Abstract
Preterm infants have an immature antioxidant system; however, they frequently require supplemental oxygen. Oxygen-free radicals cause both pulmonary and systemic inflammation, and they are associated with increased morbidity and mortality. Consequently, screening of metabolite profiles representing the amount of lipid peroxidation is considered of great relevance for the evaluation of in vivo oxidative stress and derived inflammation and damage. Ranges for total relative contents of isoprostanes (IsoPs), isofurans (IsoFs), neuroprostanes (NeuroPs), and neurofurans (NeuroFs) within targeted SpO2 ranges were determined in urine samples of 254 preterm infants <32 weeks of gestation within the frame of two randomized, controlled, and blinded clinical trials employing ultra-performance liquid chromatography–tandem mass spectrometry. A total of 536 serial urine samples collected during the first 4 weeks after birth in recruited infants who did not develop free radical associated conditions were analyzed. A reference range for lipid peroxidation byproducts, including isoprostanes, isofurans, neuroprostanes, and neurofurans, was calculated and possible correlations with neonatal conditions were investigated. Urinary elimination of isofurans in the first 4 days after birth correlated with later development of bronchopulmonary dysplasia. Our observations lead to the hypothesis that early urinary determination of lipid peroxidation byproducts, especially isofurans, is relevant to predict development of chronic lung conditions. Antioxid. Redox Signal. 23, 178–184.
Introduction
Fetal-to-neonatal transition abruptly raises tissue oxygenation, thereby generating a burst of reactive oxygen species and resulting in physiologic oxidative stress. Preterm infants with immature lungs are predisposed to respiratory insufficiency and the need for oxygen therapy immediately after birth (8). As a consequence, oxygen-free radicals are generated. Oxygen-free radicals may react with nonradical molecules in chain reactions, causing damage to DNA, proteins, and lipids or lead to the formation of DNA and protein adducts. Remarkably, free radical associated conditions such as bronchopulmonary dysplasia (BPD) causing severe morbidity and mortality have been described in the perinatal period (7).
Innovation.
A reference profile for urinary (noninvasive) lipid peroxidation biomarkers using a straightforward high-throughput ultra-performance liquid chromatography–tandem mass spectrometry method in the newborn period has been developed. Preterm infants with high isofurans in the first days after birth are more prone to develop chronic lung conditions such as bronchopulmonary dysplasia.
Isoprostanes (IsoPs) and isofurans (IsoFs) are chemically stable compounds formed in vivo via the nonenzymatic peroxidation of arachidonic acid (AA), associated with oxidant injury, and have been detected in fluids and tissues. IsoPs have been associated with normoxic, while IsoFs are generated under hyperoxic conditions. Docosahexaenoic acid (DHA) is a relevant structural component of the central nervous system. During lipid peroxidation, neuroprostanes (NeuroPs) and neurofurans (NeuroFs) arise from DHA oxidation in a similar manner to IsoPs and IsoFs from AA. NeuroPs and NeuroFs have been suggested as sensitive and specific markers of neuronal oxidative damage (1).
Analysis of lipid peroxidation products is highly complex due to the large number of metabolites, including isomers with highly similar molecular structures, physicochemical properties, and chromatographic behavior. Furthermore, for IsoFs, NeuroPs, and NeuroFs, no analytical standards are commercially available, hindering the implementation of their use as biomarkers (4).
We propose a reliable, noninvasive ultra-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) method for recording profiles of relative total contents of IsoPs, IsoFs, NeuroPs, and NeuroFs in urine of preterm infants, enabling a semi-quantitative comparison between samples. We aimed at establishing a reference range of urinary lipid peroxidation byproducts in the first 4 weeks after birth (average period of clinical stabilization) in very preterm infants who did not develop free radical-associated conditions, and therefore this could be valuable information for a neonatologist. We investigated whether there were positive correlations between lipid peroxidation byproducts and free radical-associated conditions.
