Abstract
The administration of hesperidin elicits an antidepressant-like effect in mice by a mechanism dependent on an interaction with the l-arginine-nitric oxide (NO)-cyclic guanosine monophosphate (cGMP) pathway, whose stimulation is associated with the activation of potassium (K+) channels. Thus, this study investigated the involvement of different types of K+ channels in the antidepressant-like effect of hesperidin in the mice tail suspension test (TST). The intracerebroventricular administration of tetraethylammonium (TEA, a nonspecific blocker of K+ channels), glibenclamide (an ATP-sensitive K+ channel blocker), charybdotoxin (a large- and intermediate-conductance calcium-activated K+ channel blocker) or apamin (a small-conductance calcium-activated K+ channel blocker) combined with a subeffective dose of hesperidin (0.01 mg/kg, intraperitoneally [i.p.]) was able to produce a synergistic antidepressant-like effect in the mice TST. Moreover, the antidepressant-like effect elicited by an effective dose of hesperidin (0.3 mg/kg, i.p.) in TST was abolished by the treatment of mice with pharmacological compounds K+ channel openers (cromakalim and minoxidil). Results showed that the antidepressant-like effect of hesperidin in TST may involve, at least in part, the modulation of neuronal excitability through inhibition of K+ channels and may act through a mechanism dependent on the inhibition of l-arginine-NO pathway.
Key Words: : depression, flavonoid, mechanisms of action, nitric oxide, serotonin
Introduction
Depression has become one of the most common neuropsychiatric disorders in the modern world. It is the most disabling medical condition, in terms of years lost to disability. It has also been projected that by 2030, depression will be the foremost contributor to the worldwide burden of disease.1 In recent years, herbal medicines with antidepressant effects and high safety margins have become a novel pharmacotherapy in the treatment of depression.2
Hesperidin (4′-methoxy-7-O-rutinosyl-3′,5-dihydroxyflavanone), a naturally occurring flavanone glycoside, is predominant in citrus fruits3 and exerts a variety of pharmacological effects, including antioxidant3 and neuroprotective activities.4 The potential therapeutic value of hesperidin for depression has been increasingly demonstrated by our initial research; it has exhibited antidepressant-like effect in mice, possibly dependent on the interaction with the serotonergic 5-HT1A and kappa-opioid receptors.5,6 Furthermore, our recent findings suggest that acute and chronic treatment with hesperidin produces an antidepressant-like effect by a mechanism that modulates the inhibition of the l-arginine-nitric oxide (NO)-cyclic guanosine monophosphate (cGMP) pathway. This effect may also be partially mediated through the increase of hippocampal brain-derived neurotrophic factor levels.7
Potassium (K+) channels are a key component of this electrical circuit and are controlled either by an electrical impulse or through signaling molecules.8 The association of K+ channels in the modulation of depression has been suggested by several preclinical studies. The administration of different K+ channel inhibitors such as tetraethylammonium (TEA), gliquidone and glibenclamide, charybdotoxin and apamin decreases the duration of immobility in the forced swimming test (FST) in mice, which is an indicative of an antidepressant-like effect.9,10 On the other hand, K+ channel openers, such as minoxidil or cromakalim, increase the immobility time, which is an indicative of a depressive-like effect.9,10
NO exerts its neural effects through several mechanisms, including modulation of ionic conductance.11 K+ channels have been shown to be targets of NO signaling.12 It has also been reported that both NO and cGMP, produced through the activation of nitric oxide synthase (NOS) and soluble guanylate cyclase (sGC), respectively, may activate different types of K+ channels in several tissues.2,10 Consequently, the therapeutic potential of K+ channels as drug targets in medicine is widely recognized because the dysfunction in K+ channels is associated with neurological disorders such as depression. Thus, the pharmacological modulation of these channels may potentially represent a powerful way of controlling central nervous system disorders.8,10
Based on the consideration above, the aim of this study was to investigate whether different types of K+ channels are involved in the antidepressant-like effect of hesperidin in the tail suspension test (TST) in mice. Thus, this study may be helpful to the understanding of the mechanisms underlying the antidepressant-like effect of hesperidin, which are not fully established.
