Abstract
Although ovarian hormones are thought to have a potential role in the well-known sex difference in mood and anxiety disorders, the mechanisms through which ovarian hormone changes contribute to stress regulation are not well understood. One mechanism by which ovarian hormones might impact mood regulation is by mediating the effect of psychosocial stress, which often precedes depressive episodes and may have mood consequences that are particularly relevant in women. In the current study, brain activity and mood response to psychosocial stress was examined in healthy, normally cycling women at either the high or low estradiol phase of the menstrual cycle. Twenty eight women were exposed to the Montreal Imaging Stress Task (MIST), with brain activity determined through functional magnetic resonance imaging, and behavioral response assessed with subjective mood and stress measures. Brain activity responses to psychosocial stress differed between women in the low versus high estrogen phase of the menstrual cycle: women with high estradiol levels showed significantly less deactivation in limbic regions during psychosocial stress compared to women with low estradiol levels. Additionally, women with higher estradiol levels also had less subjective distress in response to the MIST than women with lower estradiol levels. The results of this study suggest that, in normally cycling premenopausal women, high estradiol levels attenuate the brain activation changes and negative mood response to psychosocial stress. Normal ovarian hormone fluctuations may alter the impact of psychosocially stressful events by presenting periods of increased vulnerability to psychosocial stress during low estradiol phases of the menstrual cycle. This menstrual cycle – related fluctuation in stress vulnerability may be relevant to the greater risk for affective disorder or post-traumatic stress disorder in women.
Keywords: Psychosocial stress, menstrual cycle, estradiol, fMRI
1. Introduction
The sex difference in affective and stress-related disease rates (2 to 3 times greater incidence and prevalence of major depression in women compared to men (Bromet et al., 2011; Kessler et al., 2005)) emerges at puberty and remains until menopause (Kessler et al., 1994). The stress exposure model of depression suggests that MDD is the result of a vulnerability to depression, combined with the trigger of stressful life events (Hankin et al., 2007; Liu and Alloy, 2010). Accordingly, psychosocial stressors are among the top reported antecedents to depression episodes (Frank et al., 1994; Kendler et al., 1999; Kendler et al., 2000), and dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis response is a consistent finding in major depression (Burke et al., 2005; Lupien et al., 2009). Psychosocial stress may be especially important in the etiology of mood disorders in women as the depressogenic effects of life stressors are reportedly greater in women than men (Mezulis et al., 2010), even when there is no difference in the number of stressful life events or in the subjective perception of these events (Korszun., 2009). Although this finding is consistent and has been replicated by studies across the globe (Bromet et al., 2011), the reasons for the sex difference in incidence and prevalence of mood and anxiety disorders are not well understood.
The cortisol response to stress shows consistent sex differences (Vamvakopoulos, 1995), and changes across the menstrual cycle, decreasing during the late follicular phase when circulating estrogen is high (Kirschbaum C, 1999). Women are more likely than men to show variations in HPA function in response to stressors (Weiss et al., 1999) and during depressive episodes (Korszun., 2009). These differences in stress system response likely contribute to mood disorder risk in women (Weiss et al., 1999), and may be modulated by ovarian hormone fluctuations across the menstrual cycle (Kajantie E, 2006; Roca et al., 2005). The role of corticosteroids in stress response and regulation is well known, however the effects of gonadal steroids (e.g. estradiol) –which may be specifically important in women- are less well characterized.
The concurrence of increased major depression disorder (MDD) and post-traumatic stress disorder (PTSD) risk in women during the reproductive period of life suggests that the cyclic fluctuation of ovarian hormones during this period may contribute to the risk for psychopathology. Understanding the role of ovarian hormones in emotional processing and mood regulation in women may provide important insight into the mechanisms underlying the stress response and potentially the increased incidence of MDD and PTSD in women.
Ovarian hormones may modulate the effects of stress on mood. Brain areas that are central to mood regulation (including the amygdala and hippocampus) show some of the largest densities of estrogen receptors in the human brain (Merchenthaler et al., 2004; Ostlund et al., 2003); interestingly, those areas are also very rich in cortisol receptors. Estrogen may modulate the activity of these areas; large community and clinic based studies indicate that negative mood complaints (Davydov et al., 2005; Gonda et al., 2008) (even in healthy women) and suicidal behavior (Baca-Garcia et al., 2004; Saunders and Hawton, 2006) increase in women during low estrogen phases of the menstrual cycle. Brain activity related to processing negative emotional information is also modulated by changing estradiol levels across the menstrual cycle (Goldstein, 2005; Merz et al., 2012), suggesting that estrogen may alter the mood response to negative information, making this information more or less salient to cognitive processes and subsequently mood states.
Although there is strong evidence that estrogen modulates the response to negatively valenced stimuli, such as negative images (Andreano and Cahill, 2010; Goldstein, 2005), the effect of estrogen on the response to psychosocial stress is less well understood. Animal models suggest that female rodents do not gain the same beneficial effect of acute stress on hippocampally-mediated or prefrontal tasks as male animals, and that high estrogen levels enhance the negative effects of stress in these areas(Shansky et al., 2004; Shors and Leuner, 2003). In contrast studies in ovariectomized female rats indicate that estrogen replacement increases resilience to stress in earned helplessness models and support hippocampal plasticity(Bredemann and McMahon, 2014; Smith et al., 2010). The modulation of stress effects on brain activity and function remain unclear and likely depend on the type of stress and measure of function(Shansky, 2009). The effects of estradiol on the cognitive consequences or brain activity in women have not been widely investigated. Previous studies have generally compared stress response in women during the early follicular phase and mid luteal phase. However, this approach precludes examining the effects of estradiol separately from progesterone (Kirschbaum et al., 1992; Kirschbaum C, 1999). Additionally, few of these studies have included subjective mood measures, and the measurement of stress response solely through free circulating (salivary) cortisol is complicated by menstrual effects on cortisol binding globulin and adrenocorticotropic hormone sensitivity. Psychosocial stress differs from processing emotional information in a number of ways - stress includes elements of self-esteem, uncontrollability, and personal threat. Further, studies using performance–based stressors provide evidence that there is a sex difference in the endocrine (cortisol) response to this type of stress, and that cycling ovarian hormones may modulate this response in women (Kirschbaum et al., 1992; Kirschbaum C, 1999). Social-evaluative threat is one element of psychosocial stress that may be especially salient for women, and has face validity for the real life stressors women experience and that contribute to mood disorder risk (Kendler et al., 1999; Kendler et al., 2001; Stroud et al., 2002). Understanding the effect of ovarian hormones on the response to these types of stressors is thus important in understanding the role of ovarian hormones in the brain mechanisms of emotional processing and mood in women. Investigating the stress response, under controlled laboratory conditions, provides an opportunity to examine the effects of ovarian hormones on emotional processing, stress response, and subsequent mood.
