Abstract
Infiltration by immunosuppressive myeloid cells helps tumors to overcome immune surveillance and can render patients less responsive to therapeutic intervention. Several recent studies have demonstrated that reprogramming myeloid responses can effectively enhance cancer immunotherapy, suggesting several new potential combination therapies for clinical testing.
Keywords: cancer immunotherapy, immune checkpoints, PD-1, macrophages, MDSCs
Therapeutics that activates antitumor immune responses has demonstrated significant potential for the treatment of solid tumors. One of the most promising strategies targets immune checkpoint molecules, such as programmed death 1 (PD1) or cytotoxic T lymphocyte-associated antigen 4 (CTLA4).1 These immune checkpoint molecules counteract pro-inflammatory signals and block antitumor T cell activities. The potential of this type of strategies was demonstrated by the efficacy of CTLA4 antagonistic antibody, ipilimumab, in the treatment of subsets of metastatic melanoma,2 as well as recent FDA approval of PD1 for the same indication. Another category of immunotherapies involves tumor vaccination through adoptive transfer of tumor antigen-specific T cells or dendritic cells.3 An example is Sipuleucel-T, an autologous dendritic cell-based vaccination designed to activate T cells targeting a prostate cancer antigen, which significantly improved patient overall survival in a phase III trial.4 Despite clear efficacy in subsets of human cancer, these approaches are not effective in all patients or all cancer types. For example, although ipilimumab achieved impressive response rates in melanoma patients, it failed as a monotherapy to improve clinical outcome of patients with pancreatic cancer.5
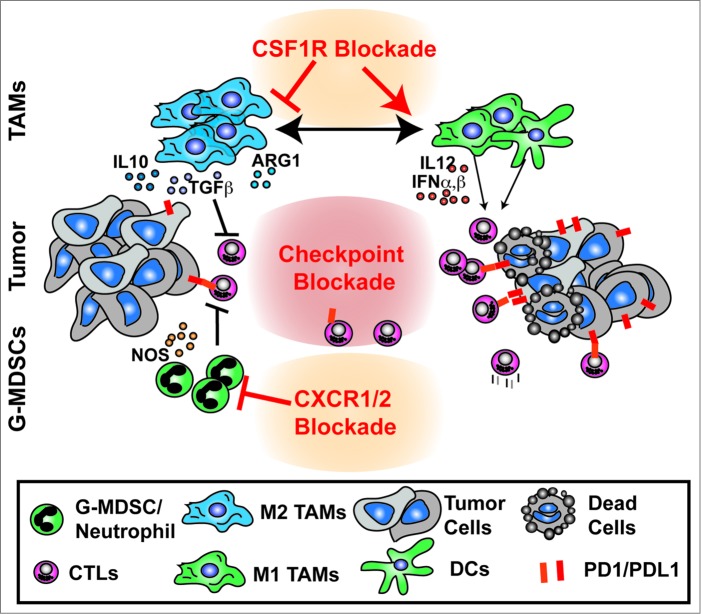
One possible explanation for the lack of responses in many patients to immunotherapy is the presence of a suppressive immune microenvironment. While tumor antigen-specific T cells may be present in many cancers, the immune infiltrate is often dominated by various subsets of myeloid cells. Tumor-infiltrating suppressive myeloid cells include macrophages, immature dendritic cells, and monocytic or granulocytic myeloid-derived suppressor cells (MDSCs). These suppressive cells can silence adaptive immune responses by blocking the recruitment of cytotoxic T lymphocytes (CTLs) to the tumor tissue, metabolically inhibiting CTL functions, chemically modifying T cell receptors to hinder the recognition of tumor antigens, and/or amplifying immune suppression via the expansion of regulatory T cells.6,7 Altogether, these myeloid cell activities can allow tumor cells to evade endogenous and treatment-elicited immune surveillance. Therefore, these subsets of suppressive myeloid cells could impose significant limitations on efficient immunotherapies (Fig. 1). Correspondingly, strategies to manipulate suppressive myeloid cells may also provide opportunities to improve the efficacy of immunotherapy. Several recent studies demonstrated that combining therapeutics that alleviates immune suppression by targeting myeloid cell activities could improve the outcome of immunotherapy in mouse models.
Figure 1.
Reprogramming of myeloid responses to enhance antitumor immunity. Tumor tissues contain extensive infiltration of suppressive myeloid cells, such as tumor-associated macrophages (TAMs), immature dendritic cells (DCs), and granulocytic myeloid-derived suppressor cells (G-MDSCs), which inhibit antitumor activities of cytotoxic T lymphocytes (CTLs). Strategies to alleviate immune suppression mediated by these myeloid cells, such as using CSF1R inhibition or CXCR1/2 signal blockade, could reprogram these myeloid cells to activate the adaptive immune system and enhance the efficacy of immunotherapeutics to eliminate tumor cells.
