Abstract
Most hematopoietic stem cell gene therapy studies require host conditioning to allow for efficient engraftment of gene-modified cells. Conditioning regimens with lower treatment-related toxicities are especially relevant for the treatment of nonmalignant blood disorders, such as hemoglobinopathies and immunodeficiencies, and for patients who are otherwise ineligible for conventional high-dose conditioning. Radioimmunotherapy, which employs an α- or a β-emitting radionuclide conjugated to a targeting antibody, is effective for delivering cytotoxic doses of radiation to a cell type of interest while minimizing off-target toxicity. Here, we demonstrate the feasibility of using a nonmyeloablative dose of a monoclonal anti-CD45 antibody conjugated to the α-emitter Astatine-211 (211At) to promote engraftment of an autologous gene-modified stem cell graft in the canine model. The doses used provided myelosuppression with rapid autologous recovery and minimal off-target toxicity. Engraftment levels were low in all dogs and reflected the low numbers of gene-modified cells infused. Our data suggest that a cell dose exceeding 1×106 cells/kg be used with nonmyeloablative doses of 211At-anti-CD45 monoclonal antibodies for sustained engraftment in the dog model.
Introduction
Hematopoietic stem cell transplant (HSCT) is an effective treatment for many congenital and acquired malignant and nonmalignant diseases of the blood. A pretransplantation regimen with high-dose conditioning provides a means for reducing or eradicating the malignant burden, while providing sufficient myelosuppression to prevent rejection of the stem cell graft. For nonmalignant blood diseases, and in patients where significant comorbidities preclude the use of a high-dose regimen, nonmyeloablative conditioning can be used to lower transplant-related complications at the risk of increased graft rejection. Nonmyeloablative conditioning regimens have been used in conjunction with allogeneic HSCT to achieve improved B cell function in severe combined immunodeficiency patients,1 and high stable mixed chimerism in patients for sickle cell disease.2
In contrast to external beam γ-irradiation, which is associated with off-target and late-toxic effects, radioimmunotherapy (RIT) is an approach that selectively delivers high-energy radiation directly to a cell type of interest via the conjugation of a radionuclide to a targeting antibody. The majority of RIT clinical studies for hematologic disease have utilized β-emitting radionuclides, such as yttrium-90 (90Y), iodine-131 (131I), and rhenium-188 (188Re), conjugated to monoclonal antibodies that bind hematopoietic antigens CD33, CD45, and CD66.3 These radioimmunoconjugates have been used at myeloablative doses primarily for the treatment of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS).4,5 β-Emitting radionuclides conjugated to CD20 have also been shown to be particularly efficacious against non-Hodgkin's lymphoma (NHL),6,7 in part because of the “cross-fire” effect of β-emissions on malignant cells that are not expressing antigen,8 a property that also makes them attractive for solid tumors. However, the characteristically long path-length of β-emitters (0.8–4 mm), which makes them suitable in the context of solid tumor and NHL treatment, poses an increased risk for off-target cytotoxicity. In contrast, α-emitting radionuclides have a shorter path length (approximately 40–80 μm) and higher linear energy transfer (LET; approximately 100 keV/μM) than the β-emitting equivalents,9 providing a stronger cytotoxic insult and higher target specificity to blood cells when conjugated to hematopoietic antigens. Clinical experience with α-emitting RIT for hematologic malignancies is limited to a smaller number of trials, in particular, the monoclonal bismuth-213-labeled (213Bi)-anti-CD33 radioimmunoconjugate for AML.10 However, because of the short half-life of 213Bi (45.6 min), other α-emitters are currently under investigation for RIT, including astatine-211 (211At) and actinium-225 (225Ac), which have half-lives of 7.21 hr and 10 days, respectively. 211At-anti-CD45 RIT was recently shown to prolong the survival of leukemic mice in a dose-dependent manner when used as a preparative regimen for HSCT.11
The CD45 antigen is a protein tyrosine phosphatase that is ubiquitously expressed on leukocytes, including the hematopoietic stem cell,12 making CD45 immunoconjugates particularly suitable as a conditioning regimen before HSCT.13 The safety and efficacy of both 213Bi-anti-CD45 and 211At-anti-CD45 RIT as a sole replacement for γ-beam total body irradiation (TBI) has been demonstrated in a dog model of dog leukocyte antigen-identical allogeneic bone marrow transplantation.14,15 In these studies, nonmyeloablative doses of anti-CD45 RIT were established that permitted stable donor chimerism at a level equivalent to 200–300 cGy TBI. Off-target toxicity in both studies was limited to liver injury at doses that exceeded 0.405 mCi/kg, likely because of the high number of CD45-expressing blood cells in the liver.15
In patients where allogeneic HSCT is not an option because of the lack of a suitable donor, autologous hematopoietic stem cell gene therapy holds promise as an alternative treatment, which eliminates complications stemming from graft-versus-host disease. The transfer of drug-resistance genes, such as the methylguanine methyltransferase variant P140K (MGMTP140K), into hematopoietic stem cells by viral vectors has been evaluated in preclinical studies and clinical trials for two important outcomes. First, in the context of malignancy, the expression of MGMTP140K in hematopoietic stem cells enables for an increase in the dose and/or frequency of administration of alkylating chemotherapy that would otherwise exceed the dose-limiting hematopoietic toxicity, when given in combination with the wild-type MGMT inhibitor, O6-benzylguanine (O6BG).16 The efficacy of this strategy was recently validated in a phase I clinical trial for temozolomide dose intensification for gliobastoma multiforme (NCT #00669669).17 Second, the expression of MGMTP140K in hematopoietic stem cells can be used to expand the hematopoietic contribution of the gene-modified stem cell population following in vivo selection with an alkylating agent and O6BG. MGMTP140K-mediated chemoselection has been shown to increase the fraction of gene-modified cells in peripheral blood in primary and secondary dog recipients,18,19 as well as the nonhuman primate.20
In the present study we explored whether engraftment of autologous hematopoietic stem cells gene-modified with a lentiviral vector containing the MGMTP140K transgene could be sustained after nonmyleoablative conditioning with the pan-hematopoietic anti-CD45 monoclonal antibody (MAb) conjugated to 211At (211At-anti-CD45 MAb).