Materials and Methods
Animals
The behavioral experiments were conducted using male adult Swiss mice (25–35 g) maintained at 22–25°C with free access to water and food, under a 12-h light/12-h dark cycle, with lights on at 6:00 a.m. The animals were used according to the guidelines of the Institutional Ethics Committee (CEUA/UNIPAMPA) under number (001/2013), and all efforts were made to minimize animal suffering and to reduce the number of animals used in the experiments.
Tail suspension test
The TST has become one of the most widely used models for assessing the antidepressant-like activity in mice. The test is based on the fact that animals subjected to the short-term inescapable stress of being suspended by their tail will develop an immobile posture.13 Mice both acoustically and visually isolated were suspended 50 cm above the floor by adhesive tape placed ∼1 cm from the tip of the tail during a 6-min period.10,13
Open-field test
To assess the possible effects of hesperidin on the locomotor and exploratory activities, mice were evaluated in the open-field test (OFT). The floor of the open-field, 45 cm in length and 45 cm in width, was divided by masking tape markers into 9 squares (3 rows of 3). Each animal was placed individually at the center of the apparatus and observed for 6 min to record the locomotor (number of segments crossed with the four paws).10,14
Drugs and treatment
The following drugs were used: l-arginine, glibenclamide, charybdotoxin, apamin, cromakalim, and minoxidil (Tocris Cookson, Ballwin, MO, USA), hesperidin, TEA, and all other chemicals were purchased from Sigma Chemical (St. Louis, MO, USA). Cromakalim and minoxidil were dissolved in saline with 10% Tween 80, whereas all the other drugs were dissolved in a saline solution (NaCl 0.9%) immediately before use, except hesperidin which was dissolved by the sequential addition of dimethyl sulfoxide (DMSO) up to a final concentration of 5%, a water solution of 0.25% Tween 80 up to a final concentration of 20% and saline to complete 100% volume. The choice of the doses of hesperidin was based on published data and on preliminary experiments.5–7,15 Drugs were administered intraperitoneally (i.p.) in a constant volume of 10 mL/kg body weight.
To test the hypothesis that the antidepressant-like effect of hesperidin is mediated through the inhibition of K+ channels, animals were pretreated with a subeffective dose of hesperidin (0.01 mg/kg, i.p.) or vehicle 30 min before the intracerebroventricular (i.c.v.) administration of TEA (a nonspecific blocker of K+ channels, 25 pg/site), glibenclamide (an ATP-sensitive K+ channel blocker, 0.5 pg/site), charybdotoxin (a large- and intermediate-conductance calcium-activated K+ channel blocker, 25 pg/site), apamin (a small-conductance calcium-activated K+ channel blocker, 10 pg/site), or vehicle before being tested in the OFT and TST.
In another set of experiments, mice were pretreated with an effective dose of hesperidin (0.3 mg/kg, i.p.) or vehicle 30 min before the administration of cromakalim (a K+ channel opener, 10 μg/site, i.c.v.) or minoxidil (a K+ channel opener, 10 μg/site). TST or the OFT was carried out 15 min later.
