Abstract
Inflammation and the presence of pro-inflammatory cytokines are associated with numerous chronic diseases such as type-2 diabetes mellitus, cardiovascular disease, Alzheimer's disease, and cancer. An overwhelming amount of data indicates that curcumin, a polyphenol obtained from the Indian spice turmeric, Curcuma longa, is a potential chemopreventive agent for treating certain cancers and other chronic inflammatory diseases. However, the low bioavailability of curcumin, partly due to its low solubility and stability in the digestive tract, limits its therapeutic applications. Recent studies have demonstrated increased bioavailability and health-promoting effects of a novel solid lipid particle formulation of curcumin (Curcumin SLCP, Longvida®). The goal of this study was to evaluate the aqueous solubility and in vitro anti-inflammatory effects of solid lipid curcumin particle (SLCP) formulations using lipopolysaccharide (LPS)-stimulated RAW 264.7 cultured murine macrophages. SLCPs treatment significantly decreased nitric oxide (NO) and prostaglandin-E2 (PGE2) levels at concentrations ranging from 10 to 50 μg/mL, and reduced interleukin-6 (IL-6) levels in a concentration-dependent manner. Transient transfection experiments using a nuclear factor-kappa B (NF-κB) reporter construct indicate that SLCPs significantly inhibit the transcriptional activity of NF-κB in macrophages. Taken together, these results show that in RAW 264.7 murine macrophages, SLCPs have improved solubility over unformulated curcumin, and significantly decrease the LPS-induced pro-inflammatory mediators NO, PGE2, and IL-6 by inhibiting the activation of NF-κB.
Key Words: : curcumin, inflammation, interleukin-6, interleukin-1β, Longvida®, NF-κB, nitric oxide, prostaglandin-E2
Introduction
Inflammation and associated pro-inflammatory processes are centrally linked to several chronic human diseases, including cancer, diabetes, obesity, arthritis, and cardiovascular and neurodegenerative diseases.1–6 During inflammation, macrophages play a critical role in managing various immunopathological phenomena, including the overproduction of inflammatory markers such as nitric oxide (NO), prostaglandin-E2 (PGE2), tumor necrotic factor α (TNFα), and cytokines such as interleukin-6 (IL-6) and interleukin-1β (IL-1β). A number of inflammatory stimuli, for example, lipopolysaccharides (LPS) and pro-inflammatory cytokines, activate immune cells to produce inflammatory mediators, and these are, therefore, useful targets in the development of novel anti-inflammatory drugs and in the evaluation of the molecular mechanisms of potential anti-inflammatory drugs.7,8 Thus, dietary agents that can suppress inflammatory markers, such as plant natural products, can potentially prevent, delay the onset, and/or treat inflammation and inflammatory-mediated diseases. A growing body of research suggests that plant phenolic compounds which possess antioxidant and anti-inflammatory properties offer an attractive dietary strategy to combat inflammation and promote human health and wellness.9,10
Curcumin (diferuloylmethane) is derived from the ground rhizomes of the Curcuma longa L. plant and is the most active curcuminoid in the Indian curry spice, turmeric.11,12 Curcumin is a lipophilic, water-insoluble, low-molecular-weight polyphenol (MW=368 g/moL) that has been used for culinary applications in many parts of the world. In Ayurveda, turmeric is widely used to treat a variety of conditions ranging from infections, wounds, and injuries, and has been implicated in the prevention of chronic diseases such as diabetes, asthma, and various inflammatory diseases.13–15 A vast number of published research studies support a wide range of pharmacological effects of curcumin, including anti-oxidant, anti-cancer, anti-Alzheimer's disease, and anti-inflammatory effects both in vitro and in vivo.13,16 On a molecular level, curcumin targets many transcription factors (including nuclear factor-kappa B [NF-κB], AP-1, and STAT-3), inflammatory mediators (PGE2, cytokines), enzymes, growth factors, protein kinases, and cell-cycle regulatory proteins.13,17–19
Despite the promising health benefits of curcumin, its low water solubility limits its oral delivery in aqueous-based formulations that are popular among consumers and widely used in the nutraceutical and functional food industries. Moreover, the poor bioavailability and extensive phase-II metabolism of curcumin are limiting factors for the oral dosage of unformulated curcumin, which limits its potential as a preventive and/or therapeutic agent.19,20 Various clinical trials have reported low systemic bioavailability of curcumin even after oral administration of doses for approximately 12 g/day.16,21 Several research strategies have been undertaken to improve the bioavailability of curcumin, including novel drug delivery systems and formulations such as liposomes, nanoparticles, and phospholipid complexes.13,22 In a clinical study, a combination of curcumin and piperine, a nonspecific CYP-450 and UGT inhibitor, resulted in increased curcumin bioavailability, but with an absorption half life of only 7 min.23 Other in vitro studies suggest that polymer-based nanoparticle formulations of curcumin, also known as “nano-curcumin,” exhibit pharmacological activity at slightly lower concentrations than those of pure curcumin in human pancreatic cancer cell lines.24 However, the potential increase in bioavailability of these latter formulations is offset by the lack of data and clinical trials on the safety and metabolism of nanoparticle formulations in humans.