Materials and Methods
Animal care and procedures
Dogs were raised and housed at the Fred Hutchinson Cancer Research Center under conditions approved by the American Association of Accreditation of Laboratory Animal Care. All experimental procedures were performed in compliance with the Fred Hutchinson Cancer Research Center Institutional Animal Care and Use Committee file 1289.
Conjugation and radiolabeling of the anti-CD45 monoclonal antibody
The dog anti-CD45 MAb CA12.10C12 was conjugated with closo-decaborate(2-), and astatination was carried out as previously described.15,21 Astatine-211 was generated by irradiation of bismuth metal with a Scanditronix MC-50 cyclotron at the University of Washington (Seattle, WA) as described previously.22 The injection was prepared by combining the radiolabeled 211At-anti-CD45 MAb with additional nonlabeled closo-decaborate-conjugated MAb for a final concentration of 0.5 mg/kg in 4 ml PBS.
Lentiviral vectors
The HIV-based lentiviral vectors were self-inactivating (SIN) pRRL vector backbones containing a central polypurine tract, and a woodchuck posttranscriptional regulatory element. The internal promoter used was the cellular elongation factor 1α EF1α promoter expressing a drug-resistant variant of methylguanine methyltransferase (MGMTP140K). The vectors were pseudotyped with the vesicular stomatitis virus glycoprotein envelope and produced essentially as described.23
Dog CD34+ cell isolation, lentivirus transduction, and transplantation
CD34+ cell enrichment was performed as previously described.24 Briefly, bone marrow samples were subjected to red cell lysis in an ammonium chloride buffered solution of which the resulting white blood cells were incubated with a CD34 antibody conjugated to biotin (Clone 1H6, produced in-house), and subsequently incubated with streptavidin microbeads (Miltenyi Biotech, Auburn, CA). CD34+ cells were enriched by magnetic-activated cell sorting on LS columns (Miltenyi Biotech) with purities ranging from 70% to 90%. Following enrichment, cells were transduced for 18–20 hr in Iscove's modified Dulbecco's medium (Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (Atlas Biologicals, Fort Collins, CO), 100 U/ml penicillin, and 100 μg/ml streptomycin (Life Technologies). Recombinant human thrombopoietin (rhTPO; Peprotech, Rocky Hill, NJ), FLT-3 ligand (rhFLT3; Miltenyi Biotech, San Diego, CA), recombinant dog granulocyte-colony stimulating factor (rcG-CSF), and recombinant dog stem cell factor (rcSCF; Amgen, Thousand Oaks, CA) were added to the transduction medium each at a final concentration of 100 ng/ml. Transduction occurred in the presence of 4 μg/ml protamine sulfate in flasks precoated with the CH296 fragment of RetroNectin (TaKaRa, Mountain View, CA) at a multiplicity of infection of 20. Transduced cells were harvested and infused following nonmyeloablative conditioning.
Conditioning regimen
Two days before transplant, bone marrow was harvested from the dogs for stem cell enrichment and gene modification. Dogs were given intravenous injections of the unlabeled dog anti-CD45 MAb (clone CA12.10C12, 10% by volume of total injected antibody) following bone marrow harvest. One hour later, dogs were injected with anti-CD45 MAb labeled with 211At at doses of 0.49 mCi/kg (H458), 0.46 mCi/kg (H461), 0.40 mCi/kg (H469), and 0.49 mCi/kg (H472). Two days following bone marrow harvest (day 0), the dogs were given autologous gene-modified CD34+ cells intravenously and monitored by regular blood counts and blood chemistry. When necessary, dogs were supported with cG-CSF and blood transfusions to facilitate engraftment of neutrophils and platelets, respectively.
