Microarrays represent an efficient way to rapidly express large numbers of compounds in highly condensed and easily operated formats by using robotics and computer-guided instrumentation. These arrays are widely employed as tools to perform multiple analyses in single assays. In particular, arrays of DNA/RNA, peptides, proteins, and cells have found substantial application in modern biology and medicine.[1–6] Carbohydrate microarrays show particularly high potential in this respect, although there has only recently been an upsurge in their development.[7,8] The need for new techniques in the area of glyco-science is, however, immense, since details of complex carbohydrate-recognition patterns are, in the main, unresolved. In addition, probing the properties of the intricate glycosylation patterns of proteins and cells is an intense research area in significant need of efficient tools for further progress. Efficient microarrays will therefore greatly enhance development in the functional glycomics field for rapid resolution of analytical and functional details of glycosylation and recognition.
In this study, a new strategy for making carbohydrate micro-arrays is presented. It is a controllable and robust method to array fabrication, both on the carbohydrate-chemistry and on the surface-chemistry levels, and the resulting carbohydrate arrays can be efficiently used to reveal the recognition patterns of carbohydrate-binding proteins.
The microarrays are based on the specific photochemistry of aryl azides. Upon light irradiation, the azide functionality in these structures becomes converted to a highly reactive nitrene species that inserts into C–H and N–H bonds.[9] In particular, perfluorophenylazides (PFPAs), which produce markedly enhanced insertion yields, have been used to photochemically introduce functional groups in, for example, proteins, ful-lerenes, and polymers.[10–13]
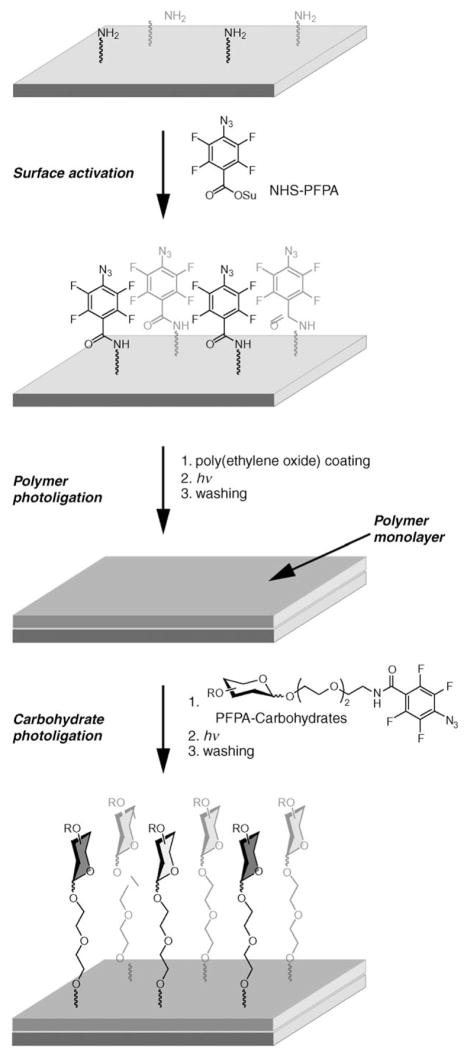
The versatility of PFPA chemistry makes it especially useful for surface modification. Surfaces can either be globally modified by the technique, or specific areas can be addressed by arraying techniques such as photomasking and conventional printing devices. Furthermore, the technique is applicable to a variety of polymers.[11, 12,14–16] In this study, PFPA chemistry was first used to covalently attach poly(ethylene oxide) (PEO) to amino-functionalized glass slides. By treating the amino groups on the array glass slide with N-hydroxylsuccinimide-de-rivatized PFPA (NHS-PFPA),[17] a monolayer of PFPA was formed on the surface (Scheme 1). Coating the slide with a solution of PEO and subsequent UV irradiation produced a thin layer of PEO that was efficiently attached to the surface.
Scheme 1.
Array generation by double photoligation. A layer of poly(ethylene oxide) was photoligated to a perfluorophenylazide (PFPA)-activated surface. Photoprobe-conjugated carbohydrates were subsequently arrayed and photoligated to the resulting polymer surface.
