Abstract
Background: We have demonstrated that acute alcohol intoxication (AAI) increases the magnitude of Ca2+ transients in pumping lymphatic vessels. We tested the contribution of extracellular Ca2+ via L-type Ca2+ channels and intracellular Ca2+ release from the sarcoplasmic reticulum (SR) to the AAI-induced increase in Ca2+ transients.
Methods and Results: AAI was produced by intragastric administration of 30% alcohol to conscious, unrestrained rats; isovolumic administration of water served as the control. Mesenteric lymphatic vessels were isolated, cannulated, and loaded with Fura-2 AM to measure changes in intracellular Ca2+. Measurements were made at intraluminal pressures of 2, 6, and 10 cm H2O. L-type Ca2+ channels were blocked with nifedipine; IP-3 receptors were inhibited with xestospongin C; and SR Ca2+ release and Ca2+ pool (Ca2+ free APSS) were achieved using caffeine. Nifedipine reduced lymphatic Ca2+ transient magnitude in both AAI and control groups at all pressures tested, but reduced lymphatic contraction frequency only in the control group. Xestospongin C did not significantly change any of the Ca2+ parameters in either group; however, fractional shortening increased in the controls at low transmural pressure. RyR (ryanodine receptor) activation with caffeine resulted in a single contraction with a greater Ca2+ transient in lymphatics from AAI than those from controls. SR Ca2+ pool was also greater in lymphatics isolated from AAI- than from control animals.
Conclusions: These data suggest that 1) L-type Ca2+ channels contribute to the AAI-induced increase in lymphatic Ca2+ transient, 2) blockage of IP-3 receptors could increase calcium sensitivity, and 3) AAI increases Ca2+ storage in the SR in lymphatic vessels.
Introduction
In humans, more than 50% of the total lymph is formed within the gastrointestinal (GI) tract.1 In addition to regulating fluid homeostasis and facilitating immune surveillance, lymphatic vessels also selectively transport newly formed lipids and fat-soluble vitamins. The collecting lymphatic vessels that exit the intestine are composed of an inner endothelial layer with one-way valves to prevent backflow, and a smooth muscle layer that generates spontaneous tone and phasic contractions to transport lymph centrally toward the mesenteric lymph nodes.2–5 In previous studies, we demonstrated that acute alcohol intoxication (AAI) modulates the pump function of mesenteric lymphatics.6,7
We have chosen to study AAI-induced changes in lymphatic pumping because AAI complicates recovery from injury and infection by impairing cardiovascular function, fluid homeostasis, and host defense.8 The gut lymphatics are a primary route for nonbacterial, tissue injury factors released from the GI tract. These factors contribute to the development of multiple organ injury and failure following traumatic injury.9–12 Clinical data show a positive correlation between excessive gut permeability and the extent of the injury in trauma in patients.12–14 Given the role of collecting lymphatics in the transport of gut-derived bacterial products, cytokines, and inflammatory mediators, it is possible that disrupted pumping of mesenteric collecting lymphatics in intoxicated trauma victims may contribute to the greater prevalence of morbidity and mortality from traumatic injury in the alcohol-abusing population.15
Contractility of lymphatic smooth muscle can be modulated by extrinsic (neural and humoral) and intrinsic (myogenic) factors. Calcium transport across the sarcolemma via L-type channel contributes significantly to the contractile activity of collecting lymphatics.16,17 Moreover, modulation of Ca2+ release from intracellular stores has been suggested to be involved in the pressure-induced enhancement of lymphatic contractions.18 Pace-making in lymphatic vessels is not generated by a single current, but relies on complex interactions between multiple Ca2+ currents.19 Most likely, spontaneous transient depolarizations in mesenteric lymphatic smooth muscle arise through spontaneous or stimulated Ca2+ release from IP3-sensitive Ca2+ stores, which activates Ca2+-activated chloride channels (ClCa).20 Other evidence suggests that the Rho kinase (ROCK) pathway is involved in maintaining lymphatic pump activity and myogenic tone in lymphatic vessels.21 Previously, we demonstrated that ROCK mediates normal tonic constriction and influences phasic contractions in lymphatics by modulating Ca2+ sensitivity of contractile proteins in lymphatic vessels.22
The molecular mechanisms underlying tonic and phasic contractions in mesenteric collecting lymphatics remain poorly understood. Furthermore, how these mechanisms are altered by AAI is not well known. Our studies have shown that AAI, resulting from intragastric alcohol (2.5 g/kg) administration to conscious, unrestrained rats, produces marked alterations in lymphatic contractile function. Our results demonstrated that lymphatics isolated from AAI rats displayed decreased contraction frequency and increased amplitude of contraction, which was related to a decreased frequency and increased magnitude of spontaneous, transient Ca2+ mobilizations into the cytoplasm.6,23 Furthermore, we found that AAI uncoupled the close association in between phasic and tonic lymphatic vessel contraction. This uncoupling was reflected in the AAI-induced increased magnitude of phasic Ca+2 mobilization and attenuated lymphatic myogenic constriction by impaired RhoA/ROCK pathway-mediated Ca+2 sensitivity.6,7 To expand understanding of the molecular mechanisms that mediate the AAI-induced increase in Ca2+ transient magnitude of lymphatic smooth muscle, in the present study, we investigated the potential role of sarcolemmal Ca2+ entry through L-type Ca2+ channels and its uptake/release from the sarcoplasmic reticulum (SR) in rat isolated mesenteric lymphatic vessels.