Colony-forming cell assay
Transduced, mock-transduced, and bone marrow white blood cell samples from transplanted dogs were cultured in semisolid methylcellulose media for 12–14 days in the presence of 100 ng/ml each recombinant human TPO, human EPO (Amgen), canine SCF, canine G-CSF, and canine GM-CSF (R&D Systems, Minneapolis, MN). Colonies were enumerated and scored based on morphology. Gene marking was assessed in colonies using lentivirus-specific PCR analysis. Individual colonies from colony-forming cell assays for each dog were picked into 90 μl of water supplemented with 1.7 U of proteinase K from Triticharium album (Sigma-Aldrich, St. Louis, MO) to extract genomic DNA, and the resulting extraction was analyzed by PCR to determine percentage of colonies positive for lentiviral integration.
Gene marking assessment
Heparinized peripheral blood and bone marrow collected at various time points following transplantation were subjected to red cell lysis by ammonium chloride buffered solution. The resulting leukocytes were prepared for DNA extraction and analyzed for lentivirus integration by qPCR using a TaqMan 5′ nuclease quantitative real-time PCR assay essentially as previously described.20 Sample DNA was analyzed in duplicate with a lentivirus-specific primer–probe combination (forward, 5′-TGA AAG CGA AAG GGA AAC CA-3′; reverse, 5′-CCG TGC GCG CTT CAG-3′; probe, 5′-6-FAM-AGC TCT CTC GAC GCA GGA CTC GGC-TAMRA-3′ [Integrated DNA Technologies, Coralville, IA]) and in a separate reaction with a interleukin-3 (IL-3)-specific primer–probe combination (forward, 5′-ATG AGC AGC TTC CCC ATC C-3′; reverse, 5′-GTC GAA AAA GGC CTC CCC-3′; probe, 5′-6-FAM-TCC TGC TTG GAT GCC AAG TCC CAC-TAMRA-3′) to adjust for equal loading volume of genomic DNA per reaction.
Results
Gene therapy and autologous hematopoietic stem cell transplant
Four dogs received autologous HSCT with cells that were gene-modified with a SIN lentiviral vector expressing the chemotherapy-resistant gene MGMTP140K expressed under the EF1α promoter (see Materials and Methods). Hematopoietic stem cell transduction efficiency, as evaluated by hematopoietic colony PCR, varied among the cohort from 17.7% to 88.0% (Table 1). Following bone marrow harvest, the dogs were infused with cold (not radiolabeled) anti-CD45 MAb at 0.05 mg/kg to reduce off-target binding of the CD45 antibody.25 One to two hours following injection of the cold antibody, the dogs received a dose of 211At-anti-CD45 MAb (day −2) ranging from 0.40 to 0.49 mCi/kg, which was shown to be nonmyeloablative based on a previous dose-escalation study (Table 1).15 Two days later, the dogs received a cell infusion of autologous gene-modified CD34+ bone marrow cells. Because of low cell yields obtained from steady-state bone marrow, final cell doses ranged from 0.3×106 to 1.8×106 cells/kg. The dogs received standard care, including blood transfusions for low platelets (median 4.5 transfusions, range 3–5) and subcutaneous recombinant canine G-CSF (5 μg/kg) for prolonged neutropenia (median 3.5 treatments, range 0–11). Additionally, H461, H469, and H472 received cyclosporine (15 mg/kg twice daily) for 3–4 months following transplant to prevent an immune response to the human MGMTP140K transgene.
Table 1.
Summary of Canine Transplantations
| Dog | Age at transplant (years) | Weight at transplant (kg) | 211At dose (mCi/kg) | Cells infused (kg−1) | Transduction efficiency (%) | Days to ANC >1000/μl | Days to platelet count >100×103/μl |
|---|---|---|---|---|---|---|---|
| H458 | 2.1 | 14.5 | 0.49 | 2.8×105 | ND | 30 | 59 |
| H461 | 1.5 | 13.7 | 0.40 | 8.3×105 | 17.7 | 15 | 38 |
| H469 | 2.4 | 9.4 | 0.46 | 2.3×106 | 40.9 | 21 | 40 |
| H472 | 2.8 | 10.3 | 0.49 | 1.8×106 | 88.0 | 15 | 40 |
ANC, absolute neutrophil count; ND, not determined.