Results
The population consisted of preterm infants ≤32 weeks of gestation randomized to lower (30%; LowOx) or higher (60%–65%; HiOx) initial inspiratory fraction of oxygen (iFiO) in the delivery room (DR) (see Notes section) (5). Perinatal characteristics are described in Table 1. No significant differences in oxidative stress biomarkers, mortality, or major perinatal morbidities were found for (Table 2).
Table 1.
Basic Clinical and Obstetric Characteristics of the Population of Very Preterm Infants (<32 Weeks of Gestation) Recruited in Two Randomized, Controlled, and Blinded Studies
| Parameter | LowOx (n=133) | HiOx (120) | p |
|---|---|---|---|
| Female sex, n (%) | 69 (51.8) | 58 (48.3) | NS |
| GA, week, median (IQR) | 28 (24, 32) | 27 (23, 31) | NS |
| BW, g, median (IQR) | 944 (720, 1280) | 1040 (755, 1368) | NS |
| Umbilical cord pH, median (IQR) | 7.29 (7.25, 7.33) | 7.28 (7.23, 7.31) | NS |
| Full antenatal steroids, n (%) | 133 (100) | 120 (100) | NS |
| Vaginal/Cesarean delivery, n | 43/90 | 44/76 | NS |
| Chorioamnionitis, n (%) | 25 (18.8) | 22 (18.3) | NS |
Patients were randomly assigned to an initial FiO2=30% (LowOx) or 60%–65% (HiOx) Data retrieved from Saugstad et al. (5).
BW, birth weight (g); GA, gestational age (weeks); IQR, interquartile range; NS, nonsignificant (α=0.05).
Table 2.
Clinical Outcomes of Very Preterm Infants(<32 Weeks of Gestation) Recruited in Two Randomized, Controlled, and Blinded Studies
| Clinical outcome, n (%) | LoxOx (n=133) | HiOx (n=120) | p |
|---|---|---|---|
| Mortality | 10 (7.5) | 17 (14.1) | NS |
| BPD | 33 (24.8) | 20 (16.6) | NS |
| PDA | 58 (43.6) | 43 (35.8) | NS |
| ROP (≥grade 2) | 10 (7.5) | 6 (5.0) | NS |
| NEC (≥grade 2) | 6 (4.5) | 4 (3.3) | NS |
| IPVH (grades III/IV) | 19 (14.3) | 18 (15.0) | NS |
Infants randomly assigned to be resuscitated with an initial FiO2 of 30% (LowOx) or 60%–65% (HiOx) Data retrieved from Saugstad et al. (5).
BPD, bronchopulmonary dysplasia; PDA, patent ductus arteriosus; ROP, retinopathy of prematurity; NEC, necrotizing enterocolitis; IPVH, intra-periventricular hemorrhage.
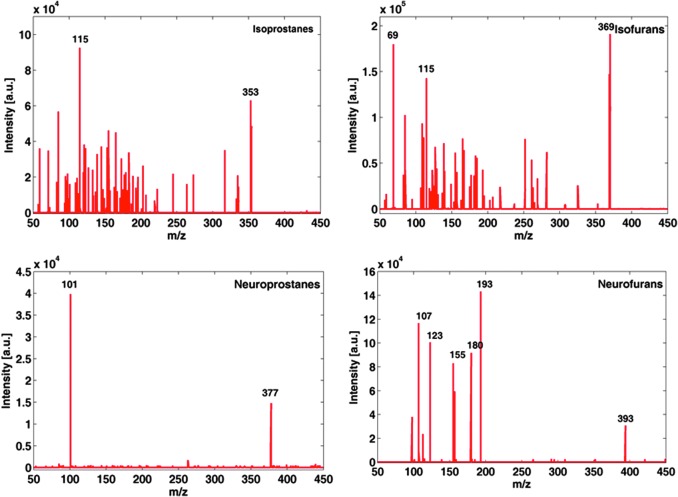
MS/MS parameters for the detection of total IsoPs, IsoFs NeuroPs, and NeuroPs were selected from the spectra depicted in Figure 1 acquired from the analysis of the in vitro oxidation products of AA and DHA. MS/MS spectra obtained from the fragmentation of 377 (NeuroPs) and 393 m/z (NeuroFs) showed fragments of 101 and 193 m/z, respectively. For the parent ions of IsoPs and IsoFs, being 353 and 369 m/z, respectively, the most intense fragment observed had m/z=115.
