02.047 [DOI] [PubMed] [Google Scholar]
- 11. Finn AV, Palacios IF, Kastrati A, Gold HK. Drug-eluting stents for diabetes mellitus: a rush to judgment? J Am Coll Cardiol. 2005;45:479–83. [DOI] [PubMed] [Google Scholar]
- 12. Yamagishi S, Nakamura K, Takeuchi M, Imaizumi T. Molecular mechanism for accelerated atherosclerosis in diabetes and its potential therapeutic intervention. Int J Clin Pharmacol Res. 2004;24:129–34. [PubMed] [Google Scholar]
- 13. Moreno PR, Fuster V. New aspects in the pathogenesis of diabetic atherothrombosis. J Am Coll Cardiol. 2004;44:2293–300. [DOI] [PubMed] [Google Scholar]
- 14. Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, et al. Changes in diabetes-related complications in the United States, 1990–2010. The New England journal of medicine. 2014;370:1514–23. 10.1056/NEJMoa1310799 [DOI] [PubMed] [Google Scholar]
- 15. Zoungas S, Chalmers J, Neal B, Billot L, Li Q, Hirakawa Y, et al. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. The New England journal of medicine. 2014;371:1392–406. 10.1056/NEJMoa1407963 [DOI] [PubMed] [Google Scholar]
- 16. Gerrity RG, Natarajan R, Nadler JL, Kimsey T. Diabetes-induced accelerated atherosclerosis in swine. Diabetes. 2001;50:1654–65. [DOI] [PubMed] [Google Scholar]
- 17. Suzuki LA, Poot M, Gerrity RG, Bornfeldt KE. Diabetes accelerates smooth muscle accumulation in lesions of atherosclerosis: lack of direct growth-promoting effects of high glucose levels. Diabetes. 2001;50:851–60. [DOI] [PubMed] [Google Scholar]
- 18. Bellinger DA, Merricks EP, Nichols TC. Swine models of type 2 diabetes mellitus: insulin resistance, glucose tolerance, and cardiovascular complications. ILAR J. 2006;47:243–58. [DOI] [PubMed] [Google Scholar]
- 19. Hasler-Rapacz JO, Nichols TC, Griggs TR, Bellinger DA, Rapacz J. Familial and diet-induced hypercholesterolemia in swine. Lipid, ApoB, and ApoA-I concentrations and distributions in plasma and lipoprotein subfractions. Arterioscler Thromb. 1994;14:923–30. [DOI] [PubMed] [Google Scholar]
- 20. Hasler-Rapacz J, Ellegren H, Fridolfsson AK, Kirkpatrick B, Kirk S, Andersson L, et al. Identification of a mutation in the low density lipoprotein receptor gene associated with recessive familial hypercholesterolemia in swine. Am J Med Genet. 1998;76:379–86. [PubMed] [Google Scholar]
- 21. Grunwald KA, Schueler K, Uelmen PJ, Lipton BA, Kaiser M, Buhman K, et al. Identification of a novel Arg—>Cys mutation in the LDL receptor that contributes to spontaneous hypercholesterolemia in pigs. Journal of lipid research. 1999;40:475–85. [PubMed] [Google Scholar]
- 22. Nichols TC, du Laney T, Zheng B, Bellinger DA, Nickols GA, Engleman W, et al. Reduction in atherosclerotic lesion size in pigs by alphaVbeta3 inhibitors is associated with inhibition of insulin-like growth factor-I-mediated signaling. Circulation research. 1999;85:1040–5. [DOI] [PubMed] [Google Scholar]
- 23. Brodala N, Merricks EP, Bellinger DA, Damrongsri D, Offenbacher S, Beck J, et al. Porphyromonas gingivalis bacteremia induces coronary and aortic atherosclerosis in normocholesterolemic and hypercholesterolemic pigs. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:1456–1. [DOI] [PubMed] [Google Scholar]
- 24. Nutrient Requirements of Swine Tenth Revised Edition ed. Subcommittee on Swine Nutrition, Committee on Animal Nutrition, Board on Agriculture, Council NR, editors: The National Academies Press; 1998. [Google Scholar]
- 25. Behme MT. Dietary fish oil enhances insulin sensitivity in miniature pigs. J Nutr. 1996;126:1549–53. [DOI] [PubMed] [Google Scholar]