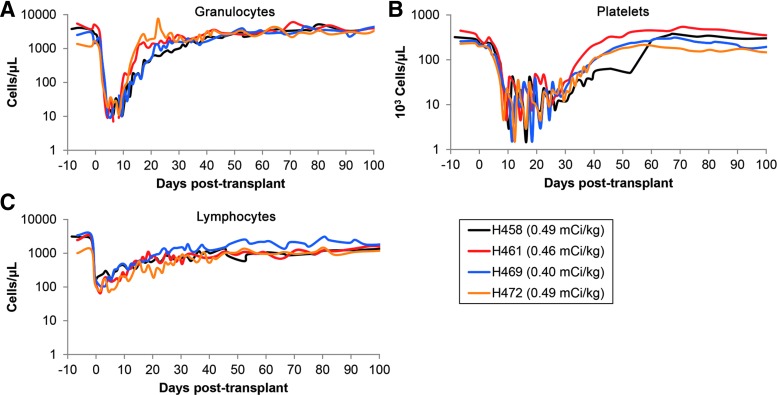
All four dogs experienced marked cytopenia after injection with 211At-anti-CD45 MAb (Fig. 1). The neutrophil nadir occurred at a median of 7 days posttransplant (range 5–9 days), with a mean neutrophil count of 9 cells/μl. The platelet nadir occurred at a median of 11.5 days posttransplant (range 9–16 days), with a mean platelet (PLT) count of 2,125 PLT/μl. Hematopoietic recovery was rapid, with a median neutrophil recovery (defined as absolute neutrophil counts >1,000 cells/μl) occurring at 18 days posttransplant (range 15–30 days), and the median platelet recovery (defined as >100,000 PLT/μl) occurring at 40 days posttransplant (range 38–59 days) (Fig. 1 and Table 1).
FIG. 1.
Hematology following conditioning with 211At-anti-CD45 monoclonal antibody (MAb). Dogs received between 0.40 and 0.49 mCi/kg of body weight on day −2, and complete blood counts were monitored for the first 100 days. All dogs experienced neutropenia, thrombocytopenia, and lymphocytopenia with a median neutrophil nadir of 9 cells/μl (A), median platelet nadir of 1,500 PTL/μl (B), and median lymphocyte nadir of 88 cells/μl (C). Color images available online at www.liebertpub.com/hum
Toxicity of 211At-anti-CD45 MAb
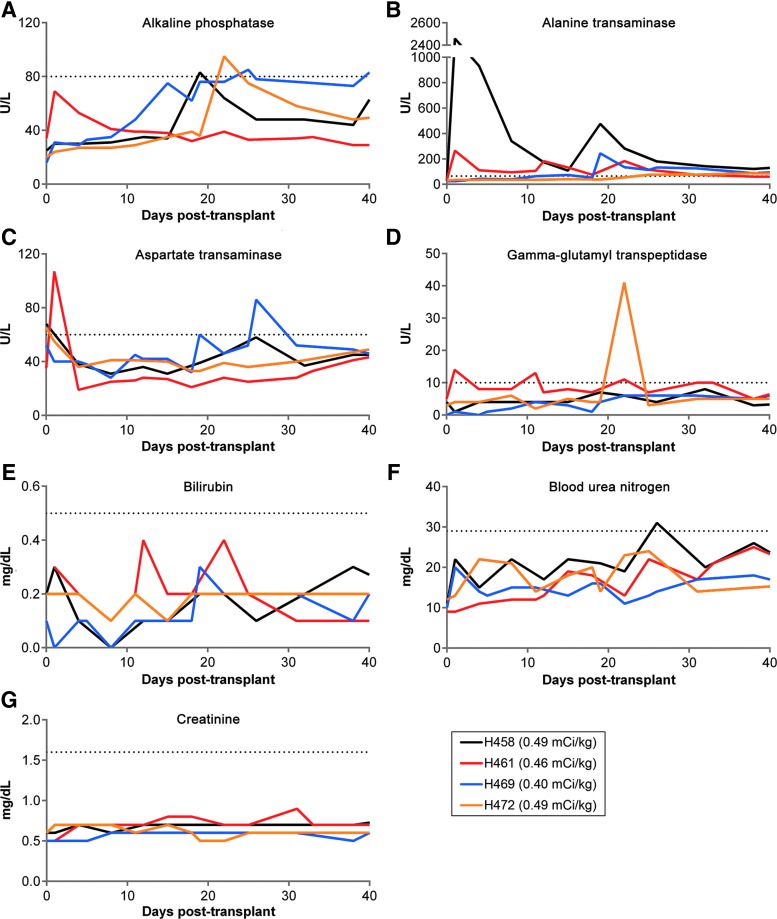
Alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl transpeptidase (GGT), bilirubin, blood urea nitrogen (BUN), and creatinine were monitored in blood serum for over 30 days following administration of the 211At-anti-CD45 MAb to evaluate renal and hepatic toxicity. As shown in Fig. 2, all dogs generally were within normal limits for ALP, AST, GGT, bilirubin, BUN, and creatinine serum levels. H458 exhibited a strong increase in ALT serum levels, reaching a maximum value of 2460 U/liter 3 days after injection of the RIT. H469 exhibited a sudden elevated ALT value of 244 U/liter at 22 days posttransplant, and levels remained slightly above the upper limit until day 45, at which ALT values returned to within normal limits until euthanasia. All four dogs demonstrated normal kidney function as evidenced by BUN and creatinine levels. Daily examination showed that the dogs experienced varying degrees of dehydration, vomiting, and diarrhea that were not dose dependent, and usually resolved within 10 days postadministration of 211At-anti-CD45 MAb. Excepting the very high initial ALT value in H459, the data suggest that the prescribed doses of 211At resulted in low extramedullary toxicity and were generally well tolerated in the dogs.
