Abstract
Cervical spinal cord injury (cSCI) disrupts bulbospinal projections to motoneurons controlling the upper limbs, resulting in significant functional impairments. Ongoing clinical and experimental research has revealed several lines of evidence for functional neuroplasticity and recovery of upper extremity function after SCI. The underlying neural substrates, however, have not been thoroughly characterized. The goals of the present study were to map the intraspinal motor circuitry associated with a defined upper extremity muscle, and evaluate chronic changes in the distribution of this circuit following incomplete cSCI. Injured animals received a high cervical (C2) lateral hemisection (Hx), which compromises supraspinal input to ipsilateral spinal motoneurons controlling the upper extremities (forelimb) in the adult rat. A battery of behavioral tests was used to characterize the time course and extent of forelimb motor recovery over a 16 week period post-injury. A retrograde transneuronal tracer – pseudorabies virus – was used to define the motor and pre-motor circuitry controlling the extensor carpi radialis longus (ECRL) muscle in spinal intact and injured animals. In the spinal intact rat, labeling was observed unilaterally within the ECRL motoneuron pool and within spinal interneurons bilaterally distributed within the dorsal horn and intermediate gray matter. No changes in labeling were observed 16 weeks post-injury, despite a moderate degree of recovery of forelimb motor function. These results suggest that recovery of the forelimb function assessed following C2Hx injury does not involve recruitment of new interneurons into the ipsilateral ECRL motor pathway. However, the functional significance of these existing interneurons to motor recovery requires further exploration.
Key words: : functional plasticity, propriospinal interneurons, SCI, upper extremity
Introduction
Although many examples of functional neuroplasticity and therapeutically driven motor recovery have been reported in the spinal cord injury (SCI) literature, the underlying neural substrates mediating post-injury behavioral changes have not been extensively documented. A detailed understanding of the neuronal circuits underlying spontaneous recovery can significantly contribute to the design of cell-based and axonal growth-promoting approaches, as well as critical evaluation and prediction of their efficacy.1,2 Unfortunately, comprehensive neuroanatomical demonstrations of functionally defined intraspinal circuits are lacking, in even the intact spinal cord.1 The more notable exceptions are studies of spinal bladder,3 autonomic,4 and respiratory1,5 networks, which have employed the transneuronal tracer, pseudorabies virus (PRV), to identify pre-motor spinal interneurons.
Treatments leading to restored upper extremity function are considered to be among the major therapeutic priorities of those who have endured cervical SCIs (cSCIs).6 Therefore, considerable attention has been directed at developing treatments to promote connectivity and functional improvements in rodent cSCI models. However, apart from demonstration of cervical motoneuron topography,7–9 the spinal neuronal substrate associated with forelimb function has not been fully investigated. Propriospinal neurons are of particular interest, because they modulate lower motoneuron activity10 and have the potential to subserve partial recovery of motor function following incomplete SCI.11–15
Accordingly, the first goal of the present work was to characterize the anatomical distribution of the intraspinal pre-motor circuitry associated with a defined forelimb muscle, the extensor carpi radialis longus (ECRL), in the adult rat. The ECRL is significantly involved in facilitating wrist extension during a number of motor behaviors including locomotion, grooming, and feeding. As in our previous definition of the spinal phrenic motor circuitry,1 PRV was used to map the distribution of ECRL motoneurons and the spinal interneurons that innervate them.
Most studies investigating function of the upper limb after cSCI have utilized experimental lesions that directly damage forelimb motoneuron pools (i.e., mid- and low-cervical contusions).16–20 Those studies clearly established that injuries damaging forelimb motoneuron pools, as well as propriospinal networks in surrounding gray matter, led to persistent forelimb motor control deficits. On the other hand, injuries rostral to forelimb motor pools, which could be considered as primarily descending tract (i.e., white matter) lesions, have received very little formal evaluation.21 The second goal of the present study was to determine the effect of C2 hemisection (C2Hx) injury on ECRL pre-motor interneuron connectivity relative to the temporal progression of forelimb behavioral improvements involving the ECRL. These results provide the first detailed documentation of spinal motor- and pre-motor interneurons associated with forelimb muscle in the intact and injured adult rat spinal cord.
Methods
Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Florida. A total of 60 adult female Sprague–Dawley rats (200–250 g; ∼16 weeks of age) were obtained from Harlan Laboratories Inc. (Indianapolis, IN). Animals were housed within the McKnight Brain Institute at the University of Florida and maintained on a 12/12 h light–dark cycle. In addition, they had free access to water and food pellets. Animals were assigned to one of two groups: spinally intact (n=22) or C2Hx groups (n=38). Animals within the spinally intact group were injected with a neuroanatomical tracer and assigned to either a 24 h (n=2), a 48 h (n=4), a 72 h (n=6), a 96 h (n=6), or a 120 h (n=4) post-injection incubation period. Within the chronic hemisection group (n=38), all animals underwent behavioral assessments prior to and at biweekly intervals following C2Hx injury. A subset of 14 animals from the C2Hx group were allocated for neuroanatomical assessments at a post-PRV incubation interval of either 72 (n=6) or 96 h (n=8). All animals assigned to the C2Hx group were assessed for lesion completeness prior to inclusion in the final analyses.
General surgical methods
Anesthesia and injury methods have been previously described.22–24 Briefly, rats were anesthetized by injection of xylazine (10 mg/kg, s.q.) and ketamine (140 mg/kg, i.p., Fort Dodge Animal Health, IA). Following completion of the surgical procedure, anesthesia was reversed via injection of yohimbine (1.2 mg/kg s.q.). Upon recovery, animals were given injections of buprenorphine (0.03 mg/kg s.q., Hospira, IL) for analgesia and sterile lactated Ringers solution (5 mL s.q.) to prevent dehydration. Postsurgical care included administration of buprenorphine (0.03 mg/kg, s.q.) for the initial 48 h post-injury and delivery of lactate Ringers solution (5 mL/day, s.q.) and oral Nutrical supplements (1–3 mL, Webster Veterinary, MA) until adequate volitional drinking and eating resumed.
Spinal cord hemisection injury
As previously described,1,23,25 a 3 cm midline dorsal incision was made from the base of the skull extending caudally to approximately the fourth cervical segment (C4). A laminectomy was performed at the second cervical segment (C2) to expose the spinal cord. A small incision was then made in the dura and a lateral Hx performed on the left side of the spinal cord using a microscalpel, followed by gentle aspiration. Using this approach, the completeness of the lesion was readily visible and the extent of the lesion was reproducible. The dura was then closed with interrupted 9-0 sutures and durafilm was placed over the dura. The overlying muscle was then sutured in layers and the skin was closed with stainless steel surgical wound clips.
Anatomical tracing protocols
Recombinants of the Bartha strain of PRV (in minimal essential medium with 10% fetal bovine serum) were used as anatomical tracers to examine the neural circuitry associated with the ECRL muscle in cohorts of spinal intact and C2Hx rats. PRV is a trans-synaptically transported tracer that will label the entire motor circuitry over time. Propagation and culture methods for PRV have been extensively detailed.1 Two recombinants of PRV were used in the present study: either PRV152 or PRV614 (each ∼2.0×108 plaque-forming units [PFU]) were used to trace the circuitry associated with the ECRL muscle.
Application of PRV was made via a small incision in the skin of the distal forelimb above the ECRL muscle in spinal intact and C2Hx rats. The skin and fascia overlying the ECRL were dissected to expose the ECRL muscle belly. PRV (10 μL) was injected into the ECRL via Hamilton syringe (Hamilton Company, Reno, NV). Animals were left to survive for 24–120 h following tracer injection. A careful time-course study was conducted in uninjured rats to determine the appropriate post-injection time point for first-order ECRL motoneuron labeling and subsequent, second-order transneuronal labeling of pre-motor ECRL spinal interneurons. Thus these data guided the selection of 72 and 96 h PRV incubation times in the chronic C2Hx group, as there was evidence of both first- and second-order labeling at these time points.
In a subset of animals (n=2 from the 72 h control group, n=2 from the 96 h control group, and n=2 from the 96 h C2Hx group), the left radial nerve was severed prior to injection of PRV, to dennervate the ECRL muscle. The muscle was then injected with PRV. These control experiments were used to determine whether any of the observed PRV labeled cells in the cervical spinal cord arose from labeling of pre-sympathetic neurons. Any neuronal PRV labeling in these rats would be the result of uptake and transport of PRV via sympathetic innervation of blood vessels, not via retrograde infection from the ECRL.
Behavioral testing of forelimb function
Prior to initiation of experimental testing, rats were handled for 3–5 min daily for 7–10 days prior to testing by laboratory personnel to familiarize the animals with test administrators. All testing was conducted at the same time of day for each behavioral test. All procedures were videotaped and subsequently quantified by a blinded observer. Rats were tested prior to injury, at 1 and 2 weeks post-injury, then at biweekly intervals thereafter, until termination of the study at 16 weeks post-injury.
Limb-use Asymmetry (Cylinder) Test
The cylinder test was conducted on awake, unrestrained animals. Testing consisted of a single trial in which rats were placed in a clear Plexiglas cylinder (20 cm in diameter, 20 cm high) for 5 min. Forelimb use was measured during vertical exploration as the number of individual and simultaneous forepaw contacts with the cylinder wall.26–28 Ipsilateral forelimb use was calculated as the proportion of ipsilateral forepaw contacts to the total number of forepaw contacts. Scores were calculated with and without dorsal paw contacts included in the quantification.
Forelimb Locomotor Scale (FLS)
Rats were placed in an open-field plastic enclosure measuring 0.85 m×1 m. and were observed for a period lasting no longer than 5 min. Trials were scored in real time by an examiner, and were also videotaped for later viewing and scoring. Scoring was conducted according to the FLS, a modified version of the Basso–Beattie–Bresnahan (BBB) Locomotor Rating Scale,29,30 as previously described.19,31,32 The FLS is 17 point scale that defines deficits based on range of motion (Table 1), level of weight support, and whether the paw is parallel to the body.
Table 1.