In this study, we examined the effect of estradiol levels across the menstrual cycle on both the brain activity and behavioral response to a laboratory-based model of psychosocial stress. We hypothesized that high circulating estrogen would reduce the effect of acute stress on brain activity and be associated with less subjective distress following the stress-inducing task.
2. Methods
2.1 Overview
This cross sectional study included 28 normally cycling women (ages18–45) who were examined during early follicular phase (day 1–2 of menstrual cycle), or at ovulation (day 12–14), and who participated in a psychological stress fMRI task. Due to the psychosocial stress task used in this study, and the requirement to debrief participants about the focus of the study once the stress task was completed, we were unable to repeat the stress task for a within subjects comparison, and were limited to between subjects/group comparisons. Approximately half of the participants were studied at the University of Vermont and half were completed at Vanderbilt University. The study coordinator was the same throughout the study and the MRI scanners were the identical make and model, with the same software version in operation at the time of the study. The study procedures were identical at both Universities, and imaging and behavior data were analyzed for study location effects. The study was reviewed and approved by both the University of Vermont and Vanderbilt University Institutional Review Boards, and all participants provided written informed consent.
2.2 Participants
Participants were recruited and told that they would be participating in a study to examine the effects of menstrual cycle hormone changes on mathematical ability in women. All participants were healthy, with regular menstrual cycles (21–35 days), no hormonal contraceptive or centrally active medication use, and no history of severe pain or mood changes related to their menstrual cycles. We excluded participants with current or past Axis I psychiatric disorders using the Structured Clinical Interview for DSM Disorders(First et al., 2001). Beck Depression Index score was required to be less than 7, and Beck Anxiety Index score less than 15 (Beck et al., 1961). Current illicit drug use and pregnancy were exclusions.
2.3 Screening and Characterization
To assure all participants were of at least average intelligence, we administered the Wechsler Abbreviated Scale of Intelligence(Wechsler, 1999). The Composite International Diagnostic Interview for premenstrual dysphoric disorder (Kessler and Ustun, 2004) was used to collect menstrual cycle history and to rule out premenstrual dysphoric disorder, and physical and psychological symptoms associated with the menstrual cycle were assessed using the Moos Menstrual Distress Questionnaire(Moos, 1968). Personality factors were assessed at screening using the NEO Five Factor Inventory (Costa and McCrae, 2000). Following screening, participants tracked their menstrual cycles for three months to determine an average cycle length and to calculate the study day date. At the University of Vermont, participants tracked using a paper calendar; at Vanderbilt University, women filled in daily surveys using the on-line REDCap system.
Women were randomly assigned to return for the study day during the early follicular phase (days 1–2 of the menstrual cycle) or at ovulation (day 12–14); before the study began, participant research ID numbers were assigned a randomly generated number, ranked according to this random number and then alternatingly assigned to the two groups. The study day took approximately 3 hours to complete. Upon arrival at the Clinical Research Center, participants completed the State and Trait Anxiety Inventories(Spielberger, 2010) and the Beck Depression and Anxiety Inventories (Beck et al., 1961).
2.4 Stress Task
We employed the Montreal Imaging Stress Task (MIST) for psychosocial stress induction (Pruessner, 2008). The MIST produces moderate psychosocial stress through a combination of motivated performance and social-evaluative threat. We presented the MIST as an arithmetic task in the MRI scanner, and instructed participants that they should achieve an 80–90% correct performance for their data to be usable in the context of this experiment. Unbeknownst to the subjects, the MIST contains an algorithm producing script that automatically adjusts the difficulty of the math tasks to the aptitude of the participant, this way maintaining a low performance rate (between 40–50%) by changing either the problem difficulty or the allotted time. A “control” condition, in which the participants solve arithmetic problems with no time limit and no performance limit, serves as a contrast to control for brain activity changes induced by arithmetic task demands (visual stimuli, motor response, and mental arithmetic). Social evaluative threat comes from scripted experimenter interaction; the experimenters enter the MRI room between runs and inform the participants that they are not doing well enough and that they need to improve their performance for the experiment to be successful.
In this study, participants completed three runs of the MIST; the MRI sessions were scheduled during the afternoon when baseline cortisol levels are low. After the first run, experimenter 1 entered the scanner room and provided the scripted feedback and asked the participant to complete a second run of the task. After the second run the experimenter 1 entered the scanner room and told the participant that the experimenter 2 (“doctor”) would like to speak with them about their performance, at which point the experimenter 2 entered the scanner room and provided the scripted feedback and asked the participant to complete a third run of the task. This protocol was designed to maintain both performance and social evaluative threat throughout the MIST and to prevent participants from habituating to the performance challenge or giving up on completing the task. Specifically the interaction of the participants and the experimenters was structured to generate psychosocial stress. To habituate the participants to the scanning environment and decrease scanner-related stress at the study day, participants entered an MRI simulator during the screening visit; in the MRI simulator, participants watched a nature video while being exposed to the MRI environment (simulated MRI sounds, head coil, and being placed in the MRI bore) and the stimulus presentation system.
The MIST was presented in a block design; each condition was presented twice per 3 runs (“stress”: 100 seconds, “control”: 50 seconds, ‘rest”: 30 seconds). Participants practiced the MIST task (control trials only) in the MRI simulator, before the MRI session.
2.5 Subjective Measures
Participants completed subjective measures: the Stress and Arousal Checklist(King et al., 1983)(before and after the MIST), the Profile of Mood States(McNair DM, 1971), and a Visual Analogue Scale for mood and task experience (both after the MIST).
2.6 Endocrine Measurements
Saliva samples were collected, using Salimetrics salivettes placed under the tongue, at seven times during the study day to assess the cortisol response to the stress task (Supp. Fig. 1). Salivettes remained under the tongue for two minutes, and then were stored at −80°C freezer until analysis. Cortisol levels were assessed using the Salimetrics High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit for quantitative determination of salivary cortisol with a sensitivity of 0.007 ug/dL for 25μl sample volume (Salimetrics LLC, Philadelphia, PA). All cortisol measurements were assayed in duplicate, with mean intra-assay coefficient of variation of 7.5% and inter-assay coefficient of variation of 14.3%. Cortisol area under the curve measurements for each participant (over the study day) were calculated using Graphpad Prism.