The role played by the l-arginine-NO pathway in the antidepressant-like effect induced by hesperidin/K+ channel blockers was investigated by the pretreatment with l-arginine, a precursor of NO (750 mg/kg, i.p.). Thirty minutes after l-arginine administration, K+ channel blockers were injected and the animals were pretreated with a subeffective dose of hesperidin (0.01 mg/kg, i.p.).5,6
Intracerebroventricular injection technique
The i.c.v. injections were performed by employing a free hand method under light ether anesthesia according to the procedure described previously,10,14 with the bregma fissure as a reference. Vehicle, K+ channel blockers, or K+ channel openers were injected in a volume of 2 μL, given over 30 sec, and the needle remained in place for another 30 sec to avoid the reflux of the substances injected.14
Statistical analysis
The results are expressed as the mean±standard error of the mean. Comparisons between experimental and control groups were performed by a two-way ANOVA (interaction of hesperidin with the pharmacological agents) followed by the Bonferroni's test when appropriate. A value of P<.05 was considered to be significant. The statistical analysis was performed using the software GraphPad Prism version 5 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Effects of combined administration of subeffective doses of the K+ channel blockers and hesperidin in the TST and OFT
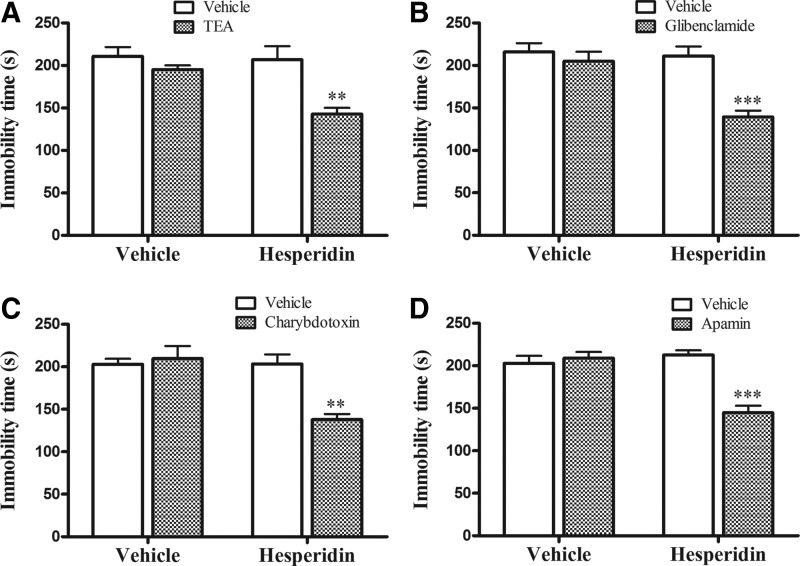
Figure 1A shows the effect of the administration of subeffective doses of hesperidin (0.01 mg/kg, i.p.), and TEA (a nonspecific blocker of K+ channels) was also able to produce a synergistic action in the TST. The two-way ANOVA revealed significant differences for hesperidin [F(1,16)=6.96; P=.0179], TEA [F(1,16)=14.04; P=.0018], and hesperidin×TEA interaction [F(1,16)=5.12; P=.0379]. Regarding locomotor activity, the two-way ANOVA revealed no differences for hesperidin [F(1,16)=0.59; P=.4525], TEA [F(1,16)=0.02; P=.8863], and hesperidin×TEA interaction [F(1.16)=0.23; P=.6380] (data not shown).
FIG. 1.
Effect of the treatment of mice with tetraethylammonium (TEA; 25 pg/site, i.c.v.; A), glibenclamide (0.5 pg/site, i.c.v.; B), charybdotoxin (25 pg/site, i.c.v.; C), or apamin (10 pg/site, i.c.v.; D) in combination with a subeffective dose of hesperidin (0.01 mg/kg, i.p.) in the TST. Each value is expressed as the mean±S.E.M. (n=7 mice in each group). Asterisks represent significant effect (**P<.01; ***P<.001) when compared with the respective vehicle-treated control group. i.c.v., intracerebroventricular; i.p., intraperitoneally; S.E.M., standard error of the mean; TST, tail suspension test.
The results depicted in Figure 1B show that glibenclamide (an ATP-sensitive K+ channel blocker) was also able to produce a synergistic action with a subeffective dose of hesperidin (0.01 mg/kg, i.p.) in the TST. The two-way ANOVA revealed significant differences for hesperidin [F(1,16)=11.99; P=.0032], glibenclamide [F(1,16)=16.59; P=.0009], and hesperidin×glibenclamide interaction [F(1,16)=8.94; P=.0086]. In addition, the administration of glibenclamide alone or in combination with hesperidin did not affect the locomotion of mice (hesperidin [F(1,16)=0.05; P=.8331], glibenclamide [F(1,16)=0.43; P=.5193], and hesperidin×glibenclamide interaction [F(1,16)=0.01; P=.9537; data not shown]).
