Abstract
The original formulation of Gate Control Theory (GCT) proposed that the perception of pain produced by spinal cord signaling to the brain depends on a balance of activity generated in large (non-nociceptive) and small (nociceptive) diameter primary afferent fibers. GCT proposed that activation of the large diameter afferent “closes” the gate by engaging a superficial dorsal horn interneuron that inhibits projection neuron firing. Activation of the nociceptors “opens” the gate through concomitant excitation of projection neurons and inhibition of inhibitory interneurons. Sixty years after publication of the GCT, we are faced with an ever-growing list of morphologically and neurochemically distinct spinal cord interneurons. This review highlights the complexity of superficial dorsal horn circuitry and addresses whether the premises outlined in GCT still have relevance today. By examining the dorsal horn circuits underlying the transmission of “pain” and “itch” messages, we also address the extent to which labeled lines can be incorporated into a contemporary view of GCT.
INTRODUCTION
In a recent review (Basbaum et al., 2009), we discussed the organization of primary afferents and highlighted several mechanisms through which tissue and nerve injury can produce a prolonged state of hypersensitivity. In the setting of injury, innocuous stimuli can now produce pain, a condition referred to as allodynia, and normally painful stimuli produce more intense pain (hyperalgesia). That review discussed several mechanisms of peripheral and central sensitization that contributes to chronic pain. Although the review indicated that many of the changes that occur in the spinal cord result from alterations in the function of dorsal horn interneurons, there was little discussion of the incredible heterogeneity of the interneurons. The present review has the interneurons as its focus, hopefully building upon several excellent reviews that appeared in the last few years (Ribeiro-da-Silva and De Koninck, 2009; Todd, 2010) and others that detailed the incredible biochemical complexity of dorsal horn organization (Gangadharan and Kuner, 2013).
Gate Control Theory revisited
It has been almost 60 years since Melzack and Wall published their Gate Control Theory (GCT) of pain (Melzack and Wall, 1965) which proposed spinal cord circuits through which pain is generated and controlled (Figure 1). GCT postulated that the extent to which a particular stimulus or ongoing injury produced pain was not merely a function of the magnitude of activity generated in a population of “pain” specific primary afferents and the brain's ability to read that input. Rather, GCT proposed that the brain monitors the activity generated by a complex, gated circuit that is located in the superficial dorsal horn of the spinal cord, a circuit that can be regulated by activity carried by nociceptive as well as nonnociceptive afferents (i.e. afferents that respond normally to innocuous stimuli). The heart of the gate of GCT consisted of a relatively simple circuit, with its literal and metaphorical key corresponding to an inhibitory interneuron in the substantia gelatinosa (SG), a region that corresponds to lamina II of the superficial dorsal horn. The gate of GCT closed when activity in large diameter (non-nociceptive) afferents “turned on” an inhibitory SG interneuron, which in turn inhibited the spinal cord projection neurons that transmit the injury message to the brain. Furthermore, and what is often misunderstood or at least not recognized, is that GCT proposed that painful stimulation not only activates the projection neuron, but also turns the “key” in the opposite direction, i.e. noxious stimulation carried by the unmyelinated nociceptors open the gate, by inhibiting the SG interneuron. As all primary afferents are excitatory, the inhibitory action of these nociceptive afferents was presumed to involve complex interneuronal circuits in the dorsal horn, the final product of which was inhibition of the SG interneuron, increased transmission of the “pain” message to the brain and thus increased pain.
Figure 1.
The original formulation of Gate Control Theory proposed that spinal cord signaling to the brain (the “Action System”) depended on the balance of activity of Large (L) and small (S) diameter primary afferent fibers. The large fibers not only can excite output/projection cells (transmission; T), but the large fibers also exert a feedforward inhibition of the T cells by concurrently activating inhibitory interneurons in the substantia gelatinosa (SG). The inhibition was presumed to involve presynaptic connections to the T cell, however, postsynaptic inhibition of the T cell by SG interneurons was not ruled out. The net effect of the large diameter afferents is to “close” the Gate. Critical to GCT, however, was the activity of small diameter (presumptive nociceptors), which not only directly excite the T cells and thus the Action System, but also engage inhibitory circuits that reduce the activity of the SG inhibitory interneurons. This presumably multisynaptic inhibitory mechanism “opens” the Gate. Finally, the GCT schematic included an illustration of the engagement of supraspinal control systems (the Central Control Trigger) that can regulate the output of the spinal cord dorsal horn.
Because there was remarkably limited knowledge of interneuron and projection neuron heterogeneity 60 years ago, the permutations that could be envisioned as to dorsal horn circuitry were also limited. Golgi studies demonstrated morphological varieties of interneurons (Gobel, 1975, 1978; Ramon y Cajal, 1909), but their function was unclear. Inhibitory GABAergic and glycinergic interneurons were appreciated and it was assumed that excitatory interneurons were glutamatergic, but there was almost no information on the circuits engaged by these interneurons, or on their heterogeneity.
Almost 60 years after the publication of GCT, we are faced with an ever-growing list of morphologically and neurochemically distinct interneurons and projection neurons, but we are only beginning to assign function to these populations. In the present review we hope to provide more than an encyclopedic version of interneuron and projection neurons’ morphological and neurochemical heterogeneity. We do introduce the major neuronal populations, but then highlight information that addresses critical questions about the circuits that are relevant to the generation of pain and itch, both in the normal state and after injury, where chronic pain and itch occur. We have tried not to reiterate what was emphasized in our recent review. Although that review pointed out that many of the injury-associated changes that occur in the spinal cord dorsal horn result from alteration in the function of interneurons, notably changes in GABAergic inhibitory controls, we did not attempt to relate function with the heterogeneity of the interneuron and projection neurons. Relating form and neurochemistry to circuitry and function is the objective of the present review.
Anatomy
Primary Afferents: nociceptive and non-nociceptive inputs to the dorsal horn
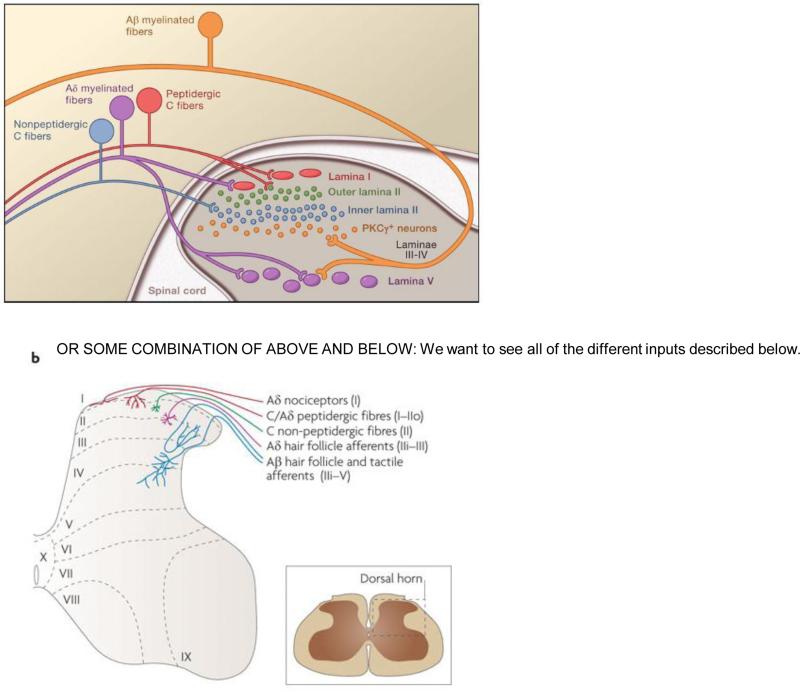
Primary afferent neurons of the dorsal root and trigeminal ganglia transmit sensory information from skin, muscle, joints and viscera to the spinal cord and its trigeminal homologue in the brainstem, respectively and information from these regions is transmitted to the brain where a pain or itch perception is generated (Figure 2). The largest diameter, myelinated (Aβ) primary afferents have a low mechanical threshold and transmit non-painful, tactile and proprioceptive information to the spinal cord. A subset of the smallest myelinated (Aδ) afferents also conveys innocuous mechanical inputs. Pain-provoking stimuli are carried by slowly conducting unmyelinated, C-fiber nociceptors and by a subset of myelinated Aδ fibers. Itch is also generated by a subset of unmyelinated afferents (the pruritoceptors). Finally, there are two populations of unmyelinated afferents that respond to low intensity (non-painful) mechanical stimuli and that have been associated, in humans as well as mice, with the generation of pleasant touch sensations (Olausson et al., 2002; Vrontou et al., 2013). The larger population of low threshold C-mechanoreceptors is characterized by its expression of tyrosine hydroxylase and the VGLUT3 subtype of vesicular glutamate transporter (Li et al., 2011; Seal et al., 2009); the second, much smaller population expresses the MrgB4 subtype of GPCR (Vrontou et al., 2013).
Figure 2.
The different populations of primary afferent fibers target different regions of the dorsal horn of the spinal cord, with the input from C and Aδ nociceptors concentrated in the superficial dorsal horn (laminae I and II). The peptidergic, TRPV1-expressing subset of nociceptors targets laminae I and outer II; the nonpeptidergic subset, many of which expresses Mrgpr GPCRs, targets inner lamina II. Aδ nociceptors target both laminae I and V. This figure also includes the PKCγ-expressing population of interneurons that have been implicated in nerve injury-evoked mechanical allodynia provoked by stimulation of large diameter (Aβ) afferents (See also Figure 5).
Although glutamate is the major excitatory neurotransmitter of all primary afferents, the C-nociceptors can also be subdivided into an NGF/TrkA- dependent, peptidergic population and a GDNF/cRet-dependent, non-peptidergic subset (Julius and Basbaum, 2001; Snider and McMahon, 1998). Many of the peptidergic neurons express substance P and CGRP as well as the capsaicin receptor, TRPV1, which confers heat pain sensitivity upon them. Other TRPV1-expressing sensory neurons appear to define a distinct population of pruritoceptors (Han et al., 2013; Imamachi et al., 2009; Liu et al., 2009). Although there is evidence for expression of gastrin-releasing peptide (GRP) by pruritoceptors (Sun and Chen, 2007), recent studies indicate that GRP may instead be concentrated in a subset of itch-relevant dorsal horn interneurons (Mishra and Hoon, 2013). The non-peptide population of C-fibers is defined anatomically by its binding of the lectin IB4 and its expression of the diverse, Mrg family of GPCRs (Dong et al., 2001).
The peptidergic and nonpeptidergic subsets of afferents are also distinguished by their spinal cord targets. The peptidergic afferents project to the most superficial laminae of the dorsal horn (I and outer II); the non-peptidergic afferents target inner lamina II. This differential targeting suggests that there are functional distinctions in the contribution of the peptidergic and non-peptidergic sensory neurons. Indeed, based on behavioral analyses after selective ablation of the peptidergic (TRPV1-expressing) or nonpeptidergic (MrgprD-expressing) nociceptors and on the different central circuits that these populations engage (Braz et al., 2005), we and others (Cavanaugh et al., 2009; Mishra et al., 2011) concluded that there is significant functional specificity in the contribution of these afferents. Specifically, the ability to transmit painful heat information requires the TRPV1 population. In contrast, a component of mechanical, but not heat, pain sensitivity is transmitted by the MrgprD subset of IB4-binding nociceptors.