Blood samples were collected from each participant at the end of the study day to determine the estradiol level and to verify menstrual phase. Estradiol was assessed from sera using the Immulite 1000 endocrine panel immunoassay system with mean intra-assay coefficient of variation of 5.9%. For analyses by menstrual phase, we required that both the participant’s menstrual cycle tracking and estradiol level indicated that they were in the assigned menstrual phase. Participants’ whose estradiol levels did not concord with their randomly assigned menstrual phase at the study day; women whose estradiol levels were higher or lower than the reference values for their assigned phase, were not included in the low or high estradiol groups, as they could not be accurately assigned to either group. These data were included in all other analyses.
Progesterone levels were assessed using the Salimetrics Salivary Progesterone (P4) Enzyme Immunoassay Kit with a sensitivity of 5pg/mL for 25μl sample volume (Salimetrics LLC, Philadelphia, PA). The first saliva sample of the day was used for progesterone measurements. All progesterone measurements were assayed in duplicate, with mean intra-assay coefficient of variation of < 1%.
2.7 MRI Scan parameters
Participants were scanned on a Philips 3.0 Tesla Achieva scanner, with an 8 channel head coil. All participants received the following MR sequences: 1) A sagittal T1-weighted 3D Turbo Field Echo Sensitivity Encoding (TFE SENSE) sequence perpendicular to the anterior commissure (AC) -posterior commissure (PC) line, repetition time (TR) of 9.9 ms, echo time (TE) of 4.6 ms, a flip angle of 8°, number signal averages (NSA) 1.0, a field of view (FOV) of 256 mm, a 256 × 256 matrix, and 1.0 mm slice thickness with no gap for 140 contiguous slices. 2) A T2- weighted Gradient and Spin Echo (GRASE) sequence was run parallel to the AC-PC line, TR 2470 ms, TE 80 ms, NSA 3.0, FOV of 230 mm, 0.7 mm slice thickness with 5.0 mm gap for 28 slices. 3) Three Echoplanar Blood Oxygenation Level Dependent (EpiBOLD) functional sequences, transverse orientation, TR 2500 ms, TE 35 ms, flip angle 90°, 1 NSA for FOV 240, 240X128 matrix, and 4.0 mm slice thickness with no gap, with ascending interleaved acquisition, for 35 contiguous slices.
2.8 fMRI Analysis
fMRI data was processed using Statistical Parametric Mapping (SPM8) (Wellcome Department of Cognitive Neurology, 2008). Preprocessing included: realignment of the three functional runs and correction for bulk-head motion, coregistration of functional and anatomical images for each participant, segmentation of the anatomical image, and normalization of the anatomical and functional images to the standard MNI template, and spatial smoothing with a Gaussian filter (8 mm at full width, half maximum).
Artifact detection was performed on functional images using the ART toolbox in SPM, and outliers for signal intensity (z>3) and motion (movement >3 mm in either translation or rotation) were entered as nuisance regressors at the first level, single-participant analysis. At first level analysis, T-maps were created from linear contrasts for task conditions and between condition comparisons; these T-maps were used in the second level whole-brain random effects analysis of participant group effects with two-sample t-tests. The preprocessed functional images had a voxel size of 2X2X2 mm and cluster threshold correction was used to control for multiple comparisons (from voxel wise p = 0.005) to corrected p < 0.001 with k = 58 (corrected voxel-wise p = 0.000001)(from alpha simulation in REST(Song et al., 2011) toolbox for SPM, using a grey matter mask).
Two sets of second level analyses were conducted, one analysis with groups defined by menstrual phase and estradiol level at the study day, and a second analysis with groups defined by subjective distress to the MIST (change in Stress Arousal Checklist score). Separate regression analyses were run for estradiol level and subjective distress by hippocampal activity (using a bilateral hippocampus region of interest mask created from the Automated Anatomical Labeling (AAL) in WFU Pickatlas (Maldjian et al., 2003)). Percent signal change in right and left hippocampus was calculated with the REX toolbox for SPM; using masks created from the average peak cluster in the hippocampus ROI (using the contrast “control” condition > “stress” condition in the full subject set) with cluster threshold correction (from voxel wise p = 0.05) to corrected p < 0.001 with k = 101 (corrected voxel-wise p = 0.001).
3. Results
3.1 Participants
Average participant age was 30.4 years (SD = 8.2 years). There was no difference in age between subject groups either by estradiol level (t (8) = −1.01, p = 0.33), or distress (t (26) = −0.83, p = 0.41). No participants had Menstrual Distress Questionnaire scores that indicated severe premenstrual somatic or affective changes. There were no differences between any of the participant groups at screening, including Beck Depression Inventory scores, Beck Anxiety Inventory scores, NEO Five Factor Inventory personality measures, or any of the Menstrual Distress Questionnaire measures, and none of the participants had elevated scores on these scales (Supp. Table 1). For the analysis of the effect of menstrual phase on stress related brain activity, we included only participants whose estradiol level at the study day confirmed their assigned menstrual phase. The reference value for median early follicular phase estradiol levels using the Immulite 1000 endocrine panel immunoassay system is 31 pg/mL. Women were included in the low estradiol phase group if they were assigned to the early follicular group and had an estradiol level on the study day less than 40 pg/mL (mean 24.91 pg/mL). Women were included in the high estradiol phase group if they were assigned to the ovulatory group and had an estradiol level on the study day greater than 50 pg/mL (mean 103.52pg/mL). This resulted in 8 participants (4 from the low estradiol, and 4 from the high estradiol group) not being included in stress-brain imaging analyses for low vs. high estradiol phase groups; these participants’ data were included in all other analyses
3.2 Brain Activity
3.2.1 Psychosocial Stress Response
Second level comparison using a one sample t–test of average images of all subjects (n = 28) (using the 1st level contrast of control condition > stress condition) revealed significantly (uncorr.p = 0.005, cluster size = 58, corr.p < .001) greater activation in cingulate, temporal (including hippocampus), and frontal regions and activation in parietal and precentral gyrus regions during the control condition compared to the stress condition (Fig. 1 and Supp. Table 2). This activity pattern is consistent with the activity pattern previously shown by studies using the MIST (Pruessner, 2008); reduced activation of hippocampus and para-limbic regions during the stress condition. These results confirm that the overall brain activity response to psychosocial stress in a sample including only women is similar to the brain activity seen in studies that include both men and women.
Figure 1.
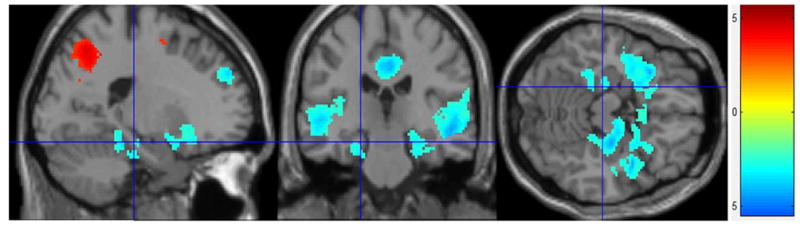
Psychosocial Stress Effect fMRI Stress Condition – Control Condition: All participants (n = 28). Greater deactivation in limbic regions during the stress condition compared to the control condition (corrected to p < 0.001).