As presented in Figure 1C, the administration of charybdotoxin (a large-and intermediate-conductance calcium-activated K+ channel blocker) produced a synergistic antidepressant-like effect when combined with a subeffective dose of hesperidin (0.01 mg/kg, i.p.) in the TST. The two-way ANOVA revealed significant differences for hesperidin [F(1,16)=11.80; P=.0034], charybdotoxin [F(1,16)=8.05; P=.0119], and hesperidin×charybdotoxin interaction [F(1,16)=12.07; P=.0031]. Additionally, charybdotoxin administration alone or in combination with hesperidin did not modify the locomotor activity of mice in the OFT, since two-way ANOVA revealed no significant differences for hesperidin [F(1,16)=0.28; P=.6043], charybdotoxin [F(1,16)=0.13; P=.7246], and hesperidin×charybdotoxin interaction [F(1,16)=0.02; P=.8957].
The results presented in Figure 1D show the effect of apamin (a small-conductance calcium-activated K+ channel blocker) in producing a synergistic anti-immobility effect with a subeffective dose of hesperidin (0.01 mg/kg, i.p.) in the TST. The two-way ANOVA revealed significant differences for hesperidin [F(1,16)=12.66; P=.0026], apamin [F(1,16)=16.44; P=.0009], and hesperidin×apamin interaction [F(1,16)=23.39; P=.0002]. Additionally, apamin administration alone or in combination with hesperidin did not modify the locomotor activity in the OFT. The two-way ANOVA revealed no significant differences for hesperidin [F(1,16)=0.42; P=.5257], apamin [F(1,16)=0.15; P=.7040], and hesperidin×apamin interaction [F(1,16)=0.02; P=.8802].
Effects of K+ channel openers on hesperidin-induced antidepressant-like effects in the TST and OFT
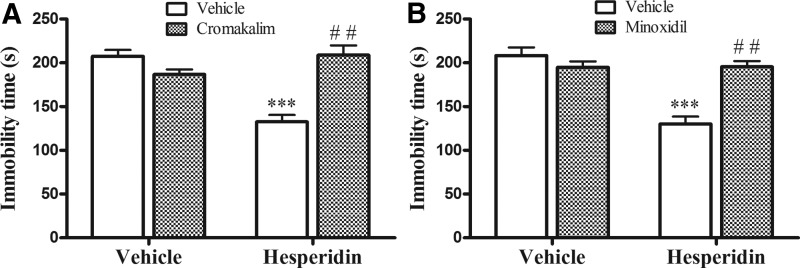
Figure 2A shows that the treatment of mice with cromakalim (a K+ channel opener) was able to reverse the antidepressant-like effect of hesperidin (0.3 mg/kg, i.p.) in the TST. The two-way ANOVA revealed significant differences for hesperidin [F(1,16)=10.56; P=.0050], cromakalim [F(1,16)=11.53; P=.0037], and hesperidin×cromakalim interaction [F(1,16)=35.36; P=.0001]. Regarding locomotor activity, cromakalim administration alone or in combination with hesperidin did not produce any change in the ambulatory behavior of mice, since the two-way ANOVA did not show significant differences for hesperidin [F(1,16)=0.18; P=.6747], cromakalim [F(1,16)=0.31; P=.5848], and hesperidin×cromakalim interaction [F(1,16)=0.28; P=.6035].
FIG. 2.
Effect of the treatment of mice with cromakalim (10 μg/site, i.c.v.; A) or minoxidil (10 μg/site, i.c.v.; B) on the antidepressant-like effect of hesperidin (0.3 mg/kg, i.p.) in the TST. Each value is expressed as the mean±S.E.M. (n=7 mice in each group). Asterisks represent significant effect (***P<.001) when compared with the respective vehicle-treated control group. (##P<.001) compared with hesperidin alone.
Figure 2B indicates that minoxidil (a K+ channel opener) also reversed the antidepressant-like effect of hesperidin (0.3 mg/kg, i.p.) in the TST. The two-way ANOVA revealed significant differences for hesperidin [F(1,16)=24.81; P=.0001], minoxidil [F(1,16)=11.26; P=.0040], and hesperidin×minoxidil interaction [F(1,16)=25.85; P=.0001]. Administration of minoxidil alone or in combination with hesperidin did not change the locomotor activity of mice, since two-way ANOVA did not show significant differences for hesperidin [F(1,16)=0.09; P=.7637], cromakalim [F(1,16)=0.14; P=.7128], and hesperidin×cromakalim interaction [F(1,16)=0.17; P=.6837].
