Abstract
We hypothesized that olive leaf extract might alleviate dyslipidemia resulting from estrogen deficiency. Serum lipid profile and mRNA expression of the related genes in the liver and adipose tissue were analyzed after providing olive leaf extract (200 or 400 mg/kg body weight; n=7 for each group) to ovariectomized rats for 10 weeks. After 10 weeks' administration, the rats in the olive leaf extract-administered groups showed significantly lower levels of serum triglyceride and very-low-density lipoprotein (VLDL)-cholesterol compared with the rats in the control group, whereas the administration of olive leaf extract did not significantly change the elevated low-density lipoprotein cholesterol levels. In addition, administration of high dose of olive leaf extract significantly decreased the liver triglyceride and increased serum estradiol levels. mRNA expressions of peroxisome proliferator-activated receptor alpha (PPAR α) and acyl-CoA oxidase (ACO) were not affected by ovariectomy, however, administration of olive leaf extract significantly increased both PPAR α and ACO mRNA expression. Expression of adiponectin mRNA in adipose tissue was significantly decreased in the ovariectomized control group. Rats administered low-dose olive leaf extract showed significantly elevated adiponectin mRNA expression compared with rats in the ovariectomized control group. Even though dose-dependent effects were not observed in most of the measurements, these results suggest that genes involved in lipid metabolism may be regulated by olive leaf extract administration in ovariectomized rats.
Key Words: : dyslipidemia, estrogen deficiency, olive leaf extract, ovariectomy, postmenopause
Introduction
Estrogen deficiency is a major risk factor for metabolic disorders such as obesity, type 2 diabetes mellitus, cardiovascular disease, and hypertension.1 Dyslipidemia, including hyperlipidemia, may be directly or indirectly associated with the metabolic states of estrogen-deficient females mentioned above.2 Dyslipidemia is mainly manifested by elevations of total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglyceride concentrations and a decrease in high-density lipoprotein (HDL) cholesterol concentration in the blood.3,4
As the increases in overweight and obesity in postmenopausal women are major public health concerns,5 natural food sources, which might potentially regulate the modified lipid metabolism of postmenopausal women such as soy genistien6 and curcuma comosa extract,7 have been investigated.
Active components of olive leaf, especially oleuropein, have been particularly studied as possible diabetes-preventive components that might decrease blood glucose concentrations and improve hyperglycemic symptoms.8 It has also been reported that olive leaf extract was effective in treating atherosclerosis by inhibiting tumor necrosis factor-α expression in rabbits with experimental atherosclerosis.9 According to Jemai et al., the rats fed cholesterol-rich diets with olive leaf extract administration showed significantly increased serum levels of HDL cholesterol and significantly decreased triglyceride, total cholesterol, and LDL cholesterol compared to those of controls fed a cholesterol-rich diet.10
Based on these previous results, we hypothesized that olive leaf extract might ameliorate dyslipidemia resulted from estrogen deficiency. Serum lipid profile and mRNA expression of the genes involved in fatty acid oxidation in the liver and adipose tissue were analyzed after providing olive leaf extract to ovariectomized rats for 10 weeks. This study sought to investigate olive leaf extract as a potential natural functional food for postmenopausal women.
Materials and Methods
Animals and diets
All animal procedures were performed in accordance with the guidelines issued by the Sookmyung Women's University for the care and use of laboratory animals (SM-IACUC-2013-0509-010). Ten-week-old female Sprague-Dawley rats (KOATECH, Gyeonggi-do, Korea) were housed in a controlled temperature (22°C±1°C) and humidity (50–60%) on a 12-h light/12-h dark cycle, with free access to water and AIN-93G diet (Research diet, New Brunswick, NJ, USA).
After 1 week of adaptation to this environment, 21 rats underwent bilateral ovariectomy,11 while 7 rats were subjected to sham operations. After 2 weeks of recovery, the rats were randomly divided into four groups as follows (n=7 for each group): Sham, nonovariectomized control; OC, ovariectomized control; LOE, ovariectomy+200 mg/kg b.w. of olive leaf extract; and HOE, ovariectomy+400 mg/kg b.w. of olive leaf extract. Rats in the LOE and HOE groups were orally administered olive leaf extract, which was dissolved in distilled water every day for 10 weeks. The rats in the OC group were administered 1 mL of distilled water as a control. Food intakes were measured every other day, and body weight was measured every week.