Materials and Methods
Animals
All procedures were approved by the Institutional Animal Care and Use Committee at the Louisiana State University Health Sciences Center and were performed in accordance with the guidelines of the NIH Guide for the Care and Use of Laboratory Animals (8th edition, 2011). Male Sprague-Dawley rats (270–350 g body wt) were housed in a controlled temperature (22°C) and controlled illumination (12:12 h light dark cycle) environment. After arrival, the rats were allowed a 1-week acclimation period and were provided standard rat chow (2018 Teklad Global 18% Protein Rodent Diet, Harlan) and water ad libitum.
Gastric catheter placement and alcohol administration protocol
AAI was produced as previously described.6,7,24,25 Briefly, rats were anesthetized with ketamine and xylazine (90 and 9 mg/kg, respectively). A sterile catheter was aseptically placed into the antrum of the stomach and exteriorized at the nape of the neck. Following a 2-day recovery period from the surgical procedure, conscious and unrestrained animals received an intragastric bolus of 30% ethyl alcohol (2.5 g/kg) via the gastric catheter. Intragastric administration of alcohol at this dose typically produces a blood alcohol level of 200–300 mg/dL within 30 min. of administration.25 A time-matched control group received isovolumic intragastric administration of vehicle (water).
Collecting lymphatic isolation and pressure step protocol
The responsiveness of lymphatic vessels to step changes in pressure was studied as previously described.25 Briefly, rats were anesthetized with ketamine and xylazine (90 and 9 mg/kg, respectively). A midline laparotomy was performed, the gut and associated mesentery were excised, and the excised mesentery was pinned in a dissection chamber containing 4°C albumin physiological salt solution (APSS). A collecting lymphatic vessel segment (1 mm length), with at least one valve, was carefully dissected from surrounding adipose and connective tissue and mounted onto two resistance-matched glass micropipettes in a vessel chamber (Living Systems Instrumentation, Burlington VT). Isolated lymphatic vessels with only one valve were used to ensure optimal pressure control in the entire segment.26 Rapid time-lapse image sets were acquired using the image acquisition software (Nikon Elements AR software) at the pressures of 2, 6, and 10 cm H2O in all lymphatic vessels studied.
Ratiometric measurement of [Ca2+]i
Measurement of [Ca2+]i in isolated lymphatic vessels was performed after loading with the ratiometric dye Fura-2-acetoxymethyl ester (Fura-2-AM; Molecular Probes, Eugene, OR) as previously described.7,23 Briefly, upon entry into the cytoplasm, AM portion of the dye is cleaved, making fura-2 membrane-impermeable. Measurements were collected in isolated lymphatics loaded with fura-2 by illuminating at alternating wavelengths of 340 and 380 nm via a dichroic mirror (400 nm; Chroma Technology Corp. 400DCLP) for durations of 50 ms each (the shortest time we could obtain sufficient signal with our hardware), over a period of 1–2 min.
A region of interest (ROI) that included the entire lymphatic vessel and surrounding area was selected for [Ca2+]i, measurements. An increase in the 340/380 ratio indicates an increase in [Ca2+]i.27 The frequency of transient increases in [Ca2+]i were identified by examining the number of peaks over time, and the mean magnitude of these transients were determined by averaging the difference between peak and basal value just prior to each peak. Baseline measurements were performed in each isolated collecting lymphatic at intraluminal pressures of 2, 6, and 10 cm H2O. After baseline measurements three experimental approaches were used; 1) L-type Ca2+ channels were blocked with application of nifedipine (10−7 M),17 2) SR Ca2+ release was inhibited by treatment with xestospongin C (10−6 M),28 or 3) the ryanodine receptor (RyR) was activated with caffeine (10 mM).29,30 All drugs were from Sigma-Aldrich and were added to the bath for 10 min prior to obtaining the measurements at 2, 6, and 10 cm H2O luminal pressure.
We determined the incubation time after pilot studies. We took the same measurements at 5, 10, and 30 min after the drug; 10 and 30 min produced very similar results. At the end of the experiment, the bath was changed to a Ca2+-free APSS to evaluate the lymphatics in a passive, relaxed state at 2, 6, and 10 cm H2O. For the caffeine trials, the same procedure was repeated after Ca2+-free albumin physiological salt solution (APSS)6 bath change to determine the relative amount of Ca2+ contained in intracellular stores. To avoid the typical errors that occur in calculating [Ca2+]i from microscopic images of tissues, raw 340/380 ratios are presented.