FIG. 1.
Mass spectrometry spectra of oxidized AA and DHA standard solutions. AA, arachidonic acid; DHA, docosahexaenoic acid. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
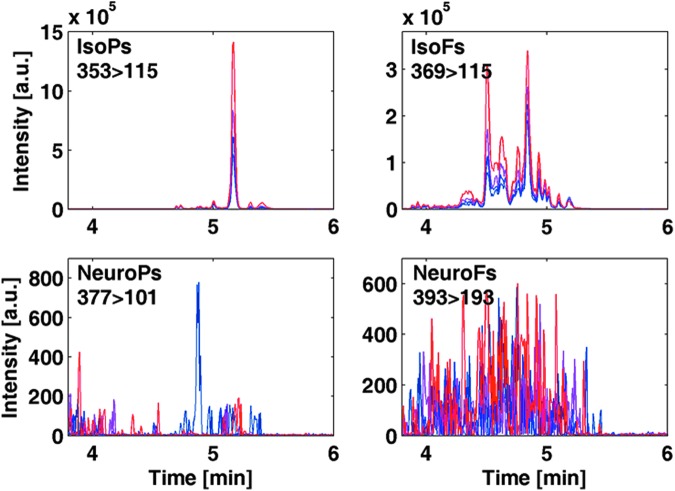
Figures 2 and 3 depict the observed changes in the chromatographic profiles during the simultaneous determination of relative total IsoPs, IsoFs, NeuroPs, and NeuroFs obtained from in vitro oxidation during the AA and DHA assays after different reaction times, respectively. For comparing the obtained chromatograms, area values were obtained using fixed retention time windows for integration selected empirically as 4.0–5.2, 4.0–5.3, 4.7–5.4, and 4.2–5.2 min for IsoPs, IsoFs, NeuroPs, and NeuroFs, respectively. The obtained area values were normalized by employing the signal of the internal standard (IS).
FIG. 2.
Evolution of total IsoPs (353>115), IsoFs (369>115), NeuroPs (377>101), and NeuroFs (393>193) during in vitro oxidation of AA (applying SPE). Peak area values of IsoPs and IsoFs were linearly regressed against reaction time by obtaining slopes significantly different from zero (α=0.05) and with R2 values≥0.95; UPLC-MS/MS responses for NeuroPs and NeuroFs remained below 0.5% of those measured for IsoPs and IsoFs. SPE, solid-phase extraction. IsoFs, isofurans; IsoPs, isoprostanes; NeuroFs, neurofurans; NeuroPs, neuroprostanes; UPLC-MS/MS, ultra-performance liquid chromatography–tandem mass spectrometry. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 3.
Evolution of total IsoPs (353>115), IsoFs (369>115), NeuroPs (377>101), and NeuroFs (393>193) during in vitro oxidation of DHA (applying SPE). Peak area values of NeuroPs and NeuroFs were linearly regressed against reaction time by obtaining slopes significantly different from zero (α=0.05) and with R2 values≥0.96; UPLC-MS/MS responses for IsoPs and IsoFs remained below 15% of those measured for NeuroPs and NeuroFs. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Solutions obtained via in vitro oxidation were subjected to a solid-phase extraction (SPE) procedure. Results comparing the analytical responses obtained with and without SPE showed recoveries of 91%±35%, 111%±39%, 86%±20%, and 93%±8%, for IsoPs, IsoFs, NeuroPs, and NeuroFs, respectively.
Total parameters were found above the limit of quantification (LOQ) (i.e., 10 times the area obtained from a blank injection) in almost all analyzed urine samples with 1.7%, 0.2%, 1.6%, and 3% of the samples giving concentrations below the LOQ for IsoPs, IsoFs, NeuroPs, and NeuroFs. Due to the lack of analytical standards, absolute concentrations could not be determined.