In contrast to the nociceptors, there is much more limited neurochemical classification of the non-nociceptive afferents. However, recent studies have demonstrated considerable heterogeneity of the myelinated afferents (Abraira and Ginty, 2013; Li et al., 2011). It is also of particular interest that the central projections of the different populations of low threshold mechanoreceptive afferents (including the low threshold C mechanoreceptors), project to the spinal cord dorsal horn in a columnar manner, with different populations targeting specific laminae. This pattern provides a modular substrate for the regulation of dorsal horn interneuron responsiveness via interactions between painful C and Aδ with non-painful Aβ, Aδ and low threshold C fiber inputs. As described below, the interneurons of the dorsal horn provide the critical link between afferent input to these modules and to the projection neurons that carry information from the spinal cord to the brain.
Projection neurons: transmitting information to the brain
The extent to which a particular stimulus is perceived as painful depends on the brain's interpretation of the activity generated in the nociresponsive output cells of the spinal cord. Many projection neurons are found in the most superficial layer of the dorsal horn (lamina I) and here the majority of the neurons, at least in the uninjured animal, are nociceptive-specific, i.e. only respond to painful stimulation. A subset of these neurons also respond to the pruritic agents, histamine and cowhage (Davidson et al., 2012). By contrast, the deeper dorsal horn (lamina V) contains so-called wide dynamic range (WDR) projection neurons that respond to both noxious and innocuous stimulation. Other nociresponsive projection neurons are scattered throughout the ventral horn, but the fact that these neurons have very large and complex receptive fields taken together with their great sensitivity to general anesthetics, which significantly alters their properties, has made their analysis more difficult (Fields et al., 1977). Somewhat surprisingly given their importance, projection neurons make up a remarkably small proportion of the total neuron count in the spinal cord, e.g. only 5% of lamina I neurons project beyond the cord (Spike et al., 2003).
Although the thalamus is the predominant target of spinal cord neurons in primates, the majority (~80%) of lamina I projection neurons in rodents terminate in the parabrachial nucleus (PB) of the dorsolateral brainstem. The PB, in turn, provides rapid access to forebrain limbic structures, including the amygdala and hypothalamus, which are presumed to contribute to the emotional quality of the pain experience. Other spinal cord projection neurons target the midbrain periaqueductal gray (PAG), reticular formation, hypothalamus and thalamus (Bernard et al., 1995; Burstein et al., 1990; Todd et al., 2000) and many spinal cord projection neurons collateralize to multiple supraspinal sites (Al-Khater and Todd, 2009; Spike et al., 2003). As to neurochemistry, most PB-projecting neurons (~80%) express the neurokinin 1 receptor (NK1), which is targeted by substance P-expressing primary afferent nociceptors (SP; (Ding et al., 1995; Todd et al., 1998) as well as SP-expressing local interneurons. As these projection neurons express neither GABA nor glycine, they are presumed to be exclusively excitatory (Littlewood et al., 1995).
Lamina I projection neurons can also be distinguished on morphological grounds, being multipolar, fusiform or pyramidal (Cheunsuang and Morris, 2000; Han et al., 1998; Lima and Coimbra, 1989). The multipolar and fusiform neurons are largely NK1-receptor expressing (Almarestani et al., 2007), and are presumed to be nociceptive, whereas the pyramidal neurons, at least in the uninjured animal, do not express NK1 receptors and appear to be non-nociceptive (Polgar et al., 2008). The latter can be defined by their expression of other markers, notably, the sst2A subtype of somatostatin receptor, the calcium binding protein, calbindin, and/or GluR4-containing AMPA receptors. Given their non-nociceptive properties, these latter neurons may receive inputs from the low threshold C mechanoreceptors and/or from unmyelinated (likely TRPM8-expressing) afferents, which confers their responsiveness to innocuous cooling. Note however that peripheral inflammation induces de novo expression of the NK1 receptor in pyramidal neurons (Almarestani et al., 2009; Saeed and Ribeiro-da-Silva, 2013). Thus, it is likely that in the setting of tissue injury, non-nociceptive pyramidal neurons also respond to noxious stimuli.
Other projection neurons derive from NK1-expressing neurons in lamina III, and many of these have long dorsally-directed dendrites that penetrate the superficial dorsal horn (lamina I and II) where they are targeted by SP-containing nociceptors (Brown et al., 1995). In contrast to the predominant nociceptive specific properties of the lamina I projection neurons, the lamina III neurons (as well others in lamina V) respond to both innocuous and noxious inputs. The innocuous input derives from large diameter non-nociceptive Aβ afferents that terminate in lamina III-V (Naim et al., 1997).
Interneurons
One of the more confusing features of the GCT model is that the critical, presumptive GABAergic inhibitory interneurons of the substantia gelatinosa, which “closed the Gate”, appeared to be directly (i.e. monosynaptically) excited and inhibited by large diameter A fibers and unmyelinated C fibers, respectively. As all primary afferent fibers are excitatory, the model was clearly representing the integrative result of the activity of complex, but at that time completely unknown, interneuronal circuits in the superficial dorsal horn. Recent studies, however, have dramatically altered our understanding not only of the heterogeneity of the interneurons and their critical contribution to inhibitory control of “pain” transmission, but also to their essential contribution to the transmission of pain messages by projection neurons to the brain. In fact, a polysynaptic circuit through which an unmyelinated C fiber can inhibit overall GABAergic control of the dorsal horn output has been described (See “Dorsal horn circuitry”).
Morphological heterogeneity of the interneurons
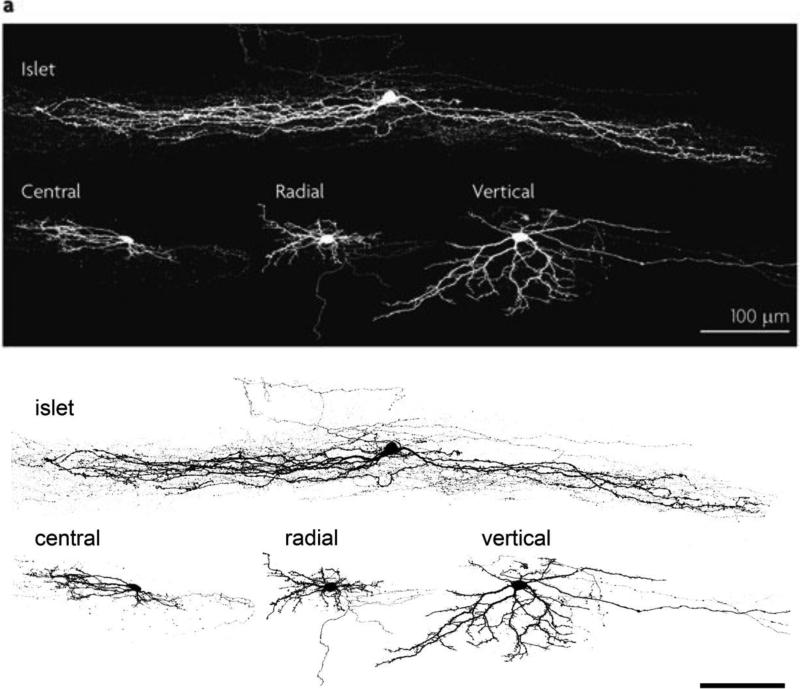
Patch clamp studies have greatly extended the early Golgi studies of superficial dorsal horn interneuron classes. Grudt and Perl (2002) described four morphologically distinguishable interneurons: islet, central, radial and vertical cells (Figure 3; (Grudt and Perl, 2002). Islet and central cells have dendritic arbors that arborize in the rostrocaudal plane (more extensively in the case of the islet cell), and that are largely limited to the lamina in which the cell body resides. The vertical cell, which likely corresponds to the stalked cell described in earlier Golgi preparations (Gobel, 1975, 1978), has its cell body in outer lamina II, near the I/II border. The ventrally-directed dendritic arbor of the vertical cell allows this neuron to integrate inputs from a column of functionally distinct (non-nociceptive and nociceptive) afferents and local interneurons (see next section). As the great majority of islet cells are GABAergic, with a subset of these coexpressing glycine, this class of interneuron is presumed to be exclusively inhibitory (Proudlock et al., 1993; Todd and McKenzie, 1989). In contrast, radial and vertical cells are predominantly glutamatergic and thus presumed to be excitatory. Central cells include both inhibitory and excitatory subsets (Maxwell et al., 2007; Yasaka et al., 2010).
Figure 3.
Four morphologically distinct subsets of interneurons predominate in lamina II of the superficial dorsal horn. Islet cells have the longest dendritic arborization, extending long axons and dendrites in the rostrocaudal plane, largely within the lamina where their cell bodies reside. Central cells also have rostrocaudally directed intralaminar arborizations, but the extent of the arborization is less than that of the islet cells. Vertical cells have complex and ramified dendritic arbors that extend largely in the dorsoventral plane, crossing multiple laminae, where they can receive convergent inputs from different populations of primary afferents. Many of the vertical cell axons arborize dorsally in lamina I. Radial cells have a restricted dendritic arbor and a variably-directed axon. Finally, whereas islets cells are exclusively inhibitory, vertical and radial cells are predominantly glutamatergic and thus excitatory. Both inhibitory and excitatory central cells have been described. (Image provided by Dr. Andrew Todd).
Neurochemistry: Differentiation of excitatory and inhibitory interneurons
The excitatory interneurons are heterogeneous and develop under the control of distinct transcription factor (Xu et al., 2008; Xu et al., 2013). The expression of the VGLUT2 vesicular glutamate transporter in many of these interneurons confirms their excitatory profile (Oliveira et al., 2003; Santos et al., 2009). However, in addition to glutamate, a host of neurochemical markers has defined distinct subpopulations of excitatory interneurons, including PKCγ (Polgar et al., 1999), the mu opioid receptor (MOR; (Eckert et al., 2003; Kemp et al., 1996; Spike et al., 2002), neurotensin (Todd et al., 1992), somatostatin (Todd et al., 2003), and neurokinin B (Polgar et al., 2006). Of particular functional interest to the development of selective pharmacological tools to regulate the firing of these interneurons is their differential expression of voltage-gated potassium channels (Marker et al., 2006) and subtypes of glutamate receptors (Albuquerque et al., 1999; Kyrozis et al., 1996). Finally, as noted above, recent studies defined a subset of presumptive excitatory interneurons that express GRP (Mishra and Hoon, 2013), a peptide implicated in the transmission of pruritoceptive (itch) messages from the periphery, and another population that expresses GRPR (Sun and Chen, 2007), which is targeted by GRP.
In some respects, the inhibitory interneurons of the superficial dorsal horn are not as heterogeneous. As noted above, the islet cell is the predominant inhibitory interneuron in the dorsal horn. All inhibitory interneurons use GABA as their neurotransmitter and many of these co-express glycine. On the other hand, although GABA and glycine are stored and can be released from the same synaptic vesicle, it is the differential expression of GABA and glycine receptors that determines which inhibitory neurotransmitter predominates. The inhibitory interneurons can also be divided on other neurochemical grounds. Thus, double label studies using antibodies directed against glutamic acid decarboxylase (GAD67), one of the enzymes required for the synthesis of GABA, identified subpopulations of GABAergic neurons that express neuropeptide Y (NPY), galanin (GAL), parvalbumin (PV) or neuronal nitric oxide synthase (nNOS). These populations do not overlap and account for ~65% of inhibitory interneurons in lamina I and ~50% in lamina II (Sardella et al., 2011). More recently, subsets of inhibitory interneurons have been defined by the different transcription factors that regulate their development (Ross et al., 2010; Wildner et al., 2013).