Recently, a single-dose human pharmacokinetic study of a standardized novel solid lipid curcumin particle (SLCP) preparation (commercially available as Longvida®) reported increased bioavailability compared with a generic curcumin extract, suggesting the potential for sustained release dosage forms of this natural product.25 Moreover, acute and sub-chronic animal toxicity studies by Dadhaniya et al. demonstrated the safety of the SLCP and the No Observed-Adverse-Effect Level (NOAEL) was determined to be 720 mg/kg bw/day, which was the highest test dose.26 In addition, DiSilvestro et al. demonstrated that a low dose of Longvida (80 mg/day) imparted potentially health-promoting effects in healthy middle-aged humans.27 In addition, in a recent investigation, Longvida selectively suppressed soluble Tau dimers and corrected molecular chaperone, synaptic, and behavioral deficits in transgenic mice, suggesting that this curcumin formulation may have potential beneficial effects against Alzheimer's disease.28 However, to date, the effects of this bioavailable curcumin preparation, namely, Longvida, on inflammation and inflammatory biomarkers remain unknown.
The goal of this study was to evaluate the effects of two novel SLCP preparations using LPS-stimulated RAW 264.7 macrophages, a well-established in vitro anti-inflammatory model. LPS increases inflammatory markers such as NO, PGE2, and IL-6. We hypothesized that the standardized SLCP formulations would block the expression of pro-inflammatory mediators.
Materials and Methods
SLCP formulations and curcumin extract
The SLCP preparation (Longvida, SLCP-1), a solution-dispersible SLCP preparation (Longvida SD, SLCP-2), and a curcumin extract (containing 95% curcuminoids) were provided to our laboratory by Verdure Sciences (Noblesville, IN, USA). The SLCP extracts were produced using patent-pending methodology as previously described25,27 and were standardized to contain ∼20% curcumin.25,26 SLCP-1 is a granular powder used for tablets and capsules, and SLCP-2 is a fine powder intended for use in other dosage forms. Briefly, turmeric root extract was mixed with pure phosphatidylcholine, vegetable stearic acid, ascorbic acid (vitamin C) palmitate, and other inert ingredients. The formulations were manufactured under cGMP standards and meet internal and external specifications for precise chemical and physical characteristics. The solubility of the two SLCP formulations and the curcumin extract are shown in Table 1 (data provided by Verdure Sciences).
Table 1.
Solubility of Curcumin Extract and the Solid Lipid Curcumin Particle Formulations in Water
| Test samples | Solubility in water (%) | Solubility in water (μg/mL) | Fold improvement |
|---|---|---|---|
| Curcumin (unformulated) | 0.00006 | 0.6 | 1 |
| SLCP-1 (Longvida®) | 14 | 140,000 | 233,000 |
| SLCP-2 (Longvida) | 76 | 760,000 | 1,270,000 |
SLCP, solid lipid curcumin particle.
Cell culture
RAW 264.7 mouse macrophage cells were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). RAW 264.7 cells were routinely cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% v/v fetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (100 μg/mL) (Sigma-Aldrich, St. Louis, MO, USA) and maintained at 37°C with 5% CO2 humidified air. Stimulated RAW 264.7 cells (1×105/100 μL) were treated with LPS (Sigma-Aldrich) with or without different concentrations of test samples (10, 25, and 50 μg/mL) for 24 h. All test samples were solubilized in dimethyl sulfoxide (DMSO; Sigma-Aldrich) with a final DMSO concentration <0.1% in the culture medium. Cell culture supernatants were collected for NO, PGE2, and cytokine assays.