Diameter measurements
Video files were converted to sequential tiff images, using a National Institutes of Health public domain software program, ImageJ 1.46r, bundled with 64-bit Java and the nifedipine to Image6D plugin, on a Windows 64 platform. Using the MATLAB (R2011b) programming language (The MathWorks Inc., Natick, Massachusetts), software was written to import and analyze the generated sequential TIFF images.
For noise reduction, each image was initially smoothed using a Gaussian low pass filter. An intensity threshold that removed most background noise was determined. A region of interest (ROI) was visualized by the most “active” portion of the lymphatic vessels. Using an edge-detection algorithm, the lymphatic vessel wall edges were calculated for each image. This allowed identification of the area between the two walls, and hence the cross-sectional area of the lymphatic vessel within the ROI.
The width of the ROI was used to calculate an average diameter for each image. From this array of diameters for each image, and knowing the time frame of the images, parameters for each contraction within the sequence were calculated. Several derived measurements for each contraction are analogous to those performed for M-mode echocardiography. For each contraction, the end diastolic diameter (EDD) and end systolic diameter (ESD) were obtained, allowing calculation of fractional shortening (FS) (ESD-EDD/EDD).
Statistical data analysis
Summarized data are presented as mean±SE and the N indicated. One-way ANOVA or two-way ANOVA followed by Bonferroni t-tests were used. Statistical significance was accepted at p<0.05.
Results
Sarcolemmal Ca2+ entry in lymphatic vessels
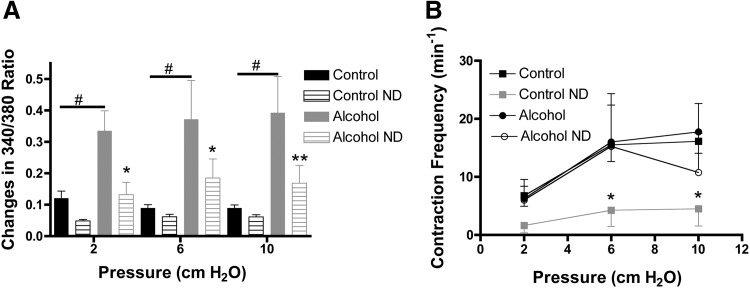
Consistent with our previous report,7 AAI significantly increased the magnitude of Ca2+ transients associated with phasic contractions at all intraluminal pressures studied (Fig. 1A). Application of nifedipine generally caused a reduction in the Ca2+ transient magnitude. These decreases were significant in lymphatic vessels from alcohol-treated animals at luminal pressures of 2, 6, and 10 cm H2O (Fig. 1A). Nifedipine also decreased contraction frequency in lymphatic vessels isolated from control animals (Fig. 1B). Interestingly, the nifedipine-induced decrease in Ca2+ transient magnitude in lymphatics from AAI animals was not associated with a decrease in contraction frequency (Fig. 1B). Nifedipine did not change the fractional shortening or other lymphatic contraction parameters within the measured time-frame (data not shown).
FIG. 1.
Impact of inhibition of L-type Ca2+ channels with nifedipine (ND) on lymphatics. (A) Nifedipine reduced amplitude of Ca2+ transients in lymphatic vessels from the AAI (N=4) but not significantly at control (N=8) groups. (B) However, nifedipine decreased CF in the control group but not in AAI lymphatics, control error bars down and alcohol up. 2-way ANOVA, Bonferroni post hoc. *p<0.05, **p<0.01, Control pre- versus Control post-nifedipine and Alcohol pre- versus Alcohol post-nifedipine. #p<0.01 Control versus Alcohol.
IP3-mediated Ca2+ release from the SR
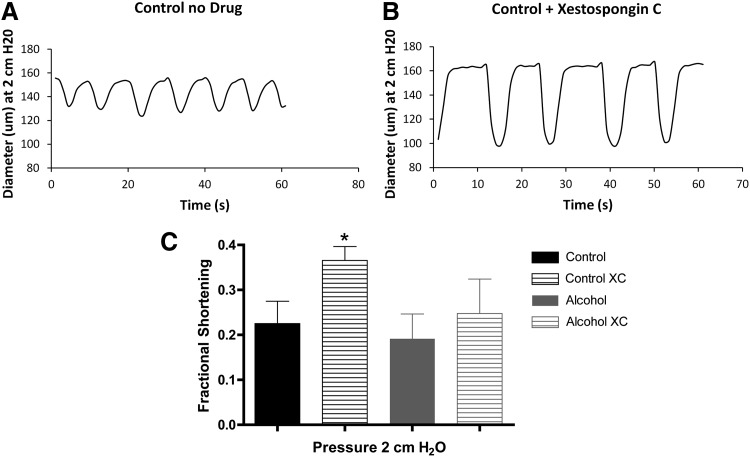
IP3 receptor-mediated Ca2+ release and its role in phasic contractions were assessed using the IP3 receptor inhibitor xestospongin C. Blockade of IP3 receptors did not significantly change Ca2+ transient magnitude or contraction frequency in either the control or alcohol group (data not shown). A surprising finding was that xestospongin C increased the mean fractional shortening in control lymphatics subjected to the 2 cm H2O luminal pressure (Fig. 2C). Representative tracings of diameter from control animals are shown on Figure 2A (before xestospongin C) and Figure 2B (after xestospongin C) at baseline pressure. This change in mean fractional shortening did not occur at 6 or 10 cm H2O luminal pressure in the control lymphatics. The fractional shortening was also unchanged following xestospongin C treatment at all pressures tested in the lymphatic vessels from the AAI group.