Forelimb Locomotor Scale (FLS) Scoring Rubric
| FLS score | Behavioral description |
|---|---|
| 0 | No movements of the forelimb (shoulder, elbow, or wrist joints) |
| 1 | Slight movements of one or two joints of the forelimb |
| 2 | Extensive movement of one joint and slight movement of another joint of the forelimb |
| 3 | Slight movement of all three joints of the forelimb |
| 4 | Extensive movement of one joint and slight movement of two joints of the forelimb |
| 5 | Extensive movement of two joints and slight movement of one joint of the forelimb |
| 6 | Extensive movement of all three joints of the forelimb |
| 7 | Plantar placement of the forelimb with no weight support |
| 8 | Dorsal stepping only |
| 9 | Dorsal stepping and/or occasional plantar stepping |
| 10 | Frequent plantar stepping |
| 11 | Continuous plantar stepping |
| 12 | Continuous plantar stepping with paw position rotated (either at initial contact, lift-off, or both) |
| 13 | Continuous plantar stepping with paw position parallel (either at initial contact, lift-off, or both) |
| 14 | Continuous plantar stepping with paw position rotated (either at initial contact, lift-off, or both) and occasional toe clearance |
| 15 | Continuous plantar stepping with paw position parallel (either at initial contact, lift-off, or both) and occasional toe clearance |
| 16 | Continuous plantar stepping with paw position rotated (either at initial contact, lift-off, or both) and continuous toe clearance |
| 17 | Continuous plantar stepping with paw position parallel (either at initial contact, lift-off, or both) and continuous toe clearance |
Numerical score values are given based on defined behavioral criteria. (Adapted from Sandrow et al.31).
Vermicelli pasta handling test
Rats were given 7 cm lengths of uncooked vermicelli pasta, marked at 1.75 cm intervals to facilitate visualization of paw and pasta movement. For 10 days prior to initiation of behavioral testing, all rats were given the vermicelli pieces in their home cages to familiarize them with the pasta handling. Testing consisted of three to five trials in which the rats were given a single piece of pasta per trial while still in the clear Plexiglas cylinder. Test trials were videotaped with a Sony Digital Camcorder for frame-by-frame analysis. Quantification of this test involved counting the number of adjustments made by each forepaw, (left vs. right), the time to eat the entire length of the pasta, and documentation of any atypical behaviors (Table 2), including atypical paw positions, failure to contact the pasta, drops, abnormal pasta grasping, or compensatory head and neck positions.33,34 Adjustments were defined as any visually confirmed release and re-grasp of the length of pasta. The time to eat was defined by when the pasta was first placed in the mouth, ending when the piece was released from both hands and completely consumed.19,33,34 Trials were ignored if the rat did not consume the entire length of pasta or turned away from the camera in such a way that the pasta and the digits were obscured from view.
Table 2.
Vermicelli Pasta Handling Test: Atypical Behaviors
| Atypical behavior | Behavioral description |
|---|---|
| a. | Paws together when long |
| b. | Guide and grasp switch |
| c. | Failure to contact |
| d. | Drop |
| e. | Paws apart when short |
| f. | Mouth pulling |
| g. | Hunched/abnormal posture |
| h. | Iron grip |
| i. | Guide around grasp |
| j. | Angling with head tilt |
Spinal cord histology
At the predefined termination point, rats were euthanized by systemic perfusion with saline followed by 4% paraformaldehyde (Sigma, St. Louis, MO). For lesion verification, the spinal cord was dissected and removed, embedded in paraffin, and 5 μm transverse sections were prepared. Spinal cord sections were mounted on glass slides (Fisher Scientific, Pittsburgh, PA), stained with cresyl violet, and evaluated using light microscopy. C2Hx lesions were considered to be anatomically complete only if there was a complete absence of apparently healthy white or gray matter in the ipsilateral spinal cord at the lesion epicenter as previously described.22–25,35,36
For anatomical tracing studies, the cervical spinal cord was removed, and 40 μm vibratome sections were made in the longitudinal plane. A subset of tissue was sectioned transversely (40 μm vibratome sections) for assessing the cross-sectional distribution of labeled neurons. For immunocytochemical analyses of PRV labeling, longitudinal vibratome sections were washed in phosphate-buffered saline (PBS) (0.1M, pH 7.4, 3×5 min), blocked against endogenous peroxidase activity (30% methanol, 0.6% hydrogen peroxide in 0.1 M PBS, incubated for 1–2 h), re-washed in PBS, and blocked against nonspecific protein labeling (10% normal goat serum in 0.1 M PBS with 0.03% Triton-X). Sections were then incubated at 4°C overnight with primary antibodies against PRV (rabbit anti-PRV; Rb133/134, raised against whole purified PRV particles that were acetone inactivated; 1:10,000; provided by Dr. Lynn Enquist). The following day, tissue was washed in PBS (0.1 M, 3×5 minutes), incubated for 2 h at room temperature in a biotinylated secondary antibody (goat anti-rabbit; Jackson Immunocytochemicals, West Grove, PA; 1:200) and rewashed in PBS (3×5 min). Sections were further incubated for 2 h in an avidin-biotin complex (ABC; Elite Vectastain Kit; Vector Laboratories, Burlingame, CA), given a third series of washes in PBS, and processed for antigen visualization with diaminobenzidine (DAB; Sigma, St. Louis, MO).1 A subset of tissue sections were counterstained with cresyl violet for visualization of neuronal cell bodies.
Microscopy and quantitative analyses
Spinal cord sections were examined using brightfield microscopy and photographs were taken using a Zeiss AxioPhot microscope with and AxioCam digital camera linked to a PC. PRV-positive cells (ECRL motoneurons and pre-motor interneurons) were quantified in serial longitudinal 40 μm sections separated by 120 μm (every fourth section) by a blind observer. Cell counts were made bilaterally in the dorsal horn, intermediate gray matter, and the ventral horn, and separated rostrocaudally into three cervical regions: C1-C3, C4-C6, and C7-T1. The average number of cells within each region (± standard error of the mean) was sampled 72 and 96 h following application of PRV. Motoneurons (primary PRV labeling) were distinguished from spinal interneurons (secondary labeling) based on their location and size. All other cells within the cervical spinal cord were considered spinal interneurons that innervate the ECRL motoneurons. In addition to reporting “raw” cell numbers, the number of PRV-positive interneurons was then expressed as a function of the number of labeled ECRL motoneurons in order to control for variations in primary labeling among animals (secondary labeling was normalized against primary labeling).1,23
Statistical analysis
All statistical analyses were performed using SigmaPlot 12.5 statistical software (SigmaPlot, SPSS, Chicago, IL). Research hypotheses were tested at an α level of 0.05 using repeated measures (RM), one, two, and three way analyses of variance (ANOVAs) to determine differences between groups. Post- hoc tests were performed using the Student–Newman–Keuls method to correct for multiple pairwise comparisons. One way RM ANOVAs were used to assess changes in body weight, cylinder test scores, time to eating, and atypical behavior scores on the vermicelli pasta handling test. Two way repeated measures ANOVA and the Student–Newman–Keuls post-hoc tests were conducted for FLS test scores and the number of adjustments in the vermicelli pasta test. For this ANOVA, factor 1 was “treatment” (i.e., pre-injury or the various post-C2Hx time points) and factor 2 was “side” (i.e., relative to the side of injury: contralateral vs. ipsilateral). All data are presented as the mean ± standard error of the mean. A p value of<0.05 was considered statistically significant.
Results
Gross histology and body mass
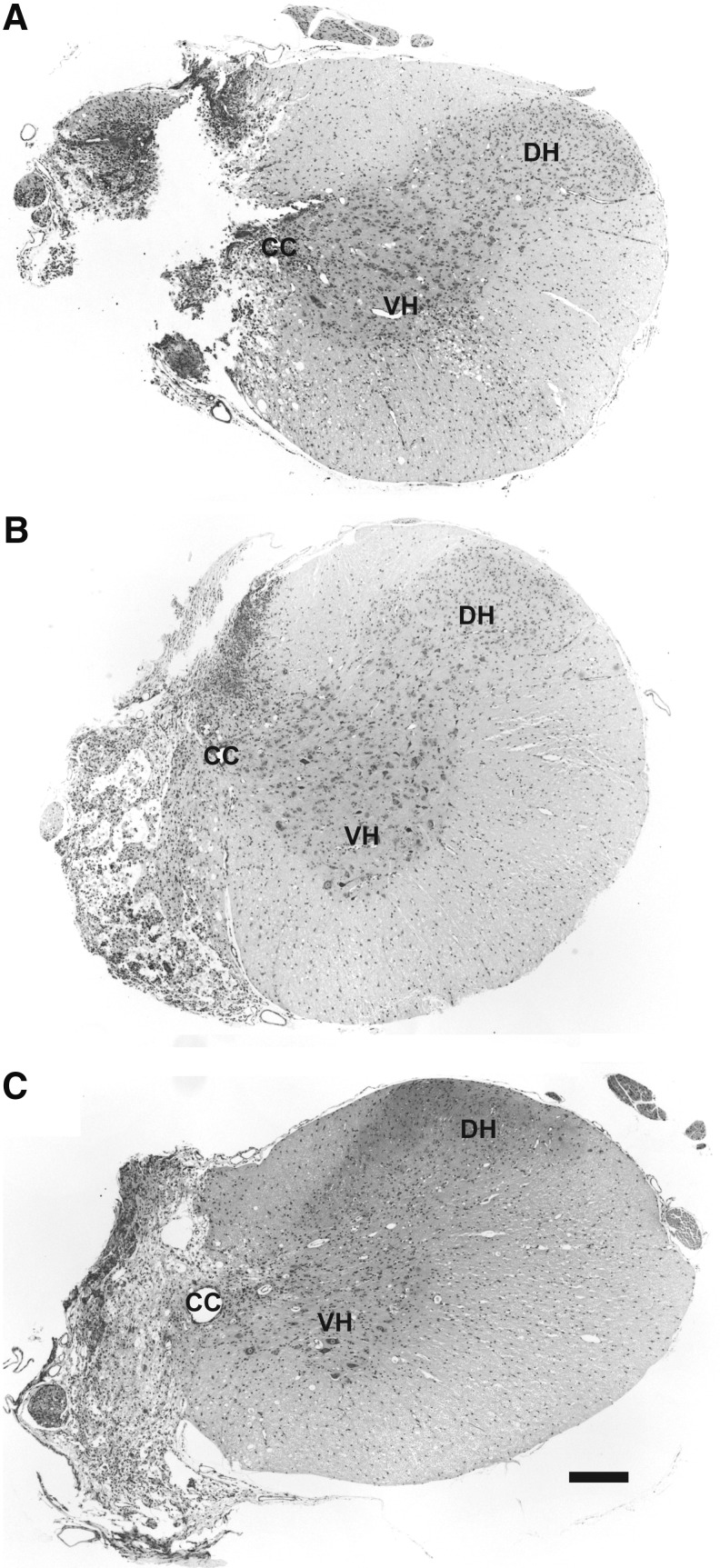
Representative histological sections of the injured cervical spinal cord are shown in Figure 1. The images show that the C2Hx lesion extended to the spinal midline; animals that did not have a histologically confirmed hemisection were excluded from the analyses (n=2 from the untraced, C2Hx group; therefore, n=38 were included in the behavioral analyses). There was a transient reduction in body weight following C2Hx. By 4 weeks post-injury, body weight had returned to pre-injury values, and rats continued to gain weight over the remainder of the study.
FIG. 1.