- 26. Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev. 1985;6:45–86. [DOI] [PubMed] [Google Scholar]
- 27. Bergman RN, Prager R, Volund A, Olefsky JM. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. The Journal of clinical investigation. 1987;79:790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moses AC, Young SC, Morrow LA, O'Brien M, Clemmons DR. Recombinant human insulin-like growth factor I increases insulin sensitivity and improves glycemic control in type II diabetes. Diabetes. 1996;45:91–100. [DOI] [PubMed] [Google Scholar]
- 29. O'Connell T, Clemmons DR. IGF-I/IGF-binding protein-3 combination improves insulin resistance by GH-dependent and independent mechanisms. J Clin Endocrinol Metab. 2002;87:4356–60. [DOI] [PubMed] [Google Scholar]
- 30. Black MH, Fingerlin TE, Allayee H, Zhang W, Xiang AH, Trigo E, et al. Evidence of interaction between PPARG2 and HNF4A contributing to variation in insulin sensitivity in Mexican Americans. Diabetes. 2008;57:1048–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sam S, Sung YA, Legro RS, Dunaif A. Evidence for pancreatic beta-cell dysfunction in brothers of women with polycystic ovary syndrome. Metabolism. 2008;57:84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Christoffersen B, Ribel U, Raun K, Golozoubova V, Pacini G. Evaluation of different methods for assessment of insulin sensitivity in Gottingen minipigs: introduction of a new, simpler method. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1195–201. 10.1152/ajpregu.90851.2008 [DOI] [PubMed] [Google Scholar]
- 33. Hausman GJ, Campion DR, Thomas GB. Adipose tissue cellularity and histochemistry in fetal swine as affected by genetic selection for high or low backfat. Journal of lipid research. 1983;24:223–8. [PubMed] [Google Scholar]
- 34. Buhlinger CA, Wangsness PJ, Martin RJ, Ziegler JH. Body composition, in vitro lipid metabolism and skeletal muscle characteristics in fast-growing, lean and in slow-growing, obese pigs at equal age and weight. Growth. 1978;42:225–36. [PubMed] [Google Scholar]
- 35. Etherton TD, Kris-Etherton PM. Characterization of plasma lipoproteins in swine with different propensities for obesity. Lipids. 1980;15:823–9. [DOI] [PubMed] [Google Scholar]
- 36. Etherton TD. Subcutaneous adipose tissue cellularity of swine with different propensities for adipose tissue growth. Growth. 1980;44:182–91. [PubMed] [Google Scholar]
- 37. McNamara JP, Martin RJ. Muscle and adipose tissue lipoprotein lipase in fetal and neonatal swine as affected by genetic selection for high or low backfat. J Anim Sci. 1982;55:1057–61. [DOI] [PubMed] [Google Scholar]
- 38. Dyson MC, Alloosh M, Vuchetich JP, Mokelke EA, Sturek M. Components of metabolic syndrome and coronary artery disease in female Ossabaw swine fed excess atherogenic diet. Comp Med. 2006;56:35–45. [PubMed] [Google Scholar]
- 39. Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25:1111–5. [DOI] [PubMed] [Google Scholar]
- 40. Mesangeau D, Laude D, Elghozi JL. Early detection of cardiovascular autonomic neuropathy in diabetic pigs using blood pressure and heart rate variability. Cardiovascular research. 2000;45:889–99. [DOI] [PubMed] [Google Scholar]
- 41. Springett R, Sakata Y, Delpy DT. Precise measurement of cerebral blood flow in newborn piglets from the bolus passage of indocyanine green. Phys Med Biol. 2001;46:2209–25. [DOI] [PubMed] [Google Scholar]
- 42. Kalela A, Ponnio M, Koivu TA, Hoyhtya M, Huhtala H, Sillanaukee P, et al. Association of serum sialic acid and MMP-9 with lipids and inflammatory markers. Eur J Clin Invest. 2000;30:99–104. [DOI] [PubMed] [Google Scholar]