Of interest, and possibly relevant to the question of modality specific spinal cord circuits, is the fact that these distinct populations of inhibitory interneurons differ, at least in the rat, in their responses to painful stimulation. For example, Polgar et al recently reported that mechanical, thermal and chemical noxious inputs activate a large number of GAL and NPY-expressing neurons (monitored by phosphorylation of extracellular signal-regulated kinase (ERK), but very few nNOS-expressing inhibitory interneurons (Polgar et al., 2013). Furthermore, there is evidence that the sst2A somatostatin receptor is expressed by virtually all GAL-positive and nNOS-positive neurons, but few NPY-expressing and none of the PV-positive neurons. Given that somatostatin is pronociceptive, and indeed directly activates the lamina I projection neurons (Gamboa-Esteves et al., 2004), it follows that concurrent engagement of inhibitory circuits by somatostatin must somehow lead to disinhibition of the output cells. Importantly, none of the noxious stimuli tested induced activity-dependent neuronal markers in PV neurons, which is in agreement with the suggestion that these cells primarily receive innocuous inputs from low threshold primary afferents (Hughes et al., 2012). Conceivably the latter circuit corresponds to the pathway through which large diameter afferents “close the Gate” of GCT (see also (Daniele and MacDermott, 2009).
Dorsal horn circuitry: from afferents to the projection neurons
The dorsal horn of the spinal cord is not merely a “relay station” where primary afferents engage the projection neurons. Rather it is the locus of incredible integration, where sensory information is subjected to local and supraspinally-derived excitatory and inhibitory regulation. Understanding how these controls are exerted clearly requires a detailed circuit map of the connectivity between primary afferents and the projection neurons.
Not long after the discovery of the lamina I contribution to pain processing (Christensen and Perl, 1970), dual recording studies in primate dorsal horn provided evidence for the direction of information flow to the output cells. Price et al used concurrent recording of neighboring neurons in laminae I and outer II and concluded that information flowed from interneurons in lamina II to projection neurons in lamina I (Price et al., 1979). As Golgi studies as well as morphological characterization of intracellularly recorded neurons had identified a population of stalked cells, with a cell body at the I-II border and an axon that arborizes in lamina I (Bennett et al., 1980; Gobel, 1975; Gobel et al., 1980; Ross et al., 2010), it was proposed that nociceptive inputs activate stalked cells and that these in turn engage the projection cells. Contemporary electrophysiological studies also combined paired recording from morphologically-characterized neurons or used laser-scanning photostimulation and identified a modular organization of circuits consisting of specific combinations of interneurons (Kato et al., 2009; Kosugi et al., 2013; Lu and Perl, 2003, 2005; Wu et al., 2010; Yasaka et al., 2007).
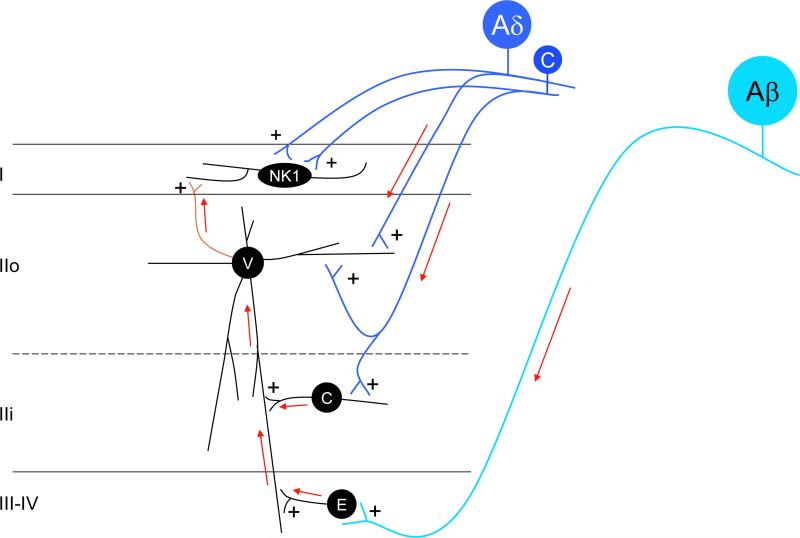
A summary of these observations (Figure 4) reveals that: central cells in inner lamina II receive an excitatory input from C nociceptors and in turn excite vertical cells of outer lamina II. The vertical cells, which receive monosynaptic primary afferent input from both nociceptive C and Aδ fibers, in turn establish excitatory connections with both interneurons and projection neurons of lamina I (including the NK1-expressing population). The latter, of course, also receive direct nociceptive C and Aδ input. Thus, noxious stimuli directly engage the projection cells and, through a dorsally-directed circuit, provide additional feedforward excitatory drive to the projection cells. Presumably loss of the excitatory interneurons that generate this “backup” excitatory drive to the projection cells underlies the dramatically reduced pain behavioral phenotype that we recently described in mice with a deletion of the gene that encodes the testicular orphan nuclear receptor (TR4; (Wang et al., 2013). Note that there are additional sources of excitatory drive generated within the interneuronal circuitry, including intralaminar excitatory connections to the central cells from radial and or other central cells (Kato et al., 2009). Finally non-nociceptive Aβ inputs that target deeper populations of excitatory interneurons (in laminae III and IV) can also engage the lamina I projection neurons, through the same ventral to dorsal circuits. Conceivably, this Aβ input contributes to the WDR properties of some lamina I neurons (the majority of which are nociceptive-specific) and to the mechanical allodynia that develops following tissue or nerve injury (Hylden et al., 1987; Hylden et al., 1989).
Figure 4.
Primary afferent-derived excitatory and inhibitory drive to the spinal cord dorsal horn.
Ventral to dorsal excitatory activation circuits: Nociceptive C- and Aδ- fibers (dark blue) directly activate vertical cells (V) of lamina IIo and these, in turn, excite NK1-expressing projection neurons of lamina I (green axon). In addition, nociceptive C-fibers activate central cells (C) of lamina IIi, and the central cells directly contact, and likely excite vertical cells in lamina IIo. Of course, projection neurons of lamina I also receive direct nociceptive C and Aδ input. Finally, non-nociceptive Aβ fibers (light blue) project to deeper laminae (III-IV) where they establish monosynaptic connections with local excitatory interneurons (E). The latter directly activate vertical cells. These dorsally-directed circuits are the route through which both noxious and innocuous primary afferent input can engage the projection neurons of lamina I. As described in Figure 5, in the setting of nerve injury, additional routes of Aβ engagement of the projection neurons are uncovered.
Feedforward inhibitory control of superficial dorsal horn circuits: These excitatory circuits are subject to profound inhibitory controls (red neurons). The majority of the inhibitory neurons (I) in lamina II are islet cells (I-i) and these can be directly engaged by input from low-threshold, mechanoreceptive C-fibers (light blue). The islet cells in turn establish monosynaptic inhibitory connections with both vertical cells of lamina IIo and NK1 receptor-expressing projection neurons of lamina I. Low threshold Aδ (D-hair; light blue) and Aβ fibers also directly engage inhibitory interneurons in laminae IIi-IV. The latter in turn exert inhibitory control of a variety of excitatory interneurons, including vertical (V) and central (C) cells. The Aβ to inhibitory cell circuit presumably underlies the circuit through which the “Gate” of Gate Control Theory can be closed. Figure 5 illustrates the neurochemical identity of critical elements in this circuit.
Interestingly, the predominantly ventral to dorsal excitatory drive to the lamina I projection cells is paralleled by a dorsal to ventral excitatory drive to projection neurons in deeper laminae, notably the WDR neurons of lamina V. Our own studies have shown that lamina II neurons that receive inputs from a subpopulation of Nav1.8-expressing, nonpeptidergic afferents, relay information to lamina V neurons (Braz et al., 2005). This view of information flow is, in fact, consonant with the early studies of Wall (Wall, 1967) who proposed that the receptive field properties of lamina V WDR neurons results from convergence of input from more dorsally located neurons. There is also evidence that activation of lamina V neurons is indirectly influenced via lamina I-derived spinal-brainstem-spinal connections (Choi et al., 2012; Kim et al., 2009).
Figure 4 further illustrates that the “pain” transmission neurons of lamina I are also subject to profound feedforward inhibitory controls (red neurons). For example, the lamina II central, and probably also the radial cells, receive an intralaminar-derived inhibitory input from islet cells. The inhibitory input to lamina I neurons, in contrast, derives predominantly from islet cell inhibition of vertical cells. There may be some direct islet cell inhibition on dendrites of projection neurons, however, as the great majority of projection neurons have dendrites restricted to lamina I, most of the islet cell inhibition is presumed to be indirect. It is also likely that some of the descending monoaminergic inhibitory control of spinal cord pain transmission neurons is mediated via connections with these inhibitory islet cells (Lu and Perl, 2007; Yasaka et al., 2010). And as postulated by GCT, Aβ afferents, via inhibitory interneurons in lamina IIi through IV, can exert profound and direct inhibitory control of lamina V pain transmission neurons, as well as an indirect inhibition through effects on vertical cells.
What is particularly difficult to unravel is the consequence to the islet cell of activity of the nociceptive, TRPV1 expressing C- and Aδ primary afferent fibers (Zheng et al., 2010). For example, while a low threshold C fiber mechanoreceptive input to the islet cell could contribute to the inhibitory effects of innocuous mechanical stimulation (Choi et al., 2012; Grudt and Perl, 2002; Yasaka et al., 2007), the nociceptive C fiber input to the islet is, at first glance, paradoxical. Specifically, existence of this connection implies that noxious stimuli engage inhibitory circuits that can reduce pain. That painful stimuli can reduce pain is not a new idea (Le Bars et al., 1992) and, in fact, Nakatsuka et al (2005) reported that primary afferent-derived substance P can activate GABAergic inhibitory circuitry in the dorsal horn. It is more likely, however, that because islet cells can inhibit other inhibitory interneurons (Kato et al., 2007; Zheng et al., 2010), disinhibitory circuitry comes into play in the ultimate transmission of the “pain” message to the projection neurons. Of course, this disinhibitory circuit that is triggered by noxious stimulation is precisely one that was promulgated in the GCT, i.e. it is the circuit through which C-fiber stimulation “opens” the Gate.
Labeled lines: the controversy continues
Gate Control Theory challenged a prevailing view, epitomized by the now famous Descartes illustration of a “pain” pathway, namely that there are labeled lines that transmit physiologically distinct information from the periphery to the brain. Recent functional and anatomical studies, however, indicate that at least at the level of the primary afferent fiber, there are labeled lines for specific somatosensory modalities (noxious heat, cold, noxious mechanical stimulation and pruritogens). As noted above, intrathecal capsaicin-induced ablation of TRPV1-expressing afferents produces heat-specific behavioral deficits in mice without affecting mechanical thresholds, whereas genetic ablation of the MrgprD subset of nonpeptidergic afferents results in a selective decrease of mechanical pain sensitivity, without altering responses to noxious heat (Cavanaugh et al., 2009). Given the polymodal properties of the great majority of unmyelinated C-fibers (Bessou and Perl, 1969; Jankowski et al., 2012; Tominaga et al., 1998), this dichotomy was unexpected. A subsequent in vivo study extended these findings by showing that the TRPV1 and MrgprD subsets of afferents only contribute to the noxious heat and mechanical responsiveness, respectively, of polymodal dorsal horn neurons (Zhang et al., 2013).
