Abstract
Background: In lymphedema, tissue fluid steadily accumulates in the subcutaneous space containing loose connective tissue. We documented previously that deformation of the structure of subcutaneous collagen bundles and fat by excess fluid leads to formation of “lakes” and interconnected channels with irregular shape. Since there is no force that could mobilize and propel stagnant fluid to the regions where lymphatics absorb and contract, this task should be taken over by external massage. The most effective in this respect seems to be the sequential intermittent pneumatic compression (IPC).
Aim: The aim of the study was to observe whether IPC would enhance and accelerate formation of tissue fluid channels.
Methods: Together with the Biocompression Systems (Moonachie, NJ), we designed a high pressure intermittent compression device and used in it our therapy protocol for patients with obstructive lymphedema of lower limbs. The study was carried out on 18 patients with lymphedema stages II–IV. The IPC was applied daily for 1–2 hours. The follow up time was 24–36 months. Lymphoscintigraphy and immunohistopathology of tissue biopsies were used for evaluation of channel formation process.
Results: The forced fluid flow brought about increase of the area of fluid channels in the thigh and groin, with a decrease in the calf. Concomitantly, with decrease of channel area in the calf, there was a decrease of calf circumference. No new lymphatic collectors were observed.
Conclusions: Compression of limb lymphedema tissues leads to formation of tissue channels as pathways for evacuation of edema fluid.
Introduction
Lymphedema of lower limbs is the effect of lack of tissue fluid drainage caused by obliteration of lymphatic collectors. The most common etiology of this condition is skin and deep tissue inflammation, soft tissues mechanical trauma, and lymphadenectomy and/or radiotherapy in cancer. Even if some fragments of lymphatics remain patent, their transport capacity is not sufficient for evacuation of excess tissue fluid. Tissue fluid steadily accumulates mostly in the subcutaneous space containing loose connective tissue. Deformation of the structure of subcutaneous collagen bundles and fat by excess fluid leads to formation of “lakes” and either blind or interconnected channels with irregular shapes.1 These channels take over the function of collectors; however, there is no force propelling fluid.
Tissue channels can be observed on lymphoscintigrams of the subcutaneous tissue. Two pictures are usually seen. One of the so-called “dermal backflow”, previously observed on color or classic oily lymphograms, is a picture of dilated subepidermal lymphatics, whereas the other honeycomb image is accumulation of tracer in the expanded subcutaneous channels.2
In advanced stages of lymphedema, tissue fluid spaces can also be found in the thickened perimuscular fascia. Since there is no force that could mobilize and propel stagnant fluid to the regions where lymphatics absorb and contract, this task should be taken over by external massage. The most effective in this respect is sequential intermittent pneumatic compression (IPC), combined with elastic garments encompassing the swollen limb.3
The hydrodynamic mechanism of pneumatic compression is still unclear. Does it mechanically mobilize and propel fluid, and if so, what are the flow pathways? Since lymphatic collectors are obliterated, fluid cannot be pushed into them and alternative paths should be created. We showed that, in lymphedema, high external forces move fluid along anatomical structures but not lymphatics.2
The question arises whether long-term application of sequential high external pressure compression may enhance and accelerate the process of formation tissue channels for edema fluid flow. A number of physical conditions should be met to propel tissue fluid toward the root of the limb. These are: generation of effective transmural pressures, overcoming low hydraulic conductivity of the subcutaneous tissue, securing unidirectional proximal flow, and prevention of backflow.4
Together with Biocompression Systems (Moonachie, NJ), we designed a compression device meeting these conditions and used it in our therapy protocol for patients with obstructive lymphedema of lower limbs. The moving fluid brought about increase of the area of fluid channels in the thigh and groin, as measured on lymphoscintigrams and histological specimens. Concomitantly, with decrease of channel area in the calf, there was a decrease of its circumference
Material and Methods
Patients
Patients studied in this project were randomly selected from a large group of individuals suffering from lymphatic circulation problems undergoing various clinical investigations.4 The study was carried out on 20 patients with diagnosis of lymphedema of one lower limb, stage I to IV, duration of 2 to 15 years. Seventeen patients reported small foot skin abrasions or light trauma of foot in the past, followed by development of foot and calf edema disappearing after rest. Larger edema developed months to years later, and in 50% of cases was complicated by one to three attacks of dermato-lymphangio-adenitis. In three patients, edema developed without any detectable reason. Cases with acute inflammation, chronic venous insufficiency, and systemic etiology of edema were excluded from the study. The contralateral healthy limbs served as controls. The consent of patients was obtained and the study was approved by Warsaw Medical University ethics committee.