Representative histological sections illustrating verified complete high cervical lateral hemisection (C2Hx) lesions 4 μm transverse sections at C2 taken from rats, 1 (A), 2 (B), and 8 weeks post-injury (C) stained with cresyl violet. The absence of healthy white and gray matter in the ipsilateral spinal cord suggests anatomically complete C2Hx lesions. CC, central canal; DH, dorsal horn; VH, ventral horn. Scale Bar: 200 μm.
The ECRL spinal motor circuitry in as defined by retrograde PRV tracing
The distribution of PRV-positive motoneurons and interneurons are first described qualitatively (Figs. 2–5), and this is followed by quantitative assessment of motoneuron and interneuron labeling (Figs. 6–8).
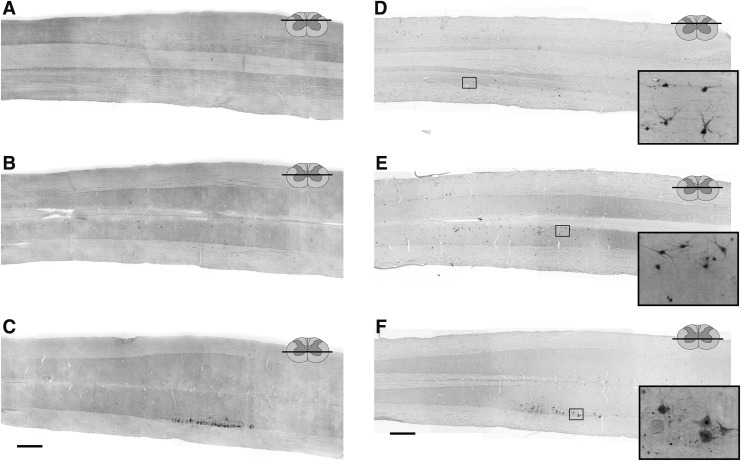
FIG. 2.
Representative longitudinal (horizontal) sections through the cervical spinal cord of uninjured adult female Sprague–Dawley rats, 72 (A–C) and 96 (D–F) h following injection of pseudorabies virus (PRV) into the left extensor carpi radialis longus (ECRL) muscle. Sections have been immunolabeled for the presence of PRV. Low resolution images (A–F) demonstrate the distribution of PRV labeling in the cervical spinal cord, and high resolution images (insets, panels D–F) demonstrate ECRL motoneuron and interneuron morphology. ECRL motoneuron labeling in the ventral horn (C and F), as well as interneuronal labeling in the intermediate gray matter (B and E) and the dorsal horn (A and D) of the cervical spinal cord at the 96 h post-injection time point. Rostrocaudal orientation is from left to right. Scale bars: 1 mm (panels A–F) and 100 μm (inset panels).
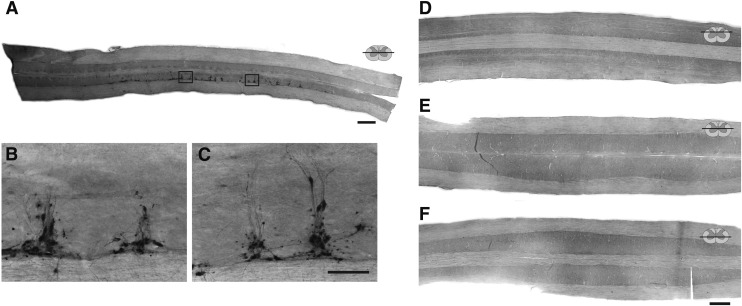
FIG. 3.
Representative longitudinal (horizontal) sections through the thoracic (A–C) and cervical spinal (D–F) cord of uninjured adult female Sprague–Dawley rats, 72 h after injection of pseudorabies virus (PRV) into the left extensor carpi radialis longus (ECRL) muscle. These control experiments were conducted to determine whether the PRV-positive labeling observed in the cervical spinal cord after injection into the ECRL muscle (see Fig. 2) was associated with non-ECRL circuitry (i.e., sympathetic labeling). In these control experiments, the left radial nerve was cut prior to injection of PRV, preventing retrograde labeling via ECRL motoneurons. Sections have been immunolabeled for the presence of PRV. Sections A–C demonstrate the distribution of sympathetic pre-ganglionic (non-ECRL) labeling associated with PRV injection into the ECRL muscle, which was concentrated within in the intermediolateral gray matter of the thoracic spinal cord. Sections D–F demonstrate the absence of PRV labeling in the dorsal horn (D), the intermediate gray matter (E), or the ventral horn (F) of the cervical spinal cord following radial nerve section, confirming that cervical labeling observed in the PRV-tracing experiments is associated with the ECRL circuitry. Rostrocaudal orientation is from left to right. Scale bars: 1 mm (A, D–F) and 100 μm (B and C).
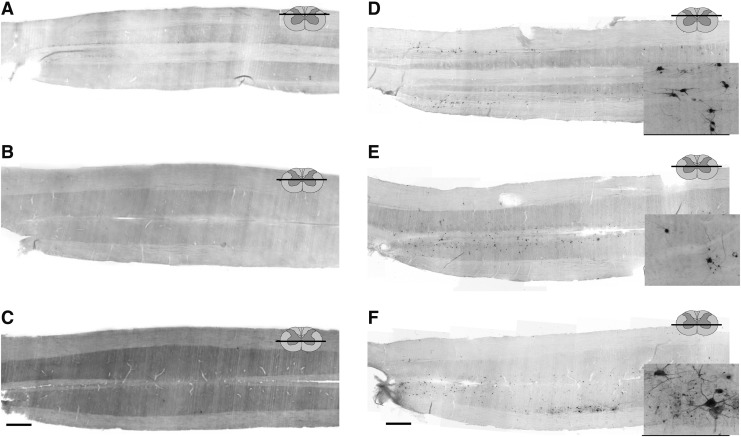
FIG. 4.
Representative longitudinal (horizontal) sections through the cervical spinal cord of rats, 16 weeks after high cervical lateral hemisection (C2Hx) injury, 72 (A–C) and 96 (D–F) h after injection of pseudorabies virus (PRV) into the left extensor carpi radialis longus (ECRL) muscle. Sections have been immunolabeled for the presence of PRV. Low resolution images (A–F) demonstrate the distribution of PRV labeling in the cervical spinal cord, and high resolution images (insets, panels D–F) demonstrate ECRL motoneuron and interneuron morphology. Motoneuron labeling was evident in the ipsilateral ventral horn (C and F), as well as bilateral interneuronal labeling in the intermediate gray matter (B and E) and dorsal horns (A and D) at the 96 h post-injection time point. As compared with uninjured controls, first-order PRV labeling at the 72 h time point was reduced, although at 96 h, significant interneuronal labeling was evident, extending throughout the cervical spinal cord in both the dorsal horn and the intermediate gray matter (D–E). Rostrocaudal orientation is from left to right. Scale bars: 1 mm (panels A–F) and 100 μm (inset panels).
FIG. 5.
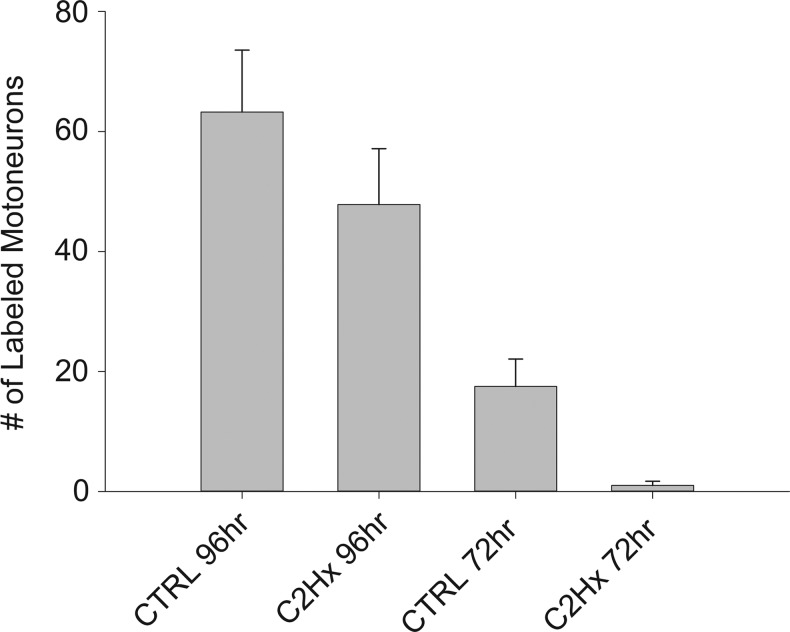
The number of pseudorabies virus (PRV)-positive extensor carpi radialis longus (ECRL) motoneurons (± standard error of the mean [SEM]) in the cervical spinal cord of control (CTRL) and high cervical lateral hemisection (C2Hx) rats. The number of labeled motoneurons in both CTRL and C2Hx groups was significantly greater (p<0.05) 96 h after delivery of PRV to the left ECRL than at 72 h (*). No evidence of contralateral motoneuron labeling was observed at any time point, in any group.
FIG. 6.
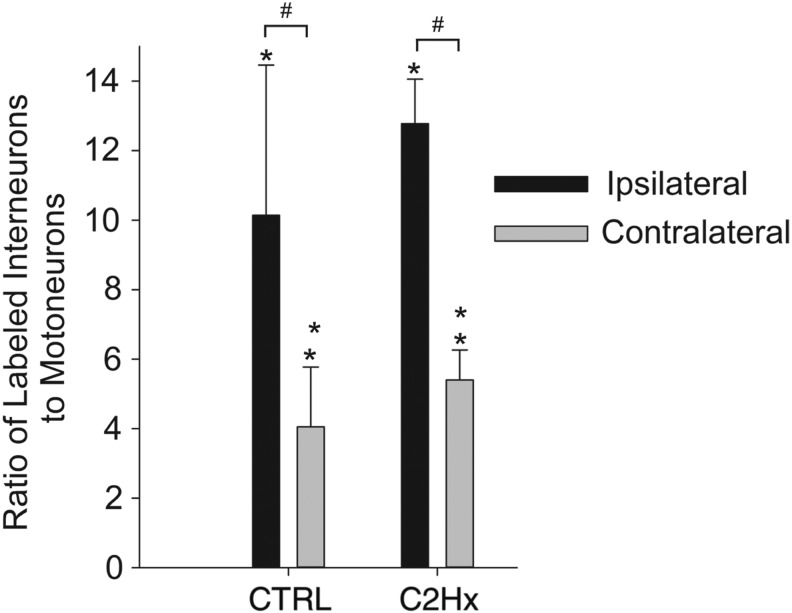
Pre-motor extensor carpi radialis longus (ECRL) interneurons in the cervical spinal cord. The number of PRV-positive cells counted are expressed relative to the number of PRV-labeled motoneurons (± standard error of the mean [SEM]). The number of labeled interneurons in both control (CTRL) and high cervical lateral hemisection (C2Hx) groups was significantly greater (p<0.05) 96 h after delivery of pseudorabies virus (PRV) to the left ECRL than at 72 h (*); 72 h after delivery of PRV, the number of ipsilateral labeled cells was no different than the number of contralateral cells in both CTRL and C2Hx groups; 96 h after delivery of PRV, the number of ipsilateral labeled cells was significantly greater (p<0.001) than the number of contralateral cells in both CTRL and C2Hx groups (#). There was no difference in the number of labeled cells between CTRL and C2Hx groups at either time point.