Cytotoxicity assay
The viability of RAW 264.7 macrophages after 24 h of continuous exposure to the test samples was determined by performing colorimetric MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymeth-oxyphenyl)-2-(4-sulphenyl)-2H-tetrazolium salt] assay (Promega, Madison, CA, USA) according to the protocol previously described.29 Briefly, after 24 h of treatment, 20 μL of MTS reagent was added to each reaction well (in a 96-well format). After 2 h of incubation, the absorbance was measured at 490 nm using a spectrophotometer (SpectraMax M2; Molecular Devices Corp, Sunnyvale, CA, USA).
Nitrite determination
RAW 264.7 macrophages were plated in a 96-well plate (1×105 cells/100 μL) and incubated at 37°C for 24 h. After 24 h, the medium was replaced and the cells were co-treated with 50 ng/mL LPS and different concentrations of the SLCP formulations (10, 25, and 50 μg/mL) for 24 h at 37°C. Cells that were not treated with LPS served as a negative control. After 24 h, the cell culture supernatants were collected and incubated (1:1, v/v) with modified Griess reagent (1% sulfanilamide in 5% phosphoric acid and 0.1% naphthylethylenediamine dihydrochloride in distilled water) (Sigma-Aldrich) for 20 min at room temperature as previously described.30 The absorbance at 540 nm was measured using a spectrophotometer (SpectraMax M2; Molecular Devices Corp). The nitrite concentration was quantified by comparison with a sodium nitrite standard curve. The assay was performed in triplicate for each concentration.
PGE2 determination
To evaluate the effects of various concentrations of the SLCP-1 and SLCP-2 formulations on PGE2 levels, PGE2 metabolites accumulated in the cell supernatants were measured using a PGE2 enzyme-immunoassay (EIA) kit (Cayman Chemical, Ann Arbor, MI, USA), according to the manufacturer's protocol. The assay was performed in triplicate for each concentration.
Determination of IL-6
The effects of the SLCP-1 and SLCP-2 formulations on the production of pro-inflammatory cytokine IL-6 were determined by Bio-Plex® Multiplex Immunoassays (BioRad Laboratories, Hercules, CA, USA), as described by the manufacturer's instructions. The experiment was performed in quadruplicate.
Transient transfection and luciferase assay
LPS-induced NF-κB upregulation accounts for a part of LPS-mediated activation of a variety of inflammatory genes, and, hence, it is important to identify the effects of SLCP formulations on the transcriptional activity of NF-κB.31 To monitor these effects of the two SLCP formulations, RAW 264.7 macrophages were transiently co-transfected with pNF-κB and pRL-CMV reporter vectors (Promega) using GenePORTER® 3000 Transfection Reagent (Genlantis, San Diego, CA, USA) according to the manufacturer's protocol. Briefly, the cells were seeded into 96-well plates (1.2×104 cells/100 μL) in complete medium without antibiotics (DMEM +10% FBS) and incubated for 24 h (∼50–70% confluency). Cells were then treated with the transfection complexes and incubated for 72 h before treatment with each of the SLCP formulations (10, 25, and 50 μg/mL). LPS was added after 2 h (1 μg/mL). After 5 h, cells were lysed with 20 μL passive lysis buffer and NF-κB activity was measured as relative firefly/renilla luciferase activity using a Dual-Luciferase® Reporter Assay System (Promega) with a GloMax™ 20/20 Luminometer (Promega) according to the manufacturer's protocol. All samples were tested in quadruplicate. Luciferase activity was recorded as relative light units and expressed as fold change relative to LPS treatment control, and the assay was performed using three replicates for each concentration.
Statistical analysis
All statistical analyses were carried out using the software program GraphPad Prism Version 5.0 (GraphPad Software, La Jolla, CA, USA). Experimental data were grouped by one variable and were analyzed by one-way ANOVA followed by a Dunnett's multiple-comparison test. A value of P<.05 was considered significant.
Results
Aqueous solubility of SLCP formulations versus curcumin
Both the SLCP-1 and SLCP-2 formulations were soluble in water, with SLCP-1 possessing ca. fourteen percent solubility and SLCP-2 having ca. seventy-six percent solubility (Table 1). On the other hand, the curcumin extract exhibited significantly less solubility in water, which was in agreement with the previous data published by Kurien et al.32 Given this solubility data as well as the previously published data supporting the increased bioavailability of the SLCPs compared with a generic curcumin extract in humans,25 we proceeded to further evaluate the SLCP formulations in targeted in vitro bioassays.
