Abstract
Traumatic brain injury (TBI) refers to brain damage resulting from external mechanical force, such as a blast or crash. Our current understanding of TBI is derived mainly from in vivo studies that show measurable biological effects on neurons sampled after TBI. Little is known about the early responses of brain cells during stimuli and which features of the stimulus are most critical to cell injury. We generated defined shear stress in a microfluidic chamber using a fast pressure servo and examined the intracellular Ca2+ levels in cultured adult astrocytes. Shear stress increased intracellular Ca2+ depending on the magnitude, duration, and rise time of the stimulus. Square pulses with a fast rise time (∼2 ms) caused transient increases in intracellular Ca2+, but when the rise time was extended to 20 ms, the response was much less. The threshold for a response is a matrix of multiple parameters. Cells can integrate the effect of shear force from repeated challenges: A pulse train of 10 narrow pulses (11.5 dyn/cm2 and 10 ms wide) resulted in a 4-fold increase in Ca2+ relative to a single pulse of the same amplitude 100 ms wide. The Ca2+ increase was eliminated in Ca2+-free media, but was observed after depleting the intracellular Ca2+ stores with thapsigargin suggesting the need for a Ca2+ influx. The Ca2+ influx was inhibited by extracellular Gd3+, a nonspecific inhibitor of mechanosensitive ion channels, but it was not affected by the more specific inhibitor, GsMTx4. The voltage-gated channel blockers, nifedipine, diltiazem, and verapamil, were also ineffective. The data show that the mechanically induced Ca2+ influx commonly associated with neuron models for TBI is also present in astrocytes, and there is a viscoelastic/plastic coupling of shear stress to the Ca2+ influx. The site of Ca2+ influx has yet to be determined.
Key words: : blast, intracellular Ca2+, mechanosensitive ion channel (MSC), shear stress, traumatic brain injury (TBI)
Introduction
Traumatic brain injury (TBI) results from an external mechanical force, such as a blast or crash. Traumatic injury is normally classified as a focal injury that results in cerebral contusions at a specific location or diffuse axonal injury over a widespread area, probably resulting from shear forces.1–4 The deformation caused by the shear forces in diffuse TBI is difficult to define because it occurs throughout the brain and is often mixed with secondary injuries resulting from subsequent biochemical and metabolic dysfunction.5,6 Most TBI patients exhibit mild or minimal observable damage upon the initial shock. However, even low-amplitude shear forces may induce biochemical processes that yield serious pathology hours and days later.7–9 To better understand the development of damage, we began an analysis of the primary causes of cellular responses to shear forces by using high-speed stimuli to establish causality.
Astrocytes are the most abundant cells in the brain and provide a critical link between the circulatory system and the maintenance of neurons (tripartite synapses, neurotransmitter processing, and so on).10 Although astrocytes have been considered less susceptible to injury than neurons, they can transmit mechanical forces to neurons and accept forces from them during TBI. Astrocytes can propagate damage signals through Ca2+ waves, signal to neurons through neurotransmitters, and alter ion concentrations in the extracellular space.10–12 Significantly, astrocyte death precedes neuronal death during TBI.13
The earliest measurable reaction of astrocytes to traumatic injury is elevation of intracellular Ca2+. Because astrocytes have cationic mechanosensitive ion channels (MSCs) that are permeable to Ca2+,12,14,15 mechanical stimuli (stretching or shear forces) can activate them and cause an increase in intracellular Ca2+.16 However, most studies have been conducted by stretching flexible substrates with adherent cultured cells17–19 or with fluid shear forces that are much slower than the shockwaves that produce TBI.18,20 Little is known about the coupling of intracellular Ca2+ responses to blast-like shear force in astrocytes.
A cell's response to mechanical insults is nonlinear, so the nature of the mechanical stimulus plays a crucial role in subsequent pathology.3,21,22 Using a blast chamber to apply rapid pressure and shear forces to cell cultures, Raven and colleagues showed that intracellular Ca2+ is more responsive to shear than to changes in pressure.23 Further, traumatic cell injury depends not only on the intensity of the loading, but also on the loading rate.24,25 Rapid shear deformations increase the membrane permeability to small dye molecules and also decrease cell viability.24 These studies suggest that cascades of cell responses are governed by multiple parameters.
In this work, we defined thresholds with multiple input parameters, including intensity, rise time, duration, duty cycle, and so on. We used tissue-cultured adult astrocytes in a microfluidic chamber where shear stress was controlled by a fast pressure servo,26 generating shear forces of known waveform while we measured the intracellular Ca2+ response. Pulsed shear with fast rise times (∼2 ms) were more effective than pulses with slower rise times defining the presence of an effective viscosity. Nonlinear summation was visible by the response of cells to repeated subthreshold stimuli. The multi-dimensional thresholds are summarized with a mathematical model. Using different pharmacological inhibitors, we found that the intracellular Ca2+ elevation involves influx pathways that do not include GsMTx4-sensitive MSCs or voltage-sensitive calcium channels.
Methods
Flow chamber and pressure servo pump
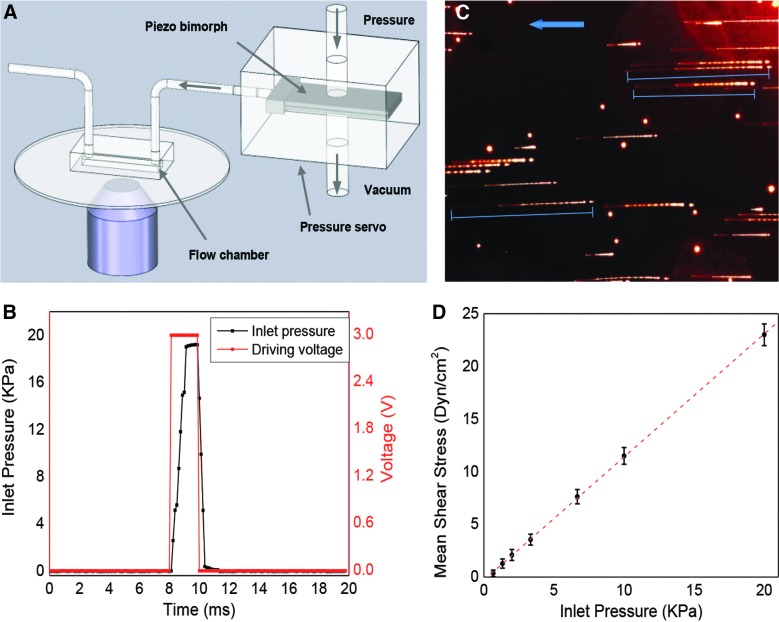
The microfluidic flow chamber is a parallel-plate flow system made of a 25-mm diameter cover-glass substrate and a 1-mm-thick glass slide separated by polydimethylsiloxane (PDMS) walls (Fig. 1A). The chamber dimensions were 1000 μm wide, 15 mm long, and 100 μm high. The device was fabricated using soft lithography following the fabrication process described previously.27 For inlet and outlet, holes were punched through the PDMS at the ends of the chamber. The chip was mounted on a home-made assembly that allowed fluorescence imaging. The chamber substrate was coated with human fibronectin (BD Biosciences, San Jose, CA) and rinsed 1 h before seeding the cells.
FIG. 1.