Effects of l-arginine (a precursor of NO) on combined administration of subeffective doses of the K+ channel blockers and hesperidin in the TST and OFT
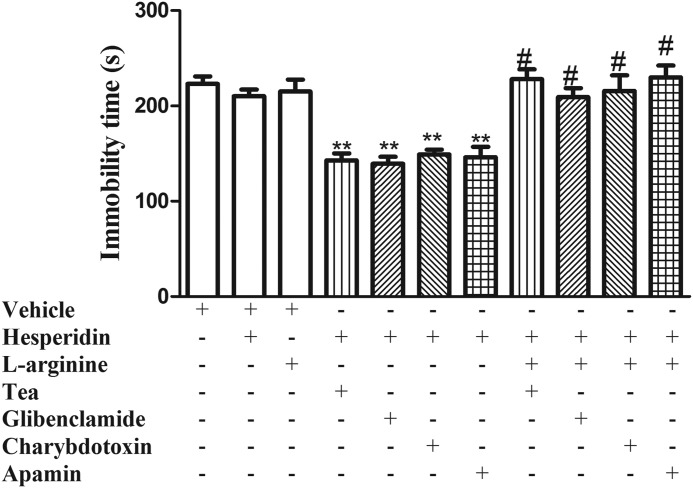
Figure 3 shows that the pretreatment of mice with l-arginine (750 mg/kg, i.p., a NO precursor) reversed the reduction in the immobility time elicited by K+ channel blockers (TEA, glibenclamide, charybdotoxin, or apamin) with a subeffective dose of hesperidin (0.01 mg/kg, i.p.) in the TST. Administration of l-arginine or K+ channel blockers alone or in combination with hesperidin did not produce any change in the ambulatory behavior of mice in the OFT.
FIG. 3.
Effect of the treatment of mice with l-arginine (750 mg/kg, i.p.) on combined administration of subeffective doses of the K+ channel blockers (TEA, glibenclamide, charybdotoxin, or apamin) with a subeffective dose of hesperidin (0.01 mg/kg, i.p.) in the TST. Each value is expressed as the mean±S.E.M. (n=7 mice in each group). Asterisks represent significant effect (**P<.01) when compared with the vehicle-treated control group. (#P<.01) compared with hesperidin/K+ channel blockers.
Discussion
Extending previous findings from our group5–7 has already contributed to the understanding of the mechanisms underlying antidepressant-like effects of hesperidin. The present study, using several pharmacological tools, has demonstrated evidences that the modulation of K+ channels contributes to the antidepressant-like effect of hesperidin in TST. The treatment of mice with subeffective doses of different K+ channel blockers (TEA, glibenclamide, charybdotoxin, and apamin) combined with a subeffective dose of hesperidin was able to produce a synergistic antidepressant-like effect in the mice TST. Additionally, to confirm our hypothesis, whereas the treatment of mice with pharmacological compounds was able to open K+ channels (cromakalim and minoxidil), the antidepressant-like effect elicited by an effective dose of hesperidin in TST was abolished.
To exclude the possibility that the synergistic effect of hesperidin and the K+ channel inhibitors in the TST is a reflection of generalized increased locomotor activity, mice were observed in an OFT for ambulation. In general, compounds that induce an increase of ambulatory behavior cause hyperactivity in the OFT together with reduced immobility in the TST and may produce a false-positive effect.16,17 Indeed, our OFT results indicate that neither the K+ channel inhibitors alone nor administered in combination with hesperidin alters the locomotor activity. Therefore, the synergistic antidepressant-like effect of hesperidin combined with the K+ channel inhibitors observed in this study could not be attributed to general hyperactivity.