Clinical diagnosis
Diagnosis and staging were based on clinical evaluation: level of edema embracing limb from foot to groin and degree of skin keratosis and fibrosis. Briefly, in stage II, pitting edema affected foot and lower half of the calf; in stage III, foot and calf were involved, with hard foot and ankle area skin; in stage IV, the whole limb was edematous with foot and calf skin hyperkeratosis and papillomatosis of toes.5
Evaluation of lymphatic pathways was done on lymphoscintigraphic images. In stage II, there was spread of tracer in foot and lower part of calf, interrupted outline of a single lymphatic, and few small inguinal nodes with irregular outline. In stage III, no draining lymphatics were seen, with some inguinal nodes of irregular outline appearing after 2 hours since tracer injection. Stage IV was characterized by spread of tracer in the foot and entire calf without visualization of collecting lymphatics and nodes. In the healthy contralateral limb, lymphoscintigraphy revealed a normal outline of superficial and deep lymphatics.6
Compression device
We used a device produced for us by Biocompression Systems (Moonachie, NJ), a pneumatic compression appliance manufacturer. The device was: multi-chamber, sequential inflation, gradient inflation pressure, time of inflation sufficient for translocation of tissue fluid to proximal region, no deflation of distal chambers to prevent fluid back-flow, and venous stasis in the superficial limb system. It was composed of eight segments 9 cm long each, sequentially inflated, inflation pressures were regulated from 50 to 125 mmHg, gradient pressures decreasing proximally by 20%, inflation time of each chamber was 50 sec, total inflation time equaled 400 sec, there was no deflation of distal chambers, deflation time of all chambers was 50 sec at the end of each cycle. The sleeve embraced the whole limb up to the inguinal crease.
Girth measurement
Limb circumference was measured at five levels as previously.4 Results of treatment were presented in percent of girth but not volume changes. Total volume change does not reflect changes at various limb levels with different soft tissue mass, from where most of the edema fluid is evacuated. False results may be obtained when fluid accumulates in the popliteal fossa and in the upper thigh with loose tissue. Presentation of girth changes provides a better picture of fluid translocation and the limb shape changes.
Lymphoscintigraphic evaluation of limb soft tissues
In lymphedema, the radioisotopic tracer injected into the foot cannot enter the obstructed lymphatic collectors. It spreads in the subepidermal lymphatic plexus and free spaces in the dermis and subcutaneous tissue. For a semiquantitative evaluation of the area occupied by the tracer, a densitometric method of Microimage (Olympus, Japan) was applied. As another parameter, distribution of radioactivity in soft tissues was measured. The limb surface was divided into three rectangles. One of the inguinal area, one of thigh, and another of the calf, on the edematous and healthy limbs. Data were expressed as percent of area occupied by the tracer in each rectangle and degree of radioactivity in this area. The pre- and post-treatment pictures were compared.
Stereoscopic lymphography of specimens stained with Paris Blue
The method has been described previously.7 Briefly, a 5×5×5 mm fragment of tissue was harvested and injected with Paris Blue in chloroform solution using a 23 gauge needle. The injected tissue was fixed in 6% formalin and subsequently dehydrated in ethanol for 6 days. To become transparent, specimens were placed in methyl salicylate (Sigma) for at least 24 h and cut into 15 micron thick slices. The area of fluid-filled blue colored spaces was measured on sections using the Microimage system (Olympus).
Immunohistopathology of tissues
To identify initial lymphatics and the outline of tissue channels, fragments of skin with subcutis and muscle fascia of 5×5×5 mm were harvested from calf and thigh, snap frozen to 70°C and sections were stained with hematoxylin-eozin. The area of fluid-filled spaces was measured on hematoxylin-stained sections using the Microimage System (Olympus). To identify the lymphatic endothelial cells (LEC), staining with monoclonal antibodies against Lyve 1, podoplanin, prox 1 (markers of LEC), CCL21 (chemokine), and VEGF-CR (vascular endothelial growth factor receptor) was done.
Electron microscopy of tissues
Skin and subcutis biopsy specimens were fixed in glutaraldehyde and processed for evaluation in the transmission electron microscope.