Calibration of shear stress in microfluidic chamber. (A) Schematic of experimental setup. High-speed pressure servo was connected to a microfluidic chamber to generate controlled fluid shear stimuli. (B) Comparison of triggering voltage pulse (red) and pressure rise (black) at inlet of the chamber, showing a rise time of 1 ms can be achieved. (C) Trajectory of microbeads under a flow pulse of 6.5 kPa for 10 ms. The image was collected from the middle plane of the chamber, ∼50 μm from the bottom. Blue arrow indicates flow direction. (D) Dependence of average shear stress on inlet pressure. Maximum flow rate was estimated from the length of the streaks and shear stress calculated as described in Methods. Each data point was averaged from >8 streaks in each frame for a total of 10 frames of four experiments. Error bar indicates standard error of the mean. Color image is available online at www.liebertpub.com/neu
An ultra-fast piezo-driven pressure servo (ALA Scientific Instruments, Farmingdale, NY) was used to generate pressure pulses of known waveform with a time resolution of ∼1 ms to drive the chamber.26 The stimuli included steps and ramps and the pressure was monitored at the chamber inlet to parameterize the shear stress. To mimic blast waves in the fluids, the waveform was chosen to closely resemble a classic Friedlander curve.28 Flow velocity was obtained experimentally by tracking fluorescent microbeads. Mean flow rate was estimated from the measured data following established theory.29 The wall shear stress, τ, was calculated using the empirical equation, 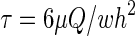 , where μ is the dynamic viscosity of the fluid, Q is the volume flow rate, and w and h are the width and the height of the chamber, respectively.30 Viscosity was assumed to be 10–3 Pa s.
, where μ is the dynamic viscosity of the fluid, Q is the volume flow rate, and w and h are the width and the height of the chamber, respectively.30 Viscosity was assumed to be 10–3 Pa s.
Cell culture
Primary adult astrocytes were isolated from gelatin-sponge implants from adult Sprague-Dawley rat brains provided by Dr. Thomas Langan (SUNY-Buffalo, Buffalo, NY). Astrocytes were maintained in Dulbecco's modified Eagle's medium, 10% fetal bovine serum, and 1% penicillin/streptomycin. Cells were transferred to the microfluidic chambers when the flasks reached 95% confluence and were cultured in the chamber for 3–5 days before the experiments. Media in the chamber were changed every 24 h to sustain the cell growth. To permit phenotypic maturation of astrocytes, they were only used in experiments between passages 4 and 12.31
Calcium dye loading and fluorescence imaging
Before the experiments, cells were gently washed with saline, followed by loading with the Ca2+-sensitive dye, Fluo-4 AM (5 μM; Invitrogen, Carlsbad, CA), and kept in an incubator (37°C with 95% air and 5% CO2) for 30 min. Chambers were then gently washed and returned to the incubator for ∼8 min to allow cleavage of the dye molecules. Normal saline was used as the shearing fluid. Fluorescence images were acquired using an inverted microscope (Axiovert 200M; Carl Zeiss, Jena, Germany) equipped with a charge-coupled device (CCD) camera (AxioCam MRm; Carl Zeiss) and a 10× objective lens. The filter set (excitation [Ex], 470/40 nm; emission [Em], 525/50 nm) was used for Ca2+ imaging. Time-lapse images were obtained using software (AxioVision; Carl Ziess). To calibrate the shear stress in the microchannel produced by the pressure pulse, fluorescent microspheres (1 μm, Sulfate Fluospheres; Invitrogen) were dissolved in the saline. The trajectories of the sphere under different pressure pulses were acquired using the filter set (Ex, 580/25; Em, 605/70).
Solutions and reagents
Normal saline containing 1 mM of CaCl2 was used as control solution. In Ca2+-free solutions, CaCl2 was replaced by MgCl2. Gadolinium chloride (Sigma-Aldrich, St. Louis, MO) was freshly prepared as 10-mM stock solution in control solution before each experiment. Nifedipine, cis-diltiazem hydrochloride, and verapamil hydrochloride (Sigma-Aldrich) were each dissolved in ethanol and then diluted in saline to 10 μM (ethanol in the final solution was less than 1:1000). The enantiomer forms of GsMTx4 were made and purified according to previously published protocols.32
Statistical analysis
Relative Ca2+ intensity was calculated using 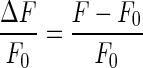 , where F and F0 are the mean fluorescence intensities of selected cells at time t and t=0, respectively. Background intensity was subtracted before the calculation. A new cell culture was used for each experiment. Mean calcium change was averaged over N selected cells from each image and over multiple experiments. The statistical analysis used the standard error of the mean. To summarize the multi-dimensional thresholds, the profile of peak Ca2+ intensity was fit with a two-state Boltzmann equation (Equation 1):
, where F and F0 are the mean fluorescence intensities of selected cells at time t and t=0, respectively. Background intensity was subtracted before the calculation. A new cell culture was used for each experiment. Mean calcium change was averaged over N selected cells from each image and over multiple experiments. The statistical analysis used the standard error of the mean. To summarize the multi-dimensional thresholds, the profile of peak Ca2+ intensity was fit with a two-state Boltzmann equation (Equation 1):
 |
where x and y are, respectively, the amplitude and duration of the shear pulse; a, m, n, and h are constants and were extracted from the fitting as a=32.55, m=0.44, n=0.096, and h=–6.54. The fidelity of the fitting was measured by R2, coefficient of determination, and R2≥0.95 for these fits.
Results
Shear stress calibration in flow chamber
We first calibrated the shear stress of various waveforms in the flow chamber without cells. Shear pulses of various amplitudes and durations were generated by the pressure servo connected to the flow chamber (Fig. 1A), and the pressure at the inlet of the chamber was measured using a pressure sensor. The pressure reached steady state value in ∼1 ms when a square pulse was applied (Fig. 1B). To visualize the flow profile, fluorescent microspheres (1 μm) were dissolved in the solution. A series of pressure pulses were applied to generate a pulsatile fluid flow. The pulse width was set to be narrower than the camera shutter open time for each frame, so that the trajectory length (marked by blue lines in Fig. 1C) represents the total traveling distance of the beads during the pulse. Figure 1C shows a typical image of bead traces with a pressure pulse of 6.5 kPa and 10-ms duration. To minimize hydraulic effects of the chamber walls, the images were taken from the midplane of the chamber (focus plane, z=50 μm) providing the maximum flow velocity. Note that there are two distinct segments along the streaks, a dimmer section (left end of the streak, Fig. 1C), representing the acceleration of the beads, and a brighter section (right end of the streak, Fig. 1C), representing the cruising and retraction. Each segment of the streaks was measured separately and the total length, averaged for all the beads in the field of view, was used to calculate maximum velocity in the chamber. The average velocity was estimated as half of the maximum velocity following the previous publication.29 Shear stress in the chamber at each applied pressure is calculated from measured velocity and shown in Figure 1D, where each data point was averaged from 10 frames of four experiments. To verify the rise time, the time required to reach the steady-state shear stress, we applied a series of narrow pressure pulses with various rise times and measured the linearity of shear force to the pulse amplitude (Supplementary Materials S1) (see online supplementary material at http://www.liebertpub.com). The data show a limiting rise time of 2 ms defining the instrumental limit. The amplitude of the shear stress was directly proportional to the amplitude of the pressure when the pulse width was longer than this limit (Fig. 1D).