- 43. Lau CL, Cantu E 3rd, Gonzalez-Stawinski GV, Holzknecht ZE, Nichols TC, Posther KE, et al. The role of antibodies and von Willebrand factor in discordant pulmonary xenotransplantation. Am J Transplant. 2003;3:1065–75. [DOI] [PubMed] [Google Scholar]
- 44. Daghini E, Chade AR, Krier JD, Versari D, Lerman A, Lerman LO. Acute inhibition of the endogenous xanthine oxidase improves renal hemodynamics in hypercholesterolemic pigs. Am J Physiol Regul Integr Comp Physiol. 2006;290:R609–15. [DOI] [PubMed] [Google Scholar]
- 45. Chade AR, Mushin OP, Zhu X, Rodriguez-Porcel M, Grande JP, Textor SC, et al. Pathways of renal fibrosis and modulation of matrix turnover in experimental hypercholesterolemia. Hypertension. 2005;46:772–9. [DOI] [PubMed] [Google Scholar]
- 46. Chade AR, Herrmann J, Zhu X, Krier JD, Lerman A, Lerman LO. Effects of proteasome inhibition on the kidney in experimental hypercholesterolemia. J Am Soc Nephrol. 2005;16:1005–12. [DOI] [PubMed] [Google Scholar]
- 47. Nichols TC, Bellinger DA, Davis KE, Koch GG, Reddick RL, Read MS, et al. Porcine von Willebrand disease and atherosclerosis. Influence of polymorphism in apolipoprotein B100 genotype. Am J Pathol. 1992;140:403–15. [PMC free article] [PubMed] [Google Scholar]
- 48. Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. The New England journal of medicine. 1987;316:1371–5. [DOI] [PubMed] [Google Scholar]
- 49. Holvoet P, Theilmeier G, Shivalkar B, Flameng W, Collen D. LDL hypercholesterolemia is associated with accumulation of oxidized LDL, atherosclerotic plaque growth, and compensatory vessel enlargement in coronary arteries of miniature pigs. Arteriosclerosis, thrombosis, and vascular biology. 1998;18:415–22. [DOI] [PubMed] [Google Scholar]
- 50. Otis CR, Wamhoff BR, Sturek M. Hyperglycemia-induced insulin resistance in diabetic dyslipidemic Yucatan swine. Comp Med. 2003;53:53–64. [PubMed] [Google Scholar]
- 51. Laber-Laird K, Smith A, Swindle MM, Colwell J. Effects of isoflurane anesthesia on glucose tolerance and insulin secretion in Yucatan minipigs. Lab Anim Sci. 1992;42:579–81. [PubMed] [Google Scholar]
- 52. Lomax MA, Karamanlidis G, Laws J, Cremers SG, Weinberg PD, Clarke L. Pigs fed saturated fat/cholesterol have a blunted hypothalamic-pituitary-adrenal function, are insulin resistant and have decreased expression of IRS-1, PGC1alpha and PPARalpha. J Nutr Biochem. 2013;24:656–63. 10.1016/j.jnutbio.2012.03.013 [DOI] [PubMed] [Google Scholar]
- 53. Fink LN, Costford SR, Lee YS, Jensen TE, Bilan PJ, Oberbach A, et al. Pro-Inflammatory macrophages increase in skeletal muscle of high fat-Fed mice and correlate with metabolic risk markers in humans. Obesity. 2014;22:747–57. 10.1002/oby.20615 [DOI] [PubMed] [Google Scholar]
- 54. Inokuchi-Shimizu S, Park EJ, Roh YS, Yang L, Zhang B, Song J, et al. TAK1-mediated autophagy and fatty acid oxidation prevent hepatosteatosis and tumorigenesis. The Journal of clinical investigation. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lee B, Qiao L, Lu M, Yoo HS, Cheung W, Mak R, et al. C/EBPalpha regulates macrophage activation and systemic metabolism. Am J Physiol Endocrinol Metab. 2014;306:E1144–54. 10.1152/ajpendo.00002.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee YS, Kim JW, Osborne O, Oh da Y, Sasik R, Schenk S, et al. Increased Adipocyte O2 Consumption Triggers HIF-1alpha, Causing Inflammation and Insulin Resistance in Obesity. Cell. 2014;157:1339–52. 