FIG. 2.
Liver and kidney function tests. Serum chemistry analysis was performed for the first month following administration of the 211At-anti-CD45 MAb. Liver enzymes alkaline phosphatase, alanine transaminase, aspartate aminotransferase, gamma-glutamyl transpeptidase, and bilirubin were monitored for liver function (A–E). Blood urea nitrogen and creatinine were followed to monitor kidney function (F, G). The dotted line in each graph represents the upper limit of normal. Color images available online at www.liebertpub.com/hum
Engraftment of gene-modified cells and chemoselection
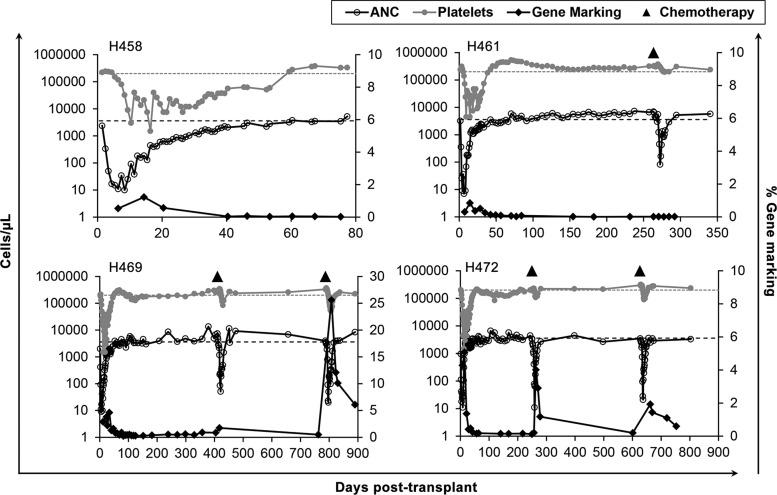
Engraftment of gene-modified cells was low in all four dogs, as determined by quantitative PCR on peripheral blood leukocytes (Fig. 3). We therefore tested whether selection for MGMTP140K gene-modified cells could be achieved by treating three of the four dogs with combination chemotherapy consisting of O6BG and the alkylating agent, BCNU. H461, H469, and H472 received an initial round of chemotherapy of 5 mg/kg O6BG, followed 1 hr later by 0.3 mg/kg BCNU. This dose has previously been shown to be sufficient for multilineage selection, as well as selection for gene-modified bone marrow CD34+ cells.26 All three dogs experienced mild neutropenia and thrombocytopenia as a result, with subsequent autologous recovery (Fig. 3). This dose was sufficient to induce a transient increase in peripheral blood gene marking in two of the three dogs (H469 and H472). No selection was observed in H461, despite a similar level of myelosuppression as compared with H469 and H472. Of note, H461 was infused with the lowest cell dose of the three dogs that received combination chemotherapy, and had the lowest preinfusion transduction efficiency as determined by an in vitro colony PCR assay (Table 1), compared with H469 and H472.
FIG. 3.
Gene marking and myelosuppression associated with chemoselection. Gene marking was performed by quantitative TaqMan PCR on peripheral blood leukocytes, and neutrophil and platelet numbers were retrieved from complete blood counts. Chemoselection was performed in H461, H469, and H472 at the indicated time points. The dotted lines represent the lower limit of normal for neutrophils and platelets.
The increase in gene marking in H469 and H472 was not sustained following the first round of chemoselection, which prompted a second round of combination chemotherapy with a 33% increase in BCNU (0.4 mg/kg) to encourage a more robust selection of gene-modified progenitor cells. The higher dosing level administered to H469 and H472 resulted in neutropenia and thrombocytopenia that was on average slightly more intense than the initial dose, although not statistically significant (Fig. 3). This dose led to a second increase in gene-modified cells in both dogs, indicating the presence of a long-term, but limited, population of gene-modified stem cells capable of hematopoietic repopulation (Fig. 3).
End of study
H458 and H461 were euthanized at 125 and 348 days posttransplant because of a loss of gene-modified cells in vivo. H469 and H472 experienced declining health and clinical complications at more than 800 days posttransplant that warranted euthanasia. H472 developed hematuria, and was suspected of having a bladder mass by ultrasonography. The dog was found to have low-grade prostatic adenocarcinoma evidenced by adenomatous hyperplasia of the prostate with foci of suspected microinvasion at necropsy 866 days posttransplant. H469 was euthanized at 890 days posttransplant, and on necropsy was discovered to have acute lobular pneumonia, renal medullary mineral castes in association with grossly detected renal stones, damaged renal medullary outer stripe, and marked increase in iron store in liver with moderate fatty change of liver.