Ca2+ response depends on both magnitude and duration of the stimuli
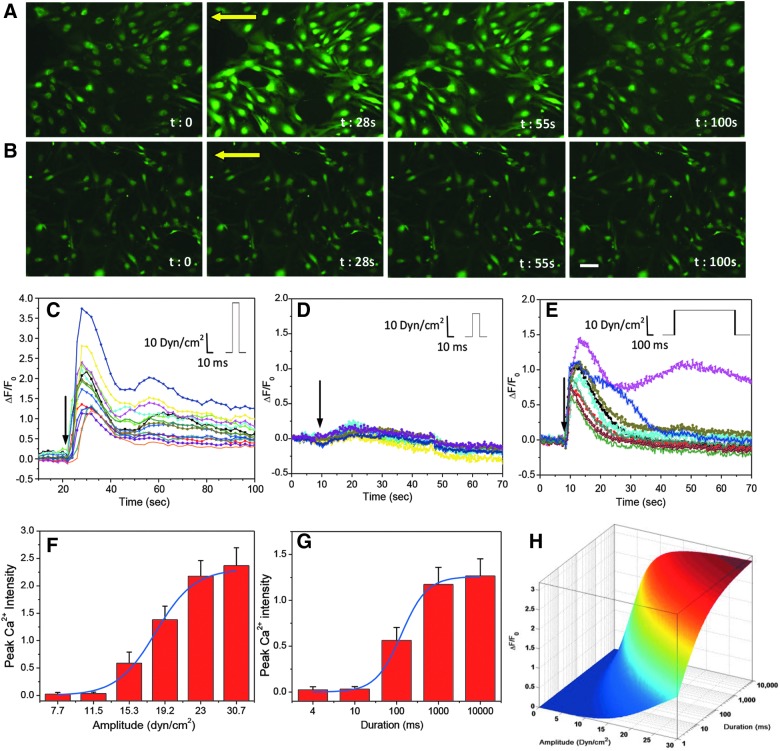
To measure whether there is a threshold of the shear force, we measured the intracellular Ca2+ changes with narrow pulses of varying magnitudes and durations. Shockwaves from a blast typically generate pulsed shear forces of ∼8-ms duration in the brain.23 We first used stimuli of 10-ms pulse width, 2-ms rise time, and magnitudes ranging from 11.5 to 23 dyn/cm2. Astrocytes responded to a shear of 23 dyn/cm2 with a transient Ca2+ increase (Fig. 2A), but clearly did not respond to 11.5 dyn/cm2 (Fig. 2B). The time course of changes in intracellular calcium concentration is shown in Figure 2C,D. The average peak change of cytosolic calcium was ∼250% at 23 dyn/cm2 (Fig. 2C), compared with ∼5% at 11.5 dyn/cm2 (Fig. 2D). Moreover, a large percentage of cells showed an instantaneous increase in calcium at the higher shear stresses (>95% at 23 dyn/cm2), whereas a small percentage responded to low stress (∼30% at 16 dyn/cm2). Thus, there are significant variations in the mechanical responses and Ca2+ coupling properties of the cells, and averaging across cell populations is unwise given the nonlinearity. At longer times, some cells showed responses from calcium waves spreading from activated neighboring cells, and we excluded those cells. We tested six amplitudes and the peak Ca2+ levels suggested an effective threshold of ∼11.5 dyn/cm2 (Fig. 2F). The molecular basis for the threshold is not known, but it may represent the force required to break some specific cytoskeletal bonds or the force required to open the Ca2+ influx channel.
FIG. 2.
Ca2+ response to shear pulses of different amplitudes and durations in astrocytes. (A and B) Fluorescence images of Fluo-4-loaded astrocytes subjected to a narrow shear pulse (10 ms duration and 2 ms rise time) of 23 (A) and 11.5 dyn/cm2 (B). Yellow arrows indicate flow direction. (C and D) Time-dependent changes in Ca2+ intensity from selected cells in (A) and (B), respectively. (E) Ca2+ responses to a pulse of lower amplitude (11.5 dyn/cm2), but with longer duration (1000 ms), showing that duration of the pulse affects the responses. (F and G) Statistics of peak Ca2+ intensity with pulses of different amplitude and duration (n=30 for each condition) and corresponding fitting curves using Equation 1. (H) Profile of stimulus thresholds (amplitude and duration), showing the lowest threshold for Ca2+ response is 11.5 dyn/cm2 for 10 ms. Scale bar represents 100 μm. Color image is available online at www.liebertpub.com/neu
Whereas the stimulus amplitude is a key parameter, the duration of the pulses also affects the response and together the two parameters establish a multi-dimensional threshold matrix for nominally square pulses. Working at a low amplitude of 11.5 dyn/cm2, we examined response to longer pulses. At 10 ms, there was still no Ca2+ response (Fig. 2D). However, when the pulse width increased to 1000 ms, there was a clear increase in Ca2+ (Fig. 2E). The peak Ca2+ level increased with duration, reaching a saturation at t=1s (Fig. 2G). We used a two-state Boltzmann equation (Equation 1) to fit the profile of peak Ca2+ intensity, and the fitting curves are shown overlapped with the experimental data for various amplitudes (Fig. 2F) and durations (Fig. 2G). The fitting constants, m=0.44 and n=0.096, respectively, indicate the significance of amplitude and duration, showing that amplitude is the dominant parameter. The multi-dimensional threshold of both the amplitude and duration is shown in Figure 2H.
Ca2+ response is sensitive to shear loading rate
In neurons cultured in three-dimensional (3D) matrices, a rapid increase of strain resulted in rapid Ca2+ elevation and a decrease in viability, but there were no significant changes in Ca2+ for slow increases in strain,25 suggesting an effective cellular viscosity. Using a similar in vitro model, Cater and colleagues showed that hippocampal cell death is independent of strain rate, suggesting that such rate dependence is not universal, or the sensitivity lies outside the experimental range.33
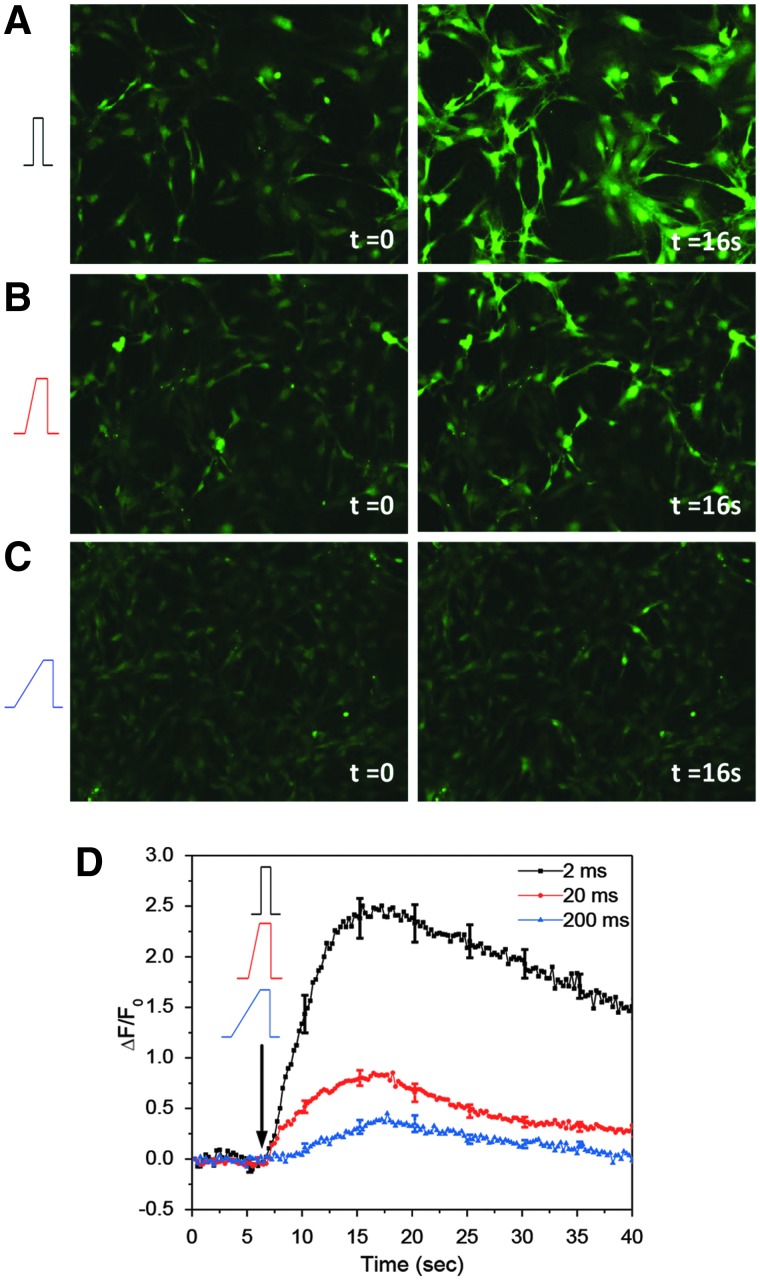
We tested Ca2+ responses to strain rate using high shear, 23 dyn/cm2, and 10-ms pulse width with rise times of 2, 20, and 200 ms. Stimuli were set so that the mean shear stress over the stimuli was constant. Responses differed drastically between rapid and slow rise times. With a rise time of 2 ms, cells showed a significant Ca2+ increase (Fig. 3A), peaking in ∼16 s (Fig. 3D, black curve). Response decreased with slower rise times (Fig. 3B,C). Peak calcium dropped from 220% with rise time of 2 ms to ∼3% with rise time of 200 ms of the same amplitude (Fig. 3D). Maximum sensitivity to strain rate was obtained for rise times lower than 20 ms and reached steady state at 200 ms. Clearly, there is an effective dashpot in series with the Ca2+ influx pathway(s).