There is also evidence for a differential expression of “analgesic” receptors on these different afferent populations. Specifically, the mu opioid receptor (MOR), which binds morphine, is concentrated in peptidergic neurons that also express substance P. In contrast, the delta opioid receptor (DOR) is expressed in non-peptidergic and myelinated afferents (Scherrer et al., 2009). Although this opioid receptor distinction countered the longstanding view that the DOR is concentrated in the peptidergic population (Guan et al., 2005; Riedl et al., 2009), our studies indicate that those conclusions were based on antibodies that were not selective for the DOR. More importantly, perhaps, and consistent with the differential distribution of MOR and DOR in primary afferents, our studies revealed a selective control of heat and mechanical pain responsiveness after intrathecal injection of MOR and DOR agonists, respectively (Scherrer et al., 2009).
With the exception of interneurons associated with the generation of itch, however, few interneuron populations have been linked to a single pain modality (e.g. painful heat or mechanical stimulation). In fact, the great majority of dorsal horn interneurons are also polymodal, making it difficult to determine the particular perceptual modality evoked by their activation. On the other hand, when the afferent-spinal cord circuitry is examined from the perspective of neurochemically-defined subsets of afferents, then distinct and functionally specific neural circuits, possibly representing labeled lines, can be demonstrated. For example using a spinal cord slice preparation, Zheng and colleagues described differential projections of TRPV1- and TRPM8-expressing primary afferents to distinct populations of inhibitory interneurons in the superficial dorsal horn (Zheng et al., 2010). Specifically, TRPV1 (i.e. heat-responsive) and TRPM8 (cold-responsive) unmyelinated afferents converge upon an inhibitory interneuron that is distinct from the traditional islet cell interneuron, which only receives TRPV1 input. Interestingly, as these two populations of inhibitory interneurons are mutually inhibitory, the authors suggested that this differential engagement of inhibitory circuitry by modality specific afferents could underlie effects of cool stimuli on heat pain processing and vice-versa (McCoy et al., 2013).
Other studies also documented selective targeting of subpopulations of interneurons by neurochemically distinct afferents. For example, Zylka and colleagues reported that the MrgprD subset of afferents activates all subtypes of excitatory interneurons, but may not directly engage the islet cell inhibitory population (Wang and Zylka, 2009). Similarly, TRPA1-expressing primary afferents, which are a subset of the nociceptive TRPV1 population and are implicated in the responsiveness to a wide variety of irritants, including mustard oil and the pruritogen, chloroquine (Wilson et al., 2011), selectively innervate vertical and radial cells (Uta et al., 2010), but not islet or central cells, raising the possibility that these morphologically distinct excitatory interneurons are part of a circuit that is dedicated to a specific pain and/or itch modality.
Specific functional associations of neurochemically distinct dorsal horn neurons/glia
NK1 receptor-expressing projection neurons: where specificity breaks down?
As noted above, up to eighty percent of the lamina I projection neurons express the NK1 receptor and these neurons unquestionably contribute to the transmission of pain messages to the brain. For example, noxious thermal stimuli induce expression of Fos in neurons that express the NK1 receptor and release of SP in the dorsal horn following peripheral noxious stimulation induces internalization of the NK1 receptor in lamina I neurons (Mantyh et al., 1995; Trafton et al., 1999). On the other hand, because NK1 receptor antagonists reduce, but do not eliminate pain in animals, and have proven disappointing in clinical studies (Borsook et al., 2012), it is certain that other inputs to the lamina I neurons are essential, including that from glutamatergic nociceptors and excitatory interneurons in lamina II (Wang et al., 2013). Most importantly, selective ablation of neurons that express the NK1 receptor using substance P-saporin based toxins reduces thermal hyperalgesia and mechanical allodynia produced by capsaicin, inflammation and nerve injury (Mantyh et al., 1997; Nichols et al., 1999; Wiley et al., 2007). Interestingly, baseline pain responses (e.g. to capsaicin) are preserved in these animals, which indicates that there are other projection neurons that contribute to acute pain sensitivity, including non-NK1 receptor-expressing neurons of lamina I and deep dorsal horn neurons. Importantly, those studies used reflex responsiveness to monitor residual “pain” processing after ablation of the NK1 receptor expressing neurons. In other words, it is possible that the reflex occurred, but that the rat, in fact, did not “experience” the noxious stimulus as painful.
An important corollary of the conclusion drawn from the SP-saporin studies is that the same population of output cells appears to transmit the chronic pain message generated in the setting of both tissue (inflammatory) and nerve injury, as well as the itch that is evoked by pruritogens (Carstens et al., 2010). It is possible that there are functionally distinct subsets of NK1 receptor-expressing lamina I neurons, or that a differential code generated in the same population of neurons underlies distinct pain and itch percepts generated under tissue and nerve injury conditions.
PKCγ interneurons: a contemporary Gate Control circuit?
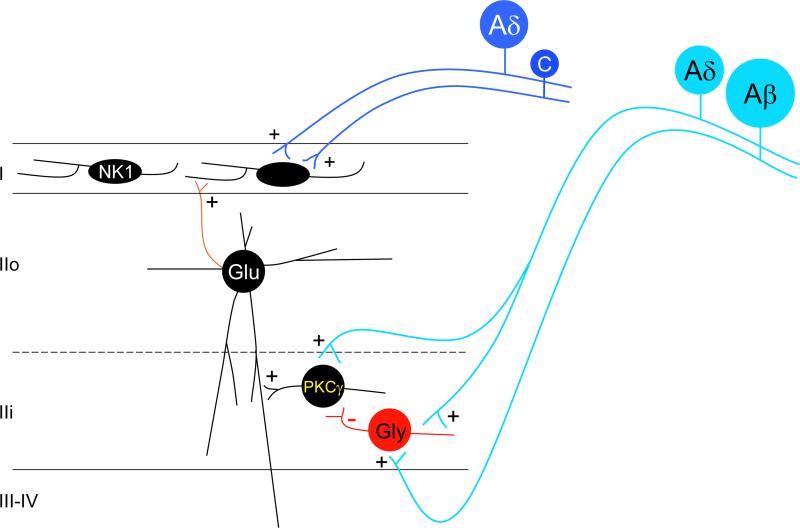
As excitatory interneurons are under strong inhibitory control (Yasaka et al., 2007) any disruption of these controls will enhance the response to noxious stimuli, leading to hyperalgesia, and can result in nociceptive circuits being engaged by innocuous stimuli. This is the condition referred to as allodynia (Coderre, 2009). In our previous review we discussed several general mechanisms through which central sensitization occurs in the setting of injury. Here we highlight several specific examples in which distinct subpopulations of dorsal horn interneurons (or glia) have been implicated. For example, Figure 5 schematizes the results from Miraucourt and colleagues who reported that intracisternal (CSF) injection of the glycine receptor antagonist, strychnine, induced a mechanical allodynia of the face of the rat, as well as light brush-induced Fos expression in interneurons and lamina I projection neurons of the trigeminal nucleus caudalis (Miraucourt et al., 2007). The latter region is the homologue of the spinal cord dorsal horn. Among the interneurons were many PKCγ-expressing cells of inner lamina II, presumptive excitatory interneurons that receive inputs from low threshold mechanoreceptors (Neumann et al., 2008) and that we previously implicated in nerve injury-induced mechanical hypersensitivity (Malmberg et al., 1997). Importantly, injection of a PKCγ antagonist not only reduced the strychnine-induced mechanical allodynia, but also the brush-induced Fos. The authors concluded that there is a tonic glycinergic inhibition of the PKCγ interneurons and when that inhibition is lifted, low threshold mechanical input carried by Aβ afferents can gain access to the pain transmission circuitry of the superficial dorsal horn. Whether the release of the PKCγ-regulated excitatory drive is exerted directly on the projection neurons could not be determined.
Figure 5.
Disruption of glycinergic inhibitory controls contributes to the development of nerve injury-induced pain hypersensitivity: Low threshold Aβ and possibly Aδ (light blue) inputs directly activate PKCγ-expressing interneurons of lamina II and these interneurons in turn activate projection neurons of lamina I, likely via polysynaptic pathways involving glutamatergic (Glu) excitatory vertical cells of lamina IIo. Under normal conditions, this excitatory drive is tonically inhibited by glycinergic interneurons, and the inhibition can be enhanced by activity of low threshold afferents. In conditions where this tonic glycinergic inhibitory control is disrupted (e.g. after peripheral nerve injury), primary afferent-derived innocuous inputs can gain access to the pain transmission circuitry of the superficial dorsal horn, leading to mechanical allodynia and thermal hyperalgesia.
Differential contribution of GABAergic and glycinergic inhibitory controls
Administration of GABA and glycine elicits powerful antinociception under most circumstances (Dirig and Yaksh, 1995; Zeilhofer et al., 2012) and conversely, pharmacological blockade of both glycinergic and GABAergic neurotransmission induces many behavioral signs of hypersensitivity and pain (Sherman and Loomis, 1995; Sivilotti and Woolf, 1994; Yaksh, 1999). Other studies indicate that even though the great majority of inhibitory glycinergic neurons in the superficial dorsal horn co-release GABA, injury-induced biochemical changes in the spinal cord can selectively influence the postsynaptic consequences of glycine co-release. Of particular interest is the report that inflammation-induced spinal cord release of prostaglandins facilitates pain transmission through a blockade of glycinergic inhibitory controls (Ahmadi et al., 2002). Specifically, release of prostaglandin E2, perhaps from primary afferent-stimulated astrocytes, acts on PGE2 receptors and this, in turn, can counteract the inhibitory action of glycine via its binding to the GlyR3 subtype of strychnine sensitive glycine receptors (Figure 6).
Figure 6.
Prostaglandins induce hypersensitivity in the setting of inflammation by regulating glycinergic inhibitory circuits: Peripheral inflammation evokes cyclooxygenase (Cox)-dependent release into the superficial dorsal horn of prostaglandin E2 (PGE2), likely from astrocytes and/or microglia. The PGE2 binds to neuronal EP2 receptors expressed by many interneurons, including those under inhibitory glycinergic control. Through a cAMP-dependent phosphorylation of the GlyR3 subunit of glycine receptors, the PGE2 reduces the effects of glycine on what are presumed to be excitatory interneurons that ultimately engage neurons in lamina I. The net result is that innocuous stimuli can provoke pain (allodynia) and noxious stimuli exacerbate pain (hyperalgesia) in the setting of tissue injury. In contrast to the effects of PGE2 on glycinergic signaling, there is no influence on GABAergic signaling, even though GABA and glycine are co-released from many superficial dorsal horn inhibitory interneurons.
