Abstract
We recently showed that, after traumatic spinal cord injury (TSCI), laminectomy does not improve intraspinal pressure (ISP), spinal cord perfusion pressure (SCPP), or the vascular pressure reactivity index (sPRx) at the injury site sufficiently because of dural compression. This is an open label, prospective trial comparing combined bony and dural decompression versus laminectomy. Twenty-one patients with acute severe TSCI had re-alignment of the fracture and surgical fixation; 11 had laminectomy alone (laminectomy group) and 10 had laminectomy and duroplasty (laminectomy+duroplasty group). Primary outcomes were magnetic resonance imaging evidence of spinal cord decompression (increase in intradural space, cerebrospinal fluid around the injured cord) and spinal cord physiology (ISP, SCPP, sPRx). The laminectomy and laminectomy+duroplasty groups were well matched. Compared with the laminectomy group, the laminectomy+duroplasty group had greater increase in intradural space at the injury site and more effective decompression of the injured cord. In the laminectomy+duroplasty group, ISP was lower, SCPP higher, and sPRx lower, (i.e., improved vascular pressure reactivity), compared with the laminectomy group. Laminectomy+duroplasty caused cerebrospinal fluid leak that settled with lumbar drain in one patient and pseudomeningocele that resolved completely in five patients. We conclude that, after TSCI, laminectomy+duroplasty improves spinal cord radiological and physiological parameters more effectively than laminectomy alone.
Key words: : decompression, duroplasty, perfusion pressure, spinal cord injury
Introduction
Traumatic spinal cord injury (TSCI) is a devastating condition that affects about 40 people per million per year in the United States.1 About a third of these patients have no motor or sensory function below the level of injury on admission (i.e., they have a complete injury). The prognosis of complete cervical spinal cord injury is poor; about 80% remain paralyzed2 and nearly 40% remain ventilator dependent.3 In the U.S., the annual cost of caring for TSCI patients was estimated at $19 billion in 2011.4
The surgical and neuro intensive care unit (NICU) management of acute severe TSCI is variable. There is no consensus on the optimal timing of surgery: half of U.K. neurosurgeons operate within 3 d of the injury5 and 80% of international spinal surgeons within 24 h of the injury.6 There also is no consensus amongst U.K. neuroanesthesiologists on the optimal values of physiological parameters, such as mean arterial pressure (MAP) or arterial partial pressure of oxygen (paO2) and carbon dioxide (paCO2).5 Published guidelines form the Joint Section on Spine and Peripheral Nerves of the American Association of Neurological Surgeons and Congress of Neurological Surgeons recommend MAP of 85-90 mm Hg for 5-7 d after TSCI.7 The management of TSCI is inadequate because there is no monitoring from the injured spinal cord to allow the treating doctors to define the optimal values of physiological parameters.
We recently reported a novel method to monitor intraspinal pressure (ISP) and spinal cord perfusion pressure (SCPP) at the site of injury in TSCI patients in NICU.8 The procedure involves inserting a Codman pressure sensor intradurally between the swollen spinal cord and the dura. Our data show that after severe TSCI, ISP is high (typically 20-40 mm Hg) and SCPP low (typically 40-60 mm Hg). By intervening to increase SCPP, we could improve outcome in some patients as assessed using motor evoked potentials and a limb motor score. Mannitol administration, reduction in PaCO2, and increase in sevoflurane dose had little effect on ISP after TSCI, even though these maneuvers have a major effect on intracranial pressure (ICP) in traumatic brain injury (TBI).9 Increasing the dose of inotropes caused an increase in ISP and MAP but with a net increase in SCPP. Together, our findings show that it is possible to safely monitor ISP and SCPP after TSCI in NICU for up to a week and that intervening to change ISP and SCPP can influence outcome.
The role of early bony decompression after TSCI to reduce spinal cord ischemia is controversial.10 The Surgical Timing in Acute Spinal Cord Injury Study concluded that early bony decompression improves outcome after TSCI11 but has been criticized on methodological grounds.10 Our recent study showed that the dura is a major cause of spinal cord compression after TSCI.8 This finding may explain why studies of bony decompression without dural opening have not convincingly shown a beneficial effect on outcome.10 Bony decompression without opening the dura in TSCI is analogous to a decompressive craniectomy without durotomy for TBI, which is largely ineffective at reducing ICP.12 Here, we investigate whether bony decompression combined with dural decompression (expansion duroplasty) safely and effectively improves ISP, SCPP and spinal cord pressure reactivity after TSCI.
Methods
Institution research board approval
Approvals for the study, including the consent form and patient information sheet, were obtained from the St. George's Joint Research Office and the National Research Ethics Service London—Camberwell St Giles Committee (No. 10/H0807/23).
Patient recruitment and follow-up
We recruited patients ages 18 to 70 years old with severe TSCI (American Spinal Injury Association [ASIA] A-C). Exclusion criteria were: 1) patients who could not consent or had other major injuries or co-morbidities; 2) time from injury to surgery more than 72 h; and 3) penetrating spinal cord injury. For the duration of the study (i.e., up to a week), patients were nursed in the NICU and were turned regularly to prevent pressure sores. Neurological examination was conducted by a neurosurgery resident in accordance with the International Standards for Neurological Classification of Spinal Cord Injury13 pre-operatively, at the time of transfer to a rehabilitation facility (at about three to four weeks), and at six months in the outpatient clinic.
Surgical technique
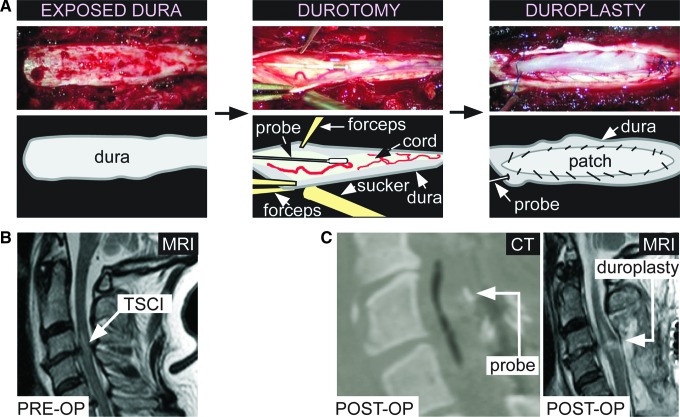
All patients had a posterior approach to realign and stabilize the spine. Spinal stabilization involved lateral mass screws for cervical injuries and pedicle screws for thoracic injuries. The first 11 patients had laminectomy without opening the dura (termed the laminectomy group). The extent of laminectomy was based on the extent of cord edema seen on magnetic resonance imaging (MRI). Four patients in the laminectomy group also had anterior cervical fixation and fusion. The last 10 patients (termed the laminectomy+duroplasty group) had laminectomy, followed by incising the posterior aspect of the dura longitudinally in the midline under a microscope. The length of the laminectomy and dural incision was estimated from the length of the swollen spinal cord edema on the pre-operative MRI. No patients in the laminectomy+duroplasty group had anterior cervical fixation and fusion. After opening the dura in the laminectomy+duroplasty group, we could see the ISP probe. In each of the 10 patients, the ISP probe was in the subdural space, thus confirming that our insertion technique was safe and did not result in intraparenchymal placement of the ISP probe. We then sutured an elliptical patch of artificial dura (Durepair®; Medtronic, Hertfordshire, UK) to the dural edges to expand the intradural space. The duroplasty was supplemented with fibrin glue (Tisseel®; Baxter, Newbury, Berkshire, UK). The surgical procedure is summarized in Figure 1A.