Study setting
The IPC was applied in each patient daily for 45 min for 24–36 months. The sleeve inflation compression started at foot level with 120 mmHg and a gradual decrease by 20% in the groin. The inflation time was 50 sec/chamber amounting to 400 sec for the whole sleeve. It was followed by 50 sec deflation. Each compression procedure was composed of 9–10 cycles. The measurements were taken after the first use of IPC, then following 1, 12, and 24–36 months, at monthly intervals. Patients wore the same type of semi-elastic noncustomized compression stockings grade 2, standard factory size fitting to limb dimensions, advised by an experienced fitter. They changed stockings every 6 months. The limb circumference was also measured in the contralateral healthy leg of each patient. Lack of lymphedema was confirmed on lymphoscintigraphy.
Statistical evaluation
The pre- and post-treatment data were compared. The channel surface area was presented in percent of the stained area. Circumference was expressed in cm and for all patients as mean±SD. For statistical evaluation of differences in channels' surface area and limb circumference over various time periods, a double tail Student t-test was applied with significance at <0.05 level.
Results
Lymphoscintigraphic area of tissue channels
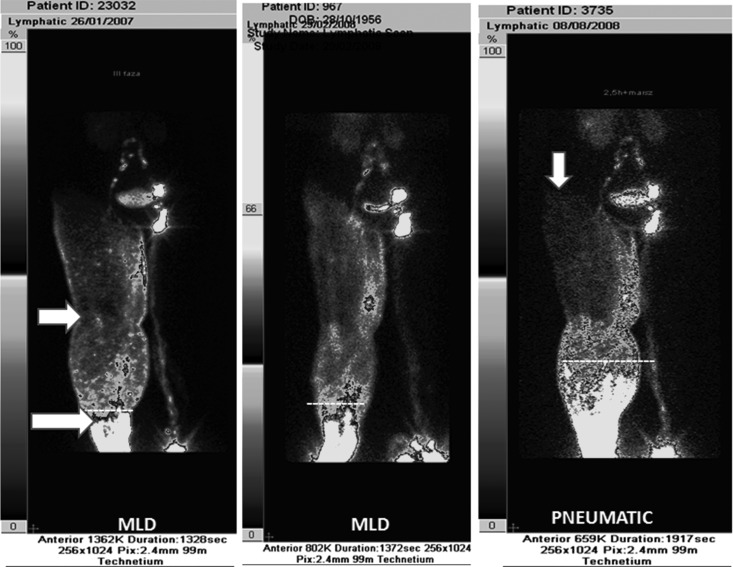
Tracer injected into the toe web spreads first in the subepidermal plexus, then flows along the subcutaneous and perifascial spaces. This creates two types of images, a “low intensity” subepidermal reaching proximal levels of the limb and “high intensity” opacifying mostly the subcutaneous distal parts of the limb (Fig. 1). The “high intensity” area was quantitated as it presented the dilated tissue spaces. It ranged between 32% and 80% in the calf before and 23% and 71% after the 1–3 years therapy. In the thigh, it was 5% to 9% before and up to 37% after the therapy. In some cases the tracer was seen after therapy in the hip area as well as entering the femoral channel (Figs.1 and 2). Example data of two patients developing channels in the thigh are presented on Figure 3.
FIG. 1.
Lymphoscintigram of lower limbs in lymphedema stage III. Edema of the right limb. Left panel: upper arrow shows area of “low intensity.” This is a picture of “dermal backflow.” Lower arrow at the site of “ high intensity” with tissue channels. Left and mid panels show pictures after manual lymphatic drainage. Right panel: intermittent pneumatic compression; tracer moved proximally into dilated subcutaneous tissue spontaneously formed channels. Vertical arrow points at tissue fluid flow to the lumbar region.
FIG. 2.
Lymphoscintigram of lower limbs in lymphedema stage III. Edema of the right limb. Left panel: no flow to the femoral canal. Mid panel: manual lymphatic drainage only some more proximal flow. Right panel: intermittent pneumatic compression brought about flow to the thigh subcutaneous tissue and femoral canal. The “high intensity” area in the thigh: biopsy showed dilated fluid channels (see Results).