10.1016/j.cell.2014.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oh DY, Walenta E, Akiyama TE, Lagakos WS, Lackey D, Pessentheiner AR, et al. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nature medicine. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jais A, Einwallner E, Sharif O, Gossens K, Lu TT, Soyal SM, et al. Heme oxygenase-1 drives metaflammation and insulin resistance in mouse and man. Cell. 2014;158:25–40. 10.1016/j.cell.2014.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Spijkerman AM, Dekker JM, Nijpels G, Adriaanse MC, Kostense PJ, Ruwaard D, et al. Microvascular complications at time of diagnosis of type 2 diabetes are similar among diabetic patients detected by targeted screening and patients newly diagnosed in general practice: the hoorn screening study. Diabetes care. 2003;26:2604–8. [DOI] [PubMed] [Google Scholar]
- 60. Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16:2134–40. [DOI] [PubMed] [Google Scholar]
- 61. Sosenko JM, Hu D, Welty T, Howard BV, Lee E, Robbins DC. Albuminuria in recent-onset type 2 diabetes: the Strong Heart Study. Diabetes care. 2002;25:1078–84. [DOI] [PubMed] [Google Scholar]
- 62. Kohler KA, McClellan WM, Ziemer DC, Kleinbaum DG, Boring JR. Risk factors for microalbuminuria in black americans with newly diagnosed type 2 diabetes. Am J Kidney Dis. 2000;36:903–13. [DOI] [PubMed] [Google Scholar]
- 63. Lucove J, Vupputuri S, Heiss G, North K, Russell M. Metabolic syndrome and the development of CKD in American Indians: the Strong Heart Study. Am J Kidney Dis. 2008;51:21–8. [DOI] [PubMed] [Google Scholar]
- 64. Bonnet F, Marre M, Halimi JM, Stengel B, Lange C, Laville M, et al. Waist circumference and the metabolic syndrome predict the development of elevated albuminuria in non-diabetic subjects: the DESIR Study. Journal of hypertension. 2006;24:1157–63. [DOI] [PubMed] [Google Scholar]
- 65. Howard BV. Insulin resistance and lipid metabolism. Am J Cardiol. 1999;84:28J–32J. [DOI] [PubMed] [Google Scholar]
- 66. Taskinen MR. Insulin resistance and lipoprotein metabolism. Curr Opin Lipidol. 1995;6:153–60. [DOI] [PubMed] [Google Scholar]
- 67. Selvin E, Rawlings AM, Grams M, Klein R, Sharrett AR, Steffes M, et al. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. The lancet Diabetes & endocrinology. 2014;2:279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Higgins PJ, Garlick RL, Bunn HF. Glycosylated hemoglobin in human and animal red cells. Role of glucose permeability. Diabetes. 1982;31:743–8. [DOI] [PubMed] [Google Scholar]
- 69. Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem. 1987;33:2153–63. [PubMed] [Google Scholar]
- 70. Wahid ST, Sultan J, Handley G, Saeed BO, Weaver JU, Robinson AC. Serum fructosamine as a marker of 5-year risk of developing diabetes mellitus in patients exhibiting stress hyperglycaemia. Diabet Med. 2002;19:543–8. [DOI] [PubMed] [Google Scholar]
- 71. Herdzik E, Safranow K, Ciechanowski K. Diagnostic value of fasting capillary glucose, fructosamine and glycosylated haemoglobin in detecting diabetes and other glucose tolerance abnormalities compared to oral glucose tolerance test. Acta Diabetol. 2002;39:15–22. [DOI] [PubMed] [Google Scholar]
- 72. Thornalley PJ. Glycation, receptor-mediated cell activation and vascular complications of diabetes. Diab Vasc Dis Res. 2004;1:21–2. [DOI] [PubMed] [Google Scholar]
- 73. Zhang YF, Hong J, Zhan WW, Li XY, Gu WQ, Yang YS, et al. Hyperglycaemia after glucose loading is a major predictor of preclinical atherosclerosis in nondiabetic subjects. Clin Endocrinol (Oxf). 2006;64:153–7. [DOI] [PubMed] [Google Scholar]