Effect of SLCP formulations on LPS-induced NO production
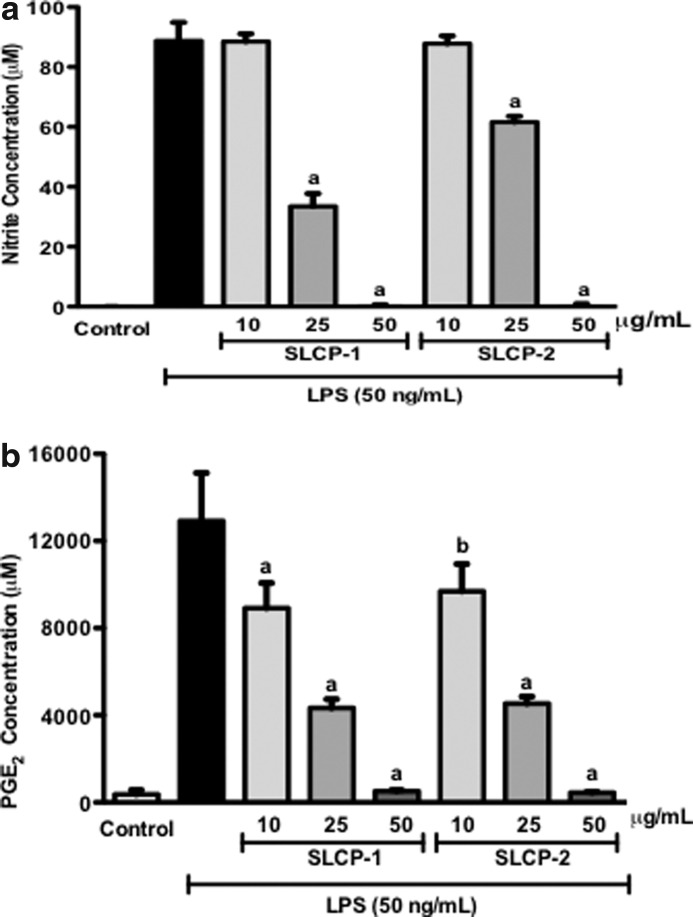
NO and PGE2 are secreted into cell culture supernatant by RAW 264.7 murine macrophages when they are treated with LPS. Since NO is highly unstable, the accumulation of nitrite (a stable oxidized product of NO) in culture media is often used as a biomarker for NO production in LPS-activated macrophages.33 An NO assay was used to evaluate the effect of the SLCP formulations on NO production as described in the “Nitrite determination” section (see Materials and Methods). RAW 264.7 cells were incubated with or without LPS (50 ng/mL) in the presence or absence of test samples (10, 25, and 50 μg/mL) for 24 h. In LPS-stimulated macrophages, nitrite levels increased significantly to 88.7±6.2 μM as compared with the solvent control (Fig. 1a). In Figure 1a, treatment with SLCP formulations suppressed nitrite concentration in LPS-activated macrophages in a concentration-dependent manner. At 25 μg/mL, both SLCP formulations significantly decreased medium nitrite to 33.5±4.2 μM and 61.6±2.0 μM, respectively. At 50 μg/mL, both SLCP-1 and SLCP-2 inhibited LPS-induced NO production in macrophages by 100%. No nitrite production could be detected in cells treated with test samples without LPS (data not shown).
FIG. 1.
Effects of SLCP formulations on: (a) Nitrite and (b) PGE2 production in LPS-stimulated RAW 264.7 cells. Cells were co-treated with LPS (50 ng/mL) and samples (10, 25, and 50 μg/mL) for 24 h. SLCP-1 and SLCP-2 inhibited NO and PGE2 levels in LPS-stimulated macrophages in a concentration-dependent manner. Data are expressed as mean values±SD. aP<.001 and bP<.01 indicate a significant difference as compared with the LPS-treated group. LPS, lipopolysaccharide; NO, nitric oxide; PGE2, prostaglandin-E2; SLCP, solid lipid curcumin particle.