FIG. 7.
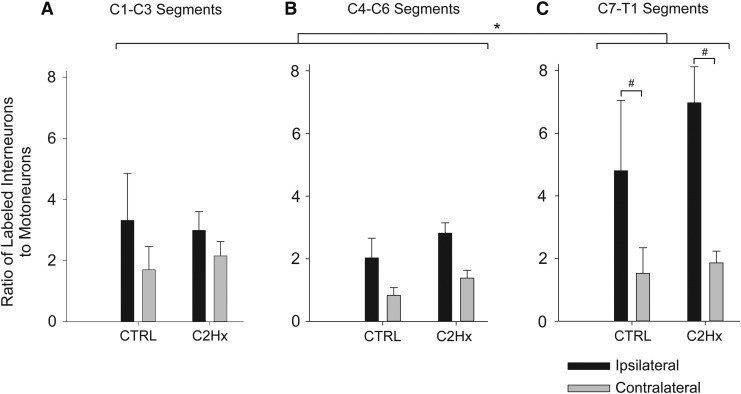
The rostrocaudal distribution of pre-motor extensor carpi radialis longus (ECRL) Interneurons in the cervical spinal cord. The number of pseudorabies (PRV)-positive cells counted in each segment are expressed relative to the number of PRV-labeled motoneurons (± standard error of the mean [SEM]). The number of labeled interneurons in both control (CTRL) and high cervical lateral hemisection (C2Hx) groups was significantly greater (p<0.05) 96 hours after delivery of PRV to the left ECRL than at 72 h at all levels (*); 72 h after delivery of PRV, the number of ipsilateral labeled cells was no different than the number of contralateral cells at any level in both CTRL and C2Hx groups; 96 h after delivery of PRV, the number of ipsilateral labeled cells was significantly greater (p<0.001) than the number of contralateral cells at C7-T1 in both CTRL and C2Hx groups (#). There was no difference in the number of labeled cells between CTRL and C2Hx groups at any level, at either time point.
FIG. 8.
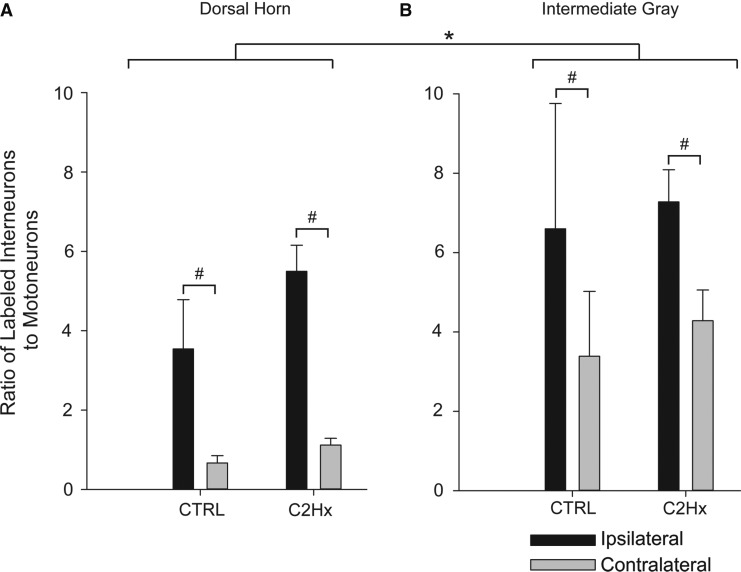
The regional distribution of pre-motor extensor carpi radialis longus (ECRL) interneurons in the cervical spinal cord. The number of pseudorabies virus (PRV)-positive cells counted in the dorsal horn (A) and in intermediate gray matter (B) are expressed relative to the number of PRV-labeled motoneurons (± standard error of the mean [SEM]). The number of labeled interneurons in both control (CTRL) and high cervical lateral hemisection (C2Hx) groups was significantly greater (p<0.05) 96 h after delivery of PRV to the left ECRL than at 72 hin both the dorsal horn and in the intermediate gray matter (*); 72 h after delivery of PRV, the number of ipsilateral labeled cells was no different than the number of contralateral cells in either region in both CTRL and C2Hx groups; 96 h after delivery of PRV, the number of ipsilateral labeled cells was significantly greater (p<0.001) than the number of contralateral cells in both the CTRL and the C2Hx groups (#). There was no difference in the number of labeled cells between CTRL and C2Hx groups in either region, at either time point.
ECRL spinal motor circuitry in spinally intact rats
No PRV-positive cells were evident in the spinal cord 24 h following PRV delivery to the ECRL. Primary motoneuron infection was in the earliest stages after 48 h, with only a single PRV-positive motoneuron identified across all animals (not shown). Motoneuron labeling became prominent after 72 h, and was restricted to the spinal cord, ipsilateral to the injection (Fig. 2). Labeling of motoneurons was even more robust after 96 h (Fig. 2), and by 120 h, there was evidence of lysis in PRV-positive motoneurons (not shown). Accordingly, quantitative analyses of motoneuron labeling (described subsequently) were restricted to the 72–96 h time points.
Interneuronal labeling was absent at 24–48 h, and minimal at 72 h following PRV delivery (Fig. 2). At 96 h, however, robust interneuronal labeling was observed in the intermediate gray matter (laminae VII and X, Fig. 2E) as well as the dorsal horn (laminae I-VI, Fig. 2D). Interneuronal PRV labeling extended throughout the upper, middle, and lower cervical spinal cord, and the majority of labeled cells were located ipsilateral to the infected ECRL motor nucleus.
Control for sympathetic neuronal labeling
Sympathetic pre-ganglionic neurons in the ipsilateral upper thoracic intermediolateral gray matter were also PRV-positive following ECRL injection (Fig. 3 A–C). Accordingly, control experiments were conducted to determine if cervical interneuron infection (e.g., Fig. 2) occurred via a pathway other than the ECRL motor circuit. Therefore, the left radial nerve was sectioned immediately prior to PRV injection into the ECRL in uninjured rats as well as those with C2Hx. Following radial nerve section, no PRV-infected cells were detected in the cervical region in either group, even after 96 h (Fig. 3 D–F). However, clear labeling of putative sympathetic neurons in the intermediolateral thoracic gray matter was still evident in these animals. Therefore, at a time point associated with robust cervical interneuron labeling with the radial nerve intact (i.e., 96 h, Fig. 2 D–F), cervical labeling never occurred if the radial nerve was sectioned. This confirms that cervical neuronal labeling was associated with the ECRL motor circuitry.
ECRL spinal motor circuit following chronic C2Hx
Spinal cord PRV labeling was assessed in tissues harvested 16 weeks following C2Hx injury (Fig. 4). At 72 h post-PRV delivery, motoneuron and interneuron labeling was very sparse (Fig. 4 A–C). Motoneuron labeling was robust after 96 h, and was qualitatively similar to labeling in uninjured animals. Bilaterally distributed pre-motor interneurons were observed throughout all cervical segments at 96 h post-injection of PRV. The extent of post-C2Hx interneuron labeling was similar to the spinally intact condition, and was most prominent in laminae VII and X (Fig. 4B, E) and also throughout the dorsal horn (Fig. 4 A, D). There were no apparent regional (i.e., across lamina) or rostrocaudal differences in the distribution of interneuronal labeling when comparing tissues from uninjured and spinally injured rats.
Quantitative analyses of PRV labeling
The number of PRV-positive ECRL motoneurons increased between 72 and 96 h in both uninjured and C2Hx rats (Fig. 5, p<0.001). Motoneuron labeling appeared to be more robust in the uninjured group, but quantification of labeled neurons did not show significant differences (p=0.054 vs. C2Hx). Interneuronal labeling was limited 72 h post-PRV in both groups (Table 3), which prevented meaningful statistical comparisons between 72 and 96 h. Quantitative analyses of interneuronal distributions were, therefore, restricted to the 96 h group. Consistent with our qualitative impressions, there was no effect of C2Hx on the total number of PRV-positive interneurons regardless of whether the results were normalized to motoneuron labeling (p=0.362, Fig. 6) or presented as raw counts (p=0.733, Table 3). Although bilaterally distributed, the number of PRV-labeled interneurons was greatest on the side of the PRV injection (p<0.007, Fig. 6). There was no significant difference in lateral distribution, however, between the control and C2Hx groups.
Table 3.
ECRL Motoneuron and Pre-Motor Interneuron Raw Counts
| Group | Side | Motoneurons (Raw count) | Interneurons (Raw count) |
|---|---|---|---|
| 72 h CTRL | Ipsilateral | 18±5 | 18±3 |
| Contralateral | n/a | 11±2 | |
| 72 h C2Hx | Ipsilateral | 1±1 | 6±2 |
| Contralateral | n/a | 5±3 | |
| 96 h CTRL | Ipsilateral | 63±10* | 555±170# |
| Contralateral | n/a | 220±63 | |
| 96 h C2Hx | Ipsilateral | 48±9* | 638±163# |
| Contralateral | n/a | 253±49 |
Values are means ± standard error of the mean (SEM). Statistical analyses of motoneuron counts were conducted using two way ANOVA. No differences in motoneuron counts were observed between CTRL and C2Hx groups. Statistical analyses of interneuron counts were limited to the 96 h time point using a two way ANOVA. No differences in labeling were observed between CTRL and C2Hx groups.
p<0.05 difference from 72 h. #p<0.05 difference from contralateral.
ECRL, extensor carpi radialis longus; CRTL, control; C2Hx, high cervical lateral hemisection.
In order to assess segmental distribution, the number of PRV-labeled interneurons was quantified within the upper- (C1-C3), mid- (C4-C6), and low-cervical, high-thoracic (C7-T1) spinal segments. This analysis revealed a significant difference in labeling across the three cervical regions (p=0.015; Fig. 7). PRV labeling in the lower cervical/upper thoracic cord was greater than in both the upper and middle cervical cord (Fig. 7). Similar results were obtained when the data were analyzed as total cell counts (Table 4). The spinal cord ipsilateral to the PRV injection again showed the greatest number of PRV-positive interneurons in each cervical region analyzed (p<0.001 vs. contralateral). There was no impact of C2Hx injury on the lateral distribution of interneuron labeling (Fig. 7; p=0.202).