- 74. Kilhovd BK, Juutilainen A, Lehto S, Ronnemaa T, Torjesen PA, Birkeland KI, et al. High serum levels of advanced glycation end products predict increased coronary heart disease mortality in nondiabetic women but not in nondiabetic men: a population-based 18-year follow-up study. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:815–20. [DOI] [PubMed] [Google Scholar]
- 75. Furusyo N, Koga T, Ai M, Otokozawa S, Kohzuma T, Ikezaki H, et al. Plasma glycated albumin level and atherosclerosis: results from the Kyushu and Okinawa Population Study (KOPS). Int J Cardiol. 2013;167:2066–72. 10.1016/j.ijcard.2012.05.045 [DOI] [PubMed] [Google Scholar]
- 76. Song SO, Kim KJ, Lee BW, Kang ES, Cha BS, Lee HC. Serum glycated albumin predicts the progression of carotid arterial atherosclerosis. Atherosclerosis. 2012;225:450–5. 10.1016/j.atherosclerosis.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 77. Pu LJ, Lu L, Shen WF, Zhang Q, Zhang RY, Zhang JS, et al. Increased serum glycated albumin level is associated with the presence and severity of coronary artery disease in type 2 diabetic patients. Circ J. 2007;71:1067–73. [DOI] [PubMed] [Google Scholar]
- 78. Nin JW, Ferreira I, Schalkwijk CG, Prins MH, Chaturvedi N, Fuller JH, et al. Levels of soluble receptor for AGE are cross-sectionally associated with cardiovascular disease in type 1 diabetes, and this association is partially mediated by endothelial and renal dysfunction and by low-grade inflammation: the EURODIAB Prospective Complications Study. Diabetologia. 2009;52:705–14. 10.1007/s00125-009-1263-5 [DOI] [PubMed] [Google Scholar]
- 79. Negrean M, Stirban A, Stratmann B, Gawlowski T, Horstmann T, Gotting C, et al. Effects of low- and high-advanced glycation endproduct meals on macro- and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2007;85:1236–43. [DOI] [PubMed] [Google Scholar]
- 80. Thornalley PJ. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch Biochem Biophys. 2003;419:31–40. [DOI] [PubMed] [Google Scholar]
- 81. Carson AP, Steffes MW, Carr JJ, Kim Y, Gross MD, Carnethon MR, et al. Hemoglobin A1c and the Progression of Coronary Artery Calcification Among Adults Without Diabetes. Diabetes care. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gokulakrishnan K, Deepa R, Velmurugan K, Ravikumar R, Karkuzhali K, Mohan V. Oxidized low-density lipoprotein and intimal medial thickness in subjects with glucose intolerance: the Chennai Urban Rural Epidemiology Study-25. Metabolism. 2007;56:245–50. [DOI] [PubMed] [Google Scholar]
- 83. Yegin A, Ozben T, Yegin H. Glycation of lipoproteins and accelerated atherosclerosis in non-insulin-dependent diabetes mellitus. Int J Clin Lab Res. 1995;25:157–61. [DOI] [PubMed] [Google Scholar]
- 84. Nilsson J, Nordin Fredrikson G, Schiopu A, Shah PK, Jansson B, Carlsson R. Oxidized LDL antibodies in treatment and risk assessment of atherosclerosis and associated cardiovascular disease. Curr Pharm Des. 2007;13:1021–30. [DOI] [PubMed] [Google Scholar]
- 85. Meisinger C, Baumert J, Khuseyinova N, Loewel H, Koenig W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation. 2005;112:651–7. [DOI] [PubMed] [Google Scholar]
- 86. Zhang L, Zalewski A, Liu Y, Mazurek T, Cowan S, Martin JL, et al. Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation. 2003;108:472–8. [DOI] [PubMed] [Google Scholar]
- 87. Ishigaki Y, Katagiri H, Gao J, Yamada T, Imai J, Uno K, et al. Impact of plasma oxidized low-density lipoprotein removal on atherosclerosis. Circulation. 2008;118:75–83. 10.1161/CIRCULATIONAHA.107.745174 [DOI] [PubMed] [Google Scholar]