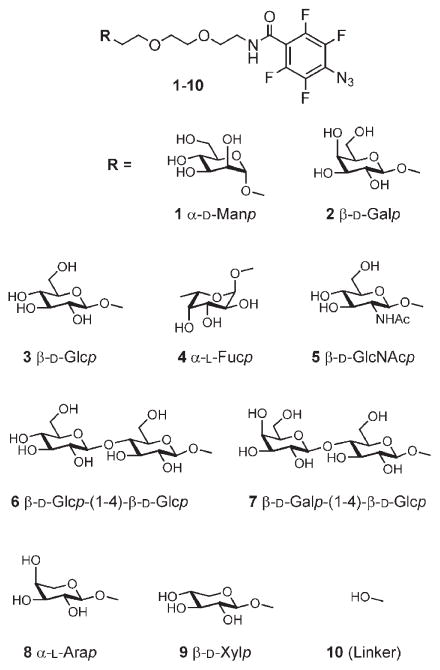
PFPA-derivatized carbohydrates were subsequently immobilized in an array format on the PEO surface by photoinitiated insertion chemistry (Scheme 1). A prototype library of carbohydrate structures was designed and synthesized, composed of nine different carbohydrate structures (Scheme 2, compounds 1–9), including monosaccharides (α-D-mannose, β-D-glucose, β-D-galactose, N-acetyl-β-D-glucosamine, α-L-fucose, α-L-arabinose, β-D-xylose), and disaccharides (lactose, cellobiose). This collection of carbohydrate structures was primarily chosen to demonstrate the efficiency of the array preparation and function, but can relatively easily be adapted to larger collections of more complex structures. In addition to the carbohydrates, the linker structure 10, which does not carry a carbohydrate head group, was coupled to the PFPA structure and used as reference.
Scheme 2.
Compounds used in array generation.
The resulting array structures were then applied to the surfaces coated with the PEO thin film. By using a high-density DNA-array machine, the slides were first spotted with the compounds. Subsequently, photochemical UV activation was employed to immobilize the carbohydrates on the substrate surface by insertion reactions of PFPA to the polymer film. The general design of the arrays produced is displayed in Figure 1. A three-by-four array, with every structure printed in quadruplicate, was employed
Figure 1.
Carbohydrate microarray results with four different lectins: Griffonia simplicifolia lectin II (GSII), peanut agglutinin (PNA), concanavalin A (ConA), and soybean agglutinin (SBA). Active carbohydrates for each lectin are easily identified from the arrays, and carbohydrates recognized are listed. The design of the library is indicated at the lower left, all carbohydrates were repeated in quadruplicate [2 × 2].
Following array development, binding analysis of the surfaces was addressed. The primary targets were known lectins of different specificity;[18–21] here fluorescence-tagged lectins were used, and fluorescence imaging was employed in developing the array binding patterns. Four different lectins were thus targeted by the arrays, the lectin from Griffonia simplicifolia II (GSII), peanut agglutinin (PNA), jack bean lectin (ConA), and soybean agglutinin (SBA).
The results from scanning the arrays are displayed in Figure 1. As can be seen, the arrays were efficient in demonstrating the specific binding patterns of the chosen lectins; both the primary binding partners and the secondary ligands could be identified. The first lectin, GSII, showed a clear preference for binding to its major binding partner, β-D-GlcNAc (5), in agreement with the ligand specificity for this lectin, and with no detectable binding to the other structures. The primary ligand for PNA was revealed to be lactose (7), followed by lower binding of β-D-galactose (2) and cellobiose (6). The preference of ConA for interacting with α-D-mannoside structures is clearly demonstrated, with efficient binding to the carbohydrate part of compound 1, the α-D-mannoside PFPA. This lectin also showed low binding to the β-D-glucoside (3) and very low binding to the N-acetyl-β-D-glucosamine (5) structure. Finally, the soybean agglutinin is specific for terminal β-D-galactoside units and thus showed binding to both β-D-galactose (2) and lactose (7). Thus, these results indicate that the method can be used to specify the pattern of binding to different carbohydrate structures for specific lectins in a single analysis.