FIG. 3.
Ca2+ responses to shear pulses of different rise times. (A–C) Images of Fluo-4-loaded astrocytes before (t=0) and after (t=16 sec) a shear stimulus (11.5 dyn/cm2, 10 ms) with rise times of 2 (A), 20 (B), and 200 ms (C). (D) Changes in Ca2+ intensity averaged over 30 cells from four experiments for each condition, showing that high strain rate (2-ms rise time) produces larger response, whereas the response is drastically reduced when the rise time is longer than 20 ms. Scale bar is 100 μm. Color image is available online at www.liebertpub.com/neu
Ca2+ response to repeated shear pulses
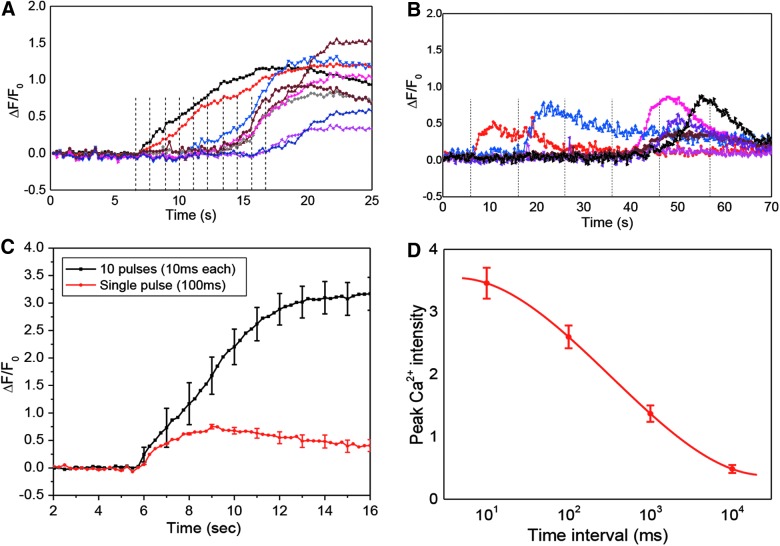
To determine whether cells have a memory of the previous events, we applied repetitive stimuli. The pulse train contained low-amplitude pulses of 11.5 dyn/cm2, with a rise time of 2 ms and duration of 10 ms that alone were not able to produce a Ca2+ rise (Fig. 2D). Each pulse train consisted of 10 pulses, separated by 10, 100, 1000, and 10,000 ms. Typical Ca2+ responses to a pulse train with 1-sec intervals is shown in Figure 4A. Individual cells were activated at different times with this train. At this frequency, ∼20% of cells responded after the second pulse, whereas a majority of cells (60%) responded to the sixth and later pulses. This finding shows that the mechanical effect is cumulative, so the internal stresses take a measurable time to relax. The frequency of the pulse train plays an important role. With short intervals of 10 ms, the pulse train caused a 340% increase of Ca2+, a response 4 times higher than a single pulse of the same amplitude with the same total duration of 100 ms (Fig. 4C). This suggests that an abrupt mechanical shock imposes a more activating effect than a sustained lower shear force, consistent with our observations from rise time studies (Fig. 3). This emphasizes the viscoelastic/plastic nature of the transduction process. At an interval of 10 sec, a time in which most of Ca2+ peaks would have fallen to the baseline, cells failed to respond with up to 10 consecutive pulses (Fig. 4B). Cells showed similar responses to a single pulse at low amplitude. Figure 4D shows peak Ca2+ with 10 consecutive pulses of various intervals, where we estimate the relaxation time to be ∼100 ms.
FIG. 4.
Ca2+ responses to repeated shear pulses. (A and B) Ca2+ responses to a train of 10 narrow pulses of 11.5 dyn/cm2 and 10-ms duration with rest intervals of 1 (A) and 10 sec (B). Dashed lines indicate the times when a pulse arrived. Note that cells can be activated by the subsequent pulses as shown in (A) and (B). (C) Comparison of Ca2+ responses to a train of 10 pulses of 11.5 dyn/cm2 and 10 ms wide, and a single pulse of the same amplitude and 100 ms wide, showing that abrupt shear force imposes more response to a sustained force. Each curve was averaged from 30 cells in four experiments. (D) Average peak Ca2+ intensity triggered by pulse trains of different time intervals (n=30) showing a relaxation time at ∼ 100 ms. Error bars indicate standard error of the mean. Color image is available online at www.liebertpub.com/neu
We conducted finite element analysis on a hyperviscoelastic cell model,34,35 and it showed that a shear pulse of 20 dyn/cm2 and 10-ms width produced a maximum strain of 16%, followed by an oscillatory decay with relaxation time of ∼100 ms (Supplementary Materials S2) (see online supplementary material at http://www.liebertpub.com), consistent with our experimental observations.
The response involves both Ca2+ influx and internal store release
It is postulated that the force-induced Ca2+ influx involves cationic MSCs14–16 because the Ca2+ influx can be triggered by many mechanical stimuli, including fluid shear stress,36 osmotic swelling,37 and stretching.18 The influx alone appears insufficient to account for the peak levels of Ca2+ given that the response is reduced in the presence of thapsigargin (Tg), suggesting a correlated Ca2+ release from internal stores.38 To determine whether the Ca2+ influx was through known mechanosensitive ion channels, we tried Gd3+, a nonspecific MSC blocker, and GsMTx4, a specific inhibitor for MSCs.14 Cells treated with 100 μM of Gd3+ or in Ca2+-free solution did not respond to the acute shear stimulus (23 dyn/cm2 for 10 ms; n=30; Fig. 5A). Disappointingly, 5 μM of GsMTx4 showed no significant inhibition, suggesting that there are other pathways for mechanically induced Ca2+ influx in astrocytes (Fig. 5A). Although there are MSCs that are sensitive to GsMTx4 in astrocytes,32,39 we have previously shown that there are multiple MSCs in cells, and swelling-induced Ca2+ entry is not sensitive to GsMTx4 in astrocytes.40 Shear responses were totally inhibited when extracellular calcium was removed and recovered after restoring calcium (Fig. 5B). These results consistently show that shear stress induces Ca2+ influx through currently undefined pathways.