- 88. Lopes-Virella MF, Hunt KJ, Baker NL, Lachin J, Nathan DM, Virella G, et al. Levels of oxidized LDL and advanced glycation end products-modified LDL in circulating immune complexes are strongly associated with increased levels of carotid intima-media thickness and its progression in type 1 diabetes. Diabetes. 2011;60:582–9. 10.2337/db10-0915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Schwenke DC, D'Agostino RB Jr., Goff DC Jr., Karter AJ, Rewers MJ, Wagenknecht LE. Differences in LDL oxidizability by glycemic status: the insulin resistance atherosclerosis study. Diabetes care. 2003;26:1449–55. [DOI] [PubMed] [Google Scholar]
- 90. Hussein OA, Gefen Y, Zidan JM, Karochero EY, Luder AS, Assy NN, et al. LDL oxidation is associated with increased blood hemoglobin A1c levels in diabetic patients. Clin Chim Acta. 2007;377:114–8. [DOI] [PubMed] [Google Scholar]
- 91. Veiraiah A. Hyperglycemia, lipoprotein glycation, and vascular disease. Angiology. 2005;56:431–8. [DOI] [PubMed] [Google Scholar]
- 92. de Castro SH, Castro-Faria-Neto HC, Gomes MB. Association of Postprandial Hyperglycemia with in Vitro LDL Oxidation in Non-Smoking Patients with Type 1 Diabetes—a Cross-Sectional Study. Rev Diabet Stud. 2005;2:157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Schindhelm RK, Alssema M, Scheffer PG, Diamant M, Dekker JM, Barto R, et al. Fasting and postprandial glycoxidative and lipoxidative stress are increased in women with type 2 diabetes. Diabetes care. 2007;30:1789–94. [DOI] [PubMed] [Google Scholar]
- 94. Serin O, Konukoglu D, Firtina S, Mavis O. Serum oxidized low density lipoprotein, paraoxonase 1 and lipid peroxidation levels during oral glucose tolerance test. Horm Metab Res. 2007;39:207–11. [DOI] [PubMed] [Google Scholar]
- 95. Okopien B, Stachura-Kulach A, Kulach A, Kowalski J, Zielinski M, Wisniewska-Wanat M, et al. The risk of atherosclerosis in patients with impaired glucose tolerance. Res Commun Mol Pathol Pharmacol. 2003;113–114:87–95. [PubMed] [Google Scholar]
- 96. Edwards IJ, Wagner JD, Litwak KN, Rudel LL, Cefalu WT. Glycation of plasma low density lipoproteins increases interaction with arterial proteoglycans. Diabetes Res Clin Pract. 1999;46:9–18. [DOI] [PubMed] [Google Scholar]
- 97. Edwards IJ, Terry JG, Bell-Farrow AD, Cefalu WT. Improved glucose control decreases the interaction of plasma low-density lipoproteins with arterial proteoglycans. Metabolism. 2002;51:1223–9. [DOI] [PubMed] [Google Scholar]
- 98. Inoue I, Shinoda Y, Nakano T, Sassa M, Goto S, Awata T, et al. Acarbose ameliorates atherogenecity of low-density lipoprotein in patients with impaired glucose tolerance. Metabolism. 2006;55:946–52. [DOI] [PubMed] [Google Scholar]
- 99. Paniagua JA, Lopez-Miranda J, Perez-Martinez P, Marin C, Vida JM, Fuentes F, et al. Oxidized-LDL levels are changed during short-term serum glucose variations and lowered with statin treatment in early Type 2 diabetes: a study of endothelial function and microalbuminuria. Diabet Med. 2005;22:1647–56. [DOI] [PubMed] [Google Scholar]
- 100. Verhamme P, Quarck R, Hao H, Knaapen M, Dymarkowski S, Bernar H, et al. Dietary cholesterol withdrawal reduces vascular inflammation and induces coronary plaque stabilization in miniature pigs. Cardiovascular research. 2002;56:135–44. [DOI] [PubMed] [Google Scholar]
- 101. Knopp RH, Paramsothy P. Oxidized LDL and abdominal obesity: a key to understanding the metabolic syndrome. Am J Clin Nutr. 2006;83:1–2. [DOI] [PubMed] [Google Scholar]