Preparation of olive leaf extract
Dried olive leaf was purchased from Aju Pharm Co. Ltd. (Seoul, Korea) and was ground to 16 meshes using a grinder (MF 10 Basic; IKA-WERKE, Staufen, Germany). Ground olive leaf (50 g) was refluxed with 250 mL of 80% ethanol at 55°C for 5 h.12,13 The mixture was filtered and completely evaporated in a rotary evaporator (N-21NS; EYELA, Tokyo, Japan) at 40°C before the concentrated mixture was freeze-dried.
Biochemical analysis
After an overnight fast, final body weights were measured and rats were euthanized using CO2. Blood was collected by cardiocentesis to determine serum lipid profiles and serum GOT (glutamic oxaloacetic transaminase), GPT (glutamic pyruvic transaminase) levels. Sera and livers were stored at −70°C until analysis. Serum GOT and GPT levels were determined using an assay kit (3I1010; Asanpharm, Hwaseong, Korea), triglyceride, total cholesterol, and HDL cholesterol levels were determined using the Cleantech TG-S kit (3I1570; Asanpharm), and T-CHO kit (3I2020; Asanpharm), and HDL-CHO (3I2030; Asanpharm) with an Epoch microplate spectrophotometer (BIOTEK, Inc., Winooski, VT, USA). Serum LDL cholesterol and very-low-density lipoprotein (VLDL) cholesterol levels were calculated using the following formula14:
LDL cholesterol (mg/dL)=total cholesterol level−(HDL cholesterol level+triglyceride level/5) (mg/dL)
VLDL cholesterol (mg/dL)=triglyceride level/5 (mg/dL)
Liver TG levels were determined using the Folch method. Liver tissue was extracted in 3 mL of chloroform:methanol (2:1) and homogenized. The suspension was vortexed at room temperature for 20 min. The top layer was discarded and the bottom layer was used for lipid analysis. The liver TG content was determined using the TG-S kit (3I1570; Asanpharm).
mRNA expression analysis
RNA was isolated from homogenized liver lobes and epididymis adipose tissue using an easy-spin™ (DNA free) Total RNA Extraction kit (iNtRON Biotechnology, Inc., Gyeonggi-do, Korea). Reverse transcription–polymerase chain reaction (RT-PCR) was performed in a PCR machine (Genomicbase, Seoul, Korea) using a Maxim RT-PCR Premix kit (iNtRON Biotechnology, Inc.). Details of primers and optimal cycling conditions are shown in Table 1. PCR products were visualized after electrophoresis in 2% agarose gels. All gene expressions were normalized to GAPDH, which served as an internal control for the quality of isolated RNA from each homogenized liver and adipose tissue sample.
Table 1.
RT-PCR Primer Sequences
| Genes | Sequence (5′-3′) | Annealing (°C) | Cycle |
|---|---|---|---|
| Adiponectin | |||
| Forward | TGTTCCTCTTAATCCTGCCC | 50 | 35 |
| Reverse | CAACATCTCCTGTCTCACCC | ||
| PPAR α | |||
| Forward | GGCTCGGAGGGCTCTGTCATC | 58 | 33 |
| Reverse | ACATGCACTGGCAGCAGTGGA | ||
| PPAR γ | |||
| Forward | TGGGGATGTCTCACAATGCCA | 54 | 40 |
| Reverse | TTCCTGTCAAGATCGCCCTCG | ||
| ACO | |||
| Foward | CTTTCTTGCTTGCCTTCCTTCTCC | 60 | 40 |
| Reverse | GCCGTTTCACCGCCTCGTA | ||
| CPT1a | |||
| Forward | GGAGACAGACACCATCCAACATA | 60 | 35 |
| Reverse | AGGTGATGGACTTGTCAAACC | ||
| LPL | |||
| Forward | CAGCTGGGCCTAACTTTGAG | 60 | 40 |
| Reverse | CCTCTCTGCAATCACACGAA | ||
| ATGL | |||
| Forward | CACTTTAGCTCCAAGGATGA | 60 | 40 |
| Reverse | TGGTTCAGTAGGCCATTCCT | ||
| GAPDH | |||
| Forward | CCTCTCTCTTGCTCTCAGTAT | 56 | 33 |
| Reverse | GTATCCGTTGTGGATCTGACA |
PPAR α, peroxisome proliferator-activated receptor alpha; PPAR γ, peroxisome proliferator-activated receptor gamma; ACO, acyl-CoA oxidase; CPT1a, carnitine palmitoyltransferase 1a; LPL, lipoprotein lipase; ATGL, adipose triglyceride lipase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RT-PCR, reverse transcription–polymerase chain reaction.