In a syngeneic murine rhabdomyosarcoma model, Highfill et al. demonstrated that an immunosuppressive microenvironment driven by granulocytic MDSC populations suppresses the efficacy of anti-PD1 treatment.8 In human sarcoma patients and mouse models, tumor cells often overexpress a family of C-X-C motif chemokines, including CXCL1, 2, and 8. Their predominant receptor, CXCR2, is expressed on granulocytes and promotes granulocytic MDSC trafficking into tumor sites. Inhibition of CXCR2 signaling blocked the recruitment of granulocytic MDSCs to the tumor site and significantly enhanced the efficacy of PD1 blockade. These data suggest that responses to immune checkpoint blockade are limited by the suppressive microenvironment driven by granulocytes, and that alleviation of this suppression could improve the efficacy of checkpoint-based therapies.
Work from our own group assessed if targeting tumor-associated macrophages (TAMs) could mitigate immune suppression and improve immunotherapy in pancreatic ductal adenocarcinoma (PDAC) models.9 We targeted TAMs through the inhibition of macrophage colony-stimulating factor receptor (CSF1R) signaling, which plays an essential role in macrophage differentiation, trafficking, and survival. Blockade of CSF1R signaling not only reduced the total number of suppressive macrophages in the tumor tissue, but also reprogrammed the remaining TAMs to support antitumor T cell responses, as shown by elevated interferon expression, reduced immunosuppressive activities, and improved antigen presentation capacity in the remaining TAMs. One unwanted consequence of CSF1R signal blockade is the upregulation of programmed death ligand 1 (PDL1) in tumor cells and CTLA4 in T cells, which potentially poses a significant limitation on the efficacy of CSF1R blockade. However, this may also provide an opportunity to convert tumors that are unresponsive to PD1/CTLA4 antagonists to be more sensitive to checkpoint-based immunotherapeutics. Based on this rationale, we designed a combination therapy by coupling CSF1/CSF1R signal blockade with immune checkpoint antagonists in murine PDAC models. While checkpoint inhibitors alone achieved limited efficacy in restraining tumor growth, the addition of CSF1R blockade markedly improved the efficacy of PD1 and CTLA4 antagonists and led to regression of well-established tumors.9 These data demonstrated that CSF1R signal blockade could render tumors more responsive to checkpoint antagonist-based therapies. Similarly, work by Mok et al., showed that targeting TAMs through CSF1R blockade could also enhance the efficacy of adoptive cell transfer (ACT)-based immunotherapy to reduce tumor burden in a mouse melanoma model.10 Interestingly, these tumor restraining effects correlated with increased expansion of adoptively transferred T cells both in the tumor and in peripheral lymphoid tissues, suggesting that reprograming myeloid responses could lead to increased antitumor T cell function systemically. Taken together, these studies indicate that mitigation of immune suppression through depletion or reprogramming of TAMs could enhance the clinical outcomes of checkpoint-based therapeutics and adoptive cell transfer-based immunotherapies.
In conclusion, the suppressive tumor microenvironment driven by myeloid cells may pose a major limitation on the efficiency of immunotherapy. Therefore, combining immunotherapy with strategies that reprogram the suppressive tumor microenvironment holds significant promises in cancer treatment (Fig. 1). Development of such strategies will require careful evaluation, as tumor cells, immune responses, and chosen therapeutic strategies all interact in a complex and dynamic manner. Future work is needed to determine which myeloid populations mediate suppression in specific tumor types, and what immunotherapeutic strategies are optimal for combination.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Quezada SA, Peggs KS. Exploiting CTLA-4, PD-1 and PD-L1 to reactivate the host immune response against cancer. Br J Cancer 2013; 108:1560-5; PMID:; http://dx.doi.org/ 10.1038/bjc.2013.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 2008; 8:299-308; PMID:; http://dx.doi.org/ 10.1038/nrc2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363:411-22; PMID:; http://dx.doi.org/ 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 5. Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 2010; 33:828-33; PMID:; http://dx.doi.org/ 10.1097/CJI.0b013e3181eec14c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immuno 2009; 9:162-74; PMID:; http://dx.doi.org/ 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010; 141:39-51; PMID:; http://dx.doi.org/ 10.1016/j.cell.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, Kaplan RN, Mackall CL. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Translat Med 2014; 6:237ra67; PMID:; http://dx.doi.org/ 10.1126/scitransl-med.3007974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res 2014; 74(18):5057-69; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mok S, Koya RC, Tsui C, Xu J, Robert L, Wu L, Graeber TG, West BL, Bollag G, Ribas A. Inhibition of CSF-1 receptor improves the antitumor efficacy of adoptive cell transfer immunotherapy. Cancer Res 2014; 74:153-61; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-1816 [DOI] [PMC free article] [PubMed] [Google Scholar]