FIG. 5.
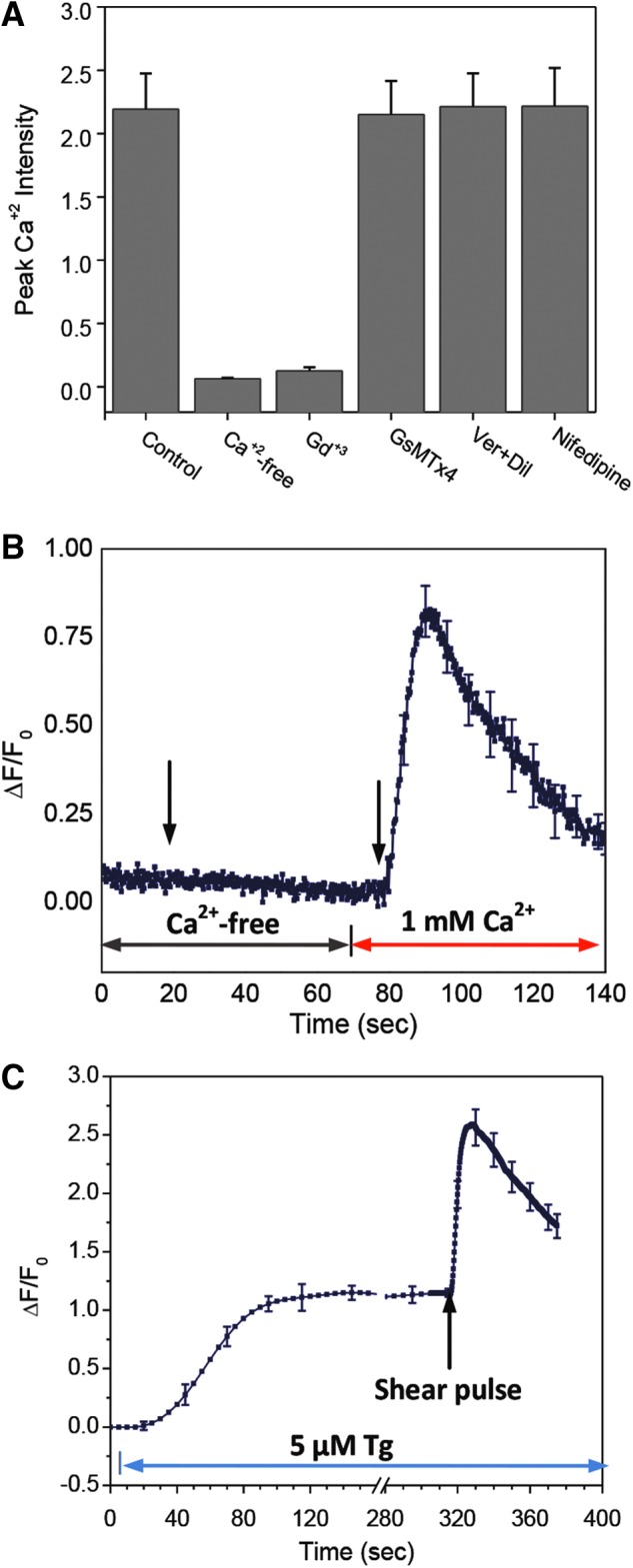
Effect of inhibitors on shear induced Ca2+ response. (A) Peak Ca2+ intensity in control, Ca2+-free, 10 μM Gd3+, 5 μM GsMTx4, 10 μM verapamil and 10 μM nifedipine solution. A shear pulse of 23 dyn/cm2 and 10 ms duration and 2 ms rise time was used for all experiments. The means are from n=30 cells from four experiments under each condition. (B) Ca2+ response to the same shear pulse in Ca2+-free solution, followed by the second pulse after adding 1 mM Ca2+ to the solution. The curve is averaged over 30 cells in 3 experiments. (C) Ca2+ response to 5 μM Tg. A shear stimulus was applied after the intensity reached a plateau, showing that the stimulus was able to induce significant Ca2+ influx after release of Ca2+ stores. The curve is averaged over 30 cells from 3 experiments of identical conditions. Error bars indicate standard error of the mean. Color image is available online at www.liebertpub.com/neu
To check on the role of Ca2+ stores, we depleted the stores with 5 μM of Tg before stimulation. Tg reduced the amplitude of the Ca2+ elevation, suggesting that both Ca2+ influx and store release contribute to response to shear stimulus. To estimate the role of each component, we treated cells with Tg (5 μM) to near total depletion, then applied the shear pulse. A superthreshold shear stimulus caused a transient Ca2+ peak (that fell within 60 sec) on top of the Tg-induced Ca2+ plateau (Fig. 5C). The ratio of the influx peak to the height of Tg-induced Ca2+ release was near 1:1, and the total Ca2+ level with shear (integrated over background) was comparable to controls under the same stimulation (Fig. 2C), showing that the transient Ca2+ influx contributed ∼50% of the response. The Ca2+ increase, not surprisingly, had two components; the first peak occurred immediately poststimulation as a result of the influx of Ca2+ and slowly inactivated (or desensitized) over ∼60 sec; the second peak (in many cases, a shoulder of the first peak) was mainly from the release of Ca2+ from intracellular stores.
Discussion
Our results show that there is a threshold shear stress (∼11.5 dyn/cm2 for 10 ms) for Ca2+ activation in cultured astrocytes (Fig. 2F). Although the magnitude of the shear forces is the dominant parameter of the stimulus, there are finite relaxation times present so that changes in stimulus duration or rise time can lead to a response from subthreshold stimuli. A complex map of thresholds containing amplitude and duration is shown in Figure 2H. The significance of each stimulus property was evaluated by fitting the profile of Ca2+ response with a two-state Boltzmann equation (Equation 1).
The force loading kinetics (rise time of the pulse) plays an essential role in Ca2+ response. This is evidenced by two experiments: First, an abrupt increase in force caused the Ca2+ rise, but a slow increase to the same amplitude failed to activate the cells (Fig. 3D). Second, Ca2+ intensity after 10 narrow pulses is 4 times the intensity for one pulse of the same total duration (Fig. 4C). These results demonstrate that the cell's response to mechanical inputs is highly nonlinear, and the viscoelastic/plastic nature of cells plays a role in the activation of the mechanosensitive Ca2+ pathway. Cells withstand mechanical loading through support from cytoskeleton, a heterogeneous, anisotropic collection of dynamically cross-linked structural proteins.41–45 The deformation of a cell depends on the intrinsic elastic deformation of fibrous cytoskeletal proteins and plastic deformation involving dynamics of reversible cross-linking and cytoskeleton reorganization.46,47 Slow shear loading engages the plastic processes that significantly modify the local force around the Ca2+ transducers acting like dashpots in series with the transducer. The strain-rate dependence of cell injury has been observed in 3D matrix cultured neurons and astrocytes, showing an increase in membrane permeability to small molecules and an increase in post-insult cell death.24,29 However, this effect was not observed in hippocampal tissues under biaxial stretch at strain rates ranging from 0.1 to 50 s–1,33 and we have showed that dye leakage in stretched dystrophic myocytes could be inhibited by GsMTx4, suggesting that the membrane breakdown was secondary to the applied stress.48 Different cell types have different properties, which is not surprising.49 Our results show that the sensitivity to strain rate decays rapidly with the increase of rise time. The strain rate used in Cater and colleagues is in the range of 0.1–50 s–1, corresponding to the rise times ranging from 20 to 10,000 ms, which is much longer than our rise times (2–200 ms; Fig. 3D).