The lack of effect of PGE2 on GABAergic functioning in the setting of tissue injury and inflammation contrasts sharply with the profound reduction of GABAergic inhibitory controls that occurs in the setting of peripheral nerve injury. It remains to be determined, however, whether the reduction of spinal GABAergic tone is a consequence of decreased GABA release (Lever et al., 2003), downregulation of GABA, GAD or pre- and postsynaptic GABA receptors (Castro-Lopes et al., 1993; Eaton et al., 1998; Fukuoka et al., 1998; Ibuki et al., 1997; Polgar et al., 2004) or even a loss of GABAergic interneurons (Moore et al., 2002; Scholz et al., 2005; Sugimoto et al., 1990), although the latter is more controversial (Polgar et al., 2003). Regardless of the mechanism, the latter changes are presumed to underlie the mechanical allodynia and thermal hyperalgesia characteristic of the neuropathic pain condition. It follows that therapeutic approaches targeting those changes have the potential to be disease modifying. Indeed, our own studies demonstrate that transplant mediated recapitulation of the spinal cord GABAergic circuitry that is altered by a peripheral nerve injury completely reverses the injury-induced mechanical hypersensitivity characteristic of the animal model of neuropathic pain (Braz et al., 2012).
Microglial-neuronal interactions
In addition to the hyperexcitability arising from nerve injury-induced disruption of islet cell inhibitory control (see Figure 7), De Koninck and colleagues provided evidence that microglia are critical intermediaries to the consequences of nerve injury (Coull et al., 2005). Specifically, the authors proposed that ATP release from injured primary afferents activates dorsal horn microglia, leading to the synthesis and release of brain-derived neurotrophic factor (BDNF) from the activated microglia. BDNF, via an action on TrkB receptors expressed by lamina I neurons, downregulates expression of the potassium-chloride exporter KCC2 (Figure 8). This event, in turn leads to a shift in the chloride gradient of lamina I neurons (Coull et al., 2003). As a result, GABAergic inhibitory controls are greatly reduced, and may even become excitatory. More recently, Smith and colleagues provided evidence that the mechanisms underlying the pronociceptive/central sensitization contribution of nerve injury-induced BDNF release from microglia are far more complex (Lu et al., 2009). Specifically, these authors reported that prior nerve injury or bath application of BDNF not only decreased synaptic excitation of inhibitory tonic islet/central neurons recorded from spinal cord slices, but also increased the activity of presumptive excitatory radial interneurons.
Figure 7.
Microglial-neuronal interactions disrupt GABAergic inhibitory control: Peripheral nerve injury activates spinal cord microglia, which in turn release numerous molecules that enhance the excitability of spinal corn neurons. Some studies indicate that chemokine ligand 2 (CCL2), which is upregulated in and released from injured primary sensory neurons, binds to the microglial chemokine receptor 2 (CCR2) to induce microglial activation. Nerve-injury-induced release of ATP, via an action on microglial P2X4 receptors, also activates the microglia. The latter release brain-derived neurotrophic factor (BDNF), which via an action on neuronal TrkB receptors, downregulates the neuronal potassium-chloride co-transporter KCC2. The net result is a reduced chloride gradient that alters the magnitude of any GABAergic inhibitory input to the neuron. In this setting there may be ongoing pain, mechanical allodynia and thermal hyperalgesia.
Figure 8.
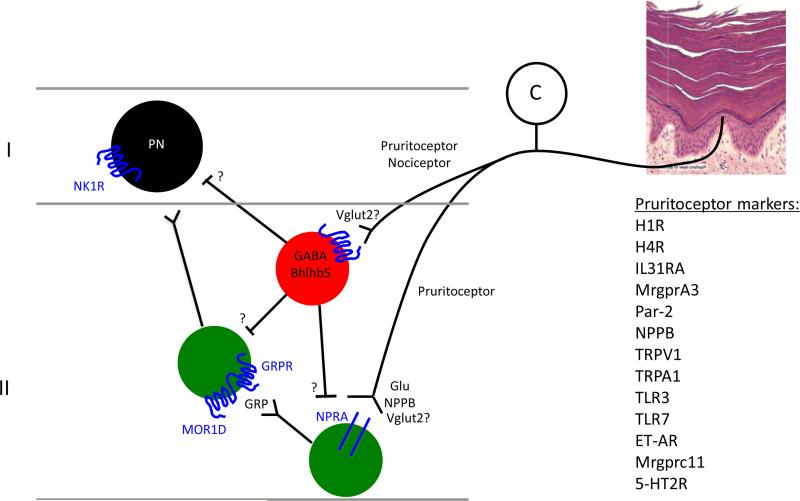
A labeled line for the transmission of itch messages: Several subpopulations of primary afferent fiber (pruritoceptors) transmit messages provoked by a host of itch-producing stimuli that engage the afferent directly or indirectly. To what extent itch and pain-producing afferents (nociceptors) overlap is unclear. Activation of the pruritoceptors evokes the release of natriuretic polypeptide B (NPPB) in the superficial dorsal horn. The receptor for NPPB, natriuretic peptide receptor A (NPRA) is expressed by presumptive excitatory interneurons of the superficial dorsal horn, and these cells in turn release gastrin-releasing peptide (GRP) and engage another interneuron population, namely one that expresses GRPR, the receptor for GRP. Whether GRPR-expressing cells project directly to the brain or signal locally to other projection neurons in the spinal cord is unclear, but our recent findings (Wang et al., 2013) suggest that the GRPR cells are interneurons. Another view holds that GRP, in fact, derives predominantly from pruritoceptors (Zhao et al., 2013), not from dorsal horn interneurons. Whether the “pain-responsive” NK1 receptor-expressing projection neurons also transmit itch messages to the brain (e.g. via inputs from GRPR cells) is also unclear. Finally, this figure illustrates that a subset of Bhlhb5-dependent inhibitory interneurons regulates the output of superficial dorsal horn itch circuits, as do primary afferent neurons that express the VGLUT2 subtype of vesicular glutamate transporter (Lagerstrom et al., 2010; Liu et al., 2010; Scherrer et al., 2010). Loss of either Bhlhb5 or of primary afferent-derived VGLUT2 generates mice with an exaggerated itch phenotype, but limited effects on pain processing.
The BDNF studies focused on the influence of the microglia on inhibitory controls. There is also considerable evidence that microglia can be activated by primary afferent-derived cytokines and that the microglia, in turn, trigger release of pronociceptive molecules. For example, neutralization of CCL2, a chemokine released by primary afferents following nerve injury prevents microglial activation and inhibits neuropathic pain (Thacker et al., 2009). Mice deficient for CCR2, the receptor for CCL2, also display impaired responses in the formalin test and do not develop mechanical allodynia in a neuropathic pain model (Abbadie et al., 2003). Dorsal horn astrocytes are yet another source of nerve injury-induced CCL2, which underscores the diversity of the mechanisms through which neuropathic pain can be generated. Finally, a very recent report indicated that nerve injury can also trigger a cyclooxygenase-1 derived synthesis of prostaglandins in microglia (Kanda et al., 2013). Missing from the great majority of these studies, however, is a specific characterization of the interneurons influenced by these nerve injury-induced biochemical changes in microglia. Clearly, as Smith and colleagues have shown, the ultimate consequence of these changes depends on the particular circuits in which the target neurons are engaged.
Pain vs itch: More questions about labeled lines
As described above, we recently reported that despite the existence of polymodal nociceptors, selective ablation studies indicate that the consequences of activity of subsets of these afferents appear selective for a particular modality. Thus, the TRPV1 population is required for noxious heat, the MrgprD subset of nonpeptidergic C-fibers for mechanical pain, and TRPM8 expressing afferents for cold (Knowlton et al., 2013). More recently, Dong and colleagues reported that a subset of MrgprD afferents also transmits the pruritic messages generated by β-alanine (Liu et al., 2012) and that a distinct subset of TRPV1-expressing nociceptors, namely those that coexpress the MrgprA3 GPCR, is also an essential driver of itch, but not pain (Han et al., 2013). One presumes that the remaining, much larger population of TRPV1 nociceptors is the pain-relevant afferent population, although discrete subsets of the remaining TRPV1 population may carry pruritogen-selective information (Imamachi et al., 2009). Although Dong used Fos expression to monitor the central consequences of selective activation of the MrgprA3 line, it could not be concluded that the neurons engaged by that afferent line are not activated by noxious, pain-producing stimuli.
On the other hand, and as noted above, it is not at all clear whether the functionality of the peripheral labeled line is retained at the level of spinal circuitry. Evidence in favor of labeled line at the spinal cord level comes from studies demonstrating a unique population of lamina I neurons that respond to innocuous cooling, but not to painful thermal or mechanical stimulation (Han et al., 1998). Other studies comparing circuits that process itch and pain also argue strongly that separate interneuronal circuits are engaged by pain and itch-producing stimuli. For example, Chen and colleagues demonstrated that ablation of gastrin-releasing peptide receptor (GRPR)-expressing neurons in the superficial dorsal horn abolishes scratching (indicative of itch) in response to a wide variety of pruritogens. Importantly, the authors found no effect of GRPR neuron deletion on pain behaviors. This finding suggested that there are separate pain and itch circuits engaged by pain and itch producing afferents. Chen and colleagues argue strongly that the afferent input to the GRPR neurons derives from unmyelinated GRP-expressing peptidergic afferents that coexpress substance P (Sun et al., 2009; Zhao et al., 2013), however, other studies suggest that natriuretic polypeptide B (NPPB) is the critical neuropeptide transmitter of primary afferent pruritoceptors and that GRP is, in fact, expressed by yet another distinct population of superficial dorsal horn excitatory interneurons (Figure 8; (Mishra and Hoon, 2013). The latter result is consistent with our finding that loss of GRP mRNA-expressing interneurons in the superficial dorsal horn of the TR4 mutant mouse is associated with a complete loss of pruritogen-induced itch (Wang et al., 2013).
Taken together with the apparently strict association of the GRP and GRPR population with the generation of itch, these studies suggest that there exists an excitatory, labeled line for itch. Furthermore, Ross et al observed spontaneous itch and exacerbated responses to the most common pruritogens in mice with a deletion of the transcription factor, Bhlhb5 (Ross et al., 2010). The behavioral phenotype was associated with an almost 25% loss of GABAergic interneurons in the superficial dorsal horn. Importantly, these mice did not display exaggerated pain responsiveness, suggesting that a different population of inhibitory neurons regulates the pain circuits. On the other hand, as many superficial dorsal horn neurons respond to both itch and pain-producing stimuli, it is possible that activity of the GRPR neurons is necessary for evoking behavioral manifestations of itch, but dispensable for inducing pain behaviors. Redundant pain transmission circuits may compensate for loss of the GRPR interneuron contribution.
The question remains whether there are subsets of projection neurons (e.g. in laminae I or V) that only transmit itch messages. Although there is some limited evidence for differential central targeting of projection neurons that respond to different pruritogens (Davidson et al., 2012), based on the very polymodal (pain and itch responsiveness) properties of projection neurons, and indeed of almost all interneurons, it seems more likely that there is convergence of pain and itch messages to the NK1 receptor-expressing projection neurons. Indeed substance P-saporin induced deletion of the NK1 receptor expressing neurons in the rat dorsal horn significantly reduces behaviors indicative of both pain and itch (Carstens et al., 2010). Furthermore, based on our studies in the TR4 mutant mice, in which the great majority of GRPR as well as GRP dorsal horn neurons were lost, with no change in the number of projection neurons, we concluded that the GRPR interneurons must communicate with the projection neuron in order to send itch signals to the brain. Apparently, polymodal interneurons that respond to pain-producing algogens and itch-producing pruritogens converge upon projection neurons that are also polymodal. Unless there are subsets of polymodal projection neurons that engage pain vs itch-relevant supraspinal targets, the perceptual distinction between pain and itch must result from a different firing code generated in the projection neurons in response to algogens and pruritogens. In this model, some critical neuronal population in the brain presumably “reads’ the patterns of activity generated by different dorsal horn circuits, resulting in a perception of pain or itch.