FIG. 2.
Effect of Xestospongin C (XC) treatment on lymphatics. Panels A and B show representative tracings of lymphatic diameter from control animals before (A) and after xestospongin C treatment (B). Xestospongin C increased the mean fractional shortening in control (N=5) lymphatics subjected to the 2 cm H2O luminal pressure, but not AAI (N=3) lymphatics (C). One-way ANOVA, Bonferroni post hoc. *p<0.05. Control pre- versus Control post-xestospongin C and Alcohol pre- versus Alcohol post-xestospongin C
RyR function and SR Ca2+ pool in lymphatic smooth muscle
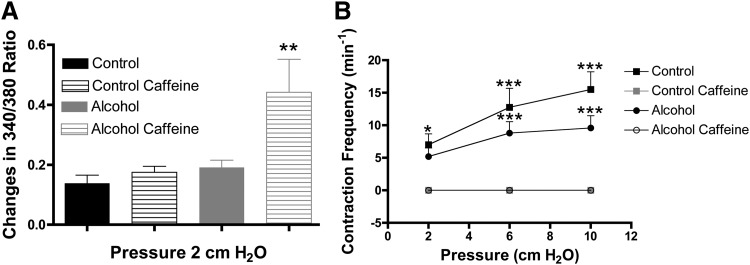
To test the role of RyR function in lymphatic pumping, we measured lymphatic pumping parameters and changes in the 340/380 ratio at luminal pressures of 2, 6, and 10 cm H2O before and after application of caffeine. Caffeine was selected because it opens RyRs and depletes Ca2+ from the SR29. Immediately after the application of caffeine, all lymphatic vessels displayed a single Ca2+ transient and associated phasic contraction, after which all Ca2+ transients and phasic contractions ceased. Figure 3A compares the magnitude of changes in 340/380 ratio associated with phasic contractions in lymphatics prior to the addition of caffeine to the magnitude of change associated with the single phasic contraction that occurred after caffeine addition. Although in this particular experiment the change in 340/380 ratio was not pronounced between the control and alcohol groups initially, immediately after caffeine was added the lymphatics from the AAI group displayed a significantly greater magnitude of Ca2+ transient magnitude in the single phasic contraction that occurred, compared to the controls (Fig. 3A).
FIG. 3.
Impact of SR depletion of Ca2+ with caffeine on lymphatics. (A) Ca2+ transient amplitude is greater in lymphatics isolated from AAI (N=5) than that of controls (N=4) after a possible RyR activation with caffeine. (B) However, the frequency of contraction is zero in lymphatics isolated from both groups post SR depletion with caffeine. (A) One-way ANOVA, Bonferroni post hoc. *p<0.05, Control pre- versus Control post-caffeine and Alcohol pre- versus Alcohol post-caffeine. (B) Two-way ANOVA, Bonferroni post hoc. *p<0.05, ***p<0.001, Control pre- versus Control post-caffeine and Alcohol pre- versus Alcohol post-caffeine.
The mean contraction frequencies at the three different intraluminal pressures, before and after the addition of caffeine, are shown in Figure 3B. These data show that prior to the addition of caffeine, the isolated lymphatics from both groups displayed the typical elevation in phasic contraction frequency due to increased luminal pressure. However, in the presence of caffeine, phasic contractions were absent in both groups.