- 102. Shin MJ, Hyun YJ, Kim OY, Kim JY, Jang Y, Lee JH. Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. Int J Obes (Lond). 2006;30:1529–34. [DOI] [PubMed] [Google Scholar]
- 103. Ho RC, Davy K, Davy B, Melby CL. Whole-body insulin sensitivity, low-density lipoprotein (LDL) particle size, and oxidized LDL in overweight, nondiabetic men. Metabolism. 2002;51:1478–83. [DOI] [PubMed] [Google Scholar]
- 104. Akanji AO, Abdella N, Mojiminiyi OA. Determinants of glycated LDL levels in nondiabetic and diabetic hyperlipidaemic patients in Kuwait. Clin Chim Acta. 2002;317:171–6. [DOI] [PubMed] [Google Scholar]
- 105. Karolkiewicz J, Pilaczynska-Szczesniak L, Maciaszek J, Osinski W. Insulin resistance, oxidative stress markers and the blood antioxidant system in overweight elderly men. Aging Male. 2006;9:159–63. [DOI] [PubMed] [Google Scholar]
- 106. Sies H, Stahl W, Sevanian A. Nutritional, dietary and postprandial oxidative stress. J Nutr. 2005;135:969–72. [DOI] [PubMed] [Google Scholar]
- 107. Tushuizen ME, Nieuwland R, Scheffer PG, Sturk A, Heine RJ, Diamant M. Two consecutive high-fat meals affect endothelial-dependent vasodilation, oxidative stress and cellular microparticles in healthy men. J Thromb Haemost. 2006;4:1003–10. [DOI] [PubMed] [Google Scholar]
- 108. Christoffersen BO, Grand N, Golozoubova V, Svendsen O, Raun K. Gender-associated differences in metabolic syndrome-related parameters in Gottingen minipigs. Comp Med. 2007;57:493–504. [PubMed] [Google Scholar]
- 109. Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nature reviews Endocrinology. 2014;10:293–302. 10.1038/nrendo.2014.29 [DOI] [PubMed] [Google Scholar]
- 110. Juutilainen A, Kortelainen S, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes care. 2004;27:2898–904. [DOI] [PubMed] [Google Scholar]
- 111. Hanssen NM, Beulens JW, van Dieren S, Scheijen JL, van der AD, Spijkerman AM, et al. Plasma advanced glycation endproducts are associated with incident cardiovascular events in individuals with type 2 diabetes: a case-cohort study with a median follow-up of 10 years (EPIC-NL). Diabetes. 2014:Available on line May 21. [DOI] [PubMed] [Google Scholar]
- 112. Song YM, Song SO, You YH, Yoon KH, Kang ES, Cha BS, et al. Glycated albumin causes pancreatic beta-cells dysfunction through autophagy dysfunction. Endocrinology. 2013;154:2626–39. 10.1210/en.2013-1031 [DOI] [PubMed] [Google Scholar]
- 113. Fu R, Wu S, Wu P, Qiu J. A Study of blood soluble P-selectin, fibrinogen, and von Willebrand factor levels in idiopathic and lone atrial fibrillation. Europace. 2011;13:31–6. 10.1093/europace/euq346 [DOI] [PubMed] [Google Scholar]
- 114. Patschan S, Chen J, Polotskaia A, Mendelev N, Cheng J, Patschan D, et al. Lipid mediators of autophagy in stress-induced premature senescence of endothelial cells. American journal of physiology. 2008;294:H1119–29. 10.1152/ajpheart.00713.2007 [DOI] [PubMed] [Google Scholar]
- 115. LaVange LM, Koch GG. Rank score tests. Circulation. 2006;114:2528–33. [DOI] [PubMed] [Google Scholar]
- 116. Dixon JL, Stoops JD, Parker JL, Laughlin MH, Weisman GA, Sturek M. Dyslipidemia and vascular dysfunction in diabetic pigs fed an atherogenic diet. Arteriosclerosis, thrombosis, and vascular biology. 1999;19:2981–92. [DOI] [PubMed] [Google Scholar]
- 117. Renard C, Van Obberghen E. Role of diabetes in atherosclerotic pathogenesis. What have we learned from animal models? Diabetes Metab. 2006;32:15–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.