Conclusions
So, how relevant is the Gate Control Hypothesis today? In 1965, it was assumed that there were only two subtypes of substantia gelatinosa inhibitory interneuron (one that is GABAergic and one that is glycinergic). As this inhibitory interneuron was the “key” that opened or closed the Gate, controversy arose as to whether circuits existed through which the Gate could be respectively opened or closed in response to small or large diameter primary afferent inputs. Contemporary studies, of course, have revealed an incredible heterogeneity to the excitatory and inhibitory interneuron populations in the dorsal horn. In our opinion, there is no question that the general concept of a dorsal horn key that can open (i.e. facilitate) or close (i.e. inhibit) information flow to the brain is a valid one. Indeed, there are probably multiple circuits, and possibly multiple gates, through which these controls can be generated.
On the other hand, despite tremendous progress in our understanding of the heterogeneity of the functional circuits that populate the dorsal horn, much remains to be learned. With respect to the question of specificity vs patterning of nociceptive (pain) and pruritoceptive (itch) information, it is clearly critical to determine the extent to which there is convergence or segregation of the information processed by the different interneuron pools described above. It is also critical to determine whether selective activation of these different projection neuron populations by different modalities of pain and itch stimuli engages different brain regions. Such questions could be addressed through a combination of, for example, optogenetic or DREADD (Designer receptors exclusively activated by designer drugs) technology (Armbruster et al., 2007), and forebrain imaging in the same animals. Of course, there is also very limited information about the contribution to pain and itch processing of the other spinal cord projection systems, including the lateral spinal nucleus (Burstein et al., 1990; Menetrey and Basbaum, 1987) and the neurons of the deeper dorsal horn. The latter region includes the wide dynamic range neurons of lamina V (Price et al., 2003), which unquestionably transmit nociceptive and probably pruritoceptive information to higher centers.
Future studies will undoubtedly reveal additional neurochemical and functional features of the major subclasses of dorsal horn interneuron, which will demonstrate even greater heterogeneity. Indeed, as noted above, although there are several subtypes of GABAergic islet cell interneuron, the functional significance of these subtypes (e.g. NPY, NOS, expressing) is unclear. A recent analysis, for example, indicates that a subset of dynorphin-expressing GABAergic interneurons is critical to the regulation of itch and, in fact, is the population lost in the Bhlhb5 mutant mouse that has exaggerated itch (Ross and Todd: paper presented at the VIIth World Congress on Itch, 2013). To what extent there is comparable heterogeneity of the excitatory interneuron population is also unclear. As the vertical cells appear critical to providing feedforward excitatory drive to the projection neurons, it will be of great interest to address their diversity. Again, even though these interneurons appear to be polymodal, as are almost all projection neurons, these interneurons could still engage different output systems depending on the firing patterns that they generate in response to the different stimuli. Because our previous reviews focused extensively on the mechanisms that operate in the setting of injury and that underlie pathological conditions of mechanical hypersensitivity and ongoing pain, in the present review we have focused primarily on the circuit organization of the normal spinal cord. Of course, there is considerable evidence that these different interneuronal populations are profoundly influenced by the changes in afferent drive that occur when there is persistent inflammation or frank nerve damage. Some cells may die, others may make new connections by sprouting of axons, and without question, there are profound molecular changes that alter the responsiveness of these interneuron populations to both noxious and innocuous inputs. Finally, we have barely touched on the diversity of the cell surface receptors that populate the neurons in the dorsal horn, including traditional excitatory and inhibitory neurotransmitter receptors, the subtypes of which can be overwhelming. We have also ignored the contribution of neurotrophin receptors, cytokine and chemokine receptors and the incredibly diverse downstream signaling pathways that can be triggered in these different interneuron populations. A better understanding of the diversity of these biochemical pathways and of the circuits in which they participate will undoubtedly direct the development of novel pharmacotherapies for the treatment of pain and itch, treatments that are hopefully devoid of the common adverse side effects of the current therapies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci (USA) 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraira VE, Ginty DD. The sensory neurons of touch. Neuron. 2013;79:618–639. doi: 10.1016/j.neuron.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi S, Lippross S, Neuhuber WL, Zeilhofer HU. PGE(2) selectively blocks inhibitory glycinergic neurotransmission onto rat superficial dorsal horn neurons. Nat Neurosci. 2002;5:34–40. doi: 10.1038/nn778. [DOI] [PubMed] [Google Scholar]
- Al-Khater KM, Todd AJ. Collateral projections of neurons in laminae I, III, and IV of rat spinal cord to thalamus, periaqueductal gray matter, and lateral parabrachial area. J Comp Neurol. 2009;515:629–646. doi: 10.1002/cne.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque C, Lee CJ, Jackson AC, MacDermott AB. Subpopulations of GABAergic and non-GABAergic rat dorsal horn neurons express Ca2+-permeable AMPA receptors. Eur J Neurosci. 1999;11:2758–2766. doi: 10.1046/j.1460-9568.1999.00691.x. [DOI] [PubMed] [Google Scholar]
- Almarestani L, Waters SM, Krause JE, Bennett GJ, Ribeiro-da-Silva A. Morphological characterization of spinal cord dorsal horn lamina I neurons projecting to the parabrachial nucleus in the rat. J Comp Neurol. 2007;504:287–297. doi: 10.1002/cne.21410. [DOI] [PubMed] [Google Scholar]
- Almarestani L, Waters SM, Krause JE, Bennett GJ, Ribeiro-da-Silva A. De novo expression of the neurokinin 1 receptor in spinal lamina I pyramidal neurons in polyarthritis. J Comp Neurol. 2009;514:284–295. doi: 10.1002/cne.22024. [DOI] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GJ, Abdelmoumene M, Hayashi H, Dubner R. Physiology and morphology of substantia gelatinosa neurons intracellularly stained with horseradish peroxidase. J Comp Neurol. 1980;194:809–827. doi: 10.1002/cne.901940407. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Dallel R, Raboisson P, Villanueva L, Le Bars D. Organization of the efferent projections from the spinal cervical enlargement to the parabrachial area and periaqueductal gray: a PHA-L study in the rat. J Comp Neurol. 1995;353:480–505. doi: 10.1002/cne.903530403. [DOI] [PubMed] [Google Scholar]
- Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophys. 1969;32:1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- Borsook D, Upadhyay J, Klimas M, Schwarz AJ, Coimbra A, Baumgartner R, George E, Potter WZ, Large T, Bleakman D, et al. Decision-making using fMRI in clinical drug development: revisiting NK-1 receptor antagonists for pain. Drug Discov Today. 2012;17:964–973. doi: 10.1016/j.drudis.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Braz JM, Sharif-Naeini R, Vogt D, Kriegstein A, Alvarez-Buylla A, Rubenstein JL, Basbaum AI. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron. 2012;74:663–675. doi: 10.1016/j.neuron.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JL, Liu H, Maggio JE, Vigna SR, Mantyh PW, Basbaum AI. Morphological characterization of substance P receptor-immunoreactive neurons in the rat spinal cord and trigeminal nucleus caudalis. J Comp Neurol. 1995;356:327–344. doi: 10.1002/cne.903560302. [DOI] [PubMed] [Google Scholar]
- Burstein R, Cliffer KD, Giesler GJ., Jr. Cells of origin of the spinohypothalamic tract in the rat. J Comp Neurol. 1990;291:329–344. doi: 10.1002/cne.902910302. [DOI] [PubMed] [Google Scholar]
- Carstens EE, Carstens MI, Simons CT, Jinks SL. Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. Neuroreport. 2010;21:303–308. doi: 10.1097/WNR.0b013e328337310a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Lopes JM, Tavares I, Coimbra A. GABA decreases in the spinal cord dorsal horn after peripheral neurectomy. Brain Res. 1993;620:287–291. doi: 10.1016/0006-8993(93)90167-l. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheunsuang O, Morris R. Spinal lamina I neurons that express neurokinin 1 receptors: morphological analysis. Neuroscience. 2000;97:335–345. doi: 10.1016/s0306-4522(00)00035-x. [DOI] [PubMed] [Google Scholar]
- Choi JI, Koehrn FJ, Sorkin LS. Carrageenan induced phosphorylation of Akt is dependent on neurokinin-1 expressing neurons in the superficial dorsal horn. Mol Pain. 2012;8:4. doi: 10.1186/1744-8069-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen BN, Perl ER. Spinal neurons specifically excited by noxious or thermal stimuli: marginal zone of the dorsal horn. J Neurophysiol. 1970;33:293–307. doi: 10.1152/jn.1970.33.2.293. [DOI] [PubMed] [Google Scholar]
- Coderre TJ. Spinal cord mechanisms of hyperalgesia and allodynia. In: Basbaum AI, Bushnell MC, editors. Science of Pain. Elsevier; Amsterdam: 2009. pp. 339–380. [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- Daniele CA, MacDermott AB. Low-threshold primary afferent drive onto GABAergic interneurons in the superficial dorsal horn of the mouse. J Neurosci. 2009;29:686–695. doi: 10.1523/JNEUROSCI.5120-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Moser HR, Honda CN, Simone DA, Giesler GJ., Jr. Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J Neurophysiol. 2012;108:1711–1723. doi: 10.1152/jn.00206.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YQ, Nomura S, Kaneko T, Mizuno N. Co-localization of mu-opioid receptor-like and substance P-like immunoreactivities in axon terminals within the superficial layers of the medullary and spinal dorsal horns of the rat. Neurosci Lett. 1995;198:45–48. doi: 10.1016/0304-3940(95)11960-5. [DOI] [PubMed] [Google Scholar]
- Dirig DM, Yaksh TL. Intrathecal baclofen and muscimol, but not midazolam, are antinociceptive using the rat-formalin model. J Pharmacol Exp Ther. 1995;275:219–227. [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Eaton MJ, Plunkett JA, Karmally S, Martinez MA, Montanez K. Changes in GAD- and GABA-immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplant of immortalized serotonergic precursors. J Chem Neuroanat. 1998;16:57–72. doi: 10.1016/s0891-0618(98)00062-3. [DOI] [PubMed] [Google Scholar]
- Eckert WA, 3rd, McNaughton KK, Light AR. Morphology and axonal arborization of rat spinal inner lamina II neurons hyperpolarized by mu-opioid-selective agonists. J Comp Neurol. 2003;458:240–256. doi: 10.1002/cne.10587. [DOI] [PubMed] [Google Scholar]
- Fields HL, Clanton CH, Anderson SD. Somatosensory properties of spinoreticular neurons in the cat. Brain Res. 1977;120:49–66. doi: 10.1016/0006-8993(77)90497-8. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Tokunaga A, Kondo E, Miki K, Tachibana T, Noguchi K. Change in mRNAs for neuropeptides and the GABA(A) receptor in dorsal root ganglion neurons in a rat experimental neuropathic pain model. Pain. 1998;78:13–26. doi: 10.1016/S0304-3959(98)00111-0. [DOI] [PubMed] [Google Scholar]