Cells become more susceptible to shear force when subjected to multiple subthreshold challenges of short interval. The data suggest that cells possess slow relaxation rates with an effective memory time of ∼10 sec (Fig. 4D). Although cytoplasmic solution viscosity may participate, it is more likely that the slow responses are due to cytoskeletal bonds breaking and reforming. The dynamic deformation of the cells was evaluated using a finite element model considering cells as hyper-viscoelastic material. The model shows that the maximum deformation occurs at the end of the pressure pulse suggesting that the cells do not use a fast adaptation system (Supplementary Materials S2) (see online supplementary material at http://www.liebertpub.com). The oscillatory relaxation of the cell has a time constant of ∼100 ms, which is consistent with our experimental results. Strain distribution clearly varies with space, time, and stimulus properties. This knowledge of the critical stimulus parameters may allow improvement of protective helmets.
It has been postulated that the force-induced Ca2+ entry is through mechanosensitive pathways, including cationic MSCs and hemichannels, followed by a release of Ca2+ from intracellular stores.14–16,50 In astrocytes, injury-induced Ca2+ elevation has been studied using a stretching model and Ca2+ variations have been reported to last for minutes to hours.18,19 We do not know the messenger pathways and effectors that are modulating this response at these slow time scales. Further, different stimuli—stretching or fluid shear—may change severity and types of injury.
Fast, controllable shear stimuli allowed us to resolve initial triggering mechanisms and assay subsequent responses. Our results show the existence of a two-step process: a primary calcium entry causing a transient Ca2+ peak and a Ca2+ release that lasts for longer times (Fig. 2C). Ca2+ response is clearly initiated by a Ca2+ influx through pathways that have yet to be defined. The response can be blocked completely by Gd3+, a general inhibitor of many MSCs51 and a modulator of bilayer structure (Fig. 5).52,53 We have shown previously that MSCs of different types exist in astrocytes, and different MSCs can be used by cells to deal with different stimuli.40 In astrocytes, cation currents activated by pressure applied to a patch can be blocked by GsMTx4, a known inhibitor specific to cationic MSCs,54 whereas Ca2+ influx activated by swelling cannot be blocked.40 The insensitivity to GsMTx4 of both shear-induced Ca2+ response and anisotonic stimulation suggests that they may have a common pathway. The insensitivity to various voltage-gated channel blockers shows that those channels are not involved in shear stimulation of Ca2+ influx in astrocytes.
The Ca2+ induced Ca2+ release from intracellular stores was common in our experiments, where the two peaks (Ca2+ entry and Ca2+ release) normally overlap. It has been shown that because of positive feedback, Ca2+ induced Ca2+ release appears as an “all-or-none” process where enough calcium is required to initiate the release of calcium from stores.55–57 This suggests an alternative explanation for the extended Ca2+ peak. Under mild shear force, if a transient Ca2+ flux does not meet the threshold of Ca2+ store release level, it may not be able to activate the release. In this case, the accumulation of small amount of Ca2+ from subthreshold influxes can be integrated in the endoplasmic reticulum (ER) to later provide Ca2+ release supporting large changes in Ca2+.
Despite these clear findings on the primary responses, the question arises as to why pathological responses can develop with such long delay times. After TBI stimuli, astrocytes swell58,59 and undergo phenotypic changes with increased expression of glial-specific proteins, such as glial fibrillary acidic protein (GFAP).60–62 The swelling appears within an hour after the stimulus.63 Whereas it is generally believed that the swelling is associated with the accumulation of potassium ions and a reduction in extracellular sodium, calcium, and chloride,58,64 most of the ion transport is through Ca2+-dependent pathways. Thus, the early Ca2+ uptake may play a key role in cell volume and cell volume regulation. Our data suggest one or multiple mechanosensitive pathways may be accountable for Ca2+ uptake and volume regulation.40 Possible candidates include transient receptor potential (TRP)V465 and/or TRPC1,16 which are reported to respond to mechanical inputs in astrocytes. The phenotypic changes, that is, the formation of reactive astrogliosis through up-regulation of intermediate filament proteins such as GFAP, occur within a few hours of TBI.66 Although the role of Ca2+ homeostasis in reactive astrogliosis is not clear, it has been shown that several Ca2+-permeable channels, including TRPV4 and L-type Ca2+ channels, are up-regulated in reactive astrocytes.65,67 A recent study showed that traumatic scratch injury to astrocytes triggers Ca2+ influx, resulting in an increase in GFAP expression and increase in the size of glial scars.68 It suggests that Ca2+ provides the early injury signal that triggers the signal transduction cascades for astrogliosis through ER Ca2+ store release and this is consistent with our results. We provided direct evidence of such a Ca2+ pathway, and we further showed that activation of such Ca2+ responses is heterogeneous, depending on the stimulus properties.
Unlike in vivo models that measure the amplitude of total disrupting force and its duration and judge the injury by concussion or cell death,13,69 our model has the ability to generate uniform and defined stimuli to a cell population and measure the earliest response before adaptation or inactivation sets in. However, the in vitro model has obvious deviations from in vivo conditions, including difference in extracellular matrix and local environments as well as lack of interaction with neurons and the chemical environment. We plan on extending the model by culturing the stimulated astrocytes for long times to see whether downstream damage occurs and under which stimulus conditions. If pathological responses do not happen, that may suggest that cellular interactions are necessary to create the pathology, and by coculturing neurons and astrocytes we can begin to address that issue.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant NS085517. The authors thank Dr. Paul Blank at the NIH for the encouragement and useful discussion at the beginning of the project.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Garman R.H., Jenkins L.W., Switzer R.C., 3rd, Bauman R.A., Tong L.C., Swauger P.V., Parks S.A., Ritzel D.V., Dixon C.E., Clark R.S., Bayir H., Kagan V., Jackson E.K., and Kochanek P.M. (2011). Blast exposure in rats with body shielding is characterized primarily by diffuse axonal injury. J. Neurotrauma 28, 947–959 [DOI] [PubMed] [Google Scholar]
- 2.Sanborn B., Nie X., Chen W., and Weerasooriya T. (2012). Inertia effects on characterization of dynamic response of brain tissue. J. Biomech. 45, 434–439 [DOI] [PubMed] [Google Scholar]
- 3.Chen Y.C., Smith D.H., and Meaney D.F. (2009). In-vitro approaches for studying blast-induced traumatic brain injury. J. Neurotrauma 26, 861–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maas A.I., Stocchetti N., and Bullock R. (2008). Moderate and severe traumatic brain injury in adults. Lancet Neurol. 7, 728–741 [DOI] [PubMed] [Google Scholar]