The pretreatment of mice compound able to block different types of K+ channels, such as TEA, a nonspecific blocker of K+ channels,18 glibenclamide, an ATP-sensitive K+ channel blocker, charybdotoxin, a blocker of large (or fast)-conductance calcium-gated K+ channels,19 and apamin, a blocker of small (or low)-conductance calcium-gated K+ channels,20 was able to produce an effect with a subeffective dose of hesperidin. Altogether, these results show an important role played by ATP-sensitive and calcium-activated K+ channels in the antidepressant-like effect of hesperidin in the TST, probably by inhibiting membrane hyperpolarization, leading to an increased excitatory response.
In many central neurons, serotonin (5-hydroxytryptamine [5-HT]) acts through 5-HT1A receptors, which are coupled to G proteins, activating an inwardly rectifying K+ current and leading to hyperpolarization.21,22 Furthermore, 5-HT hyperpolarized the medial preoptic area neurons by the activation of the G-protein-coupled inwardly rectifying K+ currents using 5-HT1A receptors.23 The inhibition of A-type K+ currents has also been reported to enhance the spontaneous basal release of [3H]5-HT in rat hippocampal slices.24 Consistent with this notion, there is substantial evidence indicating an association between K+ channels and the mechanism of action of antidepressants, especially those related to the 5-HT system, which also act on voltage-gated ion channels as inhibitors of K+ channels, including fluoxetine, sertraline, venlafaxine, and duloxetine.25,26 Hence, the synergistic action of the K+ channel blockers and hesperidin in the TST could be dependent on the activation of the serotonergic system, particularly by an interaction with the 5-HT1A receptors. This hypothesis is in agreement with the data presented by Souza et al.,5 which demonstrated that the administration of pCPA (an inhibitor of 5-HT synthesis) and WAY100635 (a selective 5-HT1A receptor antagonist) prevented the antidepressant-like effect of hesperidin in the mice TST.
To reinforce our hypothesis, we have also shown that the activation of the K+ channels produced by cromakalim or minoxidil was able to prevent the decrease in the immobility time (antidepressant-like effect) induced by an effective dose of hesperidin in the TST. These results help to support the involvement of K+ channels in the antidepressant-like effect of hesperidin in mice. Additionally, the literature data report that the administration of K+ channel openers, such as minoxidil or cromakalim, increased the immobility time.9 Additionally, the treatment of animals with cromakalim was able to antagonize the anti-immobility effect of several antidepressants, such as imipramine, amitriptyline, desipramine, and paroxetine.27
It has been suggested that the l-arginine-NO-cGMP pathway is implicated in the neurobiology of depression.2 The decrease of brain NO levels or the blocking of the synthesis of NO (blocking NOS) in the brain may induce antidepressant-like effects, thus implicating the role of endogenous hippocampal NO in the pathophysiology of major depression.28 Furthermore, some studies have reported that NO and cGMP are important modulators of some K+ channels at the central level, and the inhibition of these channels may represent an important role in the mechanisms involved in a major depressive disorder.10,29 We have recently demonstrated that hesperidin produces an antidepressant-like effect in the TST by a mechanism that modulates the inhibition of the l-arginine-NO-cGMP pathway.7 In our study, antidepressant-like effects of the combined administration of subeffective doses of the K+ channel blockers and hesperidin in the TST were reversed by pretreatment with the precursor of NO, l-arginine. These results reinforce the idea that the inhibition of l-arginine-NO-cGMP pathway prevents the activation of K+ channels and these mechanisms are involved in the antidepressant-like effect of hesperidin. As demonstrated in the present work, we extend previous findings by suggesting that the antidepressant-like effect of hesperidin in the TST may be related to a regulation of neuronal excitability modulation of K+ channels. Thus, an indirect blockade of the K+ channels by hesperidin through the l-arginine-NO-cGMP pathway could account for the behavioral results observed in this study.