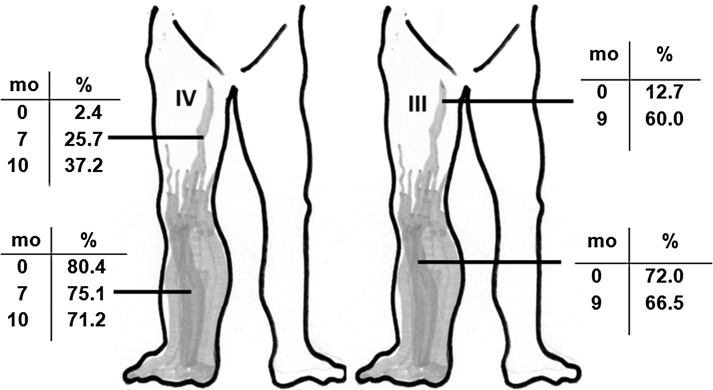
FIG. 3.
Two examples illustrating development of new tissue fluid channels in the thigh during the intermittent pneumatic compression therapy. Data derived from lymphoscintigram planimetry of areas opacified by the tracer (in percent). Mo=months of IPC therapy. Compare the calf and thigh data, clearly showing decrease of channels' area in the calf and increase in the thigh.
Color lymphography with Paris Blue
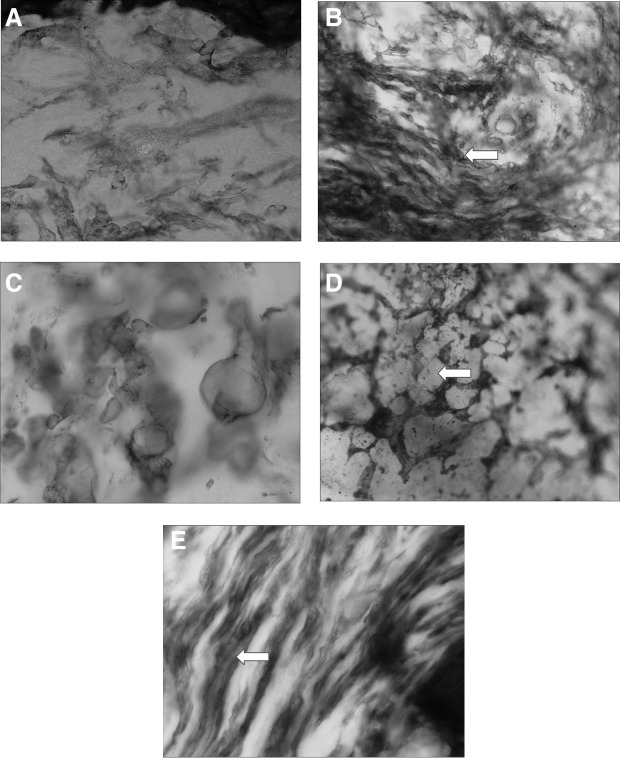
The dilatation of the subepidermal plexus was observed in all lymphedema cases already before therapy (Fig. 4A). It occupied 38%–52% of area of the papillary dermis. Multiple ramifications of minor blue-stained lymphatics reaching dermal papillae were seen. The subcutaneous compartment is composed of collagen bundles and fat tissue. The inter-collagen-bundle channels occupied 32%–48% of area before and 30%–49% after therapy both in the calf and thigh (Fig. 4B). Multiple small 100–200 μ diameter blind “what were lymphatics” could be seen (Fig. 4C). In the fat layer, the blue-stained areas were seen around fat globules (Fig. 4D). Fluid spaces were also identified along the fibers of the muscular fascia (Fig. 4E). They occupied up to 30% of area. Together, the largest channel area was observed in the subcutaneous tissue. There were no significant differences in the area of subcutaneous tissue channels in various stages of lymphedema.
FIG. 4.
Color lymphogram of subepidermal and dermal area in calf lymphedema stage III. (A) Multiple dilated irregular shaped subepidermal lymphatics denoted on lympho- scintigrams as “dermal backflow.” Paris Blue staining, X100 (see Methods). (B) Multiple vessel-like channel structures (arrow). These are spaces between collagen bundles. No lymphatic endothelial cell lining. See also histological picture Fig. 8. Paris Blue staining, X100. (C) In some areas, remaining blind fragments of lymphatic containing stagnant lymph, not to be moved without external pressure. Paris Blue staining, X200. (D) Tissue spaces around the subcutaneous tissue fat globules. Globules denoted by arrow. Paris Blue staining, X100. (E) Tissue fluid channels (arrow) along the fibrous structures of perimuscular fascia. Paris Blue staining, X200.