Menstrual Cycle Phase/Estradiol Effects
Estradiol level was significantly higher in the high estradiol phase group (mean = 103.52 pg/mL, SD = 34.09) than the low estradiol phase group (mean = 24.91 pg/mL, SD = 9.17) (t (18) = 7.04, p < 0.001). All women had salivary progesterone levels under 105 pg/mL (mean = 49.72 pg/mL, SD = 35.54), consistent with the follicular phase (Chatterton et al., 2005). Progesterone levels did not differ between the low estradiol phase group (mean = 48.90 pg/mL, SD = 36.10) and high estradiol phase group (mean = 50.54 pg/mL, SD = 36.91) (t (18) = 0.099, p = 0.92) (Supp. Fig. 2).
The fMRI estradiol phase group analysis for the effect of psychosocial stress, including participants who showed concordance of assigned menstrual phase and estradiol level, (high estradiol n = 10, low estradiol n = 10), showed a significant (uncorr.p = 0.05, cluster size = 529, corr. p <0.005) effect of estradiol level on brain activity during psychosocial stress and perceived distress following the MIST, however these findings did not survive more conservative correction for multiple comparisons. Women with low estradiol levels had significantly less activity in the left hippocampus than women with high estradiol levels during the stress condition (Fig. 2 and Supp. Table 3). This finding indicates that women in the high estradiol phase of the menstrual cycle have a greater left hippocampal activity during psychosocial stress than women in the low estradiol phase
Figure 2.
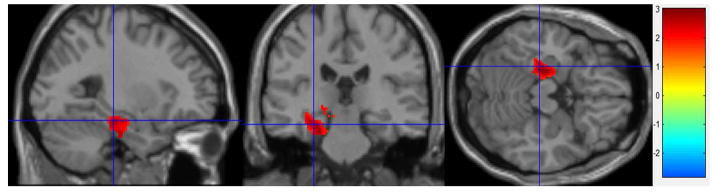
Estradiol Effects fMRI
Stress Condition – Control Condition: High estradiol (n = 10) – Low estradiol (n = 10). Greater activity in left hippocampus during the stress condition in the High estradiol group than the Low estradiol group (cluster-level corrected to p < 0.005).
Bilateral hippocampal activity during psychosocial stress was directly associated with estradiol levels in an analysis of the 20 participants included in the estradiol phase group analysis (uncorr.p = 0.005, cluster size = 58, corr.p < .001) with greater activity in the bilateral hippocampal ROI being associated with higher estradiol levels. Examining the individual right or left hippocampal ROIs showed that the correlation between estradiol level and percent signal change in either right or left hippocampus did not meet statistical significance (right hippocampus: r = −0.14, n = 28, p = 0.45; left hippocampus: r = −0.21, n =28, p = 0.37), indicating that the effect of estradiol was not lateralized.
In an analysis that included all participants grouped according to their assigned menstrual phase (without confirmation by estradiol level), there were no brain areas that showed greater activity in the periovulatory group compared to the early follicular phase group at either corrected p <0.01 or corrected p <0.05.
3.2.2 Subjective Distress Effects
To compute a group contrast for the effects of subjective distress on brain activation changes, we performed a median split of subjective distress scores; difference in pre vs. post MIST stress score on the Stress and Arousal Checklist (high distress n = 14, low distress n = 14). The subsequent fMRI analysis with the high and low distress groups showed an associated of distress and brain activity during the psychosocial stress task. Women with higher distress scores showed significantly less activity in bilateral hippocampus, midbrain, left parietal, and left frontal regions than women with higher distress scores (Fig 3 and Supp. Table 4). By contrast, greater subgenual cingulate activity was seen in women with higher distress scores compared to women with lower distress scores (Fig. 3). These differences indicate that women who respond to the MIST with high subjective distress have a greater change in brain activity during psychosocial stress than women with low subjective distress following the MIST.
Figure 3.
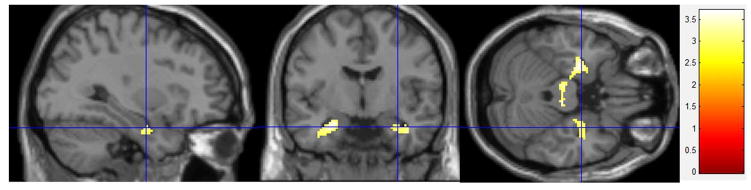
Subjective Distress Effects fMRI
Stress Condition – Control Condition: High Distress (n = 14) – Low Distress (n = 14). Greater activity in bilateral hippocampus and less subgenual cingulate activation during the stress condition in the Low Distress group than the High Distress group (cluster-level corrected to p < 0.001).
Bilateral hippocampal activity during psychosocial stress was inversely associated with distress scores in an analysis of all participants (p < 0.001) with lower activity in the hippocampal ROI being associated with greater distress. These correlations were statistically significant bilaterally (left hippocampus r = 0.51, n = 28, p = 0.006, right hippocampus r = 0.49, n = 28, p = 0.008). We re-analyzed the correlation after step-wise removal of percent signal change extreme values and association between left hippocampal percent signal change and distress remained significant (r = 0.39, n =28, p =0.04) but the right hippocampus did not (r = 0.31, n = 28, p = 0.10). A two way factorial ANOVA for E2 group and Distress group effects on hippocampal percent signal change did not show a significant interaction of estradiol phase group and distress group for right (p=0.592) or left (p=0.977) hippocampus ROIs.
There was no significant difference in brain activity between participants run at the two study locations in any of the contrasts of interest at any level of significance including uncorrected p < 0.05.
3.3 Behavioral and Mood Measures
3.3.1 Estradiol Effects
There was no effect of menstrual cycle phase or estradiol phase group on pre-MIST Stress and Arousal Checklist scores, Trait Anxiety Inventory scores, or State Anxiety scores, and no effect on any of the Profile of Mood scores, Arousal scores, or any of the scales of the Stress Task Visual Analogue Scale following the MIST (Supp. Table 5). Distress scores (difference in SACL Stress score before and after the MIST) were lower in women with high estradiol than in women with low estradiol levels (Table 1). A multiple regression was run to predict stress change from estradiol level, progesterone level, and age, F (3, 24) = 4.2, p < 0.05, R2 = .344. Only estradiol added statistically significantly to the prediction, p < 0.05.
Table 1.