- Gamboa-Esteves FO, McWilliam PN, Batten TF. Substance P (NK1) and somatostatin (sst2A) receptor immunoreactivity in NTS-projecting rat dorsal horn neurones activated by nociceptive afferent input. J Chem Neuroanat. 2004;27:251–266. doi: 10.1016/j.jchemneu.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Gangadharan V, Kuner R. Pain hypersensitivity mechanisms at a glance. Dis Model Mech. 2013;6:889–895. doi: 10.1242/dmm.011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobel S. Golgi studies in the substantia gelatinosa neurons in the spinal trigeminal nucleus. J Comp Neurol. 1975;162:397–415. doi: 10.1002/cne.901620308. [DOI] [PubMed] [Google Scholar]
- Gobel S. Golgi studies of the neurons in layer II of the dorsal horn of the medulla (trigeminal nucleus caudalis). J Comp Neurol. 1978;180:395–413. doi: 10.1002/cne.901800213. [DOI] [PubMed] [Google Scholar]
- Gobel S, Falls WM, Bennett GJ, Abdelmoumene M, Hayashi H, Humphrey E. An EM analysis of the synaptic connections of horseradish peroxidase-filled stalked cells and islet cells in the substantia gelatinosa of adult cat spinal cord. J Comp Neurol. 1980;194:781–807. doi: 10.1002/cne.901940406. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JS, Xu ZZ, Gao H, He SQ, Ma GQ, Sun T, Wang LH, Zhang ZN, Lena I, Kitchen I, et al. Interaction with vesicle luminal protachykinin regulates surface expression of delta-opioid receptors and opioid analgesia. Cell. 2005;122:619–631. doi: 10.1016/j.cell.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han ZS, Zhang ET, Craig AD. Nociceptive and thermoreceptive lamina I neurons are anatomically distinct. Nat Neurosci. 1998;1:218–225. doi: 10.1038/665. [DOI] [PubMed] [Google Scholar]
- Hughes DI, Sikander S, Kinnon CM, Boyle KA, Watanabe M, Callister RJ, Graham BA. Morphological, neurochemical and electrophysiological features of parvalbumin-expressing cells: a likely source of axo-axonic inputs in the mouse spinal dorsal horn. J Physiol. 2012;590:3927–3951. doi: 10.1113/jphysiol.2012.235655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylden JL, Nahin RL, Dubner R. Altered responses of nociceptive cat lamina I spinal dorsal horn neurons after chronic sciatic neuroma formation. Brain Res. 1987;411:341–350. doi: 10.1016/0006-8993(87)91086-9. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Nahin RL, Traub RJ, Dubner R. Expansion of receptive fields of spinal lamina I projection neurons in rats with unilateral adjuvant-induced inflammation: the contribution of dorsal horn mechanisms. Pain. 1989;37:229–243. doi: 10.1016/0304-3959(89)90135-8. [DOI] [PubMed] [Google Scholar]
- Ibuki T, Hama AT, Wang XT, Pappas GD, Sagen J. Loss of GABA-immunoreactivity in the spinal dorsal horn of rats with peripheral nerve injury and promotion of recovery by adrenal medullary grafts. Neuroscience. 1997;76:845–858. doi: 10.1016/s0306-4522(96)00341-7. [DOI] [PubMed] [Google Scholar]
- Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci (USA) 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Rau KK, Soneji DJ, Ekmann KM, Anderson CE, Molliver DC, Koerber HR. Purinergic receptor P2Y1 regulates polymodal C-fiber thermal thresholds and sensory neuron phenotypic switching during peripheral inflammation. Pain. 2012;153:410–419. doi: 10.1016/j.pain.2011.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kanda H, Kobayashi K, Yamanaka H, Noguchi K. COX-1-dependent prostaglandin D2 in microglia contributes to neuropathic pain via DP2 receptor in spinal neurons. Glia. 2013;61:943–956. doi: 10.1002/glia.22487. [DOI] [PubMed] [Google Scholar]
- Kato G, Kawasaki Y, Ji RR, Strassman AM. Differential wiring of local excitatory and inhibitory synaptic inputs to islet cells in rat spinal lamina II demonstrated by laser scanning photostimulation. J Physiol. 2007;580:815–833. doi: 10.1113/jphysiol.2007.128314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato G, Kawasaki Y, Koga K, Uta D, Kosugi M, Yasaka T, Yoshimura M, Ji RR, Strassman AM. Organization of intralaminar and translaminar neuronal connectivity in the superficial spinal dorsal horn. J Neurosci. 2009;29:5088–5099. doi: 10.1523/JNEUROSCI.6175-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp T, Spike RC, Watt C, Todd AJ. The mu-opioid receptor (MOR1) is mainly restricted to neurons that do not contain GABA or glycine in the superficial dorsal horn of the rat spinal cord. Neuroscience. 1996;75:1231–1238. doi: 10.1016/0306-4522(96)00333-8. [DOI] [PubMed] [Google Scholar]
- Kim H, Cui L, Kim J, Kim SJ. Transient receptor potential vanilloid type 1 receptor regulates glutamatergic synaptic inputs to the spinothalamic tract neurons of the spinal cord deep dorsal horn. Neuroscience. 2009;160:508–516. doi: 10.1016/j.neuroscience.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, McKemy DD. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci. 2013;33:2837–2848. doi: 10.1523/JNEUROSCI.1943-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi M, Kato G, Lukashov S, Pendse G, Puskar Z, Kozsurek M, Strassman AM. Subpopulation-specific patterns of intrinsic connectivity in mouse superficial dorsal horn as revealed by laser scanning photostimulation. J Physiol. 2013;591:1935–1949. doi: 10.1113/jphysiol.2012.244210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrozis A, Albuquerque C, Gu J, MacDermott AB. Ca(2+)-dependent inactivation of NMDA receptors: fast kinetics and high Ca2+ sensitivity in rat dorsal horn neurons. J Physiol. 1996;495:449–463. doi: 10.1113/jphysiol.1996.sp021606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerstrom MC, Rogoz K, Abrahamsen B, Persson E, Reinius B, Nordenankar K, Olund C, Smith C, Mendez JA, Chen ZF, et al. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68:529–542. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever I, Cunningham J, Grist J, Yip PK, Malcangio M. Release of BDNF and GABA in the dorsal horn of neuropathic rats. Eur J Neurosci. 2003;18:1169–1174. doi: 10.1046/j.1460-9568.2003.02848.x. [DOI] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima D, Coimbra A. Morphological types of spinomesencephalic neurons in the marginal zone (lamina I) of the rat spinal cord, as shown after retrograde labelling with cholera toxin subunit B. J Comp Neurol. 1989;279:327–339. doi: 10.1002/cne.902790212. [DOI] [PubMed] [Google Scholar]
- Littlewood NK, Todd AJ, Spike RC, Watt C, Shehab SA. The types of neuron in spinal dorsal horn which possess neurokinin-1 receptors. Neuroscience. 1995;66:597–608. doi: 10.1016/0306-4522(95)00039-l. [DOI] [PubMed] [Google Scholar]
- Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, Sun S, LaMotte RH, Dong X. Mechanisms of itch evoked by beta-alanine. J Neurosci. 2012;32:14532–14537. doi: 10.1523/JNEUROSCI.3509-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543–556. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu VB, Biggs JE, Stebbing MJ, Balasubramanyan S, Todd KG, Lai AY, Colmers WF, Dawbarn D, Ballanyi K, Smith PA. Brain-derived neurotrophic factor drives the changes in excitatory synaptic transmission in the rat superficial dorsal horn that follow sciatic nerve injury. J Physiol. 2009;587:1013–1032. doi: 10.1113/jphysiol.2008.166306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J Neurosci. 2003;23:8752–8758. doi: 10.1523/JNEUROSCI.23-25-08752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Perl ER. Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (laminae I and II). J Neurosci. 2005;25:3900–3907. doi: 10.1523/JNEUROSCI.0102-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Perl ER. Selective action of noradrenaline and serotonin on neurones of the spinal superficial dorsal horn in the rat. J Physiol. 2007;582:127–136. doi: 10.1113/jphysiol.2007.131565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCγ. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, DeMaster E, Malhotra A, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE, et al. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science. 1995;268:1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- Marker CL, Lujan R, Colon J, Wickman K. Distinct populations of spinal cord lamina II interneurons expressing G-protein-gated potassium channels. J Neurosci. 2006;26:12251–12259. doi: 10.1523/JNEUROSCI.3693-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell DJ, Belle MD, Cheunsuang O, Stewart A, Morris R. Morphology of inhibitory and excitatory interneurons in superficial laminae of the rat dorsal horn. J Physiol. 2007;584:521–533. doi: 10.1113/jphysiol.2007.140996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy ES, Taylor-Blake B, Street SE, Pribisko AL, Zheng J, Zylka MJ. Peptidergic CGRPalpha primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold. Neuron. 2013;78:138–151. doi: 10.1016/j.neuron.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Menétrey D, Basbaum AI. The distribution of substance P-, enkephalin- and dynorphin-immunoreactive neurons in the medulla of the rat and their contribution to bulbospinal pathways. Neuroscience. 1987;23:173–187. doi: 10.1016/0306-4522(87)90281-8. [DOI] [PubMed] [Google Scholar]
- Miraucourt LS, Dallel R, Voisin DL. Glycine inhibitory dysfunction turns touch into pain through PKCγ interneurons. PLoS One. 2007;2:e1116. doi: 10.1371/journal.pone.0001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340:968–971. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. TRPV1-lineage neurons are required for thermal sensation. EMBO J. 2011;30:582–593. doi: 10.1038/emboj.2010.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim M, Spike RC, Watt C, Shehab SA, Todd AJ. Cells in laminae III and IV of the rat spinal cord that possess the neurokinin-1 receptor and have dorsally directed dendrites receive a major synaptic input from tachykinin-containing primary afferents. J Neurosci. 1997;17:5536–5548. doi: 10.1523/JNEUROSCI.17-14-05536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka T, Chen M, Takeda D, King C, Ling J, Xing H, Ataka T, Vierck C, Yezierski R, Gu JG. Substance P-driven feed-forward inhibitory activity in the mammalian spinal cord. Mol Pain. 2005;1:20. doi: 10.1186/1744-8069-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Braz JM, Skinner K, Llewellyn-Smith IJ, Basbaum AI. Innocuous, not noxious, input activates PKCγ interneurons of the spinal dorsal horn via myelinated afferent fibers. J Neurosci. 2008;28:7936–7944. doi: 10.1523/JNEUROSCI.1259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, et al. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, Ekholm S, Strigo I, Worsley K, Vallbo AB, et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci. 2002;5:900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Oliveira AL, Hydling F, Olsson E, Shi T, Edwards RH, Fujiyama F, Kaneko T, Hokfelt T, Cullheim S, Meister B. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse. 2003;50:117–129. doi: 10.1002/syn.10249. [DOI] [PubMed] [Google Scholar]
- Polgar E, Al-Khater KM, Shehab S, Watanabe M, Todd AJ. Large projection neurons in lamina I of the rat spinal cord that lack the neurokinin 1 receptor are densely innervated by VGLUT2-containing axons and possess GluR4-containing AMPA receptors. J Neurosci. 2008;28:13150–13160. doi: 10.1523/JNEUROSCI.4053-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar E, Fowler JH, McGill MM, Todd AJ. The types of neuron which contain protein kinase C gamma in rat spinal cord. Brain Res. 1999;833:71–80. doi: 10.1016/s0006-8993(99)01500-0. [DOI] [PubMed] [Google Scholar]
