Abstract
A minority of patients with nonsyndromic autosomal recessive congenital ichthyosis (ARCI) display mutations in NIPAL4 (ICH-THYIN). This protein plays a role in epidermal lipid metabolism, although the mechanism is unknown. The study describes a moderate form of ARCI in an extended pedigree of American Bulldogs that is linked to the gene encoding ichthyin. The gross phenotype was manifest as a disheveled pelage shortly after birth, generalized scaling, and adherent brown scale with erythema of the abdominal skin. Pedigree analysis indicated an autosomal recessive mode of inheritance. Ultrastructurally, the epidermis showed discontinuous lipid bilayers, unprocessed lipid within corneocytes, and abnormal lamellar bodies. Linkage analysis, performed by choosing simple sequence repeat markers and single-nucleotide polymorphisms near genes known to cause ACRI, revealed an association with NIPAL4. NIPAL4 was identified and sequenced using standard methods. No mutation was identified within the gene, but affected dogs had a SINE element 5′ upstream of exon 1 in a highly conserved region. Of 545 DNA samples from American Bulldogs, 32 dogs (17 females, 15 males) were homozygous for the polymerase chain reaction fragment. All affected dogs were homozygous, with parents heterozygous for the insertion. Immunolabeling revealed an absence of ichthyin in the epidermis. This is the first description of ARCI associated with decreased expression of NIPAL4 in nonhuman species.
Keywords: animal model, genodermatosis, ichthyosis, ichthyin, NIPAL4, mutation, cornification, keratinization
Autosomal recessive congenital ichthyoses (ARCIs) are genetically heterogeneous syndromic and nonsyndromic disorders of cornification, characterized by generalized scaling with no his-tological evidence of epidermolysis.24 These disorders share an inability to correctly form the outermost layer of the epidermis—the stratum corneum. In humans, ARCI may manifest as 1 of 3 clinical forms: lamellar ichthyosis (LI), nonbullous congenital ichthyosiform erythroderma (CIE), and harlequin ichthyosis. The phenotypes of LI and CIE are often overlapping, and some patients may switch phenotypes with age and treatment. Harlequin ichthyosis is a more severe disorder of cornification that is often fatal in infancy. LI is characterized by marked scaling with brown plate-like scale in the absence of erythema, while the scale in CIE is fine and white with prominent erythema.28,31 To date, 8 genes have been associated with ACRI: transglutaminase 1 (TGM1), ABCA12, ABHD5 (CG158), 2 lipoxygenases (ALOXE3 and ALOX12B), a NIPA-like domain containing 4 (NIPAL4 or ICTHYIN), LIPN, CYP4F22, and PNPLA1.15,18,24
The stratum corneum (the final product of cornification) consists of overlapping layers of anucleate keratinocytes (corneocytes) encased in bilayers of lipid. This unassuming layer maintains the water content of the body by restricting water movement into and out of the skin. The lipid bilayers comprise an equimolar ratio of cholesterol, free fatty acids, and ceramides. The pathophysiology of several ARCIs directly or indirectly involves abnormal lipid synthesis and/or secretion, leading to impairment of the permeability barrier. Both defects in structural proteins (eg, abnormal cross-linking of the cornified envelope in TGM1 deficiency) and those disorders directly associated with lipid metabolism (eg, lipoxygenase deficiency in ALOXE3 and ALOX12B) impair the structural integrity of the lipid bilayers, albeit by different mechanisms. The phenotype is a reflection of the body’s corrective response to restore the barrier.12
ICHTHYIN (currently referred to as NIPAL4) mutations account for about 16% of patients with ACRI.14 In humans with NIPAL-4 mutations, the messenger RNA (mRNA) levels are not decreased; the mutation is thought to affect protein function rather than expression.29
While the LI phenotype is more common, some ichthyin-deficient patients display a CIE phenotype.21 The role of ichthyin in epidermal biology is poorly understood, but ichthyin is thought to function in the same lipid metabolic pathway as ALOXE3 and ALOX12B.6,20 In the normal epidermis, the LOX enzymes oxidize leukotriene intermediates from the arachadonic acid cascade into epidermal hydroxyepoxyalcohols. Ichthyin serves as a putative transmembrane lipid receptor for these ω-hydroxyalcohols, which later become esterified into phospholipids.1,2 Therefore, mutations in NIPAL4 could alter downstream signaling and/or lipid metabolism, thereby altering composition of the stratum corneum, leading to impaired barrier function.