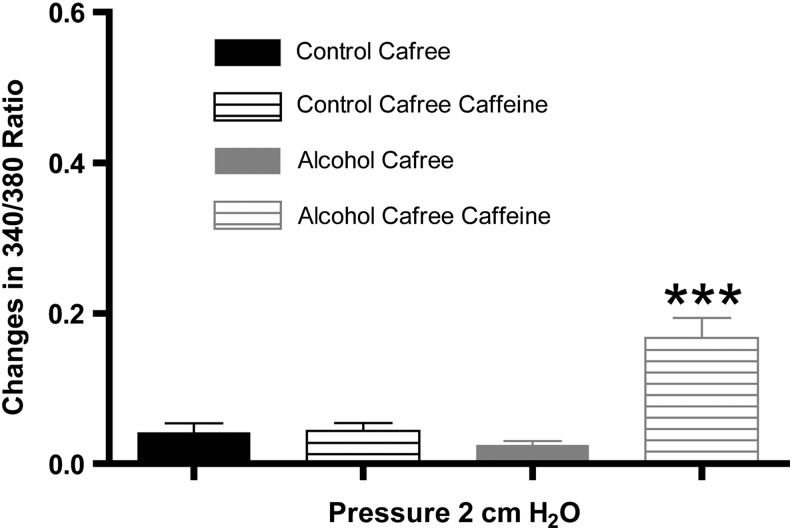
The relatively large Ca2+ transient observed in the lymphatics from the AAI group after caffeine treatment suggested that the SR was harboring higher amounts of Ca2+ in these lymphatics. To test this possibility, we used a Ca2+-free bath solution, treated the vessels with caffeine or vehicle, and measured changes in the 340/380 ratio to determine the amount of Ca2+ stored in the SR. The summarized data in Figure 4 show that lymphatic vessels from the AAI group had significantly greater Ca2+ release after treatment with caffeine than lymphatics isolated from controls, suggesting that AAI enhances Ca2+ storage in the SR in mesenteric lymphatic vessels.
FIG. 4.
The SR Ca2+ pool is elevated in lymphatics isolated from AAI (N=5) rats than from control (N=4) rats. The lymphatics were tested in a Ca2+-free APSS. One-way ANOVA, Bonferroni post hoc. ***p<0.001, Control pre- versus Control post-caffeine and Alcohol pre- versus Alcohol post-caffeine.
Discussion
Our studies examined the potential impact of alcohol on molecular mechanisms that mediate the cyclic release and uptake of Ca2+. We found that L-type Ca2+ channel blockade reduces the magnitude of Ca2+ transients, effectively bringing the AAI-induced increase in Ca2+ transient magnitude to the levels close to those of controls, without significantly changing contraction frequency. This finding suggests an important role for L-type Ca2+ channels in the alcohol-induced increase in Ca2+ transients in lymphatic vessels. Although we did not identify a clear role for IP3-mediated mobilization of Ca2+ in normal lymphatic pumping or the response to alcohol, we did observe that the IP3R antagonist xestospongin C elevated the fractional shortening of lymphatic vessels isolated from control animals at low intraluminal pressure (2 cm H2O). Lastly, caffeine-induced emptying of Ca2+ stores discontinued lymphatic vessel pumping in both the control and AAI groups, and revealed a significant elevation in the amount of Ca2+ stored in the SR after AAI, compared to controls.
The lymphatic pacemaker mechanism is driven by the oscillating opening and closing of multiple ion channels on the cell membrane and on intracellular Ca2+ stores.31,32 Ca2+ movement via the L-type channels has been thought to contribute significantly to the regulation of lymphatic pump activity, while intracellular Ca2+ flux plays a less significant role that may be modified by transmural pressure.16,17 Our observations confirm that sarcolemmal Ca2+ entry via L-type Ca2+ channels contributes to the spontaneous Ca2+ transients that precede lymphatic phasic contractions. Studies on mesenteric lymphatic vessels using wire myograph have shown that nifedipine in rats (10−6 M)16 and in humans (3×10−9 M)33 abolishes lymphatic pumping.
Our findings with the lymphatic vessels isolated from rats that did not receive alcohol reflect the results of Atchison and Johnston, who used isolated bovine mesenteric lymphatic vessels and observed that nifedipine, applied at the same concentration we used, decreased both contraction frequency and amplitude of contraction.17 However, recent findings from von der Weid and colleagues, using rat mesenteric lymphatics and very similar experimental conditions as ours, contradict our data. They reported that nifedipine applied at a concentration three times higher than ours decreases lymphatic amplitude of contraction and force without changes in contraction frequency.34
A key difference between our protocols may explain the disparity. We applied the nifedipine directly to the vessel bath via a micropipette, resulting in a near instant desired final concentration. In contrast, they added the nifedipine to a reservoir that slowly introduced nifedipine to the bath. It is possible that slowly elevating nifedipine in this way may not lead to the same type of blockade as reaching the desired concentration rapidly. Also, it is not clear from their report whether they actually reached the desired concentration at the time of their measurements. Considering that our observations essentially reproduced those of Atchison and Johnson, and we have consistently observed this type of inhibition also with diltiazem (unpublished observations), we are confident concluding that L-type Ca2+ channels have an important role in both the frequency and strength of phasic contractions of lymphatics. The decrease in contraction frequency after nifedipine was not significant in the lymphatic vessels from alcohol-treated animals. However, nifedipine significantly reduced the AAI-induced increase in the magnitude of Ca2+ transients, suggesting that L-type Ca2+ channels play a role. Furthermore, lymphatic vessels from alcohol-treated animals had unchanged contraction frequency after nifedipine treatment, suggesting that AAI activates a nifedipine-insensitive pathway to maintain contraction frequency.