Behavioral Measures at Study Day – Post MIST
| High E2 (n=10) | Low E2 (n=10) | E2Phase Groups | High Distress (n=14) | Low Distress (n=14) | Distress Groups | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | Mean | SD | Mean | SD | p | |
| Post SACL – Stress | 6.50 | 5.52 | 13.90 | 5.00 | 0.01 | 5.50 | 4.43 | 14.64 | 2.73 | <0.001 |
| Post SACL – Arousal | 8.20 | 3.01 | 8.80 | 2.70 | 0.64 | 6.86 | 3.44 | 9.00 | 2.25 | 0.60 |
| POMS Tension | 8.80 | 7.28 | 15.10 | 7.46 | 0.07 | 6.57 | 3.96 | 16.93 | 7.15 | <0.001 |
| POMS Depression | 2.40 | 2.22 | 6.90 | 7.37 | 0.08 | 2.21 | 1.76 | 9.86 | 12.29 | 0.03 |
| POMS Anger | 4.10 | 3.63 | 8.20 | 9.92 | 0.24 | 2.71 | 3.24 | 8.21 | 8.68 | 0.04 |
| POMS Fatigue | 5.90 | 5.04 | 5.30 | 3.50 | 0.76 | 5.36 | 4.73 | 6.07 | 4.95 | 0.70 |
| POMS Confusion | 6.90 | 2.69 | 9.10 | 5.30 | 0.26 | 6.00 | 3.59 | 10.29 | 5.77 | 0.03 |
| POMS Vigor | 11.70 | 5.66 | 10.90 | 7.00 | 0.78 | 10.43 | 6.20 | 10.29 | 6.27 | 0.95 |
| POMS Total-‘TMD’ | 16.30 | 14.04 | 33.20 | 29.67 | 0.12 | 12.00 | 13.97 | 41.07 | 32.95 | 0.01 |
| STVAS - Personally Involved | 83.30 | 17.29 | 81.40 | 22.76 | 0.84 | 77.21 | 22.99 | 87.93 | 10.47 | 0.12 |
| STVAS – Stressful | 86.20 | 8.99 | 87.80 | 11.32 | 0.73 | 83.43 | 9.81 | 90.29 | 8.70 | 0.06 |
| STVAS – New | 65.20 | 31.88 | 71.00 | 24.83 | 0.66 | 65.79 | 38.43 | 71.50 | 23.45 | 0.63 |
| STVAS – Uncontrollable | 69.50 | 10.32 | 73.90 | 16.11 | 0.48 | 66.29 | 14.77 | 72.86 | 24.65 | 0.40 |
| STVAS – Unpredictable | 68.20 | 28.75 | 73.40 | 13.97 | 0.61 | 67.50 | 23.12 | 72.07 | 17.75 | 0.56 |
| STVAS - Negative Consequences | 32.20 | 24.81 | 60.50 | 37.41 | 0.06 | 41.14 | 26.75 | 52.07 | 36.30 | 0.37 |
| Distress | 4.60 | 6.95 | 11.70 | 5.38 | 0.02 | 2.93 | 3.85 | 14.00 | 2.45 | <0.001 |
|
| ||||||||||
| E2 pg/mL | 103.52 | 34.09 | 24.91 | 9.17 | <0.001 | 74.49 | 40.93 | 43.47 | 35.26 | 0.04 |
E2 =Estradiol
STAI = State and Trait Anxiety Inventory
SACL = Stress and Arousal Checklist
POMS = Profile of Mood States
STVAS = Stress Task Visual Analogue Scale
Distress = Post SACL Stress – Pre SACL Stress
3.3.2 Distress Effects
There was no significant difference between groups on Stress and Arousal Checklist scores, Trait Anxiety Inventory scores, or State Anxiety scores before completing the MIST (Supp. Table 5). Women with higher distress scores had higher scores (worse mood) on all negative scales of the post-task Profile of Mood States (including tension, depression, anger, fatigue, confusion, and total mood disturbance) compared to women with lower distress scores, indicating worse mood following the MIST (Table 1). Additionally, women with higher distress scores had significantly lower estradiol levels (mean = 43.47 pg/mL, SD = 35.26) than women with lower distress scores (mean = 74.49 pg/mL, SD = 40.93) (t (26) = 2.15, p < 0.05), and there was an inverse correlation between estradiol and distress with lower estradiol levels associated with greater distress (r = −0.50, n = 29, p = < 0.01). There was no difference in progesterone levels between distress groups - low distress progesterone mean = 41.04 pg/mL, SD = 35.49, high distress progesterone mean = 58.96 pg/mL, SD = 36.84 − (t (26) = −1.31, p = 0.20) (Fig 4).
Figure 4.
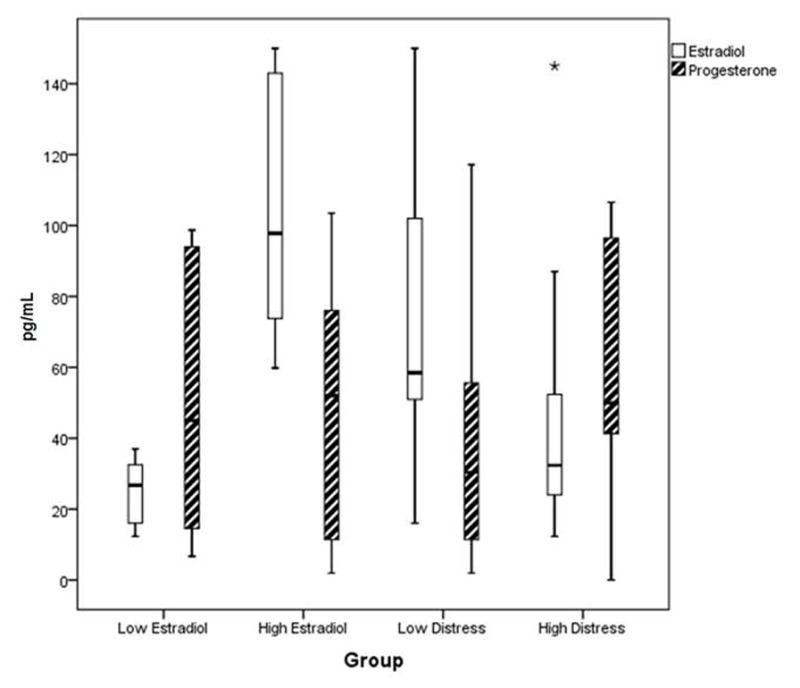
Estradiol and Progesterone Levels for Group Analyses
Low estradiol group (n =10) estradiol mean = 24.91 pg/mL, SD = 9.17, high estradiol group (n = 10) estradiol mean = 103.52 pg. mL, SD = 34.09 (t (18) = 7.04, p < 0.001). Low estradiol group progesterone mean = 48.90 pg/mL, SD = 36.10, high estradiol group progesterone mean = 50.54 pg/mL, SD = 36.91 (t (18) = 0.099, p = 0.92).