Our study extends previous findings from our group regarding the mechanism linked to the antidepressant-like effect of hesperidin in the TST. The treatment of mice with different K+ channel blockers produced an antidepressant-like effect combined with a subeffective dose of hesperidin, whereas the treatment with K+ channel openers was able to reverse the antidepressant-like effect produced by an effective dose of hesperidin. Although it is not possible to rule out other underlying mechanisms of effect, together the results herein suggest that the modulatory effects of hesperidin on modulation of neuronal excitability, through inhibition of K+ channels, may act using a mechanism dependent on the inhibition of l-arginine-NO pathway, which is a mechanism underlying its antidepressant-like effect in the TST.
Acknowledgments
Financial support by the CNPQ Research Grant #474397/2013-0. C.R.J. is a recipient of the CNPQ fellowship. L.D.F., A.T.R.G., M.G.G., L.C.S., F.D., E.E.T.A., and M.S.A. are recipients of FAPERGS, CNPQ, CAPES, or UNIPAMPA fellowships.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.WHO: Depression. World Health Organization; http://691.who.int/mental_health/management/depression/definition/2011 (cited 11 April 2014) [Google Scholar]
- 2.Freitas AE, Moretti M, Budni J, et al. : NMDA receptors and the L-arginine nitric oxide-cyclic guanosine monophosphate pathway are implicated in the antidepressant-like action of the ethanolic extract from Tabebuia avellanedade in mice. J Med Food 2013;16:1060–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang HL, Chen SC, Kumar KJS, et al. : Antioxidant and anti-inflammatory potential of hesperetin metabolites obtained from Hesperitin-administered rat serum: An ex vivo approach. J Agric Food Chem 2012;60:522–532 [DOI] [PubMed] [Google Scholar]
- 4.Hwang SL, Yen GC: Neuroprotective effects of the citrus flavanones against H2O2-induced cytotoxicity in PC12 cells. J Agric Food Chem 2008;56:859–864 [DOI] [PubMed] [Google Scholar]
- 5.Souza LC, de Gomes MG, Goes ATR, et al. : Evidence for the involvement of the serotonergic 5-HT1A receptors in the antidepressant-like effect caused by hesperidin in mice. Prog Neuropsychopharmacol Biol Psychiatry 2013;40:103–109 [DOI] [PubMed] [Google Scholar]
- 6.Filho CB, Del Fabbro L, de Gomes MG, et al. : Kappa-opioid receptors mediate the antidepressant-like activity of hesperidin in the mouse forced swimming test. Eur J Pharmacol 2013;698:286–291 [DOI] [PubMed] [Google Scholar]
- 7.Donato F, Gomes MG, Goes ATR, et al. : Hesperidin exerts antidepressant-like effects in acute and chronic treatments in mice: Possible role of L-arginine-NO-cGMP pathway and BDNF levels. Brain Res Bull 2014;104:19–26 [DOI] [PubMed] [Google Scholar]
- 8.Kowal J, Chami M, Baumgartner P, et al. : Ligand-induced structural changes in the cyclic nucleotide-modulated potassium channel MloK1. Nat Commun 2014;5:3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galeotti N, Ghelardini C, Caldari B, et al. : Effect of potassium channel modulators in mouse forced swimming test. Br J Pharmacol 1999;126:1653–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaster MP, Budni J, Binfaré RW, et al. : The inhibition of different types of potassium channels underlies the antidepressant-like effect of adenosine in the mouse forced swimming test. Prog Neuropsychopharmacol Biol Psychiatry 2007;13:690–696 [DOI] [PubMed] [Google Scholar]
- 11.Garthwaite J: Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci 2008;27:2783–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinert JR, Kopp-Scheinpflug C, Baker C, et al. : Nitric oxide is a volume transmitter regulating postsynaptic excitability at a glutamatergic synapse. Neuron 2008;60:642–656 [DOI] [PubMed] [Google Scholar]