FIG. 1.
Duroplasty technique and computed tomography (CT)/magnetic resonance imaging (MRI). (A) Left: Exposed dura after laminectomy. Middle: Durotomy held open with forceps showing injured spinal cord and intraspinal pressure (ISP) probe. Right: Sutured dural patch. (B) Pre-operative T2 MRI showing high signal at site of traumatic spinal cord injury. (C) Post-operative (left) CT showing ISP probe and (right) T2 MRI showing duroplasty. Color image is available online at www.liebertpub.com/neu
MRI scan analysis
MRI scans (1.5T; Philips Integra, Guildford, Surrey, UK) were obtained pre-operatively (Fig. 1B), at two to three weeks post-operatively (Fig. 1C), and at six to eight months post-operatively. The mid-sagittal anteroposterior (AP) diameter of the most compressed part of the dura was measured (Di) on the T2 images. The percent increase in Di (post-operative compared with pre-operative) was computed as [2×(Di(postop)–Di(preop))/(Da+Db)]×100, where Da and Db are the AP diameters of the mid-vertebral dura above and below the level of injury, respectively. In all patients, we examined the post-operative mid-sagittal T2-weighted MRI for the presence of cerebrospinal fluid (CSF) anterior and posterior to the injured spinal cord. In the laminectomy+duroplasty group, we examined the post-operative mid-sagittal T2-weighted MRIs for spinal cord expansion into the extra space created by the duroplasty. We also looked for the presence of pseudomeningocele in all post-operative scans.
ISP and arterial blood pressure (ABP) monitoring
The ISP probe was inserted in the operating theater. We first reduced and fixed the spinal fracture, inserted metalwork to stabilize the spine and performed laminectomies. At the end of this procedure, before closing the wound, a 14-gauge introducer was used to tunnel the ISP probe (Codman Microsensor Transducer, Depuy Synthes, UK and Ireland) through the skin into the wound. We used a 21-gauge needle bent at 90° to perforate the posterior aspect of the dura and arachnoid one level below the injury. The 90° bent prevents cord damage when perforating the dura. The dural perforation was widened with a blunt hook. Some CSF leaked through the dural hole, allowing the arachnoid to collapse onto the surface of the cord, thus enlarging the subdual space. To monitor ISP, the Codman probe was calibrated and advanced subdurally, under an operating microscope, through the dural hole until the probe tip was at the site of maximal spinal cord swelling according to the MRI scan. The ISP probe was secured to the skin with silk sutures and a tightening stitch around the exit site to prevent CSF leak. We monitored ABP from a radial artery with the catheter kept at the same level as the ISP probe. Videos showing the ISP probe insertion technique are shown in our earlier publication.8 The probe was connected to a Codman ICP Express monitor, in turn connected to a PowerLab data acquisition device via a ML 221 amplifier running LabChart v7.3.3 (ADInstruments, Oxford, UK). The arterial blood pressure output was taken from the bedside patient monitoring system (Philips Intellivue MX800; Philips, Guildford, Surrey, UK) and connected to the PowerLab. ISP and ABP were recorded at 100 Hz using LabChart for up to a week. Satisfactory probe position was confirmed with a post-operative CT scan within 24 h of insertion (Fig. 1C).
SCPP and sPRx
We calculated the SCPP signal, defined as MAP minus ISP. sPRx, a measure of spinal cord pressure reactivity, is the running correlation coefficient between ISP and MAP calculated over a 5-min period.8 If sPRx≤0, spinal cord pressure reactivity is intact, but if sPRx>0, spinal cord pressure reactivity is impaired. The spinal cord indices ISP, SCPP and sPRx are analogous to the brain indices ICP, cerebral perfusion pressure (CPP) and pressure reactivity index (PRx), respectively.14
Patient position
Patient position (supine or lateral) was recorded hourly on the nursing chart throughout the monitoring period and was analyzed retrospectively. In the supine position, the patient was lying directly on the wound with a pillow under the head and neck. In the lateral position, the patient was lying at about a 30° to 45° angle on the left or right, with two supporting pillows positioned away from the wound. For the laminectomy+duroplasty patients, we averaged the ISP for the lateral versus supine positions over each 24-h period.
Outcome measures
To determine whether laminectomy+duroplasty produces long-term neurological deterioration, we compared the ASIA grade, the Walking Index for Spinal Cord Injury (WISCI II),15 and bladder and bowel Spinal Cord Independence Measure (SCIM III)16 of the laminectomy versus the laminectomy+duroplasty groups.
Statistical analysis
Primary outcomes are MRI-based (increase in intradural space, CSF around the injured cord) and physiological (ISP, SCPP, sPRx). The study requires six patients in the laminectomy+duroplasty group and six in the laminectomy group to detect the following differences by two-tailed Student's t test (α=0.05; power=0.9): increase in AP dural diameter of 50 versus 10 % with standard deviation (SD)=20; ISP of 20 versus 16 mm Hg with SD=2; SCPP of 80 versus 64 mm Hg with SD=8; and sPRx of 0.2 versus 0.0 with SD=0.1. The study requires seven patients per group to detect CSF around the injured cord in 80 versus 0 % MRI scans by χ2 test (α=0.05; power=0.8). Data were analyzed using IBM SPSS Statistics (v. 20.0). For ISP, SCPP, increase in intradural space, and sPRx, we compared means of two groups using the two-tailed Student's t test. CSF around the injured spinal cord and cumulative frequencies of ISP and SCPP were compared using the χ2 test. One-sample t test was used to test the effect of patient position on ISP.