Spontaneous congenital, nonepidermolytic cornification disorders with clinical and histological features resembling ARCI in humans have been described in several dog breeds, but few causative mutations have been elucidated. To date, a novel LINE-1 insertion in TGM1 has been identified as a cause of LI in related Jack Russell Terriers.5 Recently, ACRI in Golden Retrievers has been associated with PNPLA1 mutations.15
Herein, ARCI is described in American Bulldog families from multiple continents, all of which are homozygous for an insertion upstream of NIPAL4.
Materials and Methods
Animals and Pedigree Analysis
Samples from American Bulldogs used in this study were recruited from North America, Europe, and Australia through veterinarians, breeders, and dog owners. A clinical information questionnaire, pedigree, and written informed consent form were obtained from each participant. The affected status of a dog was verified by review of a skin biopsy specimen read by one of the authors, a board-certified veterinary dermatopathologist (E.A.M.). Pedigree information was entered into a database (Pedigree/Draw; Southwest Foundation for Biomedical Research, San Antonio, TX) together with sex, numerically coded identification, and phenotype. After the pedigrees were drawn, they were scrutinized by eye. Segregation analysis was performed assuming incomplete ascertainment. Samples from the first 27 dogs with ARCI submitted to the University of Pennsylvania were used for linkage analysis. Samples from the other affected dogs that were obtained later or through the breeding studies were used to evaluate the validity of the marker assay. All dogs were cared for according to the principles outlined in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and in the International Guiding Principles for Biomedical Research Involving Animals.
DNA from 3 families (A, B, and C) of American Bulldogs was used for a small study looking for linkage between the affected animals and markers within TGM1, ALOXE3, ALOX12B, NIPAL4, ABCA12, and CYP4F22. Two of the families (B and C) were closely related, whereas the extended pedigree (beyond 6 generations) was not available for family A. In all 3 families, the parents were closely related. Litter A consisted 8 puppies (4 affected), litter B of 8 puppies (4 affected), and litter C of 6 puppies (3 affected). Sexes were not available for litter C, but for litters A and B, 3 of 7 males and 5 of 9 females were affected with ARCI. After linkage to NIPAL4 was established and a marker discovered upstream of NIPAL4, 545 DNA samples from clinically normal and 32 clinically affected American Bulldogs were obtained from referring veterinarians and breeders and DNA was extracted. Fifteen skin biopsy samples from affected dogs were available and reviewed. Most samples were received from North America and Germany, with a smaller number from other European countries, Russia, and Australia.
Blood and tissue extractions (skin samples or dewclaws) were performed using the QIAamp DNA MiniKit (Qiagen, Valencia, CA) according to the manufacturer’s recommendations, and buccal brush extractions were performed using the Puregene DNA Isolation Kit (Qiagen) according to the manufacturer’s protocol. Amplification products were extracted from the gel using the QIAquick Gel Extraction Kit (Qiagen) protocol and the reagents supplied. Samples for sequencing were submitted after amplification (see Suppl. Table S1) and gel extraction to the DNA Sequencing Center (Abramson Cancer Center, University of Pennsylvania). The resulting data were analyzed using DNAStar (DNASTAR, Inc, Madison, WI).