Statistical analysis
Statistical analysis was performed using SAS version 9.3. All data are presented as mean±SD. The results from each experimental group were compared using one-way ANOVA. Differences in mean values among the four groups were tested using Duncan's multiple tests. A P value of <.05 was considered statistically significant.
Results
Body weight, organ weight, and amount of food intake
Body and organ weight changes and amount of food intake are presented in Table 2. Food intake in ovariectomized groups was greater than the Sham group. Compared with the Sham group, all ovariectomized groups showed increased final body weights (P<.0001), liver (P=.0049), and abdominal adipose fat weights (P<.001) and significantly decreased uterus weights (P<.0001). Olive leaf extract administration regardless of doses did not significantly make the rats gain body weight or the amount of food intake that resulted from ovariectomy.
Table 2.
Body Weight and Organ Weight and Amount of Food Intake
| Sham | OC | LOE | HOE | |
|---|---|---|---|---|
| Body weight (g) | ||||
| Initial | 184.50±2.50ns | 186.89±4.18 | 186.81±4.49 | 187.29±8.02 |
| Final | 259.23±10.81b | 353.14±9.77a | 340.97±16.59a | 345.36±13.61a |
| Organ weight (g) | ||||
| Liver | 6.18±0.72b | 7.41±0.60a | 7.25±0.46a | 7.67±0.96a |
| Abdominal adipose fat | 6.08±1.93b | 12.15±1.67a | 11.95±2.14a | 11.72±3.02a |
| Uterus | 0.663±0.103a | 0.089±0.007b | 0.094±0.018b | 0.091±0.017b |
| Food intake (g/day) | 12.31±0.39b | 15.16±0.94a | 14.76±0.40a | 14.20±0.52a |
Values are mean±SD (n=7 for each group). The different letters (a, b) within a column indicate significant differences (P<.05) determined by Duncan's multiple range test.
Sham, nonovariectomized control; OC, ovariectomized control; LOE, ovariectomy+200 mg/b.w. olive leaf extract; HOE, ovariectomy+400 mg/b.w. olive leaf extract; ns, not significant.
Blood lipid profile and levels of liver triglyceride and serum estradiol
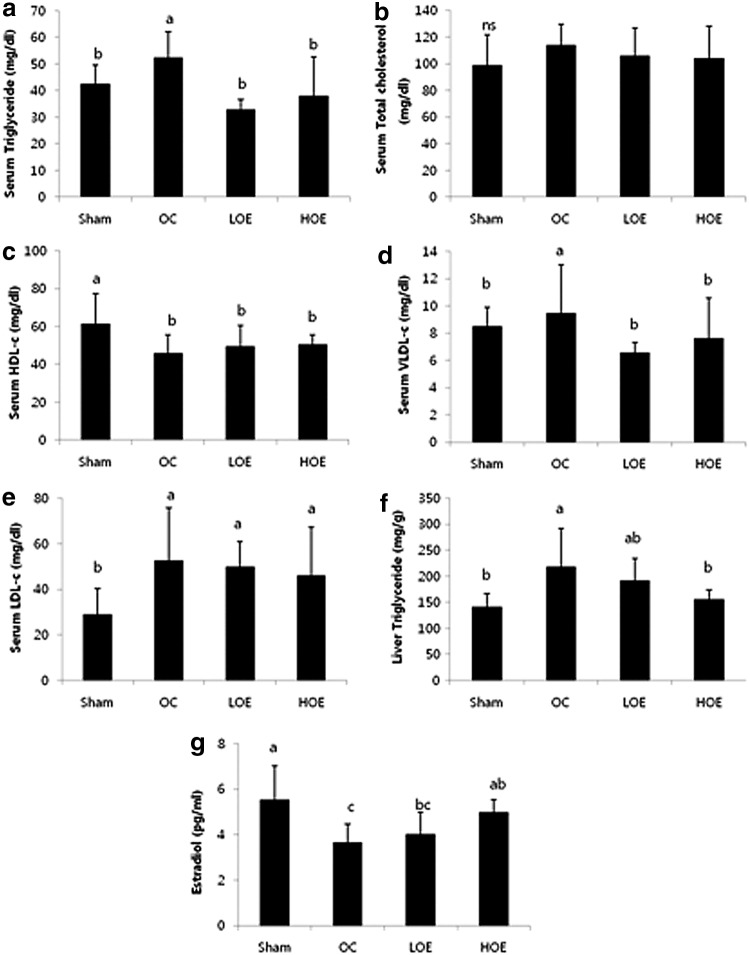
Serum lipid profile and levels of liver triglyceride and serum estradiol are shown in Figure 1. Rats in the OC group showed significantly elevated levels of triglyceride, VLDL cholesterol, and LDL cholesterol, whereas their serum HDL cholesterol levels were significantly lowered. After 10 weeks of administration, the olive leaf-administered rats showed significantly lower triglyceride and VLDL cholesterol levels compared with the rats in control groups (P=.0021 for both). Administration of olive leaf extract did not significantly change the elevated LDL cholesterol levels in the OC group. No significant changes were observed in the total cholesterol levels among all groups.
FIG. 1.