Results
Patient demographics
All eligible patients were asked to enter the study. We recruited a total of 21 patients aged 43.5±3.2 years (mean±standard error). Fifteen of 21 (70 %) were male, 16/21 (76%) had complete injuries (ASIA A) and 11/21 (52%) had cervical injury. Eleven of 21 patients (52 %) had laminectomy (laminectomy group) and 10/21 (48 %) had laminectomy and expansion duroplasty (laminectomy+duroplasty group). Several characteristics were comparable in the laminectomy versus the laminectomy+duroplasty groups, including mechanism of injury, age, sex ratio, pre-operative ASIA grade, length of spinal cord signal change on MRI, level of injury, time to surgery, surgical approach, duration of surgery, duration of ISP monitoring, and number of levels laminectomized (Table 1). There was no dural laceration in any of the patients. The size of the dural patch in the laminectomy+duroplasty group was (mean±standard error) 41.2±10.4 mm (rostro-caudal length) and 16.8±1.4 mm (transverse width).
Table 1.
Characteristics of the Laminectomy and Laminectomy + Duroplasty Groups
| Characteristic | Laminectomy | Laminectomy+duroplasty | p value |
|---|---|---|---|
| Number of patients | 11 | 10 | |
| Mechanism of injury | Fall: 7 | Fall: 5 | |
| RTA: 4 | RTA: 5 | ||
| Age, mean (range) | 43 (19 – 64) | 43 (28 – 68) | NS |
| Sex | 7 males, 4 females | 8 males, 2 females | NS |
| Pre-operative ASIA grade | A: 9, C: 2 | A: 7, B: 2, C: 1 | NS |
| Level of injury | Cervical: 7 | Cervical: 4 | NS |
| Thoracic: 4 | Thoracic: 6 | ||
| Time to surgery, mean (range) | 41.3 h (10 – 72) | 32.7 h (17 – 69) | NS |
| Surgical approach | Ant+Post: 4 | Ant+Post: 0 | NS |
| Post only: 7 | Post only: 10 | ||
| Duration of surgery, mean (range) | 337 min (196 – 433) | 301 min (206 – 490) | NS |
| Duration of ISP monitoring, mean (range) | 3.5 d (0.6 – 6.2) | 5.9 d (4.3 – 7.0) | NS |
| Length of spinal cord signal change on T2 MRI, mean (range) | 55.3 mm (16.2 – 82.8) | 62.6 mm (37.4 – 95.8) | NS |
| Number of levels laminectomized, mean (range) | 3.6 (2 – 6) | 4.6 (2 – 6) | NS |
RTA, road traffic accident; NS, not significant; ASIA, American Spinal Injury Association; Ant, anterior; Post, posterior; ISP, intraspinal pressure; MRI, magnetic resonance imaging.
MRI scans
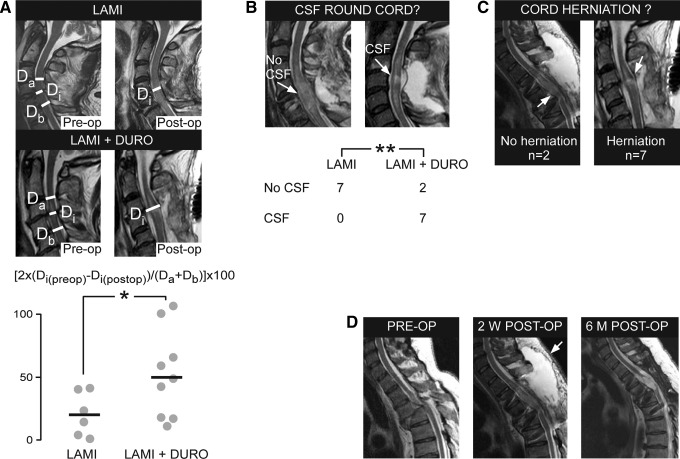
All patients had good quality pre-operative MRI scans. MRI scans performed at 2-3 weeks after surgery were uninterpretable due to artifact from the metal construct in five patients (four laminectomy, one laminectomy+duroplasty). Before surgery, the laminectomy versus the laminectomy+duroplasty groups had comparable AP diameter (mean±standard error) of the dural sac at and near the level of injury; Di(preop)=8.1±0.6 mm versus 6.6±0.7 mm and (Da+Db)/2=11.1±0.5 mm versus 10.8±0.7 mm. Comparison of the pre-operative versus post-operative (done at two to three weeks) MRI scan revealed that duroplasty increased the AP diameter of the dural canal at the injury site significantly more than laminectomy alone (Fig. 2A). CSF signal around the cord—an indicator of satisfactory decompression of the swollen spinal cord—was evident in the MRI scans done at 2-3 weeks of 78 % of laminectomy+duroplasty versus 0 % of laminectomy patients (Fig. 2B). In 78 % of laminectomy+duroplasty patients, the spinal cord expanded into the space created by the duroplasty (Fig. 2C).
FIG. 2.
Duroplasty increases space round the injured spinal cord. (A) T2 magnetic resonance imaging (MRI; top) before and after laminectomy, and (middle) before and after laminectomy+duroplasty, showing mid-sagittal anteroposterior diameter of the most compressed part of the dura (Di), the anteroposterior diameter of the mid-vertebral dura above the level of injury (Da), and the anteroposterior diameter of the mid-vertebral dura below the level of injury (Db). (Bottom) Percent increase in Di after laminectomy versus laminectomy+duroplasty. Points are patients, lines are means. (B) Top: Post-operative T2 magnetic resonance imaging (MRI) looking for cerebrospinal fluid (CSF) round the injured cord. Bottom: Numbers of patients with and without CSF around the injured cord. (C) Post-operative T2 MRI looking for expansion of the injured cord into the duroplasty. (D) T2 MRI in an American Spinal Injury Association A patient (left) before surgery, (middle) at two weeks after surgery (arrow shows pseudomeningocele), and (right) at six months after surgery (no pseudomeningocele). LAMI, laminectomy; LAMI+DURO, laminectomy+duroplasty. p<0.05*, 0.01**.