Discussion
The objective of this study was to evaluate the feasibility of using a nonmyeloablative dose of 211At-anti-CD45 MAb to facilitate engraftment of gene-modified cells in a dog model of autologous HSCT. The cohort of dogs responded to the nonmyeloablative dose as expected from a previous dose-escalation study,15 with comparable granulocyte, platelet, and lymphocyte nadirs and hematopoietic recovery. Serum chemistries immediately following the administration of the radioimmunoconjugate suggest that the conditioning regimen was well-tolerated and produced minimal off-target toxicity in three of the four dogs. The data suggest that the dose of gene-modified cell numbers infused was near the threshold for successful stem cell engraftment, as selection for a chemotherapy-resistant graft was successful when the infusion product exceeded 1×106 cells/kg. We interpret the transient increase in peripheral blood gene marking as evidence for the presence of a small number of resident gene-modified stem cells capable of hematopoietic contribution, as selection was observed in H469 and H472 upward of 2.2 and 1.7 years posttransplant, respectively. Our data suggest that nonmyeloablative doses of 211At-anti-CD45 MAb can be used to support the long-term engraftment of autologous gene-modified hematopoietic stem cells when an adequate gene-modified cell dose is infused. We further propose that the transplantation of higher doses of gene-modified cells in dogs that receive nonmyeloablative 211At-anti-CD45 MAb conditioning may allow for an in vivo expansion of chemotherapy-resistant stem cells that has been previously achieved in dogs conditioned with lethal doses of total body irradiation or reduced-intensity BCNU or temozolomide.19,27
RIT has been evaluated as a part of a preparative regimen for HSCT in several clinical studies, most notably for the treatment of AML, MDS, and NHL.4–7 However, RIT as the sole conditioning agent is so far limited to preclinical studies in animal models. In the context of conditioning for a stem cell transplant without the need for induction, α-emitting radionuclides may be preferred over the β-emitting radionuclides for conditioning because of their shorter path-length and higher LET. Both 213Bi- and 211At-anti-CD45 radioimmunoconjugates at nonmyeloablative doses have been shown to be sufficient to support stable mixed chimerism following allogeneic HSCT in the dog.14,15 However, the short half-life of 213Bi (45.6 min) poses several logistical challenges in HSCT conditioning, notably, the need to administer repeated doses to achieve sufficient myelosuppression. In a recent study comparing the biodistribution of 213Bi and 211At RIT in the mouse, 211At-radioimmunoconjugates were found at higher concentrations in the spleen, likely because of the ability of the radioimmunoconjugate to home to antigen-expressing cells before decay of the isotope, despite equivalent doses of radiation.28 Additionally, administration of 211At-labeled immunoconjugates resulted in a higher level of immunosuppression and reduced off-target cytotoxicity than the 213Bi immunoconjugates. For these reasons, this study employed the 211At-anti-CD45 MAb conditioning before autologous gene-modified HSCT.
This study used unprimed, steady-state bone marrow as the stem cell source for lentivirus transduction and HSCT. As such, our cohort received a relatively low number of cells following the conditioning regimen (Table 1). Gene-modified stem cell grafts were not detectable in the two dogs that received less than 1.0×106 cells/kg (H458 and H461). Additionally, the low transduction efficiency in H461 could have contributed to our inability to detect gene-modified progeny, even after a single cycle of chemoselection (Fig. 3). In contrast, the two dogs that received cell doses greater than 1.0×106 cells/kg had a low, but appreciable, level of gene marking that responded to combination chemotherapy consisting of O6BG and BCNU with a transient in vivo selection. The failure of a gene-modified graft in the other two animals was associated with the low cell dose infused, and we conclude that this was likely the primary limiting factor for the lack of detection of gene-modified cells in these dogs. Other factors, such as low ex vivo viral transduction efficiency (e.g., H461), may also have contributed to this result. We attribute the successful engraftment of gene-modified stem cells, as evidenced by the transient increase in peripheral blood gene marking following O6BG and BCNU combination chemotherapy, to the nonmyeloablative conditioning provided by 211At-anti-CD45 MAb, as we have previously reported the failure of gene-modified autologous stem cells to engraft in nonconditioned dogs.29211At-anti-CD45 MAb has been used at doses as low as 0.2–0.3 mCi/kg to support 30–40% stable donor chimerism for up to 1 year in an allogeneic canine HSCT model,15 suggesting that the failure of gene-modified stem cells to engraft in H458 and H461 was because of the low cell number of infused cells rather to insufficient conditioning with 0.40–0.49 mCi/kg of 211At-anti-CD45 MAb. Taken together, our data suggest that a cell dose in excess of 1.0×106 cells/kg is needed in order to overcome the threshold for engraftment following nonmyeloablative 211At-anti-CD45 MAb conditioning in the dog.