Serum lipid profile, liver triglyceride level, and serum estradiol level in the rats after administration of olive leaf extract for 10 weeks: serum levels of (a) triglyceride (b) total cholesterol, (c) HPL-c, (d) VLDC-c, (e) LDL-c, (f) liver triglyceride level, and (g) serum estradiol level. Values are mean±SD (n=7 for each group). Values with different letters are significantly different in the groups by Duncan's multiple range test (a>b). Sham, nonovariectomized control; OC, ovariectomized control; LOE, ovariectomy+200 mg/b.w. olive leaf extract; HOE, ovariectomy+400 mg/b.w. olive leaf extract; ns, not significant.
Liver triglyceride levels in the OC group were higher than the Sham group. High dose of olive leaf extract administration significantly decreased the elevated liver triglyceride levels (P=.0182), whereas low dose of olive leaf extract administration did not change them significantly.
Serum estradiol levels in the Sham group were higher than those of the OC and LOE groups. Compared with the OC group, high dose of olive leaf extract administration significantly increased serum estradiol levels, whereas low dose of olive leaf extract administration did not affect significantly (P=.0084).
mRNA expression of adiponectin, PPAR α, ACO, and CPT1 in liver
Liver mRNA expressions of adiponectin, proliferator-activated receptor alpha (PPAR α), acyl-CoA oxidase (ACO), and carnitine palmitoyltransferase type 1 (CPT1) are shown in Figure 2. Liver adiponectin mRNA expression was significantly higher in the LOE group compared with other groups (P=.0446). mRNA expressions of PPAR α and ACO were not affected by ovariectomy, however, administration of olive leaf extract significantly increased both PPAR α (P=.0191) and ACO mRNA expression (P=.0074). mRNA expression of liver CPT1 was not different among the groups.
FIG. 2.
Liver mRNA expression of (a) adiponectin, (b) PPAR α, (c) ACO, and (d) CPT1a in the rats after administration of olive leaf extract for 10 weeks. Values are mean±SD (n=7 for each group). The different letters (a, b) within a column indicate significant differences (P<.05) determined by Duncan's multiple range test. PPAR α, peroxisome proliferator-activated receptor alpha; ACO, acyl-CoA oxidase; CPT1a, carnitine palmitoyltransferase 1a; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
mRNA expression of adiponectin, PPAR γ, LPL, and ATGL in adipose tissue
Adipose tissue mRNA expressions of adiponectin, proliferator-activated receptor gamma (PPAR γ), LPL, and ATGL are shown in Figure 3. Expression of adiponectin mRNA in epididymis adipose tissue was significantly decreased in the OC group compared with the Sham group. The LOE group showed significantly elevated adiponectin mRNA expression compared with the OC group (P=.0289). mRNA expression of ATGL was significantly decreased in the OC group, however, administration of olive leaf extract did not change the expression significantly. PPAR γ and LPL mRNA expressions were not significantly different among the groups.