RNA Extraction, cDNA Synthesis, and NIPAL4 Gene Expression
For mRNA expression studies, skin biopsy samples from normal, heterozygous, and affected dogs were preserved in RNA-later (Qiagen Sciences, Gaithersburg, MD), and RNA was extracted using the QIAmp RNA Blood Mini Kit following the manufacturer’s instructions, including the step of DNase I on column digestion (Qiagen Sciences). Subsequently, complementary DNA (cDNA) was synthesized from the extracted RNA (1.0 μg RNA in a 20-μl reverse transcriptase [RT] reaction) using the High Capacity cDNA Reverse Transcription Kit with RNase Inhibitor (Applied Biosystems, Foster City, CA). Equal concentrations of cDNA from each sample were amplified by polymerase chain reaction (PCR) to assess for differences in mRNA expression of NIPAL4 (forward primer: 5′-GCGGGCTGTGGACGGAGGGTA-3′; reverse primer: 5′-CAGCAGCCATGGTGAGGAATC-3′) and of an endogenous control gene, β-actin (forward primer: 5′-ACCCCAAGGC-CAACCGTGAG-3′; reverse primer: 5′-GGATGGAGCCCC-CAATCCAC-3′), by PCR standard procedure using KOD DNA Polymerase (EMD Millipore, Billerica, MA) and gel electrophoresis analysis.
Light and Electron Microscopy
For histology, skin biopsy samples were fixed in 10% neutral buffered formalin and routinely processed, embedded in paraffin, and sectioned at 4 to 5 μm.
For electron microscopy, skin biopsy samples were obtained from a 3-week-old affected puppy and a 3-year-old dog. The samples were processed in parallel with age-matched samples from normal dogs. The tissue samples were trimmed into 1-mm pieces and fixed in modified Karnovsky’s solution at 4°C overnight. After rinsing with sodium cacodylate buffer (pH 7.3), the samples were postfixed with 1% reduced osmium-tetroxide (OsO4) for 2 hours in the dark.17 The samples were then incubated in freshly prepared 0.25% ruthenium tetroxide for 45 minutes in the dark, followed by conventional procedures of dehydration and embedding. The blocks were polymerized at 68°C for 48 hours. Sections of 80 to 90 nm were obtained from the “near-surface” location, mounted on copper grids, stained with uranyl acetate and lead citrate, and examined with a FEI Tecnai T-12 transmission electron microscope operated at 80 kV.
Indirect immunolabeling of ichthyin, using 0.2 μg/ml primary rabbit antibody (sc-133280; Santa Cruz Biotechnology, Santa Cruz, CA), was performed on paraffin-embedded skin samples from 12 affected American Bulldogs, age-matched controls, and 2 Golden Retrievers with ARCI due to an unrelated mutation (PNPLA1). A negative control was run by omission of the primary antibody. For immunodetection, a LSAB2 kit (Dako, Carpinteria, CA) was used. Visualization of antibody binding was obtained via DAB+ chromogen, 3,3′-diami-nobenzidine solution and counterstained with hematoxylin.
For immunofluorescence, skin samples obtained from normal and affected dogs were fixed 24 hours at 4°C in 4% paraformaldehyde, cryoprotected in 30% sucrose, and embedded in optimal cutting temperature compound (OCT). Then, 5-μm-thick cryosections were air-dried for 60 minutes, washed 2 × 5 minutes with 0.15M phosphate-buffered saline (PBS), and incubated for 18 hours at 4°C with rabbit anti-ichthyin (sc-133280; Santa Cruz Biotechnology) diluted 1:300 in Antibody Diluent Reagent Solution (003118; Invitrogen, Carlsbad, CA). Unbound primary antibody was removed by washing 2 × 5 minutes in PBS, and the sections were incubated for 40 minutes at 37°C with a 1:500 dilution of Alexa Fluor 568 goat anti–rabbit IgG secondary antibody (A11036; Invitrogen). After final PBS washes (2 × 5 minutes), slides were mounted in Vectashield with Dapi (H-1200; Vector Laboratories, Burlingame, CA).