- 5.Young J.A. (2012). Pharmacotherapy for traumatic brain injury: focus on sympathomimetics. Pharmacol. Ther. 134, 1–7 [DOI] [PubMed] [Google Scholar]
- 6.North S.H., Shriver-Lake L.C., Taitt C.R., and Ligler F.S. (2012). Rapid analytical methods for on-site triage for traumatic brain injury. Annu. Rev. Anal. Chem. (Palo Alto Calif) 5, 35–56 [DOI] [PubMed] [Google Scholar]
- 7.Koliatsos V.E., Cernak I., Xu L., Song Y., Savonenko A., Crain B.J., Eberhart C.G., Frangakis C.E., Melnikova T., Kim H., and Lee D. (2011). A mouse model of blast injury to brain: initial pathological, neuropathological, and behavioral characterization. J. Neuropathol. Exp. Neurol. 70, 399–416 [DOI] [PubMed] [Google Scholar]
- 8.Saljo A., Svensson B., Mayorga M., Hamberger A., and Bolouri H. (2009). Low-level blasts raise intracranial pressure and impair cognitive function in rats. J. Neurotrauma 26, 1345–1352 [DOI] [PubMed] [Google Scholar]
- 9.Kan E.M., Ling E.A., and Lu J. (2012). Microenvironment changes in mild traumatic brain injury. Brain Res. Bull. 87, 359–372 [DOI] [PubMed] [Google Scholar]
- 10.Chen Y., and Swanson R.A. (2003). Astrocytes and brain injury. J. Cereb. Blood Flow Metab. 23, 137–149 [DOI] [PubMed] [Google Scholar]
- 11.Niggel J., Sigurdson W., and Sachs F. (2000). Mechanically induced calcium movements in astrocytes, bovine aortic endothelial cells and C6 glioma cells. J. Membr. Biol. 174, 121–134 [DOI] [PubMed] [Google Scholar]
- 12.Ostrow L.W., and Sachs F. (2005). Mechanosensation and endothelin in astrocytes—hypothetical roles in CNS pathophysiology. Brain Res. Brain Res. Rev. 48, 488–508 [DOI] [PubMed] [Google Scholar]
- 13.Zhao X., Ahram A., Berman R.F., Muizelaar J.P., and Lyeth B.G. (2003). Early loss of astrocytes after experimental traumatic brain injury. Glia 44, 140–152 [DOI] [PubMed] [Google Scholar]
- 14.Suchyna T.M., Tape S.E., Koeppe R.E., Andersen O.S., Sachs F., and Gottlieb P.A. (2004). Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature 430, 235–240 [DOI] [PubMed] [Google Scholar]
- 15.Ostrow L.W., Suchyna T.M., and Sachs F. (2011). Stretch induced endothelin-1 secretion by adult rat astrocytes involves calcium influx via stretch-activated ion channels (SACs). Biochem. Biophys. Res. Commun. 410, 81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malarkey E.B., Ni Y., and Parpura V. (2008). Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia 56, 821–835 [DOI] [PubMed] [Google Scholar]
- 17.Rzigalinski B.A., Liang S., McKinney J.S., Willoughby K.A., and Ellis E.F. (1997). Effect of Ca2+ on in vitro astrocyte injury. J. Neurochem. 68, 289–296 [DOI] [PubMed] [Google Scholar]
- 18.Rzigalinski B.A., Weber J.T., Willoughby K.A., and Ellis E.F. (1998). Intracellular free calcium dynamics in stretch-injured astrocytes. J. Neurochem. 70, 2377–2385 [DOI] [PubMed] [Google Scholar]
- 19.Alford P.W., Dabiri B.E., Goss J.A., Hemphill M.A., Brigham M.D., and Parker K.K. (2011). Blast-induced phenotypic switching in cerebral vasospasm. Proc. Natl. Acad. Sci. U. S. A. 108, 12705–12710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber J.T., Rzigalinski B.A., and Ellis E.F. (2001). Traumatic injury of cortical neurons causes changes in intracellular calcium stores and capacitative calcium influx. J. Biol. Chem. 276, 1800–1807 [DOI] [PubMed] [Google Scholar]
- 21.Chung R.S., Staal J.A., McCormack G.H., Dickson T.C., Cozens M.A., Chuckowree J.A., Quilty M.C., and Vickers J.C. (2005). Mild axonal stretch injury in vitro induces a progressive series of neurofilament alterations ultimately leading to delayed axotomy. J. Neurotrauma 22, 1081–1091 [DOI] [PubMed] [Google Scholar]
- 22.Geddes-Klein D.M., Schiffman K.B., and Meaney D.F. (2006). Mechanisms and consequences of neuronal stretch injury in vitro differ with the model of trauma. J. Neurotrauma 23, 193–204 [DOI] [PubMed] [Google Scholar]
- 23.Ravin R., Blank P.S., Steinkamp A., Rappaport S.M., Ravin N., Bezrukov L., Guerrero-Cazares H., Quinones-Hinojosa A., Bezrukov S.M., and Zimmerberg J. (2012). Shear forces during blast, not abrupt changes in pressure alone, generate calcium activity in human brain cells. PLoS One 7, e39421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cullen D.K., Vernekar V.N., and LaPlaca M.C. (2011). Trauma-induced plasmalemma disruptions in three-dimensional neural cultures are dependent on strain modality and rate. J. Neurotrauma 28, 2219–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaPlaca M.C., Cullen D.K., McLoughlin J.J., and Cargill R.S., 2nd. (2005). High rate shear strain of three-dimensional neural cell cultures: a new in vitro traumatic brain injury model. J. Biomech. 38, 1093–1105 [DOI] [PubMed] [Google Scholar]
- 26.Besch S.R., Suchyna T., and Sachs F. (2002). High-speed pressure clamp. Pflügers Arch. Eur. J. Phys. 445, 161–166 [DOI] [PubMed] [Google Scholar]
- 27.Rahimzadeh J., Meng F., Sachs F., Wang J., Verma D., and Hua S.Z. (2011). Real-time observation of flow-induced cytoskeletal stress in living cells. Am. J. Physiol. Cell Physiol. 301, C646–C652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brode H.L. (1959). Blast wave from a spherical charge. Phys. Fluids 2, 217–229 [Google Scholar]
- 29.Tamayol A., and Bahrami M. (2009). Analytical solutions for laminar fully-developed flow in microchannels with non-circular cross-section. Proceedings of the ASME Fluids Engineering Division Summer Meeting (FEDSM2009), August2–5, Vail, Colorado [Google Scholar]
- 30.Wang J., Heo J., and Hua S.Z. (2010). Spatially resolved shear distribution in microfluidic chip for studying force transduction mechanisms in cells. Lab on a Chip 10, 235–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao S.L., and Chen C.J. (2001). Tyrosine kinase signaling involves in glutamate-induced astrocyte proliferation. Neuroreport 12, 3519–3522 [DOI] [PubMed] [Google Scholar]
- 32.Ostrow K.L., Mammoser A., Suchyna T., Sachs F., Oswald R., Kubo S., Chino N., and Gottlieb P.A. (2003). cDNA sequence and in vitro folding of GsMTx4, a specific peptide inhibitor of mechanosensitive channels. Toxicon 42, 263–274 [DOI] [PubMed] [Google Scholar]
- 33.Cater H.L., Sundstrom L.E., and Morrison B., 3rd (2006). Temporal development of hippocampal cell death is dependent on tissue strain but not strain rate. J. Biomech. 39, 2810–2818 [DOI] [PubMed] [Google Scholar]
- 34.Gu L.X., Chafi M.S., Ganpule S., and Chandra N. (2012). The influence of heterogeneous meninges on the brain mechanics under primary blast loading. Compos. Part B Eng. 43, 3160–3166 [Google Scholar]
- 35.Rashid B., Destrade M., and Gilchrist M.D. (2012). Mechanical characterization of brain tissue in compression at dynamic strain rates. J. Mech. Behav. Biomed. 10, 23–38 [DOI] [PubMed] [Google Scholar]
- 36.Jensen M.E., Odgaard E., Christensen M.H., Praetorius H.A., and Leipziger J. (2007). Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J. Am. Soc. Nephrol. 18, 2062–2070 [DOI] [PubMed] [Google Scholar]