In conclusion, this study has demonstrated that the versatility of photochemical immobilization chemistry can be combined with microarray techniques to create high-density and spatially addressable carbohydrate microarrays. With this approach, the photochemical properties of PFPAs can be fully employed to produce polymer thin films, and to locate the carbohydrate ligands to specific areas on the surface. Furthermore, the type and chemical nature of the polymer film on the substrate can be selected to reduce the nonspecific adsorption of ligands to the surface. The produced arrays could be employed to efficiently pinpoint the binding patterns of selected lectins for their optimal binding partners. The common specificity of the proteins could thus easily be displayed, and the relative binding efficiency for individual array compounds could be estimated. Given expanded carbohydrate repertoires, these microarrays have the potential to facilitate and accelerate various aspects of glycomics and proteomics.
Experimental Section
Experimental methods for all compounds, arrays, and analyses are given in the Supporting Information.
Supplementary Material
Acknowledgments
This study was supported by the Swedish Research Council, NIH AREA award 1R15 GM066279-01A2, the Royal Institute of Technology, and the Carl Trygger Foundation. H.Y. gratefully acknowledge a postdoctoral fellowship from the Wenner-Gren Foundations.
Footnotes
Supporting information for this article is available on the WWW under http://www.chembiochem.org or from the author.
Contributor Information
Prof. Mingdi Yan, Email: yanm@pdx.edu.
Dr. Olof Ramström, Email: ramstrom@kth.se.
References
- 1.Chen DS, Davis MM. Curr Opin Chem Biol. 2006;10:28. doi: 10.1016/j.cbpa.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Hoheisel JD. Nat Rev Genet. 2006;7:200. doi: 10.1038/nrg1809. [DOI] [PubMed] [Google Scholar]
- 3.Sievertzon M, Nilsson P, Lundeberg J. Expert Rev Mol Diagn. 2006;6:481. doi: 10.1586/14737159.6.3.481. [DOI] [PubMed] [Google Scholar]
- 4.Bryant PA, Venter D, Robins-Browne R, Curtis N. Lancet Infect Dis. 2004;4:100. doi: 10.1016/S1473-3099(04)00930-2. [DOI] [PubMed] [Google Scholar]
- 5.Espina V, Mehta AI, Winters ME, Calvert V, Wulfkuhle J, Petricoin EF, Liotta LA. Proteomics. 2003;3:2091. doi: 10.1002/pmic.200300592. [DOI] [PubMed] [Google Scholar]
- 6.Schweitzer B, Predki P, Snyder M. Proteomics. 2003;3:2190. doi: 10.1002/pmic.200300610. [DOI] [PubMed] [Google Scholar]
- 7.For reviews, see: Paulson JC, Blixt O, Collins BE. Nat Chem Biol. 2006;2:238. doi: 10.1038/nchembio785.Shin I, Park S, Lee MR. Chem Eur J. 2005;11:2894. doi: 10.1002/chem.200401030.Disney MD, Seeberger PH. Drug Discovery Today. 2004;3:151.Feizi T, Chai W. Nat Rev Mol Cell Biol. 2004;5:582. doi: 10.1038/nrm1428.Wang D. Proteomics. 2003;3:2167. doi: 10.1002/pmic.200300601.Hirabayashi J. Trends Biotechnol. 2003;21:141. doi: 10.1016/S0167-7799(03)00002-7.Love KR, Seeberger PH. Angew Chem. 2002;114:3733. doi: 10.1002/1521-3773(20021004)41:19<3583::AID-ANIE3583>3.0.CO;2-P.Angew Chem Int Ed. 2002;41:3583.Kiessling LL, Cairo CW. Nat Biotechnol. 2002;20:234. doi: 10.1038/nbt0302-234.Ortiz Mellet C, Garc%a FernQndez JM. ChemBio-Chem. 2002;3:819.