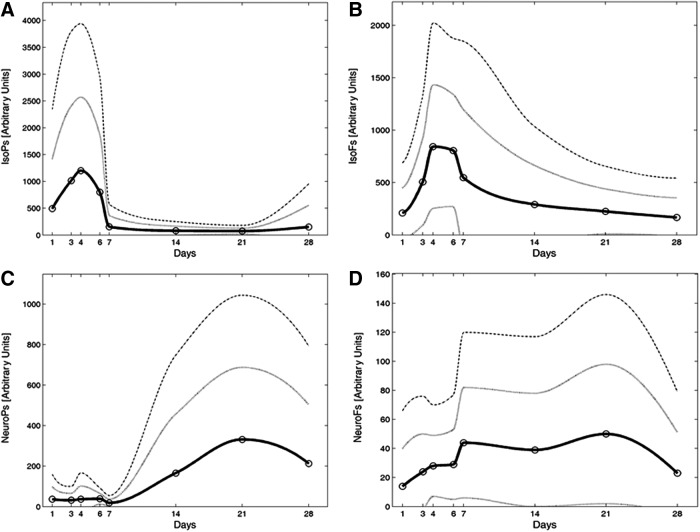
A total of 184 preterm infants pertaining to both groups (LowOx, n=96; HiOx, n=88) survived without free radical-related conditions at hospital discharge and were considered controls (5). Table 3 shows the mean and standard deviation of the urinary concentration of lipid peroxidation metabolites of controls according to the postnatal day of collection. In addition, values for IsoPs, IsoFs, NeuroPs, and NeuroFs at different postnatal days in controls and babies who later developed BPD were compared. Significant differences in IsoFs urinary elimination were found between controls and BPD babies in the first 4 days after birth. Figure 4 depicts the measured time profiles of the mean concentration and standard deviation values after birth for IsoPs, IsoFs, NeuroPs, and NeuroFs.
Table 3.
Analytical Results for Isoprostanes, Isofurans, Neuroprostanes, and Neurofurans Determined in Urine of Preterm Infants ≤32 Weeks of Gestation by Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry
| Postnatal day 1 | Postnatal day 3 | Postnatal day 4 | Postnatal day 6 | Postnatal day 7 | Postnatal day 14 | Postnatal day 21 | Postnatal day 28 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (95) | BPD (22) | Control (126) | BPD (24) | Control (93) | BPD (25) | Control (86) | BPD (18) | Control (19) | BPD (18) | Control (33) | BPD (23) | Control (26) | BPD (15) | Control (48) | BPD (28) | |
| IsoPs | 494 (926) | 534 (354) | 1016 (1391) | 955 (567) | 1201 (1370) | 1339 (1012) | 802 (1074) | 998 (562) | 154 (208) | 194 (155) | 81 (85) | 102 (66) | 75 (53) | 96 (77) | 152 (402) | 177 (234) |
| IsoFs | 211 (239) | 445 (188)a | 507 (413) | 788 (374)a | 844 (590) | 1233 (667)b | 807 (535) | 944 (667) | 547 (651) | 612 (548) | 292 (372) | 334 (185) | 225 (215) | 198 (155) | 167 (188) | 198 (219) |
| NeuroPs | 37 (61) | 46 (51) | 32 (34) | 58 (42) | 37 (65) | 58 (44) | 39 (27) | 52 (33) | 19 (18) | 25 (14) | 166 (291) | 192 (144) | 332 (356) | 379 (224) | 213 (290) | 254 (310) |
| NeuroFs | 14 (26) | 20 (18) | 24 (26) | 39 (18) | 28 (21) | 38 (31) | 29 (24) | 25 (21) | 44 (38) | 54 (44) | 39 (39) | 51 (44) | 50 (48) | 43 (38) | 23 (28) | 34 (19) |
Results of preterm infants who did not develop free radical-associated conditions (controls) are compared with patients who developed BPD defined as need of oxygen supplementation at 36 weeks postconceptional age. Results are expressed as intensity of signal units/ml of urine and expressed as mean (standard deviation).
Significance: p<0.01
Significance: p<0.05.