Immunohistopathology of tissue channels area
The images were similar to those of the Paris Blue stained. The dilated minor subepidermal lymphatics were observed in all cases already before therapy. In the subcutis on the cross- and longitudinal sections of the subcutaneous tissue, multiple interconnected channels were seen. They occupied 35%–49% of area before and 32%–52% after therapy (Fig. 5). In the fat layer, blue-stained areas were minor and only around the fat globules. Fluid spaces were also identified along the fibers of the muscular fascia (Fig. 6). The channels were not lined by cells. They were, in contrast to the few remaining lymphatics, Lyve 1, podoplanin, prox 1, CCL21, and VEGF-CR negative. There were no significant differences in the subcutaneous tissue channel area in various stages of lymphedema.
FIG. 5.
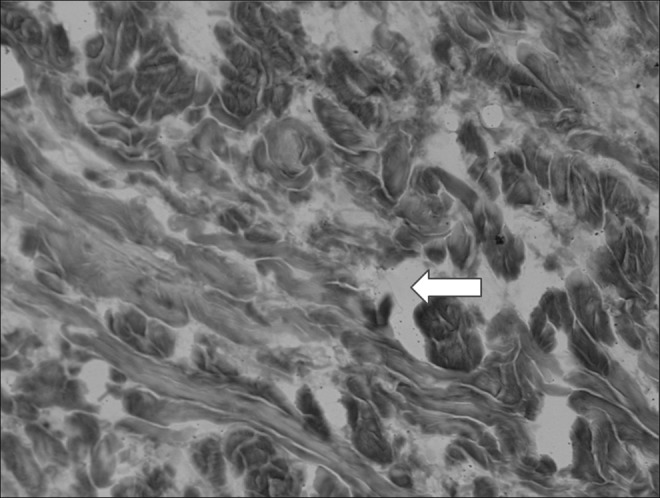
Histological picture of the subcutaneous tissue in calf lymphedema stage III. Multiple large fluid-filled (arrow) interconnected channels. No lining with endothelial cells. Hematoxylin X200.
FIG. 6.
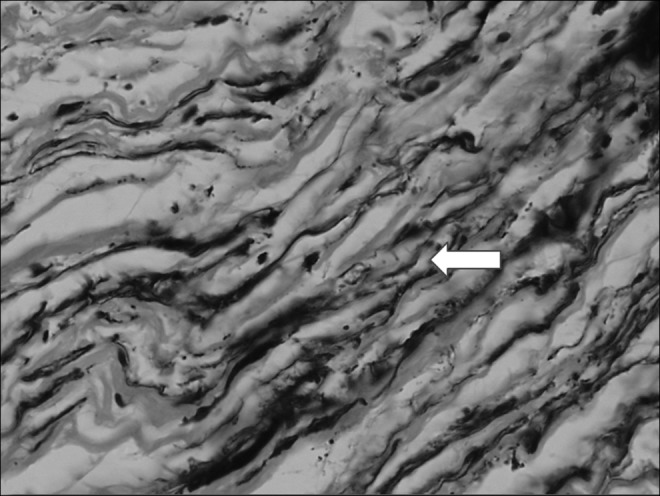
Histological picture of the perimuscular fascia in calf lymphedema stage III. Multiple large fluid-filled (arrow) longitudinal channels. No lining with endothelial cells. Hematoxylin X200.
Electron microscopy of tissues
The images showed accumulation of free fluid channels between the collagen fibers. They had an irregular shape and a diameter of a few microns (Fig. 7).
FIG. 7.
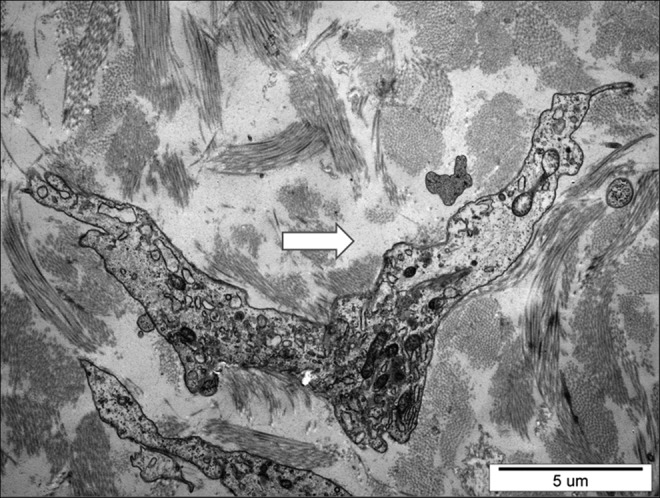
Electron microscopic picture of subcutaneous tissue in calf lymphedema stage III. Arrow at the site filled up with free fluid between collagen bundles. Excess stagnant fluid accumulated in the pericellular regions. X3000.