Low distress group (n =14) estradiol mean = 74.49 pg/mL, SD = 40.93, high distress group (n = 14) mean = 43.47 pg/mL, SD = 35.26, (t (26) = 2.15, p < 0.05). Low distress progesterone mean = 41.04 pg/mL, SD = 35.49, high distress progesterone mean = 58.96 pg/mL, SD = 36.84 − (t (26) = −1.31, p = 0.20).
There was no significant difference in any of the screening or study day subjective or behavioral measures between participants run at the two study locations at p < 0.05.
3.4 Cortisol
All subjects showed an increase in cortisol related to the MIST and cortisol AUC was associated with decreased medial prefrontal activity during psychosocial stress (Supp. Fig. 3); however there was no significant correlation between estradiol level or distress score group with cortisol response to the MIST. Cortisol AUC showed substantial inter-subject variability with an average AUC for the entire sample of 0.8 and a standard deviation of 2.8.
4. Discussion
Overall, women showed brain activity patterns during psychosocial stress that were similar to the patterns previously seen in studies that included both men and women; deactivation of limbic regions (Pruessner, 2008). However, this study showed that higher estradiol levels at periovulation were associated with greater hippocampal activity during psychosocial stress in normally cycling premenopausal women. Menstrual cycle phase and corresponding estradiol levels were directly correlated with hippocampal activity during the stress condition of the MIST; activity in the hippocampus during stress was significantly lower in women in the low estradiol levels compared to women with higher estradiol levels. These results suggest that low estradiol levels during the early follicular phase of the menstrual cycle exaggerate the effect of psychosocial stress on brain activity. Women with higher periovulatory estradiol levels also had lower distress scores following the psychosocial stress task. Group analysis based on distress following the MIST confirmed the relationship between estradiol and both brain and mood response to stress: women with higher distress had lower left hippocampal activity during the MIST, more negative mood following the MIST, and lower estradiol levels.
Previous work using the MIST has revealed deactivations during the stress condition of the task, in brain areas that are part of the limbic circuit (including hippocampus, hypothalamus, medio-orbitofrontal cortex and anterior cingulate cortex) (Pruessner, 2008). These studies have proposed that reduced limbic circuit function is associated, perhaps causally, with the stress response. Additionally, hippocampal deactivation during the stress condition of the MIST has been directly related to cortisol release and inversely correlated with measures of self-esteem, indicating that hippocampal activity is a marker of both the peripheral endocrine response to stress and related to psychological vulnerability to psychosocial stress (Pruessner, 2008). These findings are consistent with previous studies showing that the hippocampus is an important inhibitor of the HPA system (Jacobson and Sapolsky, 1991). Hippocampal function is also important in processing emotionally valenced information (Canli et al., 2002), especially in women, and in evaluating the context of social interactions (Berthoz et al., 2002). Our findings suggest that higher circulating estradiol may support continued hippocampal activity during psychosocial stress.
Although we did not see a difference in cortisol response between estradiol phase or distress groups, our finding that cortisol response to the MIST was associated with prefrontal deactivation agree with animal models showing worse performance on tasks that rely on prefrontal cortex following acute stress(Shansky et al., 2004). This study did not show an effect of estradiol on prefrontal activity, unlike animal models which show that high estrogen levels during proestrous in rodents enhance the negative effects of stress on prefrontal function. It may that we were precluded from seeing an interaction of estradiol and stress on prefrontal activity by the sample size included in this study, and future studies should examine this hypothesis through prefrontal activity and performance measures in women.
The brain regions that are commonly indicated in studies of major depression and in recent neuroimaging studies of psychosocial stress are important targets for investigating vulnerability to mood disorder and the interaction with stressful life events. That these same regions are responsive to estradiol manipulation (Andreano and Cahill, 2010; Goldstein, 2005) is interesting in light of the mood effects of estradiol in both healthy women (Gonda et al., 2008) and mood disorders related to ovarian hormone fluctuations. Estradiol has been shown to modulate activity on brain circuits important for emotional processing; estradiol level changes across the menstrual cycle are associated with changes in brain activity related to arousal for negative valenced images (Goldstein, 2005), and in fear conditioning (Zeidan et al., 2011). These findings provide evidence that estradiol may affect emotional responding through increased top-down modulation of emotional circuitry, including brain areas involved in the stress response, and may be protective against fear and anxiety. Our findings suggest that greater estradiol levels during the periovulatory phase of the menstrual cycle decrease the brain activity change in response to psychosocial stress, and reduce the acute behavioral and mood consequences of the stress experience. This interpretation is further supported by the location of estradiol receptors in the central nervous system; the hippocampus is rich in both estradiol and glucocorticoid receptors, making it an important area of interaction between these hormones. In animal models, estrogen appears to support neuroplasticity at the hippocampus (Gould et al., 1990; Woolley et al., 1990) and to be protective against the negative effects of stress (Bredemann and McMahon, 2014). High estradiol levels during the periovulatory phase of the menstrual cycle may support hippocampal function in part through these protective mechanisms.
Estradiol receptors are located in a number of brain areas, including regions important for the autonomic, hormonal, and cognitive-emotional response to psychosocial stress (Love et al., 2010). The relation of stress to depression onset (Frank et al., 1994; Kendler et al., 1999; Kendler et al., 2000) and the altered function of the stress system in major depression (Burke et al., 2005; Lupien et al., 2009) suggest that modulation of the psychosocial stress response may be a mechanism through which estradiol fluctuation may contribute to MDD and PTSD risk. The results of this study suggest that estradiol levels may modulate activity in brain areas important for processing emotional information during psychosocial stress. Increased emotional processing and physiological response to psychosocial stress, during low estrogen menstrual phases, may contribute to depressed mood in women with vulnerability to MDD. Indeed, women with MDD have greater HPA axis dysregulation than men with MDD (Young et al., 1994; Young and Ribeiro, 2006), suggesting that the stress system may be particularly important to depression in women. Estradiol may attenuate sympathetic and HPA axis activity to stress (Kajantie and Phillips, 2006; Roca et al., 2005). Although our study did not include women with mood disorders, the effect of estradiol on brain activity and mood response to psychosocial stress suggest that periods of low estradiol are associated with heightened negative emotional responding. These phases may thus present windows of increased vulnerability to the depressogenic effects of psychosocial stress in women. Further work is needed to determine whether the effects of estradiol on stress responding differ in women with vulnerability to mood and anxiety disorders, and if there is a relation between the occurrence of stressful events during different phases of the menstrual cycle and subsequent MDD or PTSD onset in vulnerable women.