Dogs H469 and H472 responded to chemoselection with a transient increase in peripheral blood cell gene marking and were followed long-term. Both succumbed to health complications at over 2.3 years following conditioning with 211At-anti-CD45 MAb. H469 was found to have a liver pathology consisting of iron stores and fatty change on necropsy. H469 showed no elevation in liver function tests immediately following the administration of 211At-anti-CD45 MAb; however, a mild and short-term elevation in ALT was noted between 22 and 45 days posttransplant. The liver is rich in blood and contains numerous CD45-positive cells, making it especially susceptible to toxicity by CD45 radioimmunoconjugates, although the short path-length of α-emitters should render the bystander effect negligible. O6BG and BCNU combination chemotherapy has been used at similar doses to those in this study for the selection of chemotherapy-resistant cells in dogs conditioned with myeloablative doses of total body irradiation without evidence for extramedullary toxicity.26 Thus, it is unlikely that 211At-anti-CD45 MAb and/or O6BG/BCNU treatments contributed to the liver finding in H469. H469 was euthanized because of declining health just 103 days following the second dose-escalated administration of BCNU (0.4 mg/kg). At the time of euthanasia, complete blood counts had returned to within normal limits, and serum values for ALP (234 U/l), ALT (128 U/l), and AST (126 U/l) were above the upper threshold.
In summary, the use of 211At-anti-CD45 MAb at nonmyeloablative doses as the sole preparative regimen is shown to be a safe and well-tolerated conditioning method for gene-modified autologous HSCT in the dog. Our results corroborate with a growing body of evidence demonstrating that nonmyeloablative doses of 211At-labeled CD45 antibodies result in a highly specific cytotoxicity for hematopoietic cells with minimal extra-hematopoietic toxicity, and allow for a rapid autologous recovery. We interpret the lack of engraftment in two dogs to be reflective of insufficient cell doses at transplant, and our data suggest that a cell dose equal to or exceeding 1.0×106 cells/kg is required for long-term engraftment in the dog when 211At-anti-CD45 MAb is used at nonmyeloablative doses for HSCT conditioning.
Acknowledgments
The authors would like to thank Dr. M. Wohlfahrt, K. Norman, and A. Adams for technical support, and G. Choi for assistance in preparing the article. We would also like to acknowledge the veterinary and technical staff who provided care and treatments for the dogs. This study was funded by NIH HL36444 and CA172582. H.-P.K. is a Markey Molecular Medicine Investigator and received support as the inaugural recipient of the José Carreras/E. Donnall Thomas Endowed Chair for Cancer Research.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Haddad E, Leroy S, Buckley RH. B-cell reconstitution for SCID: should a conditioning regimen be used in SCID treatment? (Review). J Allergy Clin Immunol 2013;131:994–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsieh MM, Fitzhugh CD, Weitzel RP, et al. . Nonmyeloablative HLA-matched sibling allogeneic hematopoietic stem cell transplantation for severe sickle cell phenotype. JAMA 2014;312:48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pagel JM, Matthews DC, Appelbaum FR, et al. . The use of radioimmunoconjugates in stem cell transplantation (Mini-review). Bone Marrow Transplant 2002;29:807–816 [DOI] [PubMed] [Google Scholar]
- 4.Appelbaum FR, Matthews DC, Eary JF, et al. . The use of radiolabeled anti-CD33 antibody to augment marrow irradiation prior to marrow transplantation for acute myelogenous leukemia. Transplantation 1992;54:829–833 [DOI] [PubMed] [Google Scholar]
- 5.Bunjes D, Buchmann I, Duncker C, et al. . Rhenium 188-labeled anti-CD66 (a, b, c, e) monoclonal antibody to intensify the conditioning regimen prior to stem cell transplantation for patients with high-risk acute myeloid leukemia or myelodysplastic syndrome: results of a phase I-II study. Blood 2001;98:565–572 [DOI] [PubMed] [Google Scholar]
- 6.Press OW, Eary JF, Appelbaum FR, et al. . Radiolabeled-antibody therapy of B-cell lymphoma with autologous bone marrow support. N Engl J Med 1993;329:1219–1224 [DOI] [PubMed] [Google Scholar]
- 7.Kaminski MS, Zasadny KR, Francis IR, et al. . Radioimmunotherapy of B-cell lymphoma with [131I] anti-B1 (anti-CD20) antibody. N Engl J Med 1993;329:459–465 [DOI] [PubMed] [Google Scholar]
- 8.Palanca-Wessels MC, Press OW. Advances in the treatment of hematologic malignancies using immunoconjugates (Review). Blood 2014;123:2293–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macklis RM, Kinsey BM, Kassis AI, et al. . Radioimmunotherapy with alpha-particle-emitting immunoconjugates. Science 1988;240:1024–1026 [DOI] [PubMed] [Google Scholar]