- Polgar E, Furuta T, Kaneko T, Todd A. Characterization of neurons that express preprotachykinin B in the dorsal horn of the rat spinal cord. Neuroscience. 2006;139:687–697. doi: 10.1016/j.neuroscience.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Polgar E, Gray S, Riddell JS, Todd AJ. Lack of evidence for significant neuronal loss in laminae I-III of the spinal dorsal horn of the rat in the chronic constriction injury model. Pain. 2004;111:144–150. doi: 10.1016/j.pain.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Polgar E, Hughes DI, Riddell JS, Maxwell DJ, Puskar Z, Todd AJ. Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 2003;104:229–239. doi: 10.1016/s0304-3959(03)00011-3. [DOI] [PubMed] [Google Scholar]
- Polgar E, Sardella TC, Tiong SY, Locke S, Watanabe M, Todd AJ. Functional differences between neurochemically defined populations of inhibitory interneurons in the rat spinal dorsal horn. Pain. 2013 doi: 10.1016/j.pain.2013.05.001. (e-Pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD, Greenspan JD, Dubner R. Neurons involved in the exteroceptive function of pain. Pain. 2003;106:215–219. doi: 10.1016/j.pain.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Price DD, Hayashi H, Dubner R, Ruda MA. Functional relationships between neurons of marginal and substantia gelatinosa layers of primate dorsal horn. Journal of neurophysiology. 1979;42:1590–1608. doi: 10.1152/jn.1979.42.6.1590. [DOI] [PubMed] [Google Scholar]
- Proudlock F, Spike RC, Todd AJ. Immunocytochemical study of somatostatin, neurotensin, GABA, and glycine in rat spinal dorsal horn. J Comp Neuro. 1993;l327:289–297. doi: 10.1002/cne.903270210. [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S. Histologie du Systèm Nerveux de l'Homme et des Vertébrés (Paris, Maloine) 1909:986. [Google Scholar]
- Ribeiro-da-Silva A, De Koninck Y. Morphological and neurochemical organization of the spinal dorsal horn. In: Basbaum AI, Bushnell MC, editors. Science of Pain. Elsevier; Amsterdam: 2009. pp. 279–310. [Google Scholar]
- Riedl MS, Schnell SA, Overland AC, Chabot-Dore AJ, Taylor AM, Ribeiro-da-Silva A, Elde RP, Wilcox GL, Stone LS. Coexpression of alpha 2A-adrenergic and delta-opioid receptors in substance P-containing terminals in rat dorsal horn. J Comp Neurol. 2009;513:385–398. doi: 10.1002/cne.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed AW, Ribeiro-da-Silva A. De novo expression of neurokinin-1 receptors by spinoparabrachial lamina I pyramidal neurons following a peripheral nerve lesion. J Comp Neurol. 2013;521:1915–1928. doi: 10.1002/cne.23267. [DOI] [PubMed] [Google Scholar]
- Santos SF, Luz LL, Szucs P, Lima D, Derkach VA, Safronov BV. Transmission efficacy and plasticity in glutamatergic synapses formed by excitatory interneurons of the substantia gelatinosa in the rat spinal cord. PLoS One. 2009;4:e8047. doi: 10.1371/journal.pone.0008047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardella TC, Polgar E, Watanabe M, Todd AJ. A quantitative study of neuronal nitric oxide synthase expression in laminae I-III of the rat spinal dorsal horn. Neuroscience. 2011;192:708–720. doi: 10.1016/j.neuroscience.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Low SA, Wang X, Zhang J, Yamanaka H, Urban R, Solorzano C, Harper B, Hnasko TS, Edwards RH, et al. VGLUT2 expression in primary afferent neurons is essential for normal acute pain and injury-induced heat hypersensitivity. Proc Natl Acad Sci (USA) 2010;107:22296–22301. doi: 10.1073/pnas.1013413108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, Moore KA, Decosterd I, Coggeshall RE, Woolf CJ. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005;25:7317–7323. doi: 10.1523/JNEUROSCI.1526-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SE, Loomis CW. Strychnine-dependent allodynia in the urethane-anesthetized rat is segmentally distributed and prevented by intrathecal glycine and betaine. Can J Physiol Pharmacol. 1995;73:1698–1705. doi: 10.1139/y95-733. [DOI] [PubMed] [Google Scholar]
- Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol. 1994;72:169–179. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- Spike RC, Puskar Z, Andrew D, Todd AJ. A quantitative and morphological study of projection neurons in lamina I of the rat lumbar spinal cord. Eur J Neurosci. 2003;18:2433–2448. doi: 10.1046/j.1460-9568.2003.02981.x. [DOI] [PubMed] [Google Scholar]
- Spike RC, Puskar Z, Sakamoto H, Stewart W, Watt C, Todd AJ. MOR-1-immunoreactive neurons in the dorsal horn of the rat spinal cord: evidence for nonsynaptic innervation by substance P-containing primary afferents and for selective activation by noxious thermal stimuli. Eur J Neurosci. 2002;15:1306–1316. doi: 10.1046/j.1460-9568.2002.01969.x. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Bennett GJ, Kajander KC. Transsynaptic degeneration in the superficial dorsal horn after sciatic nerve injury: effects of a chronic constriction injury, transection, and strychnine. Pain. 1990;42:205–213. doi: 10.1016/0304-3959(90)91164-E. [DOI] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, Thompson SW, Marchand F, McMahon SB. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain. 2009;13:263–272. doi: 10.1016/j.ejpain.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ, Hughes DI, Polgar E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci. 2003;17:13–27. doi: 10.1046/j.1460-9568.2003.02406.x. [DOI] [PubMed] [Google Scholar]
- Todd AJ, McGill MM, Shehab SA. Neurokinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. Eur J Neurosci. 2000;12:689–700. doi: 10.1046/j.1460-9568.2000.00950.x. [DOI] [PubMed] [Google Scholar]
- Todd AJ, McKenzie J. GABA-immunoreactive neurons in the dorsal horn of the rat spinal cord. Neuroscience. 1989;31:799–806. doi: 10.1016/0306-4522(89)90442-9. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Russell G, Spike RC. Immunocytochemical evidence that GABA and neurotensin exist in different neurons in laminae II and III of rat spinal dorsal horn. Neuroscience. 1992;47:685–691. doi: 10.1016/0306-4522(92)90176-3. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Spike RC, Polgar E. A quantitative study of neurons which express neurokinin-1 or somatostatin sst2a receptor in rat spinal dorsal horn. Neuroscience. 1998;85:459–473. doi: 10.1016/s0306-4522(97)00669-6. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Trafton JA, Abbadie C, Marchand S, Mantyh PW, Basbaum AI. Spinal opioid analgesia: how critical is the regulation of substance P signaling? J Neurosci. 1999;19:9642–9653. doi: 10.1523/JNEUROSCI.19-21-09642.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uta D, Furue H, Pickering AE, Rashid MH, Mizuguchi-Takase H, Katafuchi T, Imoto K, Yoshimura M. TRPA1-expressing primary afferents synapse with a morphologically identified subclass of substantia gelatinosa neurons in the adult rat spinal cord. Eur J Neurosci. 2010;31:1960–1973. doi: 10.1111/j.1460-9568.2010.07255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrontou S, Wong AM, Rau KK, Koerber HR, Anderson DJ. Genetic identification of C fibres that detect massage-like stroking of hairy skin in vivo. Nature. 2013;493:669–673. doi: 10.1038/nature11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD. The laminar organization of dorsal horn and effects of descending impulses. J Physiol. 1967;188:403–423. doi: 10.1113/jphysiol.1967.sp008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zylka MJ. Mrgprd-expressing polymodal nociceptive neurons innervate most known classes of substantia gelatinosa neurons. J Neurosci. 2009;29:13202–13209. doi: 10.1523/JNEUROSCI.3248-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang J, Eberhart D, Urban R, Meda K, Solorzano C, Yamanaka H, Rice D, Basbaum AI. Excitatory superficial dorsal horn interneurons are functionally heterogeneous and required for the full behavioral expression of pain and itch. Neuron. 2013;78:312–324. doi: 10.1016/j.neuron.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildner H, Das Gupta R, Brohl D, Heppenstall PA, Zeilhofer HU, Birchmeier C. Genome-wide expression analysis of Ptf1a- and Ascl1-deficient mice reveals new markers for distinct dorsal horn interneuron populations contributing to nociceptive reflex plasticity. J Neurosci. 2013;33:7299–7307. doi: 10.1523/JNEUROSCI.0491-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley RG, Kline R.H.t., Vierck CJ., Jr. Anti-nociceptive effects of selectively destroying substance P receptor-expressing dorsal horn neurons using [Sar9,Met(O2)11]-substance P-saporin: behavioral and anatomical analyses. Neuroscience. 2007;146:1333–1345. doi: 10.1016/j.neuroscience.2007.01.066. [DOI] [PubMed] [Google Scholar]
- Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SX, Wang W, Li H, Wang YY, Feng YP, Li YQ. The synaptic connectivity that underlies the noxious transmission and modulation within the superficial dorsal horn of the spinal cord. Prog Neurobiol. 2010;91:38–54. doi: 10.1016/j.pneurobio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Xu Y, Lopes C, Qian Y, Liu Y, Cheng L, Goulding M, Turner EE, Lima D, Ma Q. Tlx1 and Tlx3 coordinate specification of dorsal horn pain-modulatory peptidergic neurons. J Neurosci. 2008;28:4037–4046. doi: 10.1523/JNEUROSCI.4126-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Lopes C, Wende H, Guo Z, Cheng L, Birchmeier C, Ma Q. Ontogeny of excitatory spinal neurons processing distinct somatic sensory modalities. J Neurosci. 2013;33:14738–14748. doi: 10.1523/JNEUROSCI.5512-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL. Regulation of spinal nociceptive processing: where we went when we wandered onto the path marked by the gate. Pain Suppl. 1999;6:S149–152. doi: 10.1016/S0304-3959(99)00149-9. [DOI] [PubMed] [Google Scholar]
- Yasaka T, Kato G, Furue H, Rashid MH, Sonohata M, Tamae A, Murata Y, Masuko S, Yoshimura M. Cell-type-specific excitatory and inhibitory circuits involving primary afferents in the substantia gelatinosa of the rat spinal dorsal horn in vitro. J Physiol. 2007;581:603–618. doi: 10.1113/jphysiol.2006.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasaka T, Tiong SY, Hughes DI, Riddell JS, Todd AJ. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain. 2010;151:475–488. doi: 10.1016/j.pain.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilhofer HU, Wildner H, Yevenes GE. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol Rev. 2012;92:193–235. doi: 10.1152/physrev.00043.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cavanaugh DJ, Nemenov MI, Basbaum AI. The modality-specific contribution of peptidergic and non-peptidergic nociceptors is manifest at the level of dorsal horn nociresponsive neurons. J Physiol. 2013;591:1097–1110. doi: 10.1113/jphysiol.2012.242115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Huo FQ, Jeffry J, Hampton L, Demehri S, Kim S, Liu XY, Barry DM, Wan L, Liu ZC, et al. Chronic itch development in sensory neurons requires BRAF signaling pathways. J Clin Investig. 2013 doi: 10.1172/JCI70528. (e-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Lu Y, Perl ER. Inhibitory neurones of the spinal substantia gelatinosa mediate interaction of signals from primary afferents. J Physiol. 2010;588:2065–2075. doi: 10.1113/jphysiol.2010.188052. [DOI] [PMC free article] [PubMed] [Google Scholar]