Effects of SLCP on PGE2 production
We next evaluated the effects of SLCP on LPS-induced PGE2 synthesis in RAW 264.7 cells. The same supernatants were used for measurement of PGE2. After stimulation with LPS (50 ng/mL), PGE2 was released into the culture medium and rapidly converted into its metabolite. As shown in Figure 1b, at 10 μg/mL, SLCP-1and SLCP-2 lowered LPS-induced PGE2 production to about 31% and 25%, respectively. Treatment with 25 μg/mL of SLCP-1 and SLCP-2 significantly suppressed PGE2 levels by about 66% and 64%, respectively; whereas both the test samples at 50 μg/mL concentration reduced PGE2 levels to about 96% as compared with control. The SLCP formulations did not increase PGE2 levels in cells that were not treated with LPS (data not shown). Thus, our results show that SLCPs inhibited PGE2 production in a concentration-dependent manner in LPS-stimulated cells. The test samples were not responsible for altering the viability of activated macrophages as determined by MTS assay (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/jmf). Hence, the observed effects by the SLCP treatments was not attributed to cell cytotoxicity.
Effects of SLCPs on the levels of IL-6
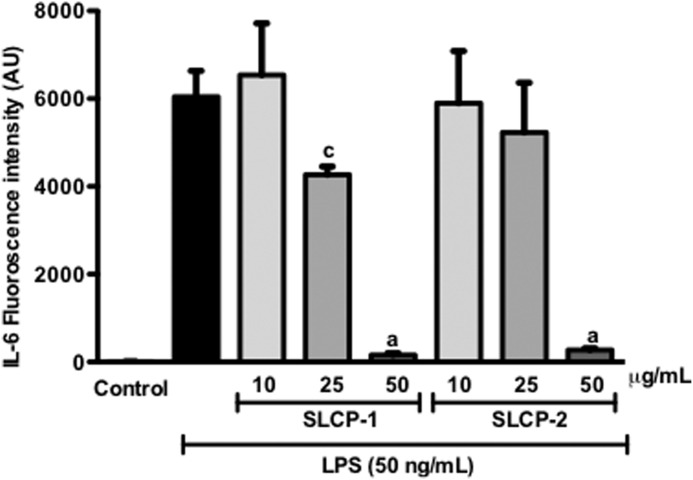
LPS induction in macrophages causes the upregulation of pro-inflammatory cytokines such as IL-6.34 Consequently, the effect of varying concentrations of the test samples on this cytokine was evaluated. LPS treatment significantly elevated levels of IL-6 (6043±589.94) in the media (Fig. 2). IL-6 levels in macrophages treated with the test samples alone, without LPS, were negligible (data not shown). The SLCP formulations suppressed IL-6 levels in a concentration-dependent manner. At 25 and 50 μg/mL of SLCP-1 treatment, IL-6 levels were significantly reduced by 29.4% and 97.4%, respectively. Similarly, treatment with 25, and 50 μg/mL of SLCP-2 decreased IL-6 production by about 13%, and 95%, respectively. These data suggested that both SLCP formulations were effective in lowering IL-6 levels in LPS-stimulated macrophages.
FIG. 2.
Effects of SLCP formulations on LPS-induced production of pro-inflammatory cytokine, IL-6, in RAW 264.7 cells. The SLCP formulations were able to suppress IL-6 levels in a concentration-dependent manner. Data are expressed as mean values±SD. aP<.001 and cP<.05 indicate a significant difference as compared with the LPS-treated group. AU, arbitrary units; IL-6, interleukin-6.
Effects of SLCP formulations on NF-κB activation
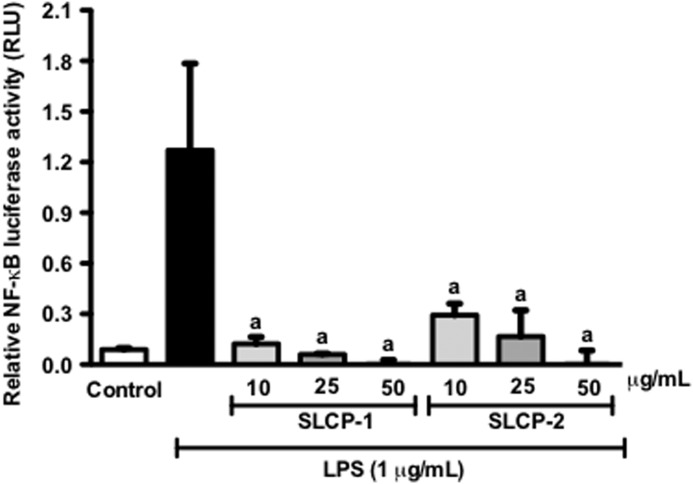
The activation of the transcription factor, NF-κB is a key step in stimulating pro-inflammatory signals. Thus, we next investigated the effect of the SLCP formulations on NF-κB activity, by transient transfection of macrophages followed by luciferase assay. After treatment with 1 μg/mL LPS, the NF-κB activity of the macrophages was significantly increased as compared with control (Fig. 3). Treatment with the SLCPs formulations significantly reduced NF-κB luciferase activity in a concentration-dependent manner, as shown in Figure 3. NF-κB luciferase activity was decreased to almost 10-fold with 10 μg/mL of SLCP-1 treatment; whereas with 10 μg/mL of SLCP-2, the activity was reduced to about 6-fold as compared with control.