FIG. 3.
Adipocyte mRNA expression of (a) adiponectin, (b) PPAR γ, (c) ATGL, and (d) LPL in the rats after administration of olive leaf extract for 10 weeks. Values are mean±SD (n=7 for each group). The different letters (a, b) within a column indicate significant differences (P<.05) determined by Duncan's multiple range test. PPAR γ, peroxisome proliferator-activated receptor gamma; LPL, lipoprotein lipase; ATGL, adipose triglyceride lipase.
Discussion
To the best of our knowledge, this is the first study to examine the effects of olive leaf extract on ovariectomy-induced dyslipidemia and mRNA expressions of related genes. Data presented in this article support previous reports by showing that estrogen deficiency is a triggering factor for dyslipidemia. Our data also demonstrated direct effects of olive leaf extract on dyslipidemia in ovariectomized rats by showing that administration of olive leaf extract modulates serum levels of TG and VLDL, and liver TG levels. In addition, the data show that olive leaf extract regulates mRNA expression of liver PPAR α and ACO genes, which are associated with fatty acid oxidation. Finally, because the mRNA expression of adiponectin in the adipose tissue was increased in the olive leaf extract-administered group, this may suggest that the olive leaf extract may affect the expression/secretion of adiponectin, which is manipulated by estrogen deficiency.
Several previous studies reported significant increases in body weight after ovariectomy in various animal models.15–18 Estrogen-deficient women also showed body fat accumulation and an average 0.8 kg weight gain every year.19 Even though the mechanism of weight gain among estrogen-deficient subjects is still unclear, it seems that the absence of estrogens may be an important obesity-triggering factor.20 The current study also showed significant increases in body weight in ovariectomized rats regardless of olive leaf extract administration. These ovariectomized rats also showed significant increases in abdominal fat accumulation and decrease of uterine weight. Supplementation of olive leaf extract did not affect weight gain, fat accumulation, or loss of uterine weight. These might be because of the short supplementation duration and/or insufficient dose to influence body weight changes.
Rats in ovariectomized groups showed significantly lower serum levels of HDL and higher TG and LDL-C compared with those of Sham rats. Dyslipidemia is one of the major complications of estrogen deficiency. Dyslipidemia among postmenopausal women and the ovariectomized animal model, especially increased serum TG and total cholesterol levels, has been reported several times. Postmenopausal women exhibit increases in LDL particles, which further increase the atherogenic potency of the profile. Serum levels of TG ≥150 mg/dL and HDL-C ≤50 mg/dL, coupled with an increase in LDL particles, constitute atherogenic dyslipidemia, which characterizes the metabolic syndrome.21 In our study, administering olive leaf extracts decreased the elevated serum TG level back to the level of Sham rats. Even though the olive leaf extract administration did not significantly affect the levels of total cholesterol, LDL-C and HDL-C, modification of serum TG and VLDL levels by olive leaf extract supplementation is important. The decrease in serum TG levels after the administration of olive leaf extract might be associated with increased mRNA expression of PPAR α. PPAR α has been well known to have a central role in the metabolism of TG-rich lipoproteins. PPAR α knockout mice showed increased hepatic TG content,22 increased VLDL production,23 and increased plasma TG levels compared to wild-type mice.24 In this study, as PPAR α expression was increased by administration of olive leaf extract, hepatic and plasma TG levels and plasma VLDL levels were decreased back to the levels of rats in the Sham group. However, there were no significant dose-dependent effects of the supplementation of olive leaf extract.
Activation of PPAR α has been also known to lead to increased tissue-specific expression of key genes involved in fatty acid uptake and β-oxidation.25 CPT1 is one of the enzymes whose expression is possibly affected by activation of PPAR α.26–28 However, in this study, mRNA expression of CPT1 was not significantly changed by the administration of olive leaf extract, whereas mRNA expression of ACO, which is the first characterized PPAR target gene, was also increased in this study.