Limb circumference
One year of IPC showed decrease of circumference in all cases (Fig. 8A). Three years of IPC showed maintenance of decreased circumferences (Fig. 8B), with major fluctuations, most likely depending on patients' compliance with the daily application of IPC. No further decrease in parameters of edema was observed that can be accounted for by restoration of normal tissue (intercellular) fluid volume but not of cells and matrix structure (fibroblasts, collagen). Major standard deviations in limb girth were due to individual reactions to IPC, presumably depending on the degree of tissue induration.
FIG. 8.
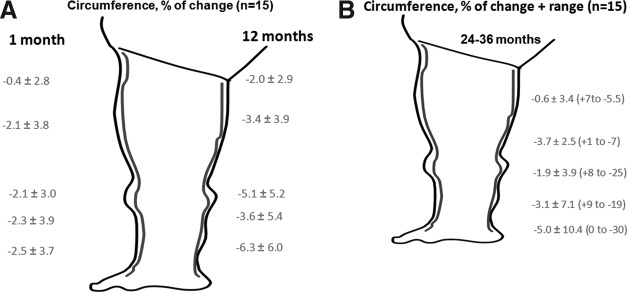
The 1–36 months results of intermittent pneumatic compression therapy of lymphedema of lower limbs. (A) 1–12 months. (B) 24–36 months. Evident decrease of calf circumference but not so expressed in the thigh. This correlates with the decrease in tissue fluid channel area in the calf but increase in the thigh.
Discussion
This study provided the following observations: a) the tissue fluid accumulating in the interstitial space in limbs with obstructed lymph flow formed spontaneous tissue channels located mostly in the subcutaneous tissue and along the muscular fascia; b) more channels running from foot to the thigh and groin were formed during long-term intermittent pneumatic compression; c) the channels were not lined by the lymphatic endothelial cells; d) decrease in limb circumference during IPC correlated with density of tissue channels in the thigh; and e) there were no significant differences in the subcutaneous tissue channel surface area in various stages of lymphedema.
One of the basic problems in clinical lymphology is that the majority of therapists are not aware of what are the pathways of edema fluid flow during compression of tissues, including manual lymphatic drainage, intermittent pneumatic compression, and bandaging. A general notion is that fluid is pushed back into lymphatics, whereas they are obliterated or at least obstructed at various levels. We wanted to show that this is not the case and that fluid flows through the spontaneously formed “channels” and formation of “channels” is enhanced by IPC therapy.
Obstruction of lymphatic collectors brings about continuous accumulation of capillary filtrate in the intercellular and subsequently interstitial space. This space expands as much as the mechanical structure of the tissue allows. Some fluid is moved to the skin surface and expands the usually non-obliterated subepidermal lymphatic plexus.1 Clinically, lymphorrhea is sometimes observed. Gradually, fluid-filled spaces can be seen on histological specimens between collagen bundles, along blood vessels and fat globules. The fluid is stagnant as there is no propelling force present in the spontaneously contracting lymphatics.8,9
Moreover, fluid hydraulic conductivity in tissues is much lower than in the lymphatic vessels. To overcome the tissue hydraulic resistance, external force moving fluid is necessary. The effectiveness of IPC was evaluated in a number of studies with use of circumference and volume measurements and lymphoscintigraphy. Using the IPC device over a long period of time (recent follow up 3 years) we could show on lymphoscintigrams that fluid spaces are gradually formed closer to the root of the limb concomitantly with decrease of calf circumference. This means IPC was helpful in formation of pathway “channels” by deformation of tissue in the thigh. A mean decrease in circumference in the calf after 1 month by 2.5% expressed in volume units is 80–100 mL in the mid portion. This is a very satisfactory result taking into account that in stage III and IV of lymphedema there is an increase in size in 40% by fluid and 20% by fibrous tissue with enormous collagen and fat deposits. Thus, we cannot expect better results in advanced stages. In the 12 months follow up the mean decrease was around 4%–6% and that would be 200–300 mL in the mid calf only. Calculated for the whole limb that would amount to 800–1000 mL.