- 10.Jurcic JG, Larson SM, Sgouros G, et al. . Targeted alpha particle immunotherapy for myeloid leukemia. Blood 2002;100:1233–1239 [PubMed] [Google Scholar]
- 11.Orozco JJ, Back T, Kenoyer A, et al. . Anti-CD45 radioimmunotherapy using 211At with bone marrow transplantation prolongs survival in a disseminated murine leukemia model. Blood 2013;121:3759–3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells (Review). Annu Rev Immunol 2003;21:107–137 [DOI] [PubMed] [Google Scholar]
- 13.Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood 2014;124:344–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandmaier BM, Bethge WA, Wilbur DS, et al. . Bismuth 213-labeled anti-CD45 radioimmunoconjugate to condition dogs for nonmyeloablative allogeneic marrow grafts. Blood 2002;100:318–326 [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Kornblit B, Hamlin DK, et al. . Durable donor engraftment after radioimmunotherapy using α-emitter astatine-211-labeled anti-CD45 antibody for conditioning in allogeneic hematopoietic cell transplantation. Blood 2012;119:1130–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis BM, Roth JC, Liu L, et al. . Characterization of the P140K, PVP(138–140)MLK, and G156A O6-methylguanine-DNA methyltransferase mutants: implications for drug resistance gene therapy. Hum Gene Ther 1999;10:2769–2778 [DOI] [PubMed] [Google Scholar]
- 17.Adair JE, Beard BC, Trobridge GD, et al. . Extended survival of glioblastoma patients after chemoprotective HSC gene therapy. Sci Transl Med 2012;4:133ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neff T, Beard BC, Peterson LJ, et al. . Polyclonal chemoprotection against temozolomide in a large-animal model of drug resistance gene therapy. Blood 2005;105:997–1002 [DOI] [PubMed] [Google Scholar]
- 19.Beard BC, Sud R, Keyser KA, et al. . Long-term polyclonal and multilineage engraftment of methylguanine methyltransferase P140K gene-modified dog hematopoietic cells in primary and secondary recipients. Blood 2009;113:5094–5103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beard BC, Trobridge GD, Ironside C, et al. . Efficient and stable MGMT-mediated selection of long-term repopulating stem cells in nonhuman primates. J Clin Invest 2010;120:2345–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilbur DS, Chyan M-K, Nakamae H, et al. . Reagents for astatination of biomolecules. 6. An intact antibody conjugated with a maleimido-closo-decaborate(2-) reagent via sulfhydryl groups had considerably higher kidney concentrations than the same antibody conjugated with an isothiocyanato-closo-decaborate(2-) reagent via lysine amines. Bioconjugate Chem 2012;23:409–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilbur DS, Vessella RL, Stray JE, et al. . Preparation and evaluation of para-[211At]astatobenzoyl labeled anti-renal cell carcinoma antibody A6H F(ab')2. In vivo distribution comparison with para-[125I]iodobenzoyl labeled A6H F(ab')2. Nucl Med Biol 1993;20:917–927 [DOI] [PubMed] [Google Scholar]
- 23.Horn PA, Keyser KA, Peterson LJ, et al. . Efficient lentiviral gene transfer to canine repopulating cells using an overnight transduction protocol. Blood 2004;103:3710–3716 [DOI] [PubMed] [Google Scholar]
- 24.Gori JL, Beard BC, Williams NP, et al. . In vivo protection of activated Tyr22-dihydrofolate reductase gene-modified canine T lymphocytes from methotrexate. J Gene Med 2013;15:233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiraly F, Kletting P, Reske S, Glatting G. Modelling radioimmunotherapy with anti-CD45 antibody to obtain a more favourable biodistribution. Nucl Med 2009;48:113–119 [DOI] [PubMed] [Google Scholar]
- 26.Neff T, Horn PA, Peterson LJ, et al. . Methylguanine methyltransferase-mediated in vivo selection and chemoprotection of allogeneic stem cells in a large-animal model. J Clin Invest 2003;112:1581–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gori JL, Beard BC, Ironside C, et al. . In vivo selection of autologous MGMT gene-modified cells following-reduced intensity conditioning with BCNU and temozolomide in the dog model. Cancer Gene Ther 2012;19:523–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamae H, Wilbur DS, Hamlin DK, et al. . Biodistribution, myelosuppression, and toxicities in mice treated with an anti-CD45 antibody labeled with the α-emitting radionuclides bismuth-213 or astatine-211. Cancer Res 2009;69:2408–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barquinero J, Kiem H-P, von Kalle C, et al. . Myelosuppressive conditioning improves autologous engraftment of genetically marked hematopoietic repopulating cells in dogs. Blood 1995;85:1195–1201 [PubMed] [Google Scholar]