- 13.Steru L, Chermat R, Thierry B, et al. : The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology (Berlin) 1985;85:367–370 [DOI] [PubMed] [Google Scholar]
- 14.Jesse CR, Wilhelm EA, Barbosa NB, et al. : Involvement of different types of potassium channels in the antidepressant-like effect of tramadol in the mouse forced swimming test. Eur J Pharmacol 2009;613:74–78 [DOI] [PubMed] [Google Scholar]
- 15.Fernández SP, Wasowski C, Paladini AC, et al. : Synergistic interaction between hesperidin, a natural flavonoid, and diazepam. Eur J Pharmacol 2005;512:189–198 [DOI] [PubMed] [Google Scholar]
- 16.Mantovani M, Pertile R, Calixto JB, et al. : Melatonin exerts an antidepressant-like effect in the tail suspension test in mice: Evidence for involvement of N-methyl-D-aspartate receptors and the L-arginine–nitric oxide pathway. Neurosci Lett 2003;343:1–4 [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues ALS, Rosa JM, Gadotti VM, et al. : Antidepressant-like and antinociceptive-like actions of 4-(4′-chlorophenyl)-6-(4″-methylphenyl)-2-hydrazinepyrimidine Mannich base in mice. Pharmacol Biochem Behav 2005;82:156–162 [DOI] [PubMed] [Google Scholar]
- 18.Halliwell JV: K+ channels in the central nervous system. In: Potassium Channels: Structure, Classification, Function and Therapeutic Potential (Cook NS, ed.). Ellis Horwood, Chichester, United Kingdom, 1990, pp. 348–381 [Google Scholar]
- 19.Gehlert DR, Gackenheimer SL: Comparison of the distribution of binding sites for the potassium channel ligands [125I]apamin, [125I]charybdotoxin and [125I] iodoglyburide in the rat brain. Neuroscience 1993;52:191–205 [DOI] [PubMed] [Google Scholar]
- 20.Hugues M, Romey G, Duval D, et al. : Apamin as a selective blocker of the calcium-dependent potassium channel in neuroblastoma cells: Voltage-clamp and biochemical characterization of the toxin receptor. Proc Natl Acad Sci USA 1982;79:1308–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takigawa T, Alzheimer C: G protein-activated inwardly rectifying K+ (GIRK) currents in dendrites of rat neocortical pyramidal cells. J Physiol 1999;517:385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong HJ, Han SH, Min BI, et al. : 5-HT1A receptor-mediated activation of G-protein-gated inwardly rectifying K+ current in rat periaqueductal gray neurons. Neuropharmacology 2001;41:175–185 [DOI] [PubMed] [Google Scholar]
- 23.Lee JJ, Hahma ET, Lee CH, et al. : 5-HT1A receptor-mediated activation of a G-protein-coupled inwardly rectifying K+ current in rat medial preoptic area neurons. Eur J Pharmacol 2008;586:114–122 [DOI] [PubMed] [Google Scholar]
- 24.Schechter LE: The potassium channel blockers 4-aminopyridine and tetraethylammonium increase the spontaneous basal release of [3H]5-hydroxytryptamine in rat hippocampal slices. J Pharmacol Exp Ther 1997;282:262–270 [PubMed] [Google Scholar]
- 25.Bortolatto CF, Jesse CR, Wilhelm EA, et al. : Involvement of potassium channels in the antidepressant-like effect of venlafaxine in mice. Life Sci 2010;86:372–376 [DOI] [PubMed] [Google Scholar]
- 26.Choi JS, Choi BH, Ahn HS, et al. : Fluoxetine inhibits A-type potassium currents in primary cultured rat hippocampal neurons. Brain Res 2004;1018:201–207 [DOI] [PubMed] [Google Scholar]
- 27.Cryan JF, Markou A, Lucki I: Assessing antidepressant activity in rodents: Recent developments and future needs. Trends Pharmacol Sci 2002;23:238–245 [DOI] [PubMed] [Google Scholar]
- 28.Joca SR, Guimaraes FS: Inhibition of neuronal nitric oxide synthase in the rat hippocampus induces antidepressant-like effects. Psychopharmacology (Berlin) 2006;185:298–305 [DOI] [PubMed] [Google Scholar]
- 29.Fujino K, Nakaya S, Wakatsuki T, et al. : Effects of nitroglycerin on ATP-induced Ca++-mobilization, Ca++-activated K channels and contraction of cultured smooth muscle cells of porcine coronary artery. J Pharmacol Exp Ther 1991;256:371–377 [PubMed] [Google Scholar]