IsoFs, isofurans; IsoPs, isoprostanes; NeuroFs, neurofurans; NeuroPs, neuroprostanes.
FIG. 4.
Nomogram representing mean values of lipid peroxidation byproducts in urine of preterm infants. Straight line represents the mean values and dotted lines above and below standard deviation of the mean. Representation: (A) Isoprostanes; (B) Isofurans; (C) Neuroprostanes; and (D) Neurofurans. Urine samples were obtained from very preterm infants (<32 weeks of gestation) that did not develop free radical-associated conditions recruited in two randomized, controlled, and blinded clinical trials (REOX trial, n=112 and ROTTERDAM trial, n=358; Ref. 5) given in AU corresponding to normalized peak area values using the IS. AU, arbitrary units; IS, internal standard.
Discussion
Results for NeuroFs and NeuroPs (Fig. 1) confirmed the use of the proposed multiple reaction monitoring (MRM) transitions of 393>193 and 377>101, respectively. For total IsoPs and IsoFs, no MRM transitions have been reported. From fragmentation patterns of IsoPs (m/z 353) and IsoFs (m/z 369) shown in Figure 1, the fragment 115 m/z was selected for the acquisition of characteristic IsoPs and IsoFs profiles. The suitability of the UPLC-MS/MS approach for the simultaneous determination of relative amounts of total IsoPs, IsoFs, NeuroPs, and NeuroFs was evaluated by monitoring the reaction products of both AA and DHA standards subjected to in vitro oxidation (Figs. 2 and 3). Retrieved UPLC-MS/MS areas of the AA standard showed a linear increase of measured responses for IsoPs and IsoFs with the time of reaction, whereas for DHA standards a linear increase of NeuroPs and NeuroFs signals was observed.
This method aims at the assessment of the status of lipid peroxidation in preterm infants. The possibility of performing sequential peroxidation byproduct analysis noninvasively could aid the neonatologist in monitoring the metabolic status of the patients and the consequences of interventions. Interestingly, when stratifying the results of the measured biomarkers by collection time points, it was observed that the relative concentrations change along the first days and weeks after birth (Fig. 4).
This study has relevant clinical implications. Recently, it has been acknowledged that preterm infants need some time to acquire a stable oxygenation after birth. Moreover, many very preterm infants require oxygen supplementation to keep oxygen saturation within established safety ranges (7). In studies by Vento et al. (9) and Kapadia et al. (3), preterm infants were randomized to an initial inspiratory fraction of oxygen of 30% versus 90%, and 21% versus 100%, respectively. In both studies (3, 9), there was a correlation between the use of higher oxygen concentrations in the DR, increased oxidative stress biomarkers, and later development of BPD. Results in this study confirm these findings. Hence, preterm babies who later on developed BPD showed significantly higher urinary elimination of IsoFs in the analytical determinations performed in the first days after birth as did the study by Vento et al. (9).
It has been previously shown that plasma IsoPs, antioxidant enzyme activity, and the ability to resist oxidative stress increase immediately after birth but decrease as infants grow older, revealing the presence of fetal-to-neonatal transition-associated oxidative stress (2). In coincidence with this, we found increased IsoPs and IsoFs during the first week after birth, whereas the increment in NeuroPs and NeuroFs elimination took place later (Fig. 4).
The access to a large sample set of this especially vulnerable population allowed to establish the time window in which lipid peroxidation can be observed. This is crucial for a correct design of future clinical trials, where lipid peroxidation is studied. In addition, we confirmed that hyperoxic resuscitation in very preterm infants leads to the formation of IsoFs, which appear to be early markers of hyperoxic lung damage.
Notes
Eligible patients were preterm of ≤32 weeks of gestation needing active intervention in the DR. The Reox Trial (EUDRACT 2088-005047-42) performed in two Spanish centers (University and Polytechnic Hospital La Fe, Valencia and Hospital Sant Joan de Deu, Barcelona) randomized 60 babies to an initial FiO2 of 30% (n=34) versus 60% (n=24). The Rotterdam Trial (NTR243 2005–2007) randomized 193 babies to an initial FiO2 of 30% (n=99) versus 65% (n=94) (5). This study was approved by the IRBs of both hospitals. Parental consent was obtained for all recruited patients.