Adiponectin is an adipocytokine that is highly expressed in human adipose tissue, circulating in serum at concentrations of 2–15 g/mL.29–31 Previous studies have investigated correlations between estrogen levels and adiponectin concentrations. One study found that postmenopausal women taking estrogen replacement therapy showed lower adiponectin levels compared with women without estrogen supplementation.32 Some animal models also indicated a negative correlation between high estrogen levels and low adiponectin secretion by adipocytes.33 However, we did not find any clear correlation between the levels of estradiol and mRNA expression of adiponectin. Adiponectin seems to be related to the secretion of estrogen, but the mechanisms remain unresolved.
There are limitations of this study that need to be addressed. First, actual hepatic lipid profiles were not measured. Measuring lipid profiles along with mRNA expressions could be interpreted along together to investigate the effects of olive leaf extract on ovariectomized-induced dyslipidemia. Second, this study was conducted with only two doses of olive leaf extract. Dose-dependent effects were not observed in most of the measurements. For further analyses, we would suggest to investigate the hepatic mRNA expression of genes involved in lipogenesis and VLDL packaging using multiplex PCR or real-time qPCR. Measuring the serum concentration of adiponectin and applying western blot would strengthen our findings.
In conclusion, the findings of this study add to the current knowledge about the effects olive leaf extract on ovariectomy-induced dyslipidemia, particularly on plasma TG and VLDL levels and liver mRNA expression of ACO along with the PPAR α expression. These results provide clinically relevant insight into the supplementation of olive leaf extract to prevent dyslipidemia resulting from estrogen deficiency.
Acknowledgment
This Research was supported by Sookmyung Women's University Research Grants 2013 (1-1303-0069).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Sun A, Ren J: Estrogen replacement therapy and cardiac function under metabolic syndrome: A treacherous art. Hypertension 2012;59:552–554 [DOI] [PubMed] [Google Scholar]
- 2.Carr MC: The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 2003;88:2404–2411 [DOI] [PubMed] [Google Scholar]
- 3.Phan BA, Toth PP: Dyslipidemia in women: Etiology and management. Int J Womens Health 2014;6:185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JJ, Choi YM: Dyslipidemia in women with polycystic ovary syndrome. Obstet Gynaecol Sci 2013;56:137–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapira N: Women's higher health risks in the obesogenic environment: A gender nutrition approach to metabolic dimorphism with predictive, preventive, and personalised medicine. EPMA J 2013;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi JS, Song J: Effect of genistein on insulin resistance, renal lipid metabolism, and antioxidative activities in ovariectomized rats. Nutrition 2009;25:676–685 [DOI] [PubMed] [Google Scholar]
- 7.Prasannarong M, Saengsirisuwan V, Piyachaturawat P, Suksamrarn A: Improvements of insulin resistance in ovariectomized rats by a novel phytoestrogen from Curcuma comosa Roxb. BMC Complement Altern Med 2012;12:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wainstein J, Ganz T, Boaz M, et al. : Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. J Med Food 2012;15:605–610 [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Geng C, Jiang L, et al. : The anti-atherosclerotic effect of olive leaf extract is related to suppressed inflammatory response in rabbits with experimental atherosclerosis. Eur J Nutr 2008;47:235–243 [DOI] [PubMed] [Google Scholar]
- 10.Jemai H, Fki I, Bouaziz M, et al. : Lipid-lowering and antioxidant effects of hydroxytyrosol and its triacetylated derivative recovered from olive tree leaves in cholesterol-fed rats. J Agric Food Chem 2008;56:2630–2636 [DOI] [PubMed] [Google Scholar]
- 11.Ho YJ, Wang CF, Hsu WY, et al. : Psychoimmunological effects of dioscorea in ovariectomized rats: Role of anxiety level. Ann Gen Psychiatry 2007;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee OH, Lee BY, Lee J, et al. : Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Bioresour Technol 2009;100:6107–6113 [DOI] [PubMed] [Google Scholar]