We recognize that menstrual cycle effects are likely not the only, or even the main, determinant of psychosocial stress responding in women; future studies are needed to examine the effects of personality factors, and lifetime trauma or chronic stress. Limitations also include that the separate roles and mechanisms of estradiol and progesterone in emotional processing and response remain to be delineated. Although we did not directly investigate the effect of differing progesterone levels on stress responding, we attempted to isolate the effects of estradiol by having women experience the psychosocial stress task during the early follicular phase or the periovulatory phases when progesterone levels are low. We excluded women whose estradiol levels were not in concordance with their assigned menstrual phase from our menstrual cycle phase/estradiol fMRI analysis, as these women were likely in the mid luteal phase, when both estradiol and progesterone levels are increasing. The periovulatory phase is a very short duration of time (2–4 days) and it is possible that some women in our study were studied after ovulation. Indeed, brain activity during the psychosocial stress task did not show a difference between groups based only on assigned menstrual phase, suggesting that the women who were omitted from the estradiol level - based analysis may not have been in the correct phase or that estradiol level rather than phase has a stronger effect on neural activity in response to psychosocial stress. Although we believe this approach was appropriate to investigate the effects of estradiol level, it resulted in a reduced sample size for the menstrual cycle phase/estradiol level analysis. As progesterone levels did not appear to have an effect on distress in this study, we included all participants in the analyses by subjective distress. This study was not designed to investigate the separate effects of estradiol and progesterone by menstrual phase, however it did reveal a linear inverse relationship between estradiol levels and subjective distress that did not exist for progesterone. Additionally, this study did not include women who experience mood disruption directly related to menstrual phase or ovarian hormones (such as Premenstrual Dysphoric Disorder or Polycystic Ovarian Syndrome), nor did this study model these disorders. The effect of ovarian hormone level/menstrual phase on brain activity during emotional processes in women with these disorders likely differs from healthy women, and the effects on stress responding in these populations should be examined separately. Another potential limitation of this study is the difference in block length between stress and control conditions of the MIST. The stress conditions of the task needed to be of sufficient length to allow the stress response to develop, however the limitations in task time (and efficient block length) did not allow for the control conditions to be of similar length. We believe the control condition was of sufficient length and had enough repetitions to use as a comparison to isolate brain activity related to the stress response.
This study is unique in that that we used a moderate psychosocial stressor in the MRI environment at different menstrual phases, which allowed us to examine both the subjective mood response and brain activity response to the stress task. Also our stress task included social evaluative threat- a type of stress that may be particularly relevant for mood in women (Kendler et al., 2001). Our data suggest that estradiol buffers the brain activity changes and negative mood response to psychosocial stress in normally cycling women. This work has important implication for an understanding of the relationship between estradiol levels and the response to stressful life events. Whether this has implications for the development of psychopathology remains to be studied. Future work should extend the investigation of ovarian hormone effects on psychosocial stress responding to women with vulnerability mood or anxiety disorders and further examine the relation of these effects to known risk factors for mood disorders.
Supplementary Material
Highlights.
We examined brain activity and subjective mood to psychosocial stress
We compared women at high and low phases of the menstrual cycle
Hippocampal activity was greater during the stressor in the high estradiol phase
Higher estradiol was associated with less distress following the stress task
Acknowledgments
Role of funding sources
This work is supported by NIAR01AG021476, GCRC MO1RR00109, Vanderbilt CTSA grant UL1 TR000445 from NCRR/NIH.
We would like to thank Dr. Terry Rabinowitz and Dr. Warren Taylor for their help in administering the MIST and suggestions in preparing the manuscript. Thanks are also due to Violet Gau for research assistance, and the research nursing staff at the University of Vermont and Vanderbilt University CRCs for their support of this study. We also thank our participant volunteers for their dedication to clinical research.
Footnotes
Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. Neuroimage. 2010;53:1286–1293. doi: 10.1016/j.neuroimage.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baca-Garcia E, Diaz-Sastre C, Ceverino A, Garcia Resa E, Oquendo MA, Saiz-Ruiz J, de Leon J. Premenstrual symptoms and luteal suicide attempts. Eur Arch Psychiatry Clin Neurosci. 2004;254:326–329. doi: 10.1007/s00406-004-0506-1. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Armony JL, Blair RJ, Dolan RJ. An fMRI study of intentional and unintentional (embarrassing) violations of social norms. Brain. 2002;125:1696–1708. doi: 10.1093/brain/awf190. [DOI] [PubMed] [Google Scholar]
- Bredemann TM, McMahon LL. 17beta Estradiol increases resilience and improves hippocampal synaptic function in helpless ovariectomized rats. Psychoneuroendocrinology. 2014;42:77–88. doi: 10.1016/j.psyneuen.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromet E, Andrade LH, Hwang I, Sampson NA, Alonso J, de Girolamo G, de Graaf R, Demyttenaere K, Hu C, Iwata N, Karam AN, Kaur J, Kostyuchenko S, Lepine JP, Levinson D, Matschinger H, Mora ME, Browne MO, Posada-Villa J, Viana MC, Williams DR, Kessler RC. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9:90. doi: 10.1186/1741-7015-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke HM, Fernald LC, Gertler PJ, Adler NE. Depressive symptoms are associated with blunted cortisol stress responses in very low-income women. Psychosom Med. 2005;67:211–216. doi: 10.1097/01.psy.0000156939.89050.28. [DOI] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JD. Sex differences in the neural basis of emotional memories. Proc Natl Acad Sci U S A. 2002;99:10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterton RT, Mateo ET, Hou N, Rademaker AW, Acharya S, Jordan VC, Morrow M. Characteristics of salivary profiles of oestradiol and progesterone in premenopausal women. Journal of Endocrinology. 2005;186:77–84. doi: 10.1677/joe.1.06025. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. Overview: innovations in assessment using the revised NEO personality inventory. Assessment. 2000;7:325–327. doi: 10.1177/107319110000700402. [DOI] [PubMed] [Google Scholar]