- 8.For recent articles, see: Stevens J, Blixt O, Tumpey TM, Tauben-berger JK, Paulson JC, Wilson IA. Science. 2006;312:404. doi: 10.1126/science.1124513.Huang CY, Thayer DA, Chang AY, Best MD, Hoffmann J, Head S, Wong CH. Proc Natl Acad Sci USA. 2006;103:15. doi: 10.1073/pnas.0509693102.de Paz JL, Noti C, See-berger PH. J Am Chem Soc. 2006;128:2766. doi: 10.1021/ja057584v.Huang GL, Zhang HC, Wang PG. Bioorg Med Chem Lett. 2006;16:2031. doi: 10.1016/j.bmcl.2005.12.063.Stevens J, Blixt O, Glaser L, Taubenberger JK, Palese P, Paulson JC, Wilson IA. J Mol Biol. 2006;355:1143. doi: 10.1016/j.jmb.2005.11.002.Brun MA, Disney MD, Seeberger PH. ChemBioChem. 2006;7:421. doi: 10.1002/cbic.200500361.Zhou X, Zhou J. Biosens Bioelectron. 2006;21:1451. doi: 10.1016/j.bios.2005.06.008.Ngundi MM, Taitt CR, McMurry SA, Kahne D, Ligler FS. Biosens Bioelectron. 2006;21:1195. doi: 10.1016/j.bios.2005.05.001.Manimala JC, Li Z, Jain A, VedBrat S, Gildersleeve JC. ChemBioChem. 2005;6:2229. doi: 10.1002/cbic.200500165.Ko KS, Jaipuri FA, Pohl NL. J Am Chem Soc. 2005;127:13 162. doi: 10.1021/ja054811k.Bochner BS, Alvarez RA, Mehta P, Bovin NV, Blixt O, White JR, Schnaar RL. J Biol Chem. 2005;280:4307. doi: 10.1074/jbc.M412378200.Lee M-r, Shin I. Org Lett. 2005;7:4269. doi: 10.1021/ol051753z.Biskup MB, Müller JU, Weingart R, Schmidt RR. ChemBio-Chem. 2005;6:1007. doi: 10.1002/cbic.200400300.Bryan MC, Fazio F, Lee HK, Huang CY, Chang A, Best MD, Calarese DA, Blixt O, Paulson JC, Burton D, Wilson IA, Wong CH. J Am Chem Soc. 2004;126:8640. doi: 10.1021/ja048433f.Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, Stevens DJ, Skehel JJ, van Die I, Burton DR, Wilson IA, Cummings R, Bovin N, Wong CH, Paulson JC. Proc Natl Acad Sci USA. 2004;101:17 033. doi: 10.1073/pnas.0407902101.Park S, Lee MR, Pyo SJ, Shin I. J Am Chem Soc. 2004;126:4812. doi: 10.1021/ja0391661.Ratner DM, Adams EW, Su J, O’Keefe BR, Mrksich M, Seeberger PH. ChemBio-Chem. 2004;5:379. doi: 10.1002/cbic.200300804.Adams EW, Ratner DM, Bokesch HR, McMahon JB, O’Keefe BR, Seeberger PH. Chem Biol. 2004;11:875. doi: 10.1016/j.chembiol.2004.04.010.
- 9.Scriven EFU, editor. Azides and Nitrenes: Reactivity and Utility. Academic Press; New York: 1984. [Google Scholar]
- 10.Joester D, Klein E, Geiger B, Addadi L. J Am Chem Soc. 2006;128:1119. doi: 10.1021/ja0537474. [DOI] [PubMed] [Google Scholar]
- 11.Yan M, Ren J. Chem Mater. 2004;16:1627. [Google Scholar]
- 12.Yan M, Bartlett M. Nano Lett. 2002;2:275. [Google Scholar]
- 13.Yan M, Cai SX, Wybourne MN, Keana JFW. J Am Chem Soc. 1993;115:814. [Google Scholar]
- 14.Liu L, Yan M. J Am Chem Soc. 2006;128:14 067. doi: 10.1021/ja060141m. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Yan M. Angew Chem. 2006;118:6353. doi: 10.1002/anie.200602097. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2006;45:6207. [Google Scholar]
- 16.Yan M. Polym News. 2002;27:6. [Google Scholar]
- 17.Keana JFW, Cai SX. J Org Chem. 1990;55:3640. [Google Scholar]
- 18.Dam TK, Brewer CF. Chem Rev. 2002;102:387. doi: 10.1021/cr000401x. [DOI] [PubMed] [Google Scholar]
- 19.Lis H, Sharon N. Chem Rev. 1998;98:637. doi: 10.1021/cr940413g. [DOI] [PubMed] [Google Scholar]
- 20.Gabius HJ, Siebert HC, André S, Jiménez-Barbero J, Rüdiger H. ChemBioChem. 2004;5:740. doi: 10.1002/cbic.200300753. [DOI] [PubMed] [Google Scholar]
- 21.Weis WI, Drickamer K. Annu Rev Biochem. 1996;65:441. doi: 10.1146/annurev.bi.65.070196.002301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.