- 13.Herrero M, Temirzoda TN, Segura-Carretero A, Quirantes R, Plaza M, Ibanez E: New possibilities for the valorization of olive oil by-products. J Chromatogr A 2011;1218:7511–7520 [DOI] [PubMed] [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 15.Kurachi H, Adachi H, Ohtsuka S, et al. : Involvement of epidermal growth factor in inducing obesity in ovariectomized mice. Am J Physiol 1993;265:E323–E331 [DOI] [PubMed] [Google Scholar]
- 16.Bray GA: Genetic, hypothalamic and endocrine features of clinical and experimental obesity. Prog Brain Res 1992;93:333–340 [DOI] [PubMed] [Google Scholar]
- 17.Lorden JF, Caudle A: Behavioral and endocrinological effects of single injections of monosodium glutamate in the mouse. Neurobehav Toxicol Teratol 1986;8:509–519 [PubMed] [Google Scholar]
- 18.Wegorzewska IN, Walters K, Weiser MJ, et al. : Postovariectomy weight gain in female rats is reversed by estrogen receptor α agonist, propylpyrazoletriol. Am J Obstet Gynecol 2008;199:67.e1–e5 [DOI] [PubMed] [Google Scholar]
- 19.Lee D, Kim S: Correlation of the bone mineral density and weight reduction therapy in estrogen replaced obese postmenopausal women. Korean J Obes 2001;10:306–313 [Google Scholar]
- 20.Clegg DJ: Minireview: The year in review of estrogen regulation of metabolism. Mol Endocrinol 2012;26:1957–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevenson JC, Crook D, Godsland IF: Influence of age and menopause on serum lipids and lipoproteins in healthy women. Atherosclerosis 1993;98:83–90 [DOI] [PubMed] [Google Scholar]
- 22.Sugden MC, Bulmer K, Gibbons GF, Knight BL, Holness MJ: Peroxisome-proliferator-activated receptor-alpha (PPARalpha) deficiency leads to dysregulation of hepatic lipid and carbohydrate metabolism by fatty acids and insulin. Biochem J 2002;364:361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linden D, Alsterholm M, Wennbo H, Oscarsson J: PPARalpha deficiency increases secretion and serum levels of apolipoprotein B-containing lipoproteins. J Lipid Res 2001;42:1831–1840 [PubMed] [Google Scholar]
- 24.Costet P, Legendre C, More J, Edgar A, Galtier P, Pineau T: Peroxisome proliferator-activated receptor alpha-isoform deficiency leads to progressive dyslipidemia with sexually dimorphic obesity and steatosis. J Biol Chem 1998;273:29577–29585 [DOI] [PubMed] [Google Scholar]
- 25.Roglans N, Bellido A, Rodriguez C, et al. : Fibrate treatment does not modify the expression of acyl coenzyme A oxidase in human liver. Clin Pharmacol Ther 2002;72:692–701 [DOI] [PubMed] [Google Scholar]
- 26.Mascaro C, Acosta E, Ortiz JA, Marrero PF, Hegardt FG, Haro D: Control of human muscle-type carnitine palmitoyltransferase I gene transcription by peroxisome proliferator-activated receptor. J Biol Chem 1998;273:8560–8563 [DOI] [PubMed] [Google Scholar]
- 27.Brandt JM, Djouadi F, Kelly DP: Fatty acids activate transcription of the muscle carnitine palmitoyltransferase I gene in cardiac myocytes via the peroxisome proliferator-activated receptor alpha. J Biol Chem 1998;273:23786–23792 [DOI] [PubMed] [Google Scholar]
- 28.Louet JF, Chatelain F, Decaux JF, et al. : Long-chain fatty acids regulate liver carnitine palmitoyltransferase I gene (L-CPT I) expression through a peroxisome-proliferator-activated receptor alpha (PPARalpha)-independent pathway. Biochem J 2001;354:189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenchik L, Register TC, Hsu FC, et al. : Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone 2003;33:646–651 [DOI] [PubMed] [Google Scholar]
- 30.Arita Y, Kihara S, Ouchi N, et al. : Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999;257:79–83 [DOI] [PubMed] [Google Scholar]
- 31.Pannacciulli N, Vettor R, Milan G, et al. : Anorexia nervosa is characterized by increased adiponectin plasma levels and reduced nonoxidative glucose metabolism. J Clin Endocrinol Metab 2003;88:1748–1752 [DOI] [PubMed] [Google Scholar]
- 32.Kunnari A, Santaniemi M, Jokela M, et al. : Estrogen replacement therapy decreases plasma adiponectin but not resistin in postmenopausal women. Metabolism 2008;57:1509–1515 [DOI] [PubMed] [Google Scholar]
- 33.Combs TP, Berg AH, Rajala MW, et al. : Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes 2003;52:268–276 [DOI] [PubMed] [Google Scholar]