- Davydov DM, Shapiro D, Goldstein IB, Chicz-DeMet A. Moods in everyday situations: effects of menstrual cycle, work, and stress hormones. J Psychosom Res. 2005;58:343–349. doi: 10.1016/j.jpsychores.2004.11.003. [DOI] [PubMed] [Google Scholar]
- First MB, RLS, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition, SCID-I/P, 2/2001. American Psychiatric Press Inc; Washington, D.C: 2001. [Google Scholar]
- Frank E, Anderson B, Reynolds CF, 3rd, Ritenour A, Kupfer DJ. Life events and the research diagnostic criteria endogenous subtype. A confirmation of the distinction using the Bedford College methods. Arch Gen Psychiatry. 1994;51:519–524. doi: 10.1001/archpsyc.1994.03950070011005. [DOI] [PubMed] [Google Scholar]
- Goldstein MJ, Poldrack Russell, Ahern Todd, Kennedy David N, Seidman Larry J, Nikos Makris. Hormonal Cycle Modulates Arousal Circuitry in Women Using Functional Magnetic Resonance Imaging. The Journal of Neuroscience. 2005;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda X, Telek T, Juhasz G, Lazary J, Vargha A, Bagdy G. Patterns of mood changes throughout the reproductive cycle in healthy women without premenstrual dysphoric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1782–1788. doi: 10.1016/j.pnpbp.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Mermelstein R, Roesch L. Sex differences in adolescent depression: stress exposure and reactivity models. Child Dev. 2007;78:279–295. doi: 10.1111/j.1467-8624.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Kajantie EPD. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Gardner CO. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the “kindling” hypothesis. Am J Psychiatry. 2000;157:1243–1251. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Prescott CA. Gender differences in the rates of exposure to stressful life events and sensitivity to their depressogenic effects. Am J Psychiatry. 2001;158:587–593. doi: 10.1176/appi.ajp.158.4.587. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Nelson CB, Hughes M, Swartz M, Blazer DG. Sex and depression in the National Comorbidity Survey. II: Cohort effects. J Affect Disord. 1994;30:15–26. doi: 10.1016/0165-0327(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Ustun TB. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) Int J Methods Psychiatr Res. 2004;13:93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MG, Burrows GD, Stanley GV. Measurement of stress and arousal: validation of the stress/arousal adjective checklist. Br J Psychol. 1983;74 (Pt 4):473–479. doi: 10.1111/j.2044-8295.1983.tb01880.x. [DOI] [PubMed] [Google Scholar]
- Kirschbaum CKB, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 1992;54:648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Korszun EYaA. Sex, trauma, stress hormones and depression. Molecular Psychiatry. 2009;15:23–28. doi: 10.1038/mp.2009.94. [DOI] [PubMed] [Google Scholar]
- Liu RT, Alloy LB. Stress generation in depression: A systematic review of the empirical literature and recommendations for future study. Clin Psychol Rev. 2010;30:582–593. doi: 10.1016/j.cpr.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love T, Smith YR, Persad CC, Tkaczyk A, Zubieta JK. Short-term hormone treatment modulates emotion response circuitry in postmenopausal women. Fertil Steril. 2010;93:1929–1937. doi: 10.1016/j.fertnstert.2008.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McNair DMLM, Droppleman LF. Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT. Neuronal correlates of extinction learning are modulated by sex hormones. Soc Cogn Affect Neurosci. 2012;7:819–830. doi: 10.1093/scan/nsr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezulis A, Funasaki K, Charbonneau A, Hyde J. Gender Differences in the Cognitive Vulnerability-Stress Model of Depression in the Transition to Adolescence. Cognitive Therapy and Research. 2010;34:501–513. [Google Scholar]
- Moos RH. The development of a menstrual distress questionnaire. Psychosom Med. 1968;30:853–867. doi: 10.1097/00006842-196811000-00006. [DOI] [PubMed] [Google Scholar]
- Ostlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Ann N Y Acad Sci. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- Pruessner KD, Khalili-Mahani Najmeh, Engert Veronika, Pruessner Marita, Buss Claudia, Renwick Robert, Dagher Alain, Meaney Michael J, Lupien Sonia. Deactivation of the Limbic System During Acute Psychosocial Stress: Evidence from Positron Emission Tomography and Functional Magnetic Resonance Imaging Studies. Biological Psychology. 2008;63:234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Roca CA, Schmidt PJ, Deuster PA, Danaceau MA, Altemus M, Putnam K, Chrousos GP, Nieman LK, Rubinow DR. Sex-related differences in stimulated hypothalamic-pituitary-adrenal axis during induced gonadal suppression. J Clin Endocrinol Metab. 2005;90:4224–4231. doi: 10.1210/jc.2004-2525. [DOI] [PubMed] [Google Scholar]
- Saunders KE, Hawton K. Suicidal behaviour and the menstrual cycle. Psychol Med. 2006;36:901–912. doi: 10.1017/S0033291706007392. [DOI] [PubMed] [Google Scholar]
- Shansky RM. Estrogen, stress and the brain: progress toward unraveling gender discrepancies in major depressive disorder. Expert Rev Neurother. 2009;9:967–973. doi: 10.1586/ern.09.46. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Glavis-Bloom C, Lerman D, McRae P, Benson C, Miller K, Cosand L, Horvath TL, Arnsten AF. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol Psychiatry. 2004;9:531–538. doi: 10.1038/sj.mp.4001435. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Leuner B. Estrogen-mediated effects on depression and memory formation in females. J Affect Disord. 2003;74:85–96. doi: 10.1016/s0165-0327(02)00428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Vedder LC, Nelson AR, Bredemann TM, McMahon LL. Duration of estrogen deprivation, not chronological age, prevents estrogen’s ability to enhance hippocampal synaptic physiology. Proc Natl Acad Sci U S A. 2010;107:19543–19548. doi: 10.1073/pnas.1009307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anxiety Inventory, The Corsini Encyclopedia of Psychology. John Wiley & Sons, Inc; 2010. [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biol Psychiatry. 2002;52:318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Vamvakopoulos NV. Sexual dimorphism of stress response and immune/inflammatory reaction: the corticotropin releasing hormone perspective. Mediators of Inflammation. 1995;4:163–174. doi: 10.1155/S0962935195000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; 1999. [Google Scholar]
- Weiss EL, Longhurst JG, Mazure CM. Childhood sexual abuse as a risk factor for depression in women: psychosocial and neurobiological correlates. Am J Psychiatry. 1999;156:816–828. doi: 10.1176/ajp.156.6.816. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Haskett RF, Grunhaus L, Pande A, Weinberg VM, Watson SJ, Akil H. Increased evening activation of the hypothalamic-pituitary-adrenal axis in depressed patients. Arch Gen Psychiatry. 1994;51:701–707. doi: 10.1001/archpsyc.1994.03950090033005. [DOI] [PubMed] [Google Scholar]
- Young EA, Ribeiro SC. Sex differences in the ACTH response to 24H metyrapone in depression. Brain Res. 2006;1126:148–155. doi: 10.1016/j.brainres.2006.05.053. [DOI] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, Milad MR. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry. 2011;70:920–927. doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