Although IP3-operated Ca2+ stores play an important role in lymphatic vessel contraction in response to vasoconstrictor stimulation,20,35,36 in our studies, IP3 receptor-mediated Ca2+ release from the SR did not significantly alter Ca2+ transients or contraction frequency in lymphatic vessels from controls or alcohol-treated animals. Moreover, our results suggest that alcohol-induced changes in Ca2+ transients are not due to a modification of IP3-mediated mobilization of Ca2+. We did observe that xestospongin C caused an increase in the fractional shortening in control lymphatics, but only at the lowest intraluminal pressure studied (2 cm H2O), without causing significant changes in the 340/380 ratio. While this finding might suggest that xestospongin C, at the concentration used, could increase calcium sensitivity, the possibility of off-target effects of the drug cannot be eliminated. To our knowledge, no other studies have made this observation.20,28,35,36 Possible explanations for this difference in results include differences in animal species, lymphatic preparations, concentration of the drug, and the pre-stimulation with constricting agents prior to use of the IP3R antagonist.
In vascular smooth muscle, caffeine has been described to act initially upon the RyR in the SR, generating an increase in intracellular Ca2+ and a transitory contraction that is followed by vasodilation.37 Caffeine is also a nonselective inhibitor of phosphodiesterase, inhibiting degradation of cAMP, causing its local accumulation and vasodilation.38 Caffeine is also described as an IP3R inhibitor.39 A study on lymphatic vessels showed that caffeine decreased spontaneous transient depolarizations due to increase in cAMP and IP3R activation, but not RyR activation. In contrast, Zhao and van Helden studies showed that caffeine inhibited guinea-pig lymphatic pumping after causing a small transient increase in intracellular Ca2+. This increase in Ca2+ was likely in consequence of RyR activation, as it was abolished following treatment with 20 mM of ryanodine.35
In our studies, caffeine discontinued lymphatic vessel pumping in both the AAI and control groups. In addition, prior to lymphatic pumping cessation there was a single phasic contraction and associated Ca2+ transient in the lymphatic vessels, which was significantly greater in the AAI group. Regardless of whether caffeine's impact is primarily due to activation of the RyR, on cAMP levels, or on the IP3R, it is clear that the cyclic release and uptake of Ca2+ from the SR is critical for lymphatic phasic contractions. Although in our studies, we cannot rule out the caffeine-induced increase in cAMP, we confirmed that there is an elevated SR storage of Ca2+, suggesting that alcohol-induced modulation of Ca2+ transients may involve altered SR Ca2+ handling in lymphatic vessels. Our results are supported by studies showing that caffeine-sensitive pools mediate alcohol-induced smooth muscle contraction.40,41 Albeit AAI increases lymphatic intracellular Ca2+, we have previously shown that it does not lead to an increased lymphatic contraction due to AAI-induced decrease in Ca2+ sensitivity.7
It is important for us to address that in our series of experiments studying the impact of caffeine on lymphatic Ca2+ transients, in this particular set of experiments we did not achieve a significant difference in the mean magnitudes of Ca2+ transients between the control and alcohol groups. This finding was in contrast to our results in Figure 1 and our previous publications,7,23 and the reason is unclear. The best possible explanations are that there is biological variability in the response that happened to manifest in this experiment, or an unidentified factor that may have been different with this particular set of experiments. Previously, we identified differences in response depending upon whether alcohol was administered in vivo or applied to lymphatics in the tissue bath.6
We can also speculate that differences may arise in response if the time from tissue harvest to the start of the experiment is highly variable, although in the current study we consistently mounted the vessels and had them pumping ex vivo within 30 min of tissue harvest. Despite the lack of difference in the magnitude of Ca2+ transients between the alcohol and control groups prior to addition of caffeine, we included this data because it provided the clue that the Ca2+ storage in the SR may be elevated, which we later confirmed in Figure 4. Also, we will be mindful in future studies to investigate the extent of variability that exists between the control and alcohol groups, and whether there are potential subpopulations of lymphatic segments that respond differently.
We aimed to investigate potential molecular mechanisms that mediate AAI-induced increase in Ca2+ transient magnitude of lymphatic smooth muscle via sarcolemmal Ca2+ entry and Ca2+ mobilization from the SR in phasic contractions. We conclude that AAI increases Ca2+ transients in mesenteric lymphatic vessels predominantly via L-type Ca2+ channels and increased Ca2+ storage in the SR. These results provide insight into mechanisms sensitive to AAI. Moreover, these results identify potential mechanisms to be targeted to modulate lymphatic pumping and in turn ameliorate the contribution of gut-derived toxins and inflammatory mediators to systemic organ injury in the setting of traumatic injury in the AAI host.