So far, no analysis of tissue hydraulic events was described in the literature. We combined lymphoscintigraphic and histological techniques in order to localize the edema fluid pathways exactly and prove that in human adults resolution of edema is not regulated by lymphangiogenesis. Our studies showed that long-term external limb compression is followed by increase in tissue channel cross surface area in the thigh, with concurrent decrease of circumference of calf. This observation suggests that the new channels developed proximally enabling the moved fluid to be absorbed in areas as upper thigh, hip, and lumbar with a normal lymphatic drainage. With respect to the question of absorption of fluid in these regions, we know from the clinical observations that within 2–3 hours after its accumulation, the swelling disappears. Moreover, radioactivity of Tc99- Nanocoll used for lymphoscintigraphy becomes in the thigh channels very low.
The question as to whether the channels were new lymphatics was answered by results of histological evaluation of sections stained against LEC markers. There was no evidence of any of Lyve 1, prox1, and podoplanin molecule, neither of the chemokine CCL21 present in normal LEC or receptor for VEGF C responsible for lymphangiogenesis.
Interestingly, no significant differences in channel formation were observed between cases in various stages of lymphedema This could be due to development of channels mostly in the expandable subcutaneous compartment containing collagen bundles and loose fat tissue but not in the dermis undergoing fibrosis, especially in later stages of lymphedema.
The mechanism of formation tissue channels for resolution of lymphedema has so far been studied only in animals. It was found in rats that interstitial fluid flow induces collagen alignment.15 In other studies, interstitial fluid channels formed before lymphatic endothelial cell organization and could direct lymphangiogenesis.16
Uzarski et al. found that interstitial fluid and edema resolution readily occur across the scar-free regenerating skin in the absence of VEGFR-3 signaling and lymphangiogenesis.17 This suggests that the resolution may be more dependent upon interstitial fluid dynamics or fluid filtration dynamics than functional lymphatic growth.
Increase in limb volume in lymphedema is partially caused growing fat tissue. The fluid conductivity of fat and of immature collagen-rich tissues is high. The modulus of adipose tissue is dictated by the collagen network that surrounds the adipocytes.18 This causes that edema fluid tries to find its way also around fat globules.
Compley and Fleck studied fat tissue hydraulic conductivity in a model of delivery of liquid into adipose tissue by the hypodermic needle. X-ray images of the injected fluid suggested that the micro-cracks are formed by the fluid pressure within the tissue and this led to an increased permeability by creation of a connecting network of micro-cracks. The volumetric flow rate was related to the injection delivery pressure.19
The open question remains whether pneumatic compression moves edema fluid through tissue spaces only or it may, at least partly, be absorbed into the venous system by the high external force load. We could not document venous absorption of edema fluid in a model of a leg segment compressed to 50 mmHg contained between two cuffs inflated to 50 mmHg occluding proximal fluid movement (unpublished). This proved that the bulk of edema fluid is moved along spontaneously formed tissue channels but not absorbed under the compressing cuff. Another evidence of tissue channels fluid pathways was provided by lymphoscintigraphy evidently showing movement of tracer either to the lumbar region or femoral channel along large blood vessels.
Taken together, in lymphedema spontaneous tissue fluid channels are formed by tissue structure deformation by mobile fluids. These channels do not have their own flow propelling force as do the normal contracting lymphatics. Pneumatic compression replaces the missing lymphatic function by providing fluid moving force, subsequently enhancing channels formation process and in effect facilitating evacuation of fluid containing excess cytokines, among them upregulating collagen synthesis. The fibrosis process is slowed down and subsequently there is no constriction of fluid channels, what may be seen in very advanced stages of lymphedema.