FIG. 3.
Effects of SLCP formulations on NF-κB activation in stimulated RAW 264.7 macrophages. The SLCP formulations significantly inhibited the transcriptional activity of NF-κB in LPS-activated murine macrophages at tested concentrations. Data are expressed as mean values±SD. aP<.001 indicates a significant difference as compared with the LPS-treated group.
Discussion
Inflammation is a key component in multiple disease states.35 Based on a review of the literature, the NF-κB signaling pathway is the predominant upstream molecular signaling pathway that causes inflammation through enhanced cytokine, NO, and prostaglandin production.36 Several studies in different animal models and in human trials support the diverse pharmacological effects of curcumin, including anti-proliferative, anti-angiogenic, anti-oxidant, anti-inflammatory, anti-microbial, hepato-, and nephro-protective properties.22 The SLCPs are novel proprietary curcumin formulations with improved solubility and bioavailability compared with generic curcumin.25 In this study, we investigated the inhibitory effects of the SLCP treatments on the inflammatory response initiated by LPS in RAW 264.7 macrophages.
In inflammation, macrophages undergo sequential steps to release pro-inflammatory mediators such as cytokines, NO, and PGE2. These molecules recruit other immune cells to the sites of inflammation. Therefore, inhibition of the release of these chemicals is a good strategy for monitoring inflammatory diseases.35
In this study, we reported that LPS-activated RAW 264.7 murine cells treated with curcumin formulations, namely, SLCP-1 and SLCP-2 exhibited concentration-dependent downregulation of NO and PGE2 production. These observations were in agreement with the previous investigations, which reported the inhibition of NO through the suppression of inducible NO synthase gene and protein expression by curcumin in LPS and IFN-γ-activated RAW 264.7 macrophages.37,38 Our study further revealed that the SLCP treatments on LPS-activated macrophages decreased IL-6 levels in a concentration-dependent manner. It has been previously demonstrated that there is a reduction in LPS-induced IL-6 levels with curcumin treatment in macrophages.39,40 Moreover, it was reported that curcumin inhibited the LPS-induced NF-κB activation through the prevention of Inhibitor κB degradation in RAW 264.7 cells.38 Thus, the downregulation of NF-κB activation can be one of the many mechanisms underlying the anti-inflammatory effects of the SLCPs.
The improved solubility of SLCP formulations compared with regular curcumin (Table 1) may be due to the amphiphilic nature of SLCP formulations that utilize phospholipids and other lipids to solubilize curcumin in aqueous solutions. In previous studies, similar types of formulations contributed to the creation of micelle-type physical structures that permit increased dissolution of lipophilic compounds. SLCP-1 and SLCP-2 differ with regard to powder size, and the differential solubility between them (Table 1) which may be due to the relative amount of surface area exposed to the aqueous medium. In this study, it was found that SLCP-2 possessed greater water solubility than SLCP-1, likely due to its decreased powder size and thus increased surface area exposed to the aqueous solution. However, both SLCPs possessed similar activity in vitro on inflammatory mediators. Future research may investigate the dissolution and micellar characteristics of these formulations and their interactions with cytokines on a molecular level. Overall, the increased aqueous solubility of the SLCP formulations compared with natural curcumin leads to a broader scope of possible formulations that can be utilized by the nutraceutical and functional food industries.
In toto, SLCP formulations concentration dependently mitigated the LPS-induced inflammatory response in macrophages, by downregulation of the production of the inflammatory markers NO, PGE2, and IL-6 through inhibition of NF-κB activation. SLCP-1 was slightly more effective in inhibiting the anti-inflammatory response than SLCP-2. Further investigations should explore the potential use of SLCP in the prevention and/or therapy of inflammation-linked diseases. However, future in vivo studies on SLCP are warranted to determine the clinical efficacy of these formulations of curcumin.