- 37.Haeberle H., Bryan L.A., Vadakkan T.J., Dickinson M.E., and Lumpkin E.A. (2008). Swelling-activated Ca2+ channels trigger Ca2+ signals in Merkel cells. PLoS One 3, e1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verkhratsky A., and Kettenmann H. (1996). Calcium signalling in glial cells. Trends Neurosci. 19, 346–352 [DOI] [PubMed] [Google Scholar]
- 39.Bowman C.L., Gottlieb P.A., Suchyna T.M., Murphy Y.K., and Sachs F. (2007). Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: history, properties, mechanisms and pharmacology. Toxicon 49, 249–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hua S.Z., Gottlieb P.A., Heo J., and Sachs F. (2010). A mechanosensitive ion channel regulating cell volume. Am. J. Physiol. Cell Physiol. 298, C1424–C1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verma D., Ye N.N., Meng F.J., Sachs F., Rahimzadeh J., and Hua S.Z. (2012). Interplay between cytoskeletal stresses and cell adaptation under chronic flow. PLoS One 7, e44167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meng F., and Sachs F. (2011). An orientation-based FRET sensor for real-time imaging of cellular forces. J. Cell Sci. 125, 743–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meng F., and Sachs F. (2011). Measuring strain of structural proteins in vivo in real time, in: Cardiac Mechano-Electric Coupling and Arrhythmia: From Pipette to Patient. Kohl P., Sachs F., and Franz M.R. (eds). Oxford University Press: Oxford, UK, pps. 431–434 [Google Scholar]
- 44.Meng F.J., and Sachs F. (2011). Visualizing dynamic cytoplasmic forces with a compliance-matched FRET sensor. J.Cell Sci. 124, 261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahimzadeh J., Meng F., Sachs F., Wang J., Verma D., and Hua S.Z. (2011). Real-time observation of flow-induced cytoskeletal stress in living cells. Am. J. Physiol. Cell Physiol. 301, C646–C652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gardel M.L., Shin J.H., MacKintosh F.C., Mahadevan L., Matsudaira P., and Weitz D.A. (2004). Elastic behavior of cross-linked and bundled actin networks. Science (New York, NY: ) 304, 1301–1305 [DOI] [PubMed] [Google Scholar]
- 47.Bursac P., Lenormand G., Fabry B., Oliver M., Weitz D.A., Viasnoff V., Butler J.P., and Fredberg J.J. (2005). Cytoskeletal remodelling and slow dynamics in the living cell. Nat. Mater. 4, 557–561 [DOI] [PubMed] [Google Scholar]
- 48.Yeung E.W., Whitehead N.P., Suchyna T.M., Gottlieb P.A., Sachs F., and Allen D.G. (2005). Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J. Physiol. 562, 367–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y., Meng F., and Sachs F. (2011). Genetically encoded force sensors for measuring mechanical forces in proteins. Commun. Integr. Biol. 4, 385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Bock M., Decrock E., Wang N., Bol M., Vinken M., Bultynck G., and Leybaert L. (2014). The dual face of connexin-based astroglial Ca communication: a key player in brain physiology and a prime target in pathology. Biochim. Biophys. Acta 1843, 2211–2232 [DOI] [PubMed] [Google Scholar]
- 51.Caldwell R.A., Clemo H.F., and Baumgarten C.M. (1998). Using gadolinium to identify stretch-activated channels: technical considerations. Am. J. Physiol. 275, C619–C621 [DOI] [PubMed] [Google Scholar]
- 52.Ermakov Y.A., Averbakh A.Z., Yusipovich A.I., and Sukharev S. (2001). Dipole potentials indicate restructuring of the membrane interface induced by gadolinium and beryllium ions. Biophys. J. 80, 1851–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ermakov Y., Averbakh A.Z., and Sukharev S.I. (1997). Lipid and cell membranes in the presence of gadolinium and other ions with high affinity to lipids. 1. Dipole and diffuse components of the boundary potential. Membr. Cell Biol. 11, 539–554 [PubMed] [Google Scholar]
- 54.Suchyna T.M., Markin V.S., and Sachs F. (2009). Biophysics and structure of the patch and the gigaseal. Biophys. J. 97, 738–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Usachev Y.M., and Thayer S.A. (1997). All-or-none Ca2+ release from intracellular stores triggered by Ca2+ influx through voltage-gated Ca2+ channels in rat sensory neurons. J. Neurosci. 17, 7404–7414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verkhratsky A., and Shmigol A. (1996). Calcium-induced calcium release in neurones. Cell Calcium 19, 1–14 [DOI] [PubMed] [Google Scholar]
- 57.Jackson J.G., and Thayer S.A. (2006). Mitochondrial modulation of Ca2+-induced Ca2+-release in rat sensory neurons. J. Neurophysiol. 96, 1093–1104 [DOI] [PubMed] [Google Scholar]
- 58.Kimelberg H.K. (1992). Astrocytic edema in CNS trauma. J. Neurotrauma 9, Suppl. 1, S71–S81 [PubMed] [Google Scholar]
- 59.Reinert M., Khaldi A., Zauner A., Doppenberg E., Choi S., and Bullock R. (2000). High extracellular potassium and its correlates after severe head injury: relationship to high intracranial pressure. Neurosurg. Focus 8, e10. [DOI] [PubMed] [Google Scholar]
- 60.Myer D.J., Gurkoff G.G., Lee S.M., Hovda D.A., and Sofroniew M.V. (2006). Essential protective roles of reactive astrocytes in traumatic brain injury. Brain 129, 2761–2772 [DOI] [PubMed] [Google Scholar]
- 61.Laird M.D., Vender J.R., and Dhandapani K.M. (2008). Opposing roles for reactive astrocytes following traumatic brain injury. Neurosignals 16, 154–164 [DOI] [PubMed] [Google Scholar]
- 62.Wilhelmsson U., Bushong E.A., Price D.L., Smarr B.L., Phung V., Terada M., Ellisman M.H., and Pekny M. (2006). Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc. Natl. Acad. Sci. U. S. A. 103, 17513–17518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dietrich W.D., Alonso O., and Halley M. (1994). Early microvascular and neuronal consequences of traumatic brain injury: a light and electron microscopic study in rats. J. Neurotrauma 11, 289–301 [DOI] [PubMed] [Google Scholar]
- 64.Kimelberg H.K., Rutledge E., Goderie S., and Charniga C. (1995). Astrocytic swelling due to hypotonic or high K+ medium causes inhibition of glutamate and aspartate uptake and increases their release. J. Cereb. Blood Flow Metab. 15, 409–416 [DOI] [PubMed] [Google Scholar]
- 65.Butenko O., Dzamba D., Benesova J., Honsa P., Benfenati V., Rusnakova V., Ferroni S., and Anderova M. (2012). The increased activity of TRPV4 channel in the astrocytes of the adult rat hippocampus after cerebral hypoxia/ischemia. PLoS One 7, e39959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pekny M., and Nilsson M. (2005). Astrocyte activation and reactive gliosis. Glia 50, 427–434 [DOI] [PubMed] [Google Scholar]
- 67.Daschil N., Obermair G.J., Flucher B.E., Stefanova N., Hutter-Paier B., Windisch M., Humpel C., and Marksteiner J. (2013). CaV1.2 calcium channel expression in reactive astrocytes is associated with the formation of amyloid-beta plaques in an Alzheimer's disease mouse model. J. Alzheimers Dis. 37, 439–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao K., Wang C.R., Jiang F., Wong A.Y., Su N., Jiang J.H., Chai R.C., Vatcher G., Teng J., Chen J., Jiang Y.W., and Yu A.C. (2013). Traumatic scratch injury in astrocytes triggers calcium influx to activate the JNK/c-Jun/AP-1 pathway and switch on GFAP expression. Glia 61, 2063–2077 [DOI] [PubMed] [Google Scholar]
- 69.Greenwald R.M., Gwin J.T., Chu J.J., and Crisco J.J. (2008). Head impact severity measures for evaluating mild traumatic brain injury risk exposure. Neurosurgery 62, 789–798; discussion, 798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.