Complications
There were no complications in the laminectomy group. In the laminectomy+duroplasty group, 1/10 patients developed CSF leak through the wound on Day 1 post-operatively (which was treated successfully with lumbar drainage for 5 d) and 5/10 of patients had pseudomeningoceles evident on the first post-operative MRI, which had resolved completely within six months (Fig. 2D). There were no other complications, such as wound infection, meningitis, spinal cord associated hematoma, or worsening neurological deficit (post-operative vs. pre-operative ASIA grade). There was no progressive post-operative kyphosis on serial MRI at a mean follow-up of 38 weeks based on the Cobb angle (immediate post-operative 28.4±3.60 vs. 27.8±3.80 at follow-up).
ISP and SCPP
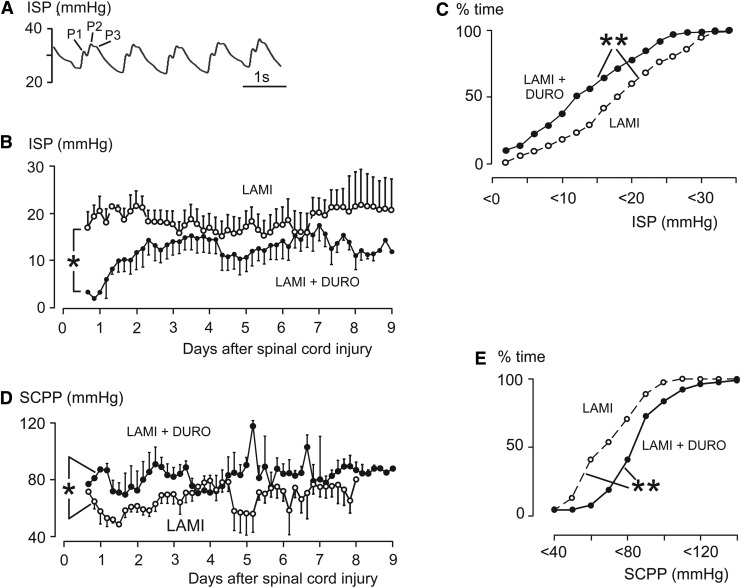
ISP recordings were taken from 11/11 laminectomy patients and 9/10 laminectomy+duroplasty patients. In one laminectomy+duroplasty patient, the probe was dislodged into the paraspinal muscles at Day 1. The period of ISP recording was comparable in the laminectomy and laminectomy+duroplasty groups (Table 1). The ISP waveform was remarkably similar to the ICP waveform with percussion, tidal, and dicrotic peaks (Fig. 3A). Plot of four-hourly mean ISP against time showed that ISP was lower in the laminectomy+duroplasty versus laminectomy group (overall mean±standard error 12.7±0.4 vs. 18.0±0.5 mm Hg; p<0.01; Fig. 3B). Compared with laminectomy, laminectomy+duroplasty caused a significant left-shift of the ISP cumulative frequency curve (Fig. 3C). This indicates that more time is spent at a lower ISP after laminectomy+duroplasty versus laminectomy. Figure 3D shows the four-hourly mean SCPP plotted against time. SCPP after laminectomy+duroplasty was higher than after laminectomy (overall mean±standard error, 83.1±1.1 mm Hg vs. 66.8±1.3; p<0.05). Compared with laminectomy, laminectomy+duroplasty caused a significant right-shift of the SCPP cumulative frequency curve (Fig. 3E). This indicates that more time is spent at a higher SCPP after laminectomy+duroplasty versus laminectomy.
FIG. 3.
Intraspinal pressure (ISP) and spinal cord perfusion pressure (SCPP). (A) Representative ISP waveform showing percussion (P1), tidal (P2) and dicrotic (P3) peaks. (B) Mean four-hourly ISP of laminectomy, and laminectomy+duroplasty patients. (C) Cumulative frequency curve of ISP. (D) Mean four-hourly SCPP of laminectomy and duroplasty patients. (E) Cumulative frequency curve of SCPP. Laminectomy (open circles, n=11); laminectomy+duroplasty (closed circles, n=9), Mean±standard error. LAM, laminectomy; LAMI+DURO, laminectomy+duroplasty. p<0.05*, 0.01**.
Spinal cord pressure reactivity
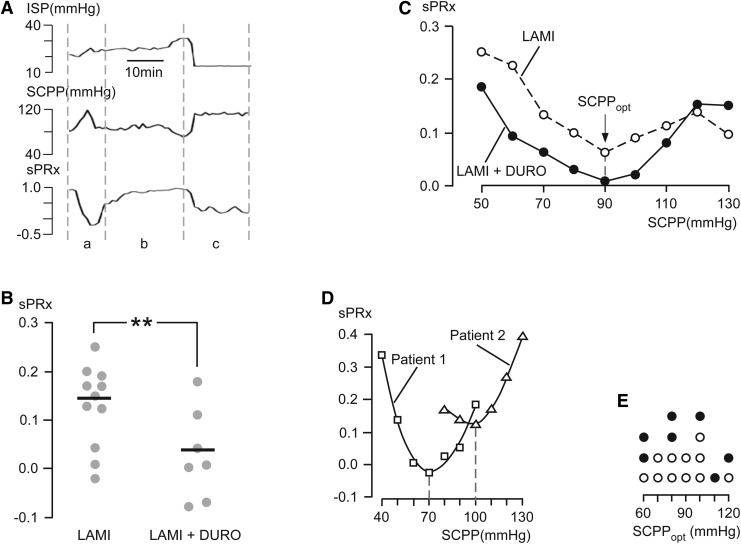
Representative ISP, SCPP, and sPRx signals recorded simultaneously from one patient are shown in Figure 4A. The signals are divided into three intervals: a, b and c. In a, there is a sudden increase in SCPP without any change in ISP (indicating increased spinal cord perfusion due to a rise in MAP), which is accompanied by a decrease in sPRx (i.e., improved spinal cord pressure reactivity). In b, there is a progressive increase in ISP and decrease in SCPP (probably indicating increasing compression of the injured, swollen spinal cord against the surrounding dura), which is associated with a rise in sPRx (i.e., impaired pressure reactivity). In c, ISP falls and SCPP rises (i.e., reduced spinal cord swelling and improved spinal cord perfusion) associated with a fall in sPRx (i.e., improvement in spinal cord pressure reactivity). For each patient, we averaged sPRx over the entire monitoring period. Compared with the laminectomy group, the laminectomy+duroplasty group had significantly lower average sPRx (i.e., improved spinal cord vascular pressure reactivity; Fig. 4B). There was a U-shaped relationship between sPRx and SCPP for the laminectomy+duroplasty group, as well as the laminectomy group (Fig. 4C). The SCPP at the minimum sPRx (i.e., the SCPP that produces the best spinal cord pressure reactivity) is termed SCPPopt. Figure 4D shows that SCPPopt=∼90 mm Hg for the laminectomy as well as the laminectomy+duroplasty groups. For SCPP 50-110 mm Hg, the sPRx of the laminectomy+duroplasty group is lower than the corresponding sPRx of the laminectomy group. At high SCPP (>110 mm Hg), the sPRx of the laminectomy+duroplasty group is higher than the corresponding sPRx of the laminectomy group. Figure 4D shows the relationship between sPRx versus SCPP for two laminectomy patients (Patient 1, Patient 2). The large difference in SCPPopt between Patient 1 and Patient 2 (70 and 100 mm Hg) suggests high inter-patient variability in SCPPopt. We, therefore, plotted SCPPopt for individual laminectomy as well as laminectomy+duroplasty patients. Two laminectomy+duroplasty patients who did not have a U-shaped relationship between sPRx versus SCPP were excluded. SCPPopt varied between 60-120 mm Hg between patients, with the group average at ∼90 mm Hg.