Supplementary Material
Acknowledgments
This project was partially supported by Verdure Sciences, who also kindly provided the SLCP formulations and curcumin extract. This work was also supported by grants from the National Institutes of Health (4R01ES016042, 5K22ES013782 to A.L.S.) for the supplies. Spectrophotometric data were acquired from an instrument located in the RI-INBRE core facility located at the University of Rhode Island (Kingston, RI, USA) obtained with Grant 5 P20 GM103430-13 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH).
Author Disclosure Statement
The authors declare that there are no competing financial interests.
References
- 1.Chen S: Natural products triggering biological targets—a review of the anti-inflammatory phytochemicals targeting the arachidonic acid pathway in allergy asthma and rheumatoid arthritis. Curr Drug Targets 2011;12:288–301 [DOI] [PubMed] [Google Scholar]
- 2.Kim MK, Kim K, Han JY, Lim JM, Song YS: Modulation of inflammatory signaling pathways by phytochemicals in ovarian cancer. Genes Nutr 2011;6:109–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ros E, Tapsell LC, Sabate J: Nuts and berries for heart health. Curr Atheroscler Rep 2010;12:397–406 [DOI] [PubMed] [Google Scholar]
- 4.Tan AC, Konczak I, Sze DM, Ramzan I: Molecular pathways for cancer chemoprevention by dietary phytochemicals. Nutr Cancer 2011;63:495–505 [DOI] [PubMed] [Google Scholar]
- 5.Tapsell LC, Hemphill I, Cobiac L, et al. : Health benefits of herbs and spices: the past, the present, the future. Med J Aust 2006;185(4 Suppl):S4–S24 [DOI] [PubMed] [Google Scholar]
- 6.Zeng H, Lazarova DL: Obesity-related colon cancer: dietary factors and their mechanisms of anticancer action. Clin Exp Pharmacol Physiol 2012;39:161–167 [DOI] [PubMed] [Google Scholar]
- 7.Zeilhofer HU, Brune K: Analgesic strategies beyond the inhibition of cyclooxygenases. Trends Pharmacol Sci 2006;27:467–474 [DOI] [PubMed] [Google Scholar]
- 8.Yoon W-J, Ham YM, Kim S-S, et al. : Suppression of pro-inflammatory cytokines, iNOS, and COX-2 expression by brown algae Sargassum micracanthum in RAW 264.7 macrophages. Eur Asian J Biosci 2009;3:130–143 [Google Scholar]
- 9.Del Rio D, Borges G, Crozier A: Berry flavonoids and phenolics: bioavailability and evidence of protective effects. Br J Nutr 2010;104 Suppl 3:S67–S90 [DOI] [PubMed] [Google Scholar]
- 10.Del Rio D, Costa LG, Lean ME, Crozier A: Polyphenols and health: what compounds are involved? Nutr Metab Cardiovasc Dis 2010;20:1–6 [DOI] [PubMed] [Google Scholar]
- 11.Ammon HP, Wahl MA: Pharmacology of Curcuma longa. Planta Med 1991;57:1–7 [DOI] [PubMed] [Google Scholar]
- 12.Bisht K, Choi WH, Park SY, Chung MK, Koh WS: Curcumin enhances non-inflammatory phagocytic activity of RAW264.7 cells. Biochem Biophys Res Commun 2009;379:632–636 [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal BB, Sung B: Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci 2009;30:85–94 [DOI] [PubMed] [Google Scholar]
- 14.Jagetia GC, Aggarwal BB: “Spicing up” of the immune system by curcumin. J Clin Immunol 2007;27:19–35 [DOI] [PubMed] [Google Scholar]
- 15.Shishodia S, Sethi G, Aggarwal BB: Curcumin: getting back to the roots. Ann NY Acad Sci 2005;1056:206–217 [DOI] [PubMed] [Google Scholar]
- 16.Olivera A, Moore TW, Hu F, et al. : Inhibition of the NF-kappaB signaling pathway by the curcumin analog, 3,5-Bis(2-pyridinylmethylidene)-4-piperidone (EF31): anti-inflammatory and anti-cancer properties. Int Immunopharmacol 2012;12:368–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao X, Kuo J, Jiang H, et al. : Immunomodulatory activity of curcumin: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production in vitro. Biochem Pharmacol 2004;68:51–61 [DOI] [PubMed] [Google Scholar]
- 18.Rasheed Z, Akhtar N, Anbazhagan AN, Ramamurthy S, Shukla M, Haqqi TM: polyphenol-rich pomegranate fruit extract (POMx) suppresses PMACI-induced expression of pro-inflammatory cytokines by inhibiting the activation of MAP Kinases and NF-kappaB in human KU812 cells. J Inflamm (Lond) 2009;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB: Bioavailability of curcumin: problems and promises. Mol Pharm 2007;4:807–818 [DOI] [PubMed] [Google Scholar]