Acknowledgments
The idea and experimental design was developed by Souza-Smith, Breslin, and Molina. Kerut created a program to analyze diameter from fluorescent tiff images and wrote it on the materials and methods. Souza-Smith performed experiments, analysis, and data interpretation, and wrote the manuscript. Breslin and Molina provided financial support and guidance. All authors read, edited, and approved the final version of the manuscript.
Author Disclosure Statement
The authors have no conflict of interest or financial ties to disclose.
This work was supported by NIH F32 (5F32AA021049-03), R21 (AA020049), and LSUHSC-NO Physiology Departmental Funds.
References
- 1.Alexander JS, Ganta VC, Jordan PA, Witte MH. Gastrointestinal lymphatics in health and disease. Pathophysiology 2010;17:315–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liao S, Padera TP. Lymphatic function and immune regulation in health and disease. Lymphat Res Biol 2013;11:136–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller MJ, McDole JR, Newberry RD. Microanatomy of the intestinal lymphatic system. Ann NY Acad Sci 2010;1207:E21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unthank JL, Bohlen HG. Lymphatic pathways and role of valves in lymph propulsion from small intestine. Am J Physiol 1988;254:G389–398 [DOI] [PubMed] [Google Scholar]
- 5.Breslin JW. Mechanical forces and lymphatic transport. Microvasc Res 2014;96:46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Souza-Smith FM, Kurtz KM, Molina PE, Breslin JW. Adaptation of mesenteric collecting lymphatic pump function following acute alcohol intoxication. Microcirculation 2010;17:514–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Souza-Smith FM, Molina PE, Breslin JW. Reduced RhoA activity mediates acute alcohol intoxication-induced inhibition of lymphatic myogenic constriction despite increased cytosolic [Ca(2+) ]. Microcirculation 2013;20:377–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molina PE, Gardner JD, Souza-Smith FM, Whitaker AM. Alcohol abuse: Critical pathophysiological processes and contribution to disease burden. Physiology (Bethesda) 2014;29:203–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deitch EA. Gut lymph and lymphatics: A source of factors leading to organ injury and dysfunction. Ann NY Acad Sci 2010;1207:E103–111 [DOI] [PubMed] [Google Scholar]
- 10.Deitch EA. Gut lymph hypotesis of early shock and trauma-induced multiple organ dysfunction syndrome: A new look at gut origin sepsis. J Organ Dysfunct 2006;2:10 [Google Scholar]
- 11.Deitch EA. Gut lymph and lymphatics: A source of factors leading to organ injury and dysfunction. Ann NY Acad Sci 1207:E103–111 [DOI] [PubMed] [Google Scholar]
- 12.Senthil M, Brown M, Xu DZ, Lu Q, Feketeova E, Deitch EA. Gut-lymph hypothesis of systemic inflammatory response syndrome/multiple-organ dysfunction syndrome: Validating studies in a porcine model. J Trauma 2006;60:958–965; discussion 965–957 [DOI] [PubMed] [Google Scholar]
- 13.Faries PL, Simon RJ, Martella AT, Lee MJ, Machiedo GW. Intestinal permeability correlates with severity of injury in trauma patients. J Trauma 1998;44:1031–1035; discussion 1035–1036 [DOI] [PubMed] [Google Scholar]
- 14.Langkamp-Henken B, Donovan TB, Pate LM, Maull CD, Kudsk KA. Increased intestinal permeability following blunt and penetrating trauma. Crit Care Med 1995;23:660–664 [DOI] [PubMed] [Google Scholar]
- 15.Hadfield RJ, Mercer M, Parr MJ. Alcohol and drug abuse in trauma. Resuscitation 2001;48:25–36 [DOI] [PubMed] [Google Scholar]
- 16.von der Weid PY, Lee S, Imtiaz MS, Zawieja DC, Davis MJ. Electrophysiological properties of rat mesenteric lymphatic vessels and their regulation by stretch. Lymphat Res Biol. 2014;12(2):66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atchison DJ, Johnston MG. Role of extra- and intracellular Ca2+ in the lymphatic myogenic response. Am J Physiol 1997;272:R326–333 [DOI] [PubMed] [Google Scholar]
- 18.Atchison DJ, Rodela H, Johnston MG. Intracellular calcium stores modulation in lymph vessels depends on wall stretch. Can J Physiol Pharmacol 1998;76:367–372 [PubMed] [Google Scholar]
- 19.Toland HM, McCloskey KD, Thornbury KD, McHale NG, Hollywood MA. Ca(2+)-activated Cl(-) current in sheep lymphatic smooth muscle. Am J Physiol Cell Physiol 2000;279:C1327–1335 [DOI] [PubMed] [Google Scholar]