Results
Pedigree Analysis
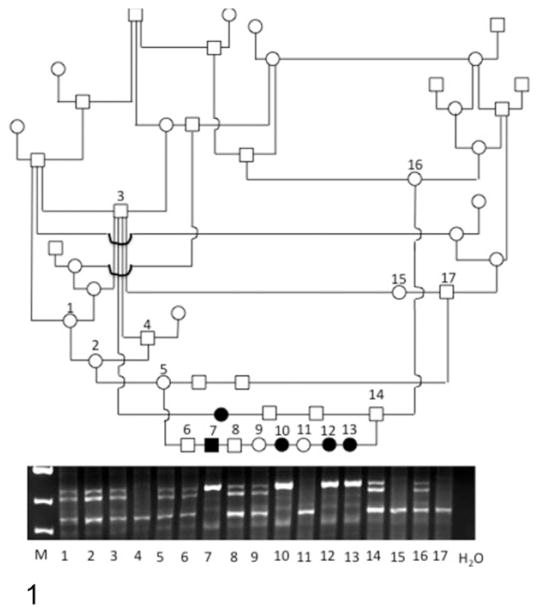
Complete family information was available for 15 affected Bulldogs. Eight males and 7 females were affected with ichthyosis, and 47 dogs (26 males and 21 females) were clinically normal in 7 litters produced by clinically normal parents. All parents of the affected dogs had at least 1 ancestor in common (Fig. 1). Segregation analysis demonstrated that 24.2% of dogs with complete pedigree information were affected (χ2 = 0.017).
Figure 1.
Partial pedigree of American Bulldogs with ichthyosis. Squares and circles represent males and females, respectively. Open and filled-in symbols designate normal dogs and affected dogs, respectively. While not always indicated, all dogs in this pedigree had common ancestors. Marker results are shown; the numbers correspond to those in the pedigree. Affected dogs have an insertion upstream of the 5′ end of NIPAL4, resulting in a larger polymerase chain reaction product.
Clinical Features
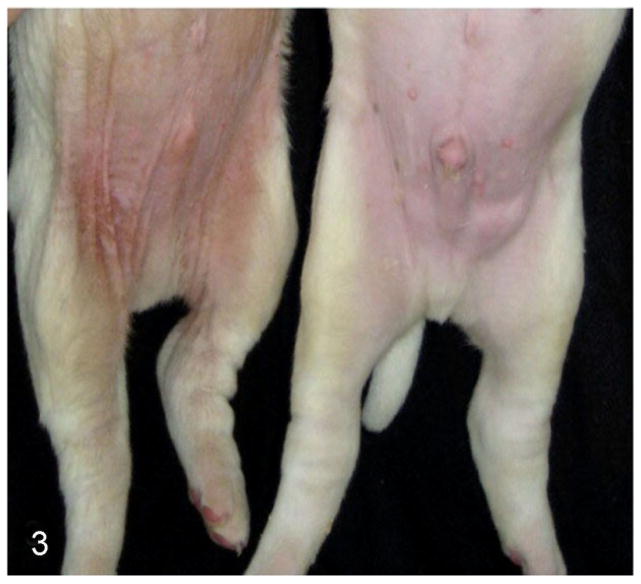
Clinical signs of ichthyosis were present in 32 American Bulldogs. Of the affected dogs, 17 were female and 15 male. All dogs had a consistent phenotype with confirmed onset before 6 weeks of age. The dogs were otherwise healthy. The gross phenotype was recognizable within 1 to 2 weeks of birth as a disheveled pelage with generalized soft, white scale (Fig. 2). The glabrous skin of the abdomen was discolored light brown and mildly erythematous with diffusely adherent light brown scale (Fig. 3). The clinical signs either did not change into adulthood or worsened slightly and often complicated by Malassezia overgrowth. Pruritus was variable and appeared to correlate with Malassezia overgrowth. After some affected puppies in litter B were humanely euthanized, a complete postmortem examination was performed and no extracutaneous abnormalities were found.
Figure 2.
Autosomal recessive ichthyosis (ARCI), 3-week-old American Bulldog puppies. The affected dog has a disheveled pelage (left) compared with the normal littermate (right).
Figure 3.
Autosomal recessive ichthyosis (ARCI), 3-week-old American Bulldog puppies. The affected puppy (left) has erythema, mild brown discoloration, and scaling of the abdominal skin.
Histology and Ultrastructure