- 20.Shehzad A, Wahid F, Lee YS: Curcumin in cancer chemoprevention: molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch Pharm (Weinheim) 2010;343:489–499 [DOI] [PubMed] [Google Scholar]
- 21.Cheng AL, Hsu CH, Lin JK, et al. : Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 2001;21:2895–2900 [PubMed] [Google Scholar]
- 22.Anand P, Nair HB, Sung B, et al. : Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem Pharmacol 2010;79:330–338 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS: Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 1998;64:353–356 [DOI] [PubMed] [Google Scholar]
- 24.Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A: Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnol 2007;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gota VS, Maru GB, Soni TG, Gandhi TR, Kochar N, Agarwal MG: Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J Agric Food Chem 2010;58:2095–2099 [DOI] [PubMed] [Google Scholar]
- 26.Dadhaniya P, Patel C, Muchhara J, et al. : Safety assessment of a solid lipid curcumin particle preparation: acute and subchronic toxicity studies. Food Chem Toxicol 2011;49:1834–1842 [DOI] [PubMed] [Google Scholar]
- 27.DiSilvestro RA, Joseph E, Zhao S, Bomser J: Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr J 2012;11:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma QL, Zuo X, Yang F, et al. : Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic, and behavioral deficits in aged human tau transgenic mice. J Biol Chem 2013;288:4056–4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Henry GE, Seeram NP: Identification and bioactivities of resveratrol oligomers and flavonoids from Carex folliculata seeds. J Agric Food Chem 2009;57:7282–7287 [DOI] [PubMed] [Google Scholar]
- 30.Legault J, Girard-Lalancette K, Grenon C, Dussault C, Pichette A: Antioxidant activity, inhibition of nitric oxide overproduction, and in vitro antiproliferative effect of maple sap and syrup from Acer saccharum. J Med Food 2010;13:460–468 [DOI] [PubMed] [Google Scholar]
- 31.Djoko B, Chiou RY, Shee JJ, Liu YW: Characterization of immunological activities of peanut stilbenoids, arachidin-1, piceatannol, and resveratrol on lipopolysaccharide-induced inflammation of RAW 264.7 macrophages. J Agric Food Chem 2007;55:2376–2383 [DOI] [PubMed] [Google Scholar]
- 32.Kurien BT, Singh A, Matsumoto H, Scofield RH: Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay Drug Dev Technol 2007;5:567–576 [DOI] [PubMed] [Google Scholar]
- 33.Hevel JM, Marletta MA: Nitric-oxide synthase assays. Methods Enzymol 1994;233:250–258 [DOI] [PubMed] [Google Scholar]
- 34.Karin M, Greten FR: NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 2005;5:749–759 [DOI] [PubMed] [Google Scholar]
- 35.Lee H, Cho H, Lim D, Kang Y, Lee K, Park J: Mechanisms by which licochalcone E exhibits potent anti-inflammatory properties: studies with phorbol ester-treated mouse skin and lipopolysaccharide-stimulated murine macrophages. Int J Mol Sci 2013;14:10926–10943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karin M: NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol 2009;1:a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brouet I, Ohshima H: Curcumin, an anti-tumour promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem Biophys Res Commun 1995;206:533–540 [DOI] [PubMed] [Google Scholar]
- 38.Pan MH, Lin-Shiau SY, Lin JK: Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IkappaB kinase and NFkappaB activation in macrophages. Biochem Pharmacol 2000;60:1665–1676 [DOI] [PubMed] [Google Scholar]
- 39.Zhao C, Yang J, Wang Y, et al. : Synthesis of mono-carbonyl analogues of curcumin and their effects on inhibition of cytokine release in LPS-stimulated RAW 264.7 macrophages. Bioorg Med Chem 2010;18:2388–2393 [DOI] [PubMed] [Google Scholar]
- 40.Guimaraes MR, Leite FR, Spolidorio LC, Kirkwood KL, Rossa C, Jr.: Curcumin abrogates LPS-induced pro-inflammatory cytokines in RAW 264.7 macrophages. Evidence for novel mechanisms involving SOCS-1, -3 and p38 MAPK. Arch Oral Biol 2013;58:1309–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.