Urinary samples were collected within 24 h of birth, on postnatal days 3, 4, 6, 7, 14, 21, and 28 by adding gauzes into the diapers or in Hollister collection bags. Samples were stored at −80°C. After thawing on ice, samples were centrifuged at 7500 rpm and at 4°C for 10 min. Two hundred ninety-seven microliters of H2O (pH 3, adjusted with formic acid):CH3OH (85:15, v/v) were added to 600 μl of supernatant and spiked with 3 μl of IS solution PGF2α-D4 (20 μM). Discovery® DSC-18 SPE 96-well plates (Sigma-Aldrich, St. Louis, MO) were conditioned with 1 ml CH3OH and 1 ml H2O before loading diluted samples. Each well was washed with 500 μl H2O and 500 μl heptane, and samples were eluted using four times 100 μl ethyl acetate. Recovered extracts were evaporated to dryness under a stream of N2 and dissolved in 60 μl of H2O (pH 3):CH3OH (85:15 v/v).
Arachidonic acid (>95%), cis-4, 7, 10, 13, 16, 19-docosahexanoic acid (>98%), 2,2′-azobis (2-methylpropionamidine) di-hydrochloride (AAPH, 97%), formic acid (analytical grade), and potassium phosphate mono- and dibasic (analytical grade) were from Sigma-Aldrich Química SA (Madrid, Spain). PGF2α-D4 was purchased from Cayman Chemical Company (Ann Arbor, MI) and used as IS. Ethanol (analytical grade), methanol (LC-MS grade), and n-heptane (analytical grade) were obtained from J.T. Baker (Avantor Performance Materials B.V., Deventer, The Netherlands), and ethyl acetate (analytical grade) was from Panreac (Barcelona, Spain).
IsoPs and IsoFs were generated from the oxidation of AA, and NeuroPs and NeuroFs were generated from the oxidation of DHA as described by Song et al. (6). Briefly, DHA and AA were dissolved separately in a mixture of ethanol:phosphate buffer (pH 7.4, 100 mM) to reach a final concentration of AA and DHA of 20 mM. Then, 50 μl of a 1 M solution of AAPH was added to each oxidation reaction and the mixtures were incubated at 37°C, withdrawing 500 μl aliquots of the reaction mixture after 0, 2, 4, 6, 8, 10, 24, and 30 h. After 30 h, the oxidation process was stopped by immersion of the reaction vials in ice water. For the monitoring of the oxidation reaction, collected aliquots were diluted 1:10 in H2O (pH 3):CH3OH (85:15, v/v) and subsequently analyzed by UPLC-MS/MS with and without a previous SPE clean up following the procedure described for urine samples.
UPLC-MS/MS analysis was carried out by employing an Acquity–Xevo TQ system (Waters, Milford, MA) in the negative electrospray ionization (ESI−) mode using the following conditions: capillary 3.5 kV, source temperature 120°C, desolvation temperature 300°C, dwell time 5 ms; nitrogen cone and desolvation gas flows were 25 and 680 L/h, respectively. Separation were carried out using a Kinetex UPLC C18 reversed-phase column (2.1×100 mm, 1.7 μm) and precolumn (2.1×2 mm) from Phenomenex (Torrance, CA), and a CH3OH (0.1% v/v HCOOH):H2O (0.1% v/v HCOOH) binary gradient. Flow rate, column temperature, and injection volume were set at 400 μl/min, 37°C, and 5 μl, respectively. The following gradient was employed: From 0 to 1 min, 30% v/v CH3OH (0.05% v/v HCOOH) (i.e., channel B) were used and from 1 to 4.0 min, %B increased till 90%. Return to initial conditions was achieved at 4.1 min, and conditions were maintained for 3.9 min.
Acquisition parameters of mass spectrometric detection carried out by MRM are summarized in Table 4. Chromatographic area values were normalized using PGF2α-D4 as IS.
Table 4.
Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry Acquisition Parameters for the Determination of Isoprostanes, Isofurans, Neuroprostanes, and Neurofurans in Urine of Preterm Infants in the First 4 Weeks After Birth
| Analyte | m/z Parent ion | m/z Daughter ion | Cone (V) | Collision (eV) | Time window (min) |
|---|---|---|---|---|---|
| IsoPs | 353.2 | 115 | 40 | 20 | 3.9–6.0 |
| IsoFs | 369.2 | 115 | 45 | 20 | 3.9–6.0 |
| NeuroPs | 377 | 101 | 35 | 20 | 3.8–6.0 |
| NeuroFs | 393 | 193 | 35 | 20 | 3.8–6.0 |
| PGF2α-D4 | 357.5 | 197.3 | 45 | 30 | 4.6–5.3 |
Abbreviations Used
- AA
arachidonic acid
- AU
arbitrary units
- BPD
bronchopulmonary dysplasia
- BW
birth weight
- DHA
docosahexaenoic acid
- DR
delivery room
- GA
gestational age
- IQR
interquartile range
- IPVH
intra-periventricular hemorrhage
- IS
internal standard
- IsoFs
isofurans
- IsoPs
isoprostanes
- LOQ
limit of quantification
- MRM
multiple reaction monitoring
- NEC
necrotizing enterocolitis
- NeuroFs
neurofurans
- NeuroPs
neuroprostanes
- PDA
patent ductus arteriosus
- ROP
retinopathy of prematurity
- SPE
solid phase extraction
- UPLC-MS/MS
ultra-performance liquid chromatography–tandem mass spectrometry
Acknowledgments
The authors would like to express their gratitude to all the parents of the babies who voluntarily allowed their sons/daughters to participate in this study as well as to the neonatologist and nurses of both NICUs in Spain and Holland. They also kindly acknowledge the support from SCSIE (Servicio Central de Soporte a la Investigación Experimental) at the University of Valencia (Spain). I.T-C, J.E., and J.K. acknowledge their personal grants (FI12/00109, Sara Borrell CD11/00154, Sara Borrell CD12/00667), respectively. M.V. acknowledges the FISPI11/0313 grant from the Instituto Carlos III (Ministry of Economy and Competitiveness) and EC11-246 grant from the Spanish Ministry of Health, Social Services, and Equality. I.L. is grateful for financial support from the Laerdal Foundation (Stavanger; Norway) and the Spanish Maternal and Infant Network (RD12/0022/0012).
References
- 1.Belik J, González-Luis GE, Perez-Vizcaino F, and Villamor E. Isoprostanes in fetal and neonatal health and disease. Free Radic Biol Med 48: 177–188, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Friel JK, Friesen RW, Harding SV, and Roberts LJ. Evidence of oxidative stress in full-term healthy infants. Pediatr Res 56: 878–882, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Kapadia VS, Chalak LF, Sparks JE, Allen JR, Savani RC, and Wyckoff MH. Resuscitation of preterm neonates with limited versus high oxygen strategy. Pediatrics 132: e1488–e1496, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts LJ. and Milne GL. Isoprostanes. J Lipid Res 50: S219–S223, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saugstad OD, Aune D, Aguar M, Kapadia V, Finer N, and Vento M. Systematic review and meta-analysis of optimal initial fraction of oxygen levels in the delivery room at ≤32 weeks. Acta Paediatr 103: 744–751, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Song WL, Lawson JA, Reilly D, Rokach J, Chang CT, Giasson B, and Fitzgerald GA. Neurofurans, novel indices of oxidant stress derived from docosahexanoic acid. J Biol Chem 283: 6–16, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Vento M. Oxygen supplementation in the neonatal period: changing the paradigm. Neonatology 105: 323–331, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Vento M, Aguar M, Escobar J, et al. Antenatal steroids and antioxidant enzyme activity in preterm infants: influence of gender and timing. Antioxid Redox Signal 11: 2945–2955, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Vento M, Moro M, Escrig R, et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics 124: e439–e449, 2009 [DOI] [PubMed] [Google Scholar]