FIG. 4.
Vascular pressure reactivity index (sPRx). (A) Representative intraspinal pressure (ISP), spinal cord perfusion pressure (SCPP), and sPRx. (a) ISP constant high, SCPP rises, sPRx falls; (b) ISP constant high; SCPP constant low; sPRx constant high; (c) ISP low; SCPP high; sPRx low (for explanation, see text). (B) sPRx after laminectomy+duroplasty and after laminectomy. The SCPP that produces the best spinal cord pressure reactivity (SCPPopt) corresponds to minimum sPRx. Points are patients, lines are means. (C) Mean sPRx vs. SCPP after laminectomy and after laminectomy+duroplasty. (D) sPRx vs. SCPP for two patients (Patient 1 SCPPopt=70 mm Hg; Patient 2 SCPPopt=100 mm Hg). (E) SCPPopt of individual laminectomy (open circles) and laminectomy+duroplasty (closed circles) patients. Laminectomy (open circles, n=11); laminectomy+duroplasty (closed circles, n=7). LAMI, laminectomy; LAMI+DURO, laminectomy+duroplasty. p<0.01**.
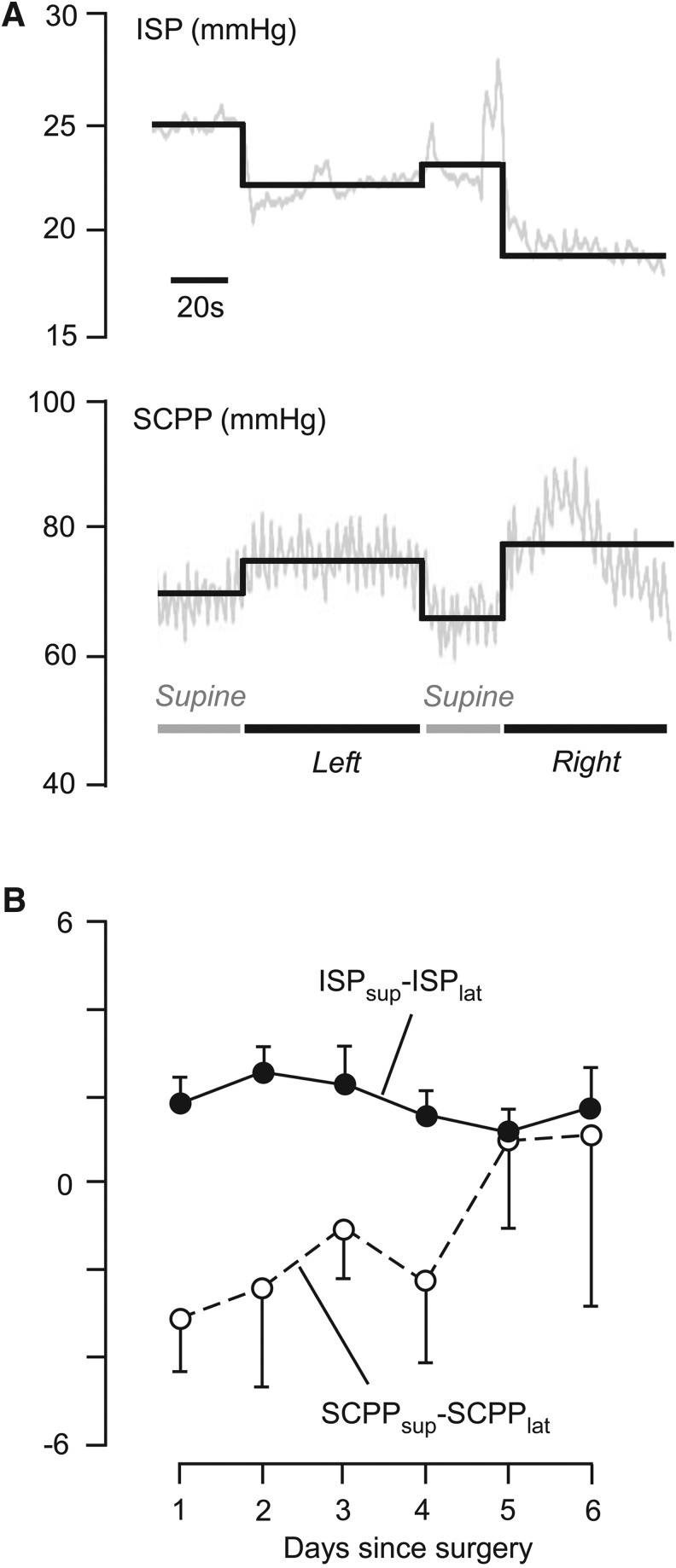
Patient position
We hypothesized that duroplasty exposes the injured spinal cord to compression forces applied to the wound. This suggests that ISP may rise in the supine versus lateral position in bed. Figure 5A shows representative ISP and SCPP signals from a patient with a thoracic spinal cord injury that had duroplasty lying in different positions. In this patient, lying supine was associated with higher ISP and lower SCPP, compared with lying laterally. The difference in ISP between the supine and lateral positions averaged over 24 h (ISPsup – ISPlat) and corresponding difference in SCPP (SCPPsup – SCPPlat) for the laminectomy+duroplasty patient group were plotted against time (Fig. 5B). Lying supine versus laterally caused, on average, a rise in ISP by ∼ 2 mm Hg in the first 4 d after surgery with a corresponding fall in SCPP. The maximum ISPsup – ISPlat observed in a patient was ∼7 mm Hg and the minimum SCPPsup – SCPPlat was−11 mm Hg. Together, our data show that lying supine produces a small increase in ISP and a small decrease in SCPP. In some patients these changes may be large enough to be clinically significant.
FIG. 5.
Intraspinal pressure (ISP) and spinal cord perfusion pressure (SCPP) in supine vs. lateral patient position after laminectomy+duroplasty. (A) Representative ISP and corresponding SCPP recorded from a patient lying supine or laterally (i.e., lying on left or right side). Recorded signal (gray line), mean (black line). (B) Mean daily difference between supine and side positions for ISP (ISPsup – ISPlat) and SCPP (SCPPsup – SCPPlat) plotted against days since surgery. n=9; mean±standard error.
Patient outcome
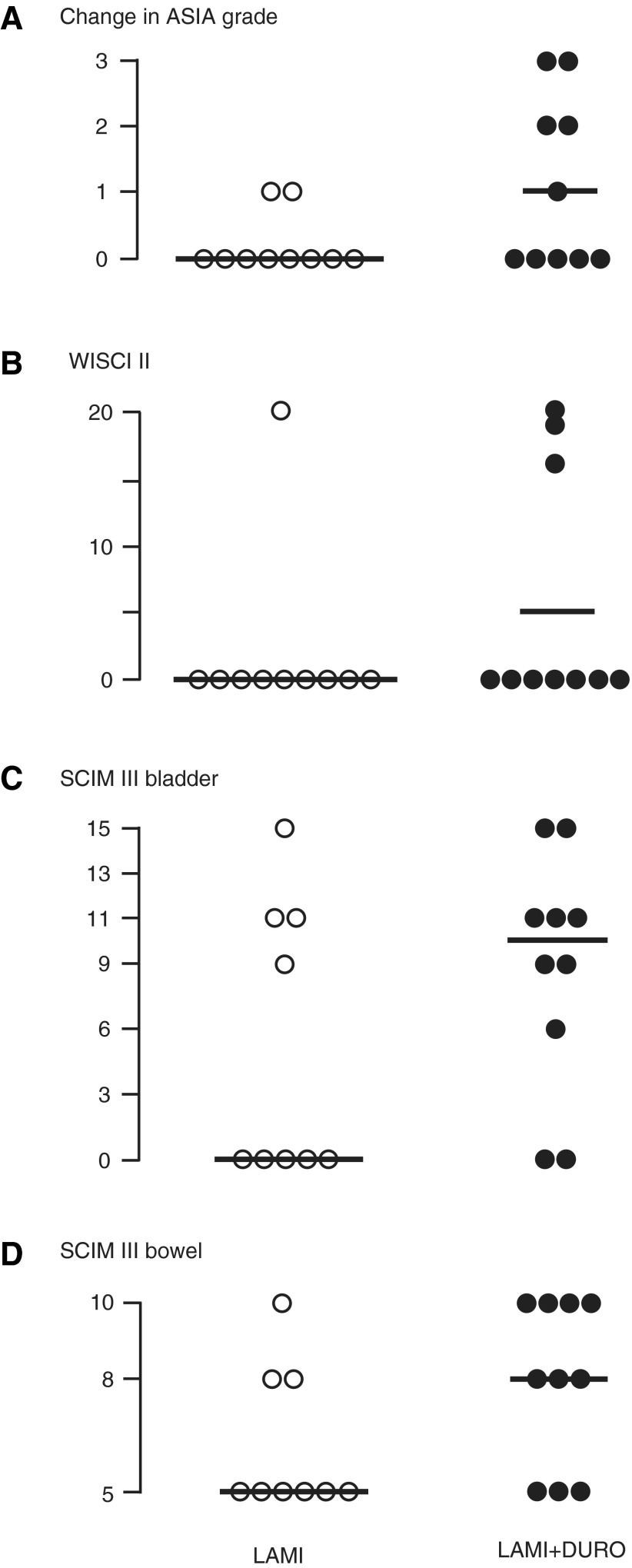
Follow-up was 25.8±4.7 months after laminectomy versus 9.4±0.7 months after laminectomy+duroplasty (mean±standard error; p<0.005). In general, all laminectomy+duroplasty patients with incomplete cervical and thoracic spinal cord injuries (ASIA B, C) and some patients with complete cervical spinal cord injuries (ASIA A) on admission improved their ASIA grade at follow-up. No recovery was seen in any of the four laminectomy+duroplasty patients who presented with a complete thoracic spinal cord injury on admission (ASIA A). Change in ASIA grade (ASIA grade at follow-up minus ASIA grade at presentation), walking ability, bladder function, and bowel function were better in the laminectomy+duroplasty versus the laminectomy group, though not significant at p<0.05 (Fig. 6). These data suggest that laminectomy+duroplasty does not cause neurological deterioration in the first few months after surgery.
FIG. 6.
Outcomes after laminectomy vs. laminectomy+duroplasty at follow-up. (A) Change in American Spinal Injury Association grade (at follow-up minus at presentation). (B) Walking Index for Spinal Cord Injury (WISCI II). (C) Spinal Cord Independence Measure (SCIM III) bladder. (D) SCIM III bowel.
Discussion
Our key finding is that in patients with acute severe TSCI, laminectomy+duroplasty safely improves ISP, SCPP, and sPRx. MRI examination revealed that the injured spinal cord is more effectively decompressed by laminectomy+duroplasty, compared with laminectomy alone. Duroplasty can be easily performed in 15 min at the end of spinal fixation surgery and is technically straightforward; it involves suturing an elliptical piece of artificial dura to the durotomy edges.
We set out to investigate laminectomy+duroplasty after TSCI following our recent study, which showed that laminectomy alone does not reduce ISP or SCPP effectively. This study identified the dura as a major cause of spinal cord compression after injury.8 Failure to open the dura does not effectively decompress the spinal cord and may explain why early bony decompression alone is controversial after TSCI.10 Laminectomy+duroplasty for TSCI involves bony and dural decompression and is analogous to decompressive craniectomy for TBI that involves craniectomy and durotomy.12
Our data show that expansion duroplasty markedly increases the space around the injured spinal cord, thus allowing the spinal cord to expand. Our earlier study showed that, after TSCI, ISP is high and SCPP is low.8 Expansion duroplasty eliminates the dural compression, thus leading to reduction in ISP and increase in SCPP at the injury site. After laminectomy, SCPP is less than 60 mm Hg about 40 % of the time, compared with less than 5% of the time after laminectomy+duroplasty. These changes in ICP and SCPP produced by laminectomy+duroplasty might reduce secondary ischemic damage to the injured spinal cord.17
Laminectomy+duroplasty did not worsen the ASIA grade of any of the patients. Though our study was not powered to detect differences in functional outcomes, there were non-significant trends of improved walking ability, and bowel and bladder functions in the laminectomy+duroplasty group versus the laminectomy group. ASIA A thoracic TSCI patients did not benefit from laminectomy+duroplasty, whereas two-thirds of ASIA A cervical TSCI recovered substantially. This indicates that ASIA A thoracic TSCI is more severe than ASIA A cervical TSCI. Possible explanations include the less extensive collateral vascular supply in the thoracic than the cervical spinal cord,18 the presence of a watershed region in the mid-thoracic spinal cord,18 and the greater force required to fracture the poorly-mobile thoracic than the cervical spine. CSF leak through the wound was seen in a patient and resolved by lumbar drainage. The most common complication after laminectomy+duroplasty was asymptomatic pseudomeningocele that resolved completely in six months.
sPRx is improved more after laminectomy+duroplasty than after laminectomy alone. In the laminectomy and in the laminectomy+duroplasty patients, the relationship between sPRx and SCPP is U-shaped, suggesting that both spinal cord hypoperfusion and hyperperfusion may be detrimental. This is similar to the U-shaped relationship between PRx and cerebral perfusion pressure after TBI.19 Decompressive craniectomy after TBI causes derangement in PRx,20 but laminectomy+duroplasty after TSCI improves sPRx. The reason for this difference between PRx and sPRx after decompression is unclear. Though SCPPopt was ∼90 mm Hg for the entire patient group, there is marked inter-patient variability, which supports individualized treatment for TSCI. The laminectomy+duroplasty group had lower sPRx than the laminectomy group at SCPP 50-110 mm Hg. At SCPP>110 mm Hg, sPRx was lower in the laminectomy than the laminectomy+duroplasty group. A possible explanation is that at very high SCPP, the extra space produced by duroplasty allows marked circumferential expansion of the injured spinal cord, which impairs its vascular reactivity.
Our data suggest that nursing care may have a major influence on outcome. In the supine position the wound is compressed, leading to a rise in ISP and a fall in SCPP, which may exacerbate spinal cord ischemic damage. We suggest that for the first 4 d after surgery, patients should be nursed in the lateral position or supine by positioning a ring-shaped pillow round the wound. After Day 5, lying supine had no effect on ISP or SCPP.
Laminectomy+duroplasty also might benefit patients with edematous transverse myelitis (e.g., associated with neuromyelitis optica).21 In neuromyelitis optica patients, the swollen spinal cord appears compressed against the surrounding dura, which may cause high ISP and low SCPP. This observation suggests that in longitudinally extensive transverse myelitis, ischemia may play a key but unappreciated role in causing secondary damage. By reducing ISP and increasing SCPP, laminectomy+duroplasty might improve outcome in this patient group.
There are a few published reports of dural decompression after TSCI. Perkins and Deane performed durotomy in six patients with TSCI in whom the theca appeared tense after bony decompression; three recovered completely and three partially.22 In a pig model of contusion TSCI, increased thecal sac dimensions were associated with reduced cord compression and reduced cord pressure at the injury site.23 Durotomy also reduced interstitial spinal cord pressure after TSCI in ex vivo pig spinal cords.24 In rodent models of TSCI, durotomy with duroplasty improves outcome more than durotomy without duroplasty.25 In rats, durotomy without duroplasty is associated with more macrophage accumulation, cystic cavitation and fibroblast proliferation than durotomy with duroplasty.26 Dural continuity also prevented epidural and spinal cord fibroblast proliferation and scar formation.27 Together, the animal experiments suggest that opening the dura is advantageous for relieving ISP, but a dural patch is required to reduce spinal cord inflammation and scarring. Our data also are suggestive of benefits, which need to be followed up with a larger randomized, controlled trial.
Acknowledgments
We thank Matthew Crocker, Tim Jones, Tony Bell, Tim Bishop, and Jason Bernard of St. George's Hospital, as well as David Bell and Nick Thomas of King's College Hospital, for help with patient recruitment.
IP and MCP were responsible for data collection, analysis and drafting of manuscript. MCW and SS were responsible for data collection and analysis. GVV and MC were responsible for data analysis. AZ was responsible for data collection. All authors reviewed and approved the final draft of the manuscript.
Author Disclosure Statement
Financial support was provided by the Neurosciences Research Foundation (MCP, MCW, IP), London Deanery (IP, MCW), the Royal College of Surgeons of England (MCW), the Housham Fund (MCW), and the Fletcher Fund (MCP). MCP is supported by the Guthy Jackson Foundation and by the Wings for Life Spinal Cord Research Foundation. MC is National Institute for Health Research principal investigator on cerebrospinal dynamics. GV is supported by an A. G. Leventis Foundation Scholarship and a Charter Studentship from St. Edmund's College, Cambridge. The funders had no input at any stage of this research.
MC received royalties from ICM+software (part of the licensing fee). For the remaining authors, no competing financial interests exist.
References
- 1.Cripps R.A., Lee B.B., Wing P., Weerts E., Mackay J., and Brown D. (2011). A global map for traumatic spinal cord injury epidemiology: towards a living data repository for injury prevention. Spinal Cord 49, 493–501 [DOI] [PubMed] [Google Scholar]
- 2.Curt A., Van Hedel H.J., Klaus D., and Dietz V. (2008). Recovery from a spinal cord injury: significance of compensation, neural plasticity, and repair. J. Neurotrauma 25, 677–685 [DOI] [PubMed] [Google Scholar]
- 3.Kornblith L.Z., Kutcher M.E., Callcut R.A., Redick B.J., Hu C.K., Cogbill T.H., Baker C.C., Shapiro M.L., Burlew C.C., Kaups K.L., DeMoya M.A., Haan J.M., Koontz C.H., Zolin S.J., Gordy S.D., Shatz D.V., Paul D.B., and Cohen M.J; Western Trauma Association Study Group. (2013). Mechanical ventilation weaning and extubation after spinal cord injury: a Western Trauma Association multicenter study. J. Trauma Acute Care Surg. 75, 1060–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Y., Chen Y., and DeVivo M. (2011). Lifetime direct costs after spinal cord injury. Topics Spinal Cord Inj. Rehabil. 16, 10–16 [Google Scholar]
- 5.Werndle M.C., Zoumprouli A., Sedgwick P., and Papadopoulos M.C. (2012). Variability in the treatment of acute spinal cord injury in the United Kingdom: results of a national survey. J. Neurotrauma 29, 880–888 [DOI] [PubMed] [Google Scholar]
- 6.Fehlings M.G., Rabin D., Sears W., Cadotte D.W., and Aarabi B. (2010). Current practice in the timing of surgical intervention in spinal cord injury. Spine (Phila Pa 1976) 35(21 Suppl), S166–S173 [DOI] [PubMed] [Google Scholar]
- 7.Walters B.C., Hadley M.N., Hurlbert R.J., Aarabi B., Dhall S.S., Gelb D.E., Harrigan M.R., Rozelle C.J., Ryken T.C., and Theodore N.; American Association of Neurological Surgeons; Congress of Neurological Surgeons. (2013). Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery 60 Suppl 1, 82–91 [DOI] [PubMed] [Google Scholar]
- 8.Werndle M.C., Saadoun S., Phang I., Czosnyka M., Varsos G.V., Czosnyka Z.H., Smielewski P., Jamous A., Bell B.A., Zoumprouli A., and Papadopoulos M.C. (2014). Monitoring of spinal cord perfusion pressure in acute spinal cord injury: initial findings of the injured spinal cord pressure evaluation study*. Crit. Care Med. 42, 646–655 [DOI] [PubMed] [Google Scholar]
- 9.Ropper A.H. (2014). Management of raised intracranial pressure and hyperosmolar therapy. Pract. Neurol. 14, 152–158 [DOI] [PubMed] [Google Scholar]
- 10.van Middendorp J.J., Hosman A.J.F., and Doi S.A.R. (2013). The effects of the timing of spinal surgery after traumatic spinal cord injury: a systematic review and meta-analysis. J. Neurotrauma 30, 1781–1794 [DOI] [PubMed] [Google Scholar]
- 11.Fehlings M.G., Vaccaro A., Wilson J.R., Singh A.W., Cadotte D., Harrop J.S., Aarabi B., Shaffrey C., Dvorak M., Fisher C., Arnold P., Massicotte E.M., Lewis S., and Rampersaud R. (2012). Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS One 7(2): e32037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang X. and Wen L. (2010). Technical considerations in decompressive craniectomy in the treatment of traumatic brain injury. Int. J. Med. Sci. 7, 385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirshblum S.C., Waring W., Biering-Sorensen F., Burns S.P., Johansen M., Schmidt-Read M., Donovan W., Graves D., Jha A., Jones L., Mulcahey M.J., and Krassioukov A. (2011). Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J. Spinal Cord Med. 34, 547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czosnyka M. and Pickard J.D. (2004). Monitoring and interpretation of intracranial pressure. J. Neurol. Neurosurg. Psychiatry 75, 813–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ditunno J.F, Jr., Ditunno P.L., Graziani V., Scivoletto G., Bernardi M., Castellano V., Marchetti M., Barbeau H., Frankel H.L., D'Andrea Greve J.M., Ko H.Y., Marshall R., and Nance P. (2001). Walking index for spinal cord injury (WISCI): an international multicenter validity and reliability study. Spinal Cord 38, 234–243 [DOI] [PubMed] [Google Scholar]
- 16.Ackerman P., Morrison S.A., McDowell S., and Vazquez L. (2010). Using the Spinal Cord Independence Measure III to measure functional recovery in a post-acute spinal cord injury program. Spinal Cord 48, 380–387 [DOI] [PubMed] [Google Scholar]
- 17.Rowland J.W., Hawryluk G.W., Kwon B., and Fehlings M.G. (2008). Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg. Focus 25, E2. [DOI] [PubMed] [Google Scholar]
- 18.Martirosyan N.L., Feuerstein J.S., Theodore N., Cavalcanti D.D., Spetzler R.F., and Preul M.C. (2011). Blood supply and vascular reactivity of the spinal cord under normal and pathological conditions. J. Neurosurg. Spine 15, 238–251 [DOI] [PubMed] [Google Scholar]
- 19.Steiner L.A., Czosnyka M., Piechnik S.K., Smielewski P., Chatfield D., Menon D.K., and Pickard J.D. (2002). Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit. Care Med. 30, 733–738 [DOI] [PubMed] [Google Scholar]
- 20.Timofeev I., Czosnyka M., Nortje J., Smielewski P., Kirkpatrick P., Gupta A., and Hutchinson P. (2008). Effect of decompressive craniectomy on intracranial pressure and cerebrospinal compensation following traumatic brain injury. J. Neurosurg. 108, 66–73 [DOI] [PubMed] [Google Scholar]
- 21.Papadopoulos M.C. and Verkman A.S. (2012). Aquaporin 4 and neuromyelitis optica. Lancet Neurol. 11, 535–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perkins P. and Deane R. (1998). Long-term follow-up of six patients with acute spinal injury following dural decompression. Injury 19, 397–401 [DOI] [PubMed] [Google Scholar]
- 23.Jone C.F., Newell R.S., Lee J.H., Cripton P.A., and Kwon B.K. (2012). The pressure distribution of cerebrospinal fluid responds to residual compression and decompression in an animal model of acute spinal cord injury. Spine (Phila Pa 1976) 37, E1422–E431 [DOI] [PubMed] [Google Scholar]
- 24.Awwad W., Bassi M., Shrier I., Al-Ahaideb A., Steele R.J., and Jarzem P.F. (2014). Mitigating spinal cord distraction injuries: the effect of durotomy in decreasing cord interstitial pressure in vitro. Eur. J. Orthop. Surg. Traumatol. 24 Suppl 1, S261–S267 [DOI] [PubMed] [Google Scholar]
- 25.Smith J.S., Anderson R., Pham T., Bhatia N., Steward O., and Gupta R. (2010). Role of early surgical decompression of the intradural space after cervical spinal cord injury in an animal model. J. Bone Joint Surg. Am. 92, 1206–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iannotti C., Zhang Y.P., Shields L.B., Han Y., Burke D.A., Xu X.M., and Shields C.B. (2006). Dural repair reduces connective tissue scar invasion and cystic cavity formation after acute spinal cord laceration injury in adult rats. J. Neurotrauma 23, 853–865 [DOI] [PubMed] [Google Scholar]
- 27.Fernandez E. and Pallini R. (1985). Connective tissue scarring in experimental spinal cord lesions: significance of dural continuity and role of epidural tissues. Acta Neurochir. (Wien) 76, 145–148 [DOI] [PubMed] [Google Scholar]