- 20.von der Weid PY, Rahman M, Imtiaz MS, van Helden DF. Spontaneous transient depolarizations in lymphatic vessels of the guinea pig mesentery: Pharmacology and implication for spontaneous contractility. Am J Physiol Heart Circ Physiol 2008;295:H1989–2000 [DOI] [PubMed] [Google Scholar]
- 21.Hosaka K, Mizuno R, Ohhashi T. Rho-Rho kinase pathway is involved in the regulation of myogenic tone and pump activity in isolated lymph vessels. Am J Physiol Heart Circ Physiol 2003;284:H2015–2025 [DOI] [PubMed] [Google Scholar]
- 22.Kurtz KH, Souza-Smith FM, Moor AN, Breslin JW. Rho kinase enhances contractions of rat mesenteric collecting lymphatics. PLoS One 2014;9:e94082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Souza-Smith FM, Kurtz KM, Breslin JW. Measurement of cytosolic Ca2+ in isolated contractile lymphatics. J Vis Exp 2012;58:3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phelan H, Stahls P, Hunt J, Bagby GJ, Molina PE. Impact of alcohol intoxication on hemodynamic, metabolic, and cytokine responses to hemorrhagic shock. J Trauma 2002;52:675–682 [DOI] [PubMed] [Google Scholar]
- 25.Souza-Smith FM, Kurtz KM, Molina PE, Breslin JW. Adaptation of mesenteric collecting lymphatic pump function following acute alcohol intoxication. Microcirculation 2010;17:514–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis MJ, Davis AM, Ku CW, Gashev AA. Myogenic constriction and dilation of isolated lymphatic vessels. Am J Physiol Heart Circ Physiol 2009;296:H293–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Souza-Smith FM, Kurtz KM, Breslin JW. Measurement of cytosolic Ca2+ in isolated contractile lymphatics. J Vis Exp 2011;58;e3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawai Y, Yokoyama Y, Kaidoh M, Ohhashi T. Shear stress-induced ATP-mediated endothelial constitutive nitric oxide synthase expression in human lymphatic endothelial cells. Am J Physiol Cell Physiol 2010;298:C647–655 [DOI] [PubMed] [Google Scholar]
- 29.Essin K, Gollasch M. Role of ryanodine receptor subtypes in initiation and formation of calcium sparks in arterial smooth muscle: comparison with striated muscle. J Biomed Biotechnol 2009;2009:135249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicolas JM, Antunez E, Thomas AP, Fernandez-Sola J, Tobias E, Estruch R, Urbano-Marquez A. Ethanol acutely decreases calcium transients in cultured human myotubes. Alcohol Clin Exp Res 1998;22:1086–1092 [DOI] [PubMed] [Google Scholar]
- 31.Van Helden DF, Zhao J. Lymphatic vasomotion. Clin Exp Pharmacol Physiol 2000;27:1014–1018 [DOI] [PubMed] [Google Scholar]
- 32.McCloskey KD, Toland HM, Hollywood MA, Thornbury KD, McHale NG. Hyperpolarisation-activated inward current in isolated sheep mesenteric lymphatic smooth muscle. J Physiol 1999;521:201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Telinius N, Mohanakumar S, Majgaard J, et al. Human lymphatic vessel contractile activity is inhibited in vitro but not in vivo by the calcium channel blocker nifedipine. J Physiol 2014;592:4697–4714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S, Roizes S, von der Weid PY. Distinct roles of L- and T-type voltage-dependent Ca2+ channels in regulation of lymphatic vessel contractile activity. J Physiol 2014;592:5409–5427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao J, van Helden DF. ET-1-associated vasomotion and vasospasm in lymphatic vessels of the guinea-pig mesentery. Br J Pharmacol 2003;140:1399–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimizuka K, Kawai Y, Maejima D, Ajima K, Kaidoh M, Ohhashi T. Sphingosine 1-phosphate (S1P) induces S1P2 receptor-dependent tonic contraction in murine iliac lymph vessels. Microcirculation 2013;20:1–16 [DOI] [PubMed] [Google Scholar]
- 37.Echeverri D, Montes FR, Cabrera M, Galan A, Prieto A. Caffeine's vascular mechanisms of action. Int J Vasc Med 2010;2010:834060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Umemura T, Ueda K, Nishioka K, et al. Effects of acute administration of caffeine on vascular function. Am J Cardiol 2006;98:1538–1541 [DOI] [PubMed] [Google Scholar]
- 39.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature 1993;361:315–325 [DOI] [PubMed] [Google Scholar]
- 40.Dondas NY, Kaplan M, Kaya D, Singirik E. The impact of extracellular and intracellular Ca2+ on ethanol-induced smooth muscle contraction. Acta Pharmacol Sin 2009;30:1421–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Werber AH, Morgan RA, Zhou P, Yang C. Intracellular mechanisms of constriction of rat aorta by ethanol. Alcohol 1997;14:351–360 [DOI] [PubMed] [Google Scholar]