Table 4.
Segmental Distribution of Pre-Motor ECRL Interneurons
| Interneurons | Interneurons | Interneurons | ||
|---|---|---|---|---|
| Group | Side | C1-C3 | C4-C6 | C7-T1 |
| 72 h CTRL | Ipsilateral | 4±1 | 7±2 | 7±1 |
| Contralateral | 3±1 | 5±1 | 4±1 | |
| 72 h C2Hx | Ipsilateral | 3±1 | 1±1 | 2±1 |
| Contralateral | 1±1 | 1±1 | 2±2 | |
| 96 h CTRL | Ipsilateral | 185±63*# | 119±32*# | 252±88* |
| Contralateral | 95±30# | 48±11# | 77± | |
| 96 h C2Hx | Ipsilateral | 146±36*# | 134±27*# | 359±115* |
| Contralateral | 101±23# | 62±10# | 90±22 |
Values are mean number of ECRL interneurons (raw counts) by segment ± standard error of the mean (SEM). Statistical analyses of interneuron counts were limited to the 96 h time point using a two way ANOVA. No differences in labeling were observed between CTRL and C2Hx groups.
p<0.05 difference from contralateral. #p<0.05 difference from C7-T1.
ECRL, extensor carpi radialis longus; CRTL, control; C2Hx, high cervical lateral hemisection.
Lastly, we assessed whether interneuronal labeling differed across the spinal laminae (i.e., dorsal horn vs. intermediate gray matter). Overall, the normalized labeling was greater in the intermediate gray matter than in the dorsal horn (p=0.005; Fig. 8). This was also reflected by the raw counts (Table 5). Within both regions, there were a greater number of labeled interneurons in the ipsilateral spinal cord in both groups (p<0.001 vs. contralateral). Consistent with the prior analyses, however, there was no effect of C2Hx injury PRV labeling when analyzed with respect to gray matter location (Fig. 8, p=0.261).
Table 5.
Regional Distribution of Pre-Motor ECRL Interneurons
| Group | Side | Dorsal horn | Intermediate gray |
|---|---|---|---|
| 72 h CTRL | Ipsilateral | 3±1 | 16±2 |
| Contralateral | 3±1 | 8±2 | |
| 72 h C2Hx | Ipsilateral | 2±1 | 4±2 |
| Contralateral | 1±1 | 3±2 | |
| 96 h CTRL | Ipsilateral | 203±62* | 352±119*# |
| Contralateral | 44±17 | 176±60# | |
| 96 h C2Hx | Ipsilateral | 270±66* | 368±100*# |
| Contralateral | 51±10 | 202±43# |
Values are mean number of ECRL interneurons (raw counts) by region ± standard error of the mean (SEM). Statistical analyses of interneuron counts were limited to the 96 h time point using a two way ANOVA. No differences in labeling were observed between CTRL and C2Hx groups.
p<0.05 difference from contralateral. #p<0.05 difference from dorsal horn.
ECRL, extensor carpi radialis longus; CRTL, control; C2Hx, high cervical lateral hemisection.
Forelimb functional testing
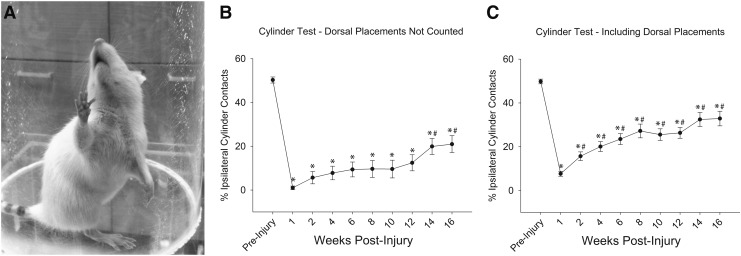
Prior to injury, rats used the left and right forelimbs equally during vertical cylinder exploration. Following C2Hx, ipsilateral forelimb use was dramatically reduced at all post-injury time points (Fig. 9). Because all rats had limited ability to extend the wrist to place the palmar surface on the cylinder following C2Hx, we also stratified the cylinder contacts to exclude (Fig. 9B) and include dorsal paw contacts (Fig. 9C). The overall recovery curve was qualitatively similar in both cases, but the deficits were more persistent if dorsal paw contacts were not counted (Fig. 9B). Inclusion of dorsal placements suggested a more rapid recovery, with improvements seen 2 weeks post-injury.
FIG. 9.
(A) The impact of high cervical lateral hemisection (C2Hx) on ipsilateral upper extremity use during vertical cylinder exploration. Ipsilateral paw use was represented as the percentage of ipsilateral placements relative to the total number of placements. Scores were calculated both with (Panel C) and without (Panel B) dorsal paw placements included in the quantification. All post-injury data points were significantly different than pre-injury values (*p<0.05). If dorsal paw placements were excluded from the quantification, progressive improvements in ipsilateral forelimb use occurred beginning in week 14 (#p<0.05). If dorsal paw placements were included in the quantification, progressive improvements in ipsilateral forelimb use occurred beginning in week 2 (#p<0.05). Values are mean ± standard error (SE) using one way repeated measures analysis of variance (RM ANOVA). p<0.05.
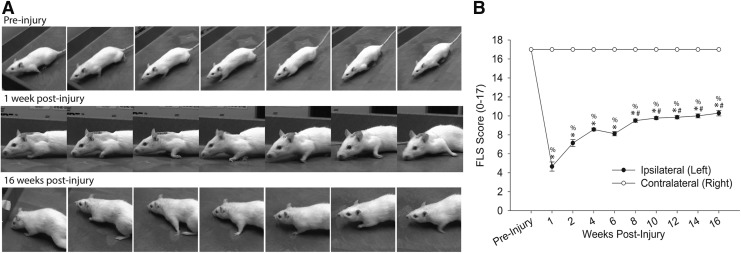
Uninjured rats demonstrated full range of motion, weight-bearing on the plantar surface of the paw during stance, and adequate toe clearance during swing (Fig. 10A), and ipsilateral FLS scores prior to injury were 17±0 out of a possible score of 17 (Fig. 10B). One week following C2Hx, there was reduced range of motion at all upper limb joints during locomotion, and inability bear weight on the plantar surface of the limb. There was also an inability to clear the toes during swing, and ipsilateral FLS scores were dramatically reduced. There was a significant effect of time on the ipsilateral FLS score (p<0.001) with values below pre-injury at all post-injury time points (p<0.05). However, a modest time-dependent recovery occurred, and by 8 weeks the FLS score was significantly greater than the immediate post-injury values (p<0.05). By 16 weeks post-injury, rats demonstrated improved toe clearance, and were able to place the limb and bear weight during stance. Persistent deficits included lack of toe clearance and internal and external limb rotation.
FIG. 10.
(A) The impact of high cervical lateral hemisection (C2Hx) on ipsilateral locomotor function was assessed during open field-locomotion using the Forelimb Locomotor Scale (FLS). Representative images depict locomotor function prior to and at 1 and 16 weeks post-injury. (B) FLS scores were determined in uninjured rats and at 1, 2, 4, 6, 8, 10, 12, 14, and 16 weeks post-C2Hx injury. All post-injury ipsilateral data points were significantly different than the contralateral (%p<0.05) scores, and were significantly different than pre-injury values (*p<0.05). Progressive improvements in forelimb locomotor function occurred beginning in week 8, with each successive data point increasing significantly from the 6 week time point (#p<0.05). Values are mean ± SE using one-way repeated measures analysis of variance (RM ANOVA). p<0.05.
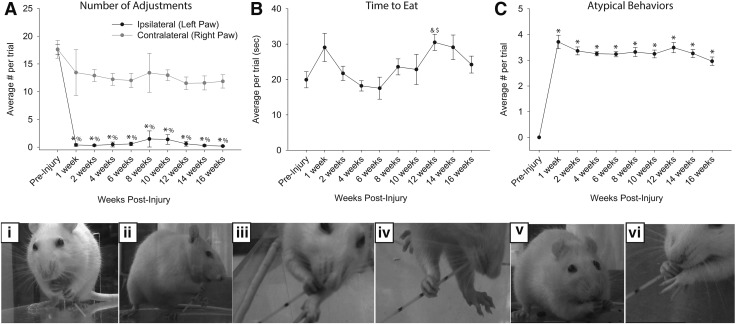
Figure 11 provides the results of the pasta handling test for characterization of a skilled forelimb task. Prior to injury, all rats used a bimanual strategy for consuming pasta. One hand was positioned proximal to the mouth and was used to grasp the pasta while the other hand (positioned more distally) was used to advance the pasta toward the mouth (Fig. 11D, panel i). Early after the injury, rats were unable to use the ipsilateral forelimb to grip the pasta, and there was little evidence of recovery in the ability for fine motor manipulations. Therefore, the number of ipsilateral paw adjustments dropped to almost zero immediately post-injury, and remained at this value (Fig. 11A). In addition, there was no evidence for a compensatory increase in the number of “pasta adjustments” with the contralateral paw following C2Hx. Some minor differences in total time to eat pasta were noted following injury; however, there was considerable variability within and between animals (Fig. 11B). C2Hx also resulted in a dramatic increase in the number of “atypical behaviors” observed during each pasta handling trial (Fig. 11C, p<0.05). For example, many rats demonstrated abnormal postures, including angled head tilt or hunched posture (Fig. 11D; panels ii-vi). Alternative methods for using the ipsilateral limb were also observed, and these included: 1) using the ipsilateral limb for weight-bearing support while eating, and 2) using the ipsilateral limb to support the contralateral limb while eating (Fig. 11D; panels iii, iv, and vi).
FIG. 11.
Vermicelli pasta handling test. The average number of paw adjustments (A), time to eat (B), and number of atypical behaviors (C and D) were quantified (mean ± standard error of the mean [SEM]). (A) The average number of adjustments made by the ipsilateral (left) paw was dramatically reduced after injury (p<0.05) and was significantly lower than the number of adjustments made by the contralateral (right) paw (p<0.05). Rats did not regain ipsilateral paw use over the course of 16 weeks (p<0.05). (B) No difference in the time to eat the pasta was observed, although there was considerable variability between animals and between testing sessions. (C) The average number of atypical behaviors observed per trial increased following high cervical lateral hemisection (C2Hx) injury (p<0.05). %significantly different from contralateral; *significantly different from pre-injury; &significantly different from 4 weeks post-C2Hx; $significantly different from 6 weeks post-C2Hx. (D) Examples of normal (i) and the most common atypical behaviors (ii-vi) demonstrated by rats during the vermicelli pasta handling test. Example images include normal eating behavior (i), head tilt with angled pasta hold (ii, iii, iv and vi), hunched posture (iii, iv, and v), and failure to contact (ii, iii, iv, and vi). Atypical behaviors were observed frequently after injury, and may represent compensatory strategies for accomplishing the pasta eating task.
Discussion
Spinal interneurons are well known for playing important roles in shaping motoneuron output.37 More recent evidence also suggests that propriospinal neurons have the potential to mediate spontaneous and therapeutically induced behavioral changes after SCI.11,13,20,38 The neuroanatomical characterization of such interneuronal plasticity, however, is limited. One possibility is that injury or treatment could result in the formation of novel interneuron–motoneuron relationships, which in turn may subserve functional improvements. The present transneuronal tracing study thus provides the first comprehensive demonstration of the localization of mid-cervical interneurons, which are networked with a specific forelimb motoneuron population and an assessment of that connectivity relative to chronic SCI and recovery of forelimb function following a lesion rostral to the motoneuron circuit of interest. Our collective results indicate that first-order interneuronal synaptic relationships with ECRL motoneurons ipsilateral to the side of C2Hx remain seemingly hard wired despite unilateral white matter damage and extensive suprasegmental denervation of the ECRL circuit.
Forelimb spinal motor circuitry in spinally intact rats
Assigning spinal interneurons as being directly or indirectly pre-motor to the ECRL motoneuron pool was supported by neuronal infection occurring in the absence of possible overlap with spinal autonomic circuitry. It should be noted, however, that definitively distinguishing between second and subsequent orders of infection (i.e., connectivity) cannot be achieved without more specific temporal analyses of interneuronal labeling. Likewise, the distribution of ECRL-related interneurons was conservative, in that we did not attempt to identify cells that may have been embedded within or close to the ECRL motoneuron pool.
Although direct inferences about functional connectivity cannot be made from PRV results alone, our neuroanatomical findings complement previous neurophysiological investigations of cervical propriospinal relationships with upper extremity motor function in animal models39–46 and humans.47–50 Trans-synaptic tracing techniques1,4,51,52 were used in the present study to anatomically characterize the distribution of interneurons within the ECRL motor circuit. The results show an extensive bilaterally distributed propriospinal network with pre-motor interneurons being prominent in the intermediate gray matter (laminae VII and X) of the mid-cervical spinal cord, ipsilateral to the motoneuron pool. Dual anterograde and retrograde tracing techniques will be required to map the suprasegmental projections onto these cells; however, both excitatory and inhibitory corticospinal, reticulospinal, rubrospinal, and tectospinal inputs have been reported,42,53–56 projecting to multiple forelimb motoneuron pools including shoulder, elbow, wrist, and digit muscles.57–60
We previously described a bilaterally distributed cervical propriospinal network that innervated both phrenic and intercostal motoneurons using a similar PRV approach.1 The similarities between interneuronal distributions within the ECRL and phrenic motor circuits suggest that these cells may be functionally important for coordinating diaphragm and limb movement (i.e., respiratory–locomotor coupling). Although several studies have demonstrated precise coupling between these two motor outputs, the underlying mechanisms are not clearly established.61–66 Cervical propriospinal networks may provide an anatomical substrate that facilitates respiratory–locomotor coupling, both with the spinal cord intact and also in the context of motor rehabilitation and recovery.67–69
Interneurons and SCI
Neuroanatomical plasticity can occur in multiple ways, and the primary focus of this study was on potential changes in interneuronal relationships associated with a specific muscle's spinal network: in this case, ECRL motoneurons. The ECRL was chosen in the present study because wrist extensor function is one of the primary movements involved in the performance of the behavioral tasks employed here. Altered number or distribution of second- or later-order PRV infection could reflect a change in the relationship between spinal interneurons and motoneurons. Previous work has suggested that such anatomical reorganization can occur within other motor circuitry following SCI.11 Contrary to our expectations, however, neither variable was altered by C2Hx in our study. Therefore, the most immediate interpretation is that neither injury nor any degree of recovery entailed a loss or addition of interneurons into the ECRL circuit. It is possible that some limited sprouting from interneurons could have occurred resulting in increased ECRL motoneuron innervation by pre-motor cells, but that this went undetected by PRV tracing. To explore this possibility, more in-depth temporal analyses of interneuron labeling relative to spinally intact controls would be required in future work.
PRV transneuronal labeling has not been shown definitively to be associated with function; however, it is noteworthy that we1,70 and others51,52 have observed delayed first-order labeling of motoneurons after SCI. Although this may be associated in part with deafferentation, it is more likely a reflection of neuromuscular junction dynamics post-injury71 or changes in PRV receptors.51,52 Irrespective of the underlying mechanism, delayed first-order infection can influence quantitative analyses, most likely resulting in an underestimation of interneuronal labeling post-injury. We found no evidence of altered interneuronal distribution post-injury; therefore, conservative interpretations of these results would conclude that either there was no impact of injury and/or plasticity on the number of neurons within the ECRL circuitry or that changes in the circuitry were underrepresented.1,51,52
The absence of robust evidence for anatomical reorganization of the cervical ECRL interneurons following C2Hx is consistent with our prior study of the phrenic motor circuit, in which minimal changes in the distribution and prevalence of pre-motor interneurons occurred after chronic C2Hx.1 That, however, does not dismiss a functional role of pre-motor, cervical interneurons in the recovery process.20,72 Alterations in the functional efficacy of existing propriospinal circuits (e.g., without forming a “new circuit”) will not necessarily be detected using the PRV trans-synaptic tracing approach. The neuroanatomical tracing methods utilized in this study characterize a very specific aspect of the intraspinal motor circuitry controlling the ECRL muscle. Specifically, PRV enables the identification of the location and distribution of pre-motor interneurons that are synaptically coupled with the ECRL motoneurons. Changes in neurotransmitter release or post-synaptic receptor density in cervical spinal neurons likely to contribute motor recovery after C2Hx73 and could be explored in future studies by using additional immunohistochemical or pharmacological approaches. Similarly, PRV tracing methods do not enable us to identify alterations in the excitability within a motor circuit, which may significantly impact motor recovery following SCI.74
The propriospinal network may also be receiving new synaptic connections not recognized in the present work. One possibility is that sprouting of axotomized and/or intact fibers from supraspinal tracts may produce new synaptic connections onto surviving motoneurons and/or interneurons.11,13,75–78 Whereas some evidence has been reported suggesting that collateral sprouting of intact descending motor fibers may not contribute to recovery of forelimb motor function, there is also compelling evidence that under certain conditions, such potential does exist.11,13,76,79 Alternatively, we cannot rule out the possibility that PRV is not able to trans-synaptically label the new synapses formed in response to SCI, although this would seem unlikely based on what is known about the viral transport. Another consideration regarding the absence of robust differences in interneuronal labeling observed after SCI may be related to the PRV tracing methods employed in this study. It is possible that this tracing method does not have sufficient accuracy or precision to identify very subtle changes in interneuronal connectivity within the ECRL motor circuitry. Conversely, it may be that there is a more extensive reorganization of spinal connectivity within motor circuits not studied in the present work (mediating control of other upper extremity muscles).
Recovery of forelimb motor function and spinal circuitry
Behavioral assessments in the present study were selected to evaluate functional recovery both qualitatively and quantitatively. This approach takes into account that scores on functional assessments may not reflect the true nature of the motor algorithm for specific tasks. Although we did not observe overt changes in the ECRL network relative to the moderate recovery of forelimb function observed, the behaviors exhibited underscore potential neural substrate changes elsewhere in the complex of forelimb motoneuron circuits. Progressive, spontaneous recovery of ipsilateral forelimb function occurred over weeks to months following C2Hx injury; recovery, however, was incomplete and reached a plateau by 2 months post-injury. Forelimb recovery was most robust in the performance of gross motor behaviors, with chronic deficits persisting for fine motor tasks. Limited recovery was noted in toe clearance and paw rotation during locomotion, and the ability to grasp and manipulate pasta with the forepaw also remained considerably impaired. Interestingly, despite persistent deficits in fine motor function, rats demonstrated a remarkable ability to perform the desired task after injury utilizing a number of alternative (compensatory) strategies. Given these functional outcomes, especially in terms of fine motor behavior, the absence of neuroanatomical change in the ECRL circuit is not surprising. On the other hand, the acquisition of compensatory strategies not only complicates the analysis of post-injury changes in forelimb function, but also highlights the challenges of defining the contribution of anatomical plasticity to functional recoveries involving multiple muscles and their respective motoneuron circuits.
Commentary on behavioral assessment methods
For the present study, we assessed a range of functional behaviors related to upper limb function including fine, gross, and locomotor abilities; therefore, functional tests were selected accordingly. Assessment of upper extremity function can be quite complicated, however, as most functional tasks of the upper extremity involve complex coordination between multiple muscles and joints within a limb, as well as the ability to stabilize the limb appropriately. Accordingly, scores on functional assessments may not necessarily reflect the true nature of motor deficits, as they do not necessarily distinguish between an inability to perform the coordinated behavior of interest and the inability to perform the component motor behaviors. Furthermore, one of the goals of using standardized assessments of functional abilities is to facilitate comparison across groups of animals or across different time points. However, applying a quantification rubric to a given test limits the ability to assess subjective measures related to the task being assessed.
For example, the vermicelli pasta test entails the quantification of the time it takes to eat a length of pasta, the number of left and right paw adjustments made on the pasta, and the number of defined atypical behaviors that were observed in a given trial. In the present study, rats were able to accomplish the pasta eating task by using a wide array of different movement strategies that we were unable to quantify using the rubric defined by the test guideline. Therefore, whereas scores on the test may have indicated a lack of recovery (e.g., time to consume pasta), it was evident to the rater that over the period of weeks and months post-injury, rats were actually modifying the way they were performing the test. This highlights the importance of selecting tests that will be sensitive to functional changes in order to provide the most accurate assessments.
The complications that arise in quantifying compensatory behavioral strategies used to accomplish a given motor task were recently highlighted in a review by Fouad and colleagues.79 One of the major issues is how to characterize and evaluate the contribution of compensatory behaviors to motor recovery. One approach is to use qualitative assessments such as the FLS, the BBBm or the “atypical behaviors” component of the vermicelli pasta test.10,28,29,31,33,80,81 These scoring systems are designed to compare movement patterns in animals with neurological deficits to defined movement patterns observed in neurologically intact animals. An obvious challenge with this approach is that one cannot necessarily determine whether a compensatory behavior occurred (vs. a true impairment). Furthermore, whether abnormal execution of a movement pattern is actually a beneficial compensation employed in order to accomplish a given task is often not taken into consideration by these assessments, nor do the defined guidelines for administering these assessments enable these behaviors to be characterized.
For example, rats that demonstrate numerous atypical behaviors during the pasta handling task may still achieve similar (or even faster) time-to-eat rates than they did pre-injury. This example also highlights the difficulties in using quantitative measures to evaluate functional recovery (e.g., time to eat pasta, number of paw placements), as rats might eat the pasta faster by using these compensatory (abnormal) strategies such as tilting the head or using the ipsilesional limb to guide and or assist the grasping hand. Therefore, in the present study, behavioral assessments were selected to evaluate functional recovery both qualitatively and quantitatively.
Conclusion
We have described an extensive spinal network of interneurons that are synaptically coupled to the ECRL muscle. These pre-motor interneurons are likely to participate in modulating ECRL motor activity and may possibly contribute to coupling respiratory and locomotor activity. The distribution and prevalence of these interneurons did not change after C2Hx injury, suggesting that the progressive, spontaneous recovery of ipsilateral forelimb function described in the present work does not involve recruitment of new interneurons into the ECRL motor pathway. We speculate that the lack of overt reorganization of interneuronal connectivity post-C2Hx in the spinal ECRL circuitry studied can be attributed in part to the limited recovery and use of the ECRL in the behaviors assessed. The compensatory strategies and atypical motor behaviors observed in these animals suggest that other upper extremity muscles are being recruited to perform behavioral tasks. It is possible that there are changes in connectivity in neural substrates mediating control of these recruited muscles. Selecting a target muscle to explore such changes, however, is complicated by the fact that individual animals adopt different compensatory atypical behaviors.
The functional significance of the extensive cervical propriospinal network1 (and current data) to functional motor recovery after SCI remains an important and unanswered question. Establishing the role of propriospinal networks to motor control presents a difficult experimental challenge; however, genetic methods are emerging that are beginning to shed light on this issue. Continued investigation of this topic, and in particular, the functional role of cervical propriospinal networks, may help guide the development of more targeted therapeutic strategies for enhancing upper limb and/or respiratory motor recovery by harnessing and enhancing endogenous neuroplasticity.
Acknowledgments
We thank Dr. John Houle for his comments on the manuscript. Support for this work and the investigators was provided by grants from the National Institutes of Health (NIH): 1R01-HD-052682 (D.D.F.), 1R01- NS054025 (P.J.R.), and R01-NS081112 (M.A.L.), and the Paralyzed Veterans of America (M.A.L.). E.G.R. was supported by the Foundation for Physical Therapy and an NIH T32 Training Grant (HD043730).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Lane M.A., White T.E., Coutts M.A., et al. (2008). Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J. Comp. Neurol. 511, 692–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reier P., Lane M., Hall E.D., Teng Y.D., and Howland D.R. (2012). Translational spinal cord injury research: preclinical guidelines and challenges. Handb. Clin. Neurol. 109, 411–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vizzard M., Erickson V., Card J., Roppolo J., and de Groat W. (1995). Transneuronal labeling of neurons in the adult rat brainstem and spinal cord after injection of pseudorabies virus into the urethra. J. Comp. Neurol. 355, 629–640 [DOI] [PubMed] [Google Scholar]
- 4.Hou S., Duale H., Cameron A.A., Abshire S.M., Lyttle T.S., and Rabchevsky A.G. (2008). Plasticity of lumbosacral propriospinal neurons is associated with the development of autonomic dysreflexia after thoracic spinal cord transection. J. Comp. Neurol. 509, 382–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billig I., Forris J.M., Enquist L.W., Card J., and Yates B.J. (2000). Definition of neuronal circuitry controlling the activity of phrenic and abdominal motoneurons in the ferret using recombinant strains of pseudorabies virus. J. Neurosci. 20, 7446–7454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson K.D. (2004). Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma 21, 1371–1383 [DOI] [PubMed] [Google Scholar]
- 7.McKenna J.E., Prusky G.T., and Whishaw IQ. (2000). Cervical motoneuron topography reflects the proximodistal organization of muscles and movements of the rat forelimb: a retrograde carbocyanine dye analysis. J. Comp. Neurol. 419, 286–296 [DOI] [PubMed] [Google Scholar]
- 8.Morris R., Tosolini A.P., Goldstein J.D., and Whishaw I.Q. (2011). Impaired arpeggio movement in skilled reaching by rubrospinal tract lesions in the rat: a behavioral/anatomical fractionation. J. Neurotrauma 28, 2439–2451 [DOI] [PubMed] [Google Scholar]
- 9.Tosolini A.P., and Morris R. (2012). Spatial characterization of the motor neuron columns supplying the rat forelimb. Neuroscience 200, 19–30 [DOI] [PubMed] [Google Scholar]
- 10.Kinoshita M., Matsui R., Kato S., et al. (2012). Genetic dissection of the circuit for hand dexterity in primates. Nature 487, 235–238 [DOI] [PubMed] [Google Scholar]
- 11.Bareyre F.M., Kerschensteiner M., Raineteau O., Mettenleiter T.C., Weinmann O., Schwab M.E. (2004). The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 7, 269–277 [DOI] [PubMed] [Google Scholar]
- 12.Courtine G., Gerasimenko Y., van den Brand R., et al. (2009). Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci. 12, 1333–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Courtine G., Song B., Roy R.R., et al. (2008). Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med. 14, 69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerasimenko Y.P., Ichiyama R.M., Lavrov I.A., et al. (2007). Epidural spinal cord stimulation plus quipazine administration enable stepping in complete spinal adult rats. J. Neurophysiol. 98, 2525–2536 [DOI] [PubMed] [Google Scholar]
- 15.Harkema S.J. (2008). Plasticity of interneuronal networks of the functionally isolated human spinal cord. Brain Res. Rev. 57, 255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson K.D., Gunawan A., and Steward O. (2005). Quantitative assessment of forelimb motor function after cervical spinal cord injury in rats: relationship to the corticospinal tract. Exp Neurol. 194:161–174 [DOI] [PubMed] [Google Scholar]
- 17.Anderson K.D., Sharp K.G., Hofstadter M., Irvine K.A., Murray M., and Steward O. (2009). Forelimb locomotor assessment scale (FLAS): novel assessment of forelimb dysfunction after cervical spinal cord injury. Exp. Neurol. 220, 23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson K.D., Sharp K.G., and Steward O. (2009). Bilateral cervical contusion spinal cord injury in rats. Exp. Neurol. 220, 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khaing Z.Z., Geissler S.A., Jiang S., Milman B.D., Aguilar S.V., and Schmidt S.E. (2012). Assessing forelimb function after unilateral cervical spinal cord injury: Novel forelimb tasks predict lesion severity and recovery. J. Neurotrauma 29, 488–498 [DOI] [PubMed] [Google Scholar]
- 20.Weishaupt N., Hurd C., Wei D., and Fouad K. (2013). Reticulospinal plasticity after cervical spinal cord injury in the rat involves withdrawal of projections below the injury. Exp. Neurol. 247, 241–249 [DOI] [PubMed] [Google Scholar]
- 21.Fujiki M., Kobayashi H., Inoue R., and Ishii K. (2004). Immediate plasticity in the motor pathways after spinal cord hemisection: implications for transcranial magnetic motor-evoked potentials. Exp. Neurol. 187, 2468–2477 [DOI] [PubMed] [Google Scholar]
- 22.Doperalski N.J., Sandhu M.S., Bavis R.W., Reier P.J., and Fuller D.D. (2008). Ventilation and phrenic output following high cervical spinal hemisection in male vs. female rats. Respir. Physiol. Neurobiol. 162, 160–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dougherty B.J., Lee K.Z., Gonzalez–Rothi E.J., Lane M.A., Reier P.J., and Fuller D.D. (2012). Recovery of inspiratory intercostal muscle activity following high cervical hemisection. Respir. Physiol. Neurobiol. 183, 186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuller D.D., Doperalski N.J., Dougherty B.J., Sandhu M.S., Bolser D.C., and Reier P.J. (2008). Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp. Neurol. 211, 97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuller D.D., Golder F.J., Olson E.B., Jr., and Mitchell G.S. (2006). Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J. Appl. Physiol. 100, 800–806 [DOI] [PubMed] [Google Scholar]
- 26.Hua Y., Schallert T., Keep R.F., Wu J., Hoff J.T., and Xi G. (2001). Behavioral tests after intracerebral hemorrhage in the rat. Stroke 33, 2478–2484 [DOI] [PubMed] [Google Scholar]
- 27.DeBow S.B., Davies M.L.A., Clarke H.L., and Colbourne F. (2003). Constraint-induced movement therapy and rehabilitation exercises lessen motor deficits and volume of brain injury after striatal hemorrhagic stroke in rats. Stroke 34, 1021–1026 [DOI] [PubMed] [Google Scholar]
- 28.Gensel J.C., Tovar A., Hamers F.P.T., Deibert R.J., Beattie M.S., and Breshnahan J.C. (2006). Behavioral and histological characterization of unilateral cervical spinal cord contusion injury in rats. J. Neurotrauma 23, 36–54 [DOI] [PubMed] [Google Scholar]
- 29.Basso D.M., Beattie M.S., and Breshnahan J.C. (1995). A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, 1–21 [DOI] [PubMed] [Google Scholar]
- 30.Basso D.M., Beattie M.S., and Breshnahan J.C. (1996). Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight–drop device versus transection. Exp. Neurol. 139:244–256 [DOI] [PubMed] [Google Scholar]
- 31.Sandrow H.R., Shumsky J.S., Amin A., and Houle J.D. (2008). Aspiration of a cervical spinal contusion injury in preparation for delayed peripheral nerve grafting does not impair forelimb behavior or axon regeneration. Exp. Neurol. 210, 489–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao Y., Shumsky J.S., Sabol M.A., et al. (2008). Nogo-66 receptor antagonist peptide (NEP1-40) administration promotes functional recovery and axonal growth after lateral funiculus injury in the adult rat. Neurorehabil. Neural Repair 22, 262–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allred R.P., Adkins D.L., Woodlee M.T., et al. (2008). The vermicelli handling test: a simple quantitative measure of dextrous forepaw function in rats. J Neurosci. Methods 170, 229–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tennant K.A., Asay A.L., Allred R.P., Ozburn A.R., Kleim J.A., and Jones T.A. (2010). The vermicelli and capellini handling tests: simple quantitative measures of dextrous forepaw function in rats and mice. J. Vis Exp 41, pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dougherty B.J., Lee K.Z., Lane M.A., Reier P.J., and Fuller D.D. (2012). Contribution of the spontaneous crossed-phrenic phenomenon to inspiratory tidal volume in spontaneously breathing rats. J. Appl. Physiol. 112, 96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuller D.D., Baker–Herman T.L., Golder F.J, Doperalski N.J., Watters J.J., and Mitchell G.S. (2005). Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. J. Neurotrauma 22, 203–213 [DOI] [PubMed] [Google Scholar]
- 37.Jankowska E. (2001). Spinal interneuronal systems: identification, multifunction character and reconfigurations in mammals. J. Physiol. 533, 31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llewellyn–Smith I.J., Weaver L.C., and Keast J. (2006). Effects of spinal cord injury on synaptic inputs to sympathetic preganglionic neurons. Prog. Brain Res. 152, 11–26 [DOI] [PubMed] [Google Scholar]
- 39.Arshavsky Y.I., Orlovsky G.N., Pavlova G.A., Popova L.B. (1986). Activity of C3-C4 propriospinal neurons during fictitous forelimb locomotion in the cat. Brain Res. 363, 354–357 [DOI] [PubMed] [Google Scholar]
- 40.Blagovechtchenski E., Pettersson L.G., Perfiliev S., Krasnochokova E., and Lundburg A. (2000). Control of digits via C3-C4 propriospinal neurones in cats: recovery after lesions Neurosci. Res. 38, 103–107 [DOI] [PubMed] [Google Scholar]
- 41.Illert M., Lundberg A., Padel Y., and Tanaka R. (1978). Integration in descending motor pathways controlling the forelimb in the cat. 5. Properties of and monosynaptic excitatory convergence on C3-C4 propriospinal neurones. Exp. Brain Res. 33, 101–130 [DOI] [PubMed] [Google Scholar]
- 42.Illert M., Lundberg A., and Tanakan R. (1977). Integration in descending motor pathways controlling the forelimb in the cat. 3. Convergence on propriosponal neurones transmitting disynaptic excitation from the corticospinal tract and other descending tracts. Exp. Brain Res. 29, 323–246 [DOI] [PubMed] [Google Scholar]
- 43.Illert M., and Tanaka R. (1978). Integration in descending motor pathways controlling the forelimb in the cat. 4. Corticospinal inhibition of forelimb motoneurones mediated by short propriospinal neurones. Exp Brain Res. 31, 131–141 [DOI] [PubMed] [Google Scholar]
- 44.Sasaki S, Isa T., Pettersson L.G., et al. (2004). Dextrous finger movements in primate without monosynaptic corticomotoneuronal excitation. J. Neurophysiol. 92, 3142–3147 [DOI] [PubMed] [Google Scholar]
- 45.Maier M.A., Illert M., Kirkwood P.A, Nielsen J.B., and Lemon R.N. (1998). Does a C3-C4 propriospinal system transmit corticospinal excitation in the primate? An investigation in the macaque monkey. J. Physiol. 511, 191–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isa T., Ohki Y., Seki K., and Alstermark B. (2006). Properties of propriospinal neurons in the C3-C4 segments mediating disynaptic pyramidal excitation to forelimb motoneurons in the macaque monkey. J. Neurophysiol. 95, 3674–3685 [DOI] [PubMed] [Google Scholar]
- 47.Pierrot–Deseilligny E., Burke D. (2005). Propriospinal relay for descending motor commands, in: The Circuitry of the Human Spinal Cord. Cambridge University Press: New York, pps. 452–510 [Google Scholar]
- 48.Burke D., Gracies J.M., Mazevet D., Meunier S., and Pierrot–Deseilligny E. (1992). Convergence of descending and various peripheral inputs onto common propriospinal-like neurones in man. J. Physiol. 449, 655–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gracies J.M., Meunier S., Pierrot–Deseilligny E. (1994). Evidence for corticospinal excitation of presumed propriospinal neurones in man. J. Physiol. 475, 509–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pierrot–Deseilligny E., Marchand–Pauvert V. (2002). A cervical propriospinal system in man. Adv. Exp. Med. Biol. 508, 273–279 [DOI] [PubMed] [Google Scholar]
- 51.Duale H., Hou S., Derbenev A.V., Smith B.N., and Rabchevsky A.G. (2009). Spinal cord injury reduces the efficacy of pseudorabies virus labeling of sympathetic preganglionic neurons. J. Neuropathol. Exp. Neurol. 68, 168–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duale H., Lyttle T.S., Smith B.N., and Rabchevsky A.G. (2010). Noxious colorectal distention in spinalized rats reduces pseudorabies virus labeling of sympathetic neurons. J. Neurotrauma 22, 203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alstermark B., and Ohlson S. (2000). Origin of corticospinal neurones evoking disynaptic excitation in forelimb motoneurones mediated via C3-C4 propriospinal neurones in the cat. Neurosci. Res. 37, 91–100 [DOI] [PubMed] [Google Scholar]
- 54.Alstermark B., and Ohlson S. (2000). Origin of corticospinal neurones evoking monosynaptic excitation in C3-C4 propriospinal neurones in the cat. Neurosci. Res. 38, 249–256 [DOI] [PubMed] [Google Scholar]
- 55.Alstermark B., Lundberg A., and Sasaki S. (1984). Integration in descending motor pathways controlling the forelimb in the cat. 10. Inhibitory pathways to forelimb motoneurons via C3-4 propriospinal neurones. Exp. Brain Res. 56, 279–292 [DOI] [PubMed] [Google Scholar]
- 56.Alstermark B., Lundberg A., and Sasaki S. (1984). Integration in descending motor pathways controlling the forelimb in the cat. 11. Inhibitory pathways from higher motor centers and forelimb afferents to C3-C4 propriospinal neurones. Exp. Brain Res. 56, 293–307 [DOI] [PubMed] [Google Scholar]
- 57.Alstermark B., and Sasaki S. (1986). Integration in descending motor pathways controlling the forelimb in the cat. 15. Comparison of the projection from excitatory C3-C4 propriospinal neurones to different species of forelimb motoneurones. Exp. Brain Res. 63, 543–556 [DOI] [PubMed] [Google Scholar]
- 58.Alstermark B., and Sasaki S. (1985). Integration in descending motor pathways controlling the forelimb in the cat. 13. Corticospinal effects in shoulder, elbow, wrist, and digit motoneurones. Exp. Brain Res. 59, 353–364 [DOI] [PubMed] [Google Scholar]
- 59.Alstermark B., Kummel H. (1990). Transneuronal transport of wheat germ agglutinin conjugated horseradish peroxidase into last order spinal interneurones projecting to acromio- and spinodeltoideus motoneurones in the cat. 1. Location of labelled interneurones and influence of synaptic activity on the transneuronal transport. Exp. Brain Res. 80, 83–95 [DOI] [PubMed] [Google Scholar]
- 60.Tantisira B., Alstermark B., Isa T., Kummel H., and Pinter M. (1996). Motoneuronal projection pattern of single C3-C4 propriospinal neurones. Can. J. Physiol. Pharmacol. 74, 518–530 [PubMed] [Google Scholar]
- 61.Viala D., Persegol L., and Palisses R. (1987). Relationship between phrenic and hindlimb extensor activities during fictive locomotion. Neurosci. Lett. 74, 49–52 [DOI] [PubMed] [Google Scholar]
- 62.Viala D., Viala G., Persegol L., and Palisses R. (1987). From alternate to synchronous bilateral pattern of the phrenic bursts entrained by fictive locomotion in the spinal rabbit preparation. Neurosci. Lett. 78, 318–322 [DOI] [PubMed] [Google Scholar]
- 63.Palisses R., Persegol L., Viala D., and Viala G. (1988). Reflex modulation of phrenic activity through hindlimb passive motion in decorticate and spinal rabbit preparation. Neuroscience 24, 719–728 [DOI] [PubMed] [Google Scholar]
- 64.Morin D., and Viala D. (2002). Coordinations of locomotor and respiratory rhythms in vitro are critically dependent on hindlimb sensory inputs. J. Neurosci. 22, 4756–4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kawahara K., Kumagai S., Nakazono Y., and Miyamoto Y. (1989). Coupling between respiratory and stepping rhythms during locomotion in decerebrate cats. J. Appl. Physiol. 67, 110–115 [DOI] [PubMed] [Google Scholar]
- 66.Kawahara K., Nakazono Y., Yamauchi Y., and Miyamoto Y. (1989). Coupling between respiratory and locomotor rhythms during fictive locomotion in decerebrate cats. Neurosci. Lett. 103, 326–330 [DOI] [PubMed] [Google Scholar]
- 67.Lovett–Barr M.R., Satriotomo I., Muir G.D., et al. (2012). Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J. Neurosci. 32, 3591–3600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trumbower R.D., Jayaraman A., Mitchell G.S., and Rymer W.Z. (2012). Exposure to acute intermittant hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil. Neural Repair 26, 143–172 [DOI] [PubMed] [Google Scholar]
- 69.Li S., Rymer W.Z. (2011). Voluntary breathing influences corticospinal excitability of nonrespiratory finger muscles. J. Neurophysiol. 105, 512–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lane M.A., Lee K.Z., Fuller D.D., and Reier P.J. (2009). Spinal circuitry and respiratory recovery following spinal cord injury. Respir. Physiol. Neurobiol. 169, 123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mantilla C.B., and Sieck G.C. (2003). Mechanisms underlying motor unit plasticity in the respiratory system. J. Appl. Physiol. 94:1230–1241 [DOI] [PubMed] [Google Scholar]
- 72.Sandhu M.S., Dougherty B.J., Lane M.A., et al. (2009). Respiratory recovery following high cervical hemisection. Respir. Physiol. Neurobiol. 169, 94–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alilain W.J., and Goshgarian H.G. (2008). Glutamate receptor plasticity and activity-regulated cytoskeletal associated protein regulation in the phrenic motor nucleus may mediate spontaneous recovery of the hemidiaphragm following chronic cervical spinal cord injury. Exp. Neurol. 212, 348–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Y., Chen L., Liu R., Wang Y., Chen X.Y., and Wolpaw J.R. (2014). Locomotor impact of beneficial or nonbeneficial H-reflex conditioning after spinal cord injury. J. Neurophysiol. 111, 1249–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fouad K., Pedersen V., Schwab M.E., and Bromsamle C. (2001). Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr. Biol. 11, 1766–1770 [DOI] [PubMed] [Google Scholar]
- 76.Ballermann M., and Fouad K. (2006). Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur. J. Neurosci. 23, 1988–1996 [DOI] [PubMed] [Google Scholar]
- 77.Weidner N., Ner A., Salimi N., and Tuszynski M.H. (2001). Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc. Natl. Acad. Sci. U. S. A. 98, 3513–3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghosh A., Sydekum E., Haiss F., et al. (2009). Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. J. Neurosci. 29, 12210–12219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fouad K., Hurd C., and Magnuson D.S. (2013). Functional testing in animal models of spinal cord injury: not as straight forward as one would think. Front. Integr. Neurosci. 7, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Azim E., Jiang J., Alstermark B., and Jessell T.M. (2014). Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature 508, 357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sooksawate T., Isa K., Matsui R., et al. (2013). Viral vector-mediated selective and reversible blockade of the pathway for visual orienting in mice. Front. Neural Circuits 7, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]