Acknowledgments
This study was supported by grants from the National Center for Science (Poland) NN404113139 and the National Center for Research and Development NR13002606.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Olszewski WL, Jain P, Ambujam G, Zaleska M, Cakala M. Topography of accumulation of stagnant lymph and tissue fluid in soft tissues of human lymphedematous lower limbs. Lymphat Res Biol 2009;7:239–245 [DOI] [PubMed] [Google Scholar]
- 2.Olszewski WL, Cwikla J, Zaleska M, Domaszewska-Szostek A, Gradalski T, Szopinska S. Pathways of lymph and tissue fluid flow during intermittent pneumatic massage of lower limbs with obstructive lymphedema. Lymphology 2011;44:54–64 [PubMed] [Google Scholar]
- 3.Morris RJ. Intermittent pneumatic compression-systems and applications. J Med Engin Technol 2008,32:179–188 [DOI] [PubMed] [Google Scholar]
- 4.Zaleska M, Olszewski WL, Jain P, Gogia S, Rekha A, Mishra S, Durlik M. Pressures and timing of intermittent pneumatic compression devices for efficient tissue fluid and lymph flow in limbs with lymphedema. Lymphat Res Biol 2013;11:227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olszewski WL. Clinical picture of lymphedema. In: Olszewski W. L. (Ed.), Lymph Stasis: Pathophysiology, Diagnosis and Treatment. CRC Press, Boca Raton, FL, 1991. p. 351 [Google Scholar]
- 6.Olszewski WL. Atlas of Lymphology. Servier, Paris, 2001 [Google Scholar]
- 7.Olszewski W, Zajac S, Machowski Z, Sokolowski J. [Staining of the lymphatic vessels with the D. D. Zerbino method] Folia Morphol (Warsz) 1968;27:397–402 [PubMed] [Google Scholar]
- 8.Olszewski WL, Engeset A. Intrinsic contractility of leg lymphatics in man. Preliminary communication. Lymphology 1979;12:81–84 [PubMed] [Google Scholar]
- 9.Olszewski WL. Contractility patterns of human leg lymphatics in various stages of obstructive lymphedema. Ann NY Acad Sci 2008;1131:110–118 [DOI] [PubMed] [Google Scholar]
- 10.Baulieu F, Baulieu JL, Vaillant L, Secchi V, Barsotti J. Factorial analysis in radionuclide lymphography: Assessment of the effects of sequential pneumatic compression. Lymphology 198;22:178–185 [PubMed] [Google Scholar]
- 11.Ketterings C, Zeddemann S. Use of the C-scan in evaluation of peripheral lymphedema. Lymphology 1997;30:49–62 [PubMed] [Google Scholar]
- 12.Miranda F, Jr, Perez MC, Castiglioni ML, Juliano Y, Amorim JE, Nakano LC, de Barros N, Jr, Lustre WG, Burihan E. Effect of sequential intermittent pneumatic compression on both leg lymphedema volume and on lymph transport as semi-quantitatively evaluated by lymphoscintigraphy. Lymphology 2001;34:135–141 [PubMed] [Google Scholar]
- 13.Pecking AP, Cluzan RV. Results of a self sequential pressotherapy as a substitute for classical decongestive therapy in a group of patients with a refractory upper limb lymphedema. XIX. International Congress of Lymphology 01-06 Sept. 2003; Freiburg/Germany [Google Scholar]
- 14.Kafejian-Haddad AP, Perez JM, Castiglioni ML, Miranda Júnior F, de Figueiredo LF. Lymphoscintigraphic evaluation of manual lymphatic drainage for lower extremity lymphedema. Lymphology 2006;39:41–48 [PubMed] [Google Scholar]
- 15.Ng CP, Hinz B, Swartz MA. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J Cell Sci 2005;118:4731–4737 [DOI] [PubMed] [Google Scholar]
- 16.Boardman KC, Swartz MA. Interstitial flow as a guide for lymphangiogenesis. Circ Res 2003;92:801–808 [DOI] [PubMed] [Google Scholar]
- 17.Uzarski J, Drelles MB, Gibbs SE, Ongstad EL, Goral JC, McKeown KK, Raehl AM, Roberts MA, Pytowski B, Smith MR, Goldman J. The resolution of lymphedema by interstitial flow in the mouse tail skin. Am J Physiol Heart Circ Physiol 2008;294:H1326–H1334 [DOI] [PubMed] [Google Scholar]
- 18.Comley K, Fleck NA. A micromechanical model for the Young's modulus of adipose tissue. Int J Solids Structures 2010;47:2982–2990 [Google Scholar]
- 19.Compley K, Fleck NA. Deep penetration and liquid injection into adipose tissue. J Mechan Material Structures 2011;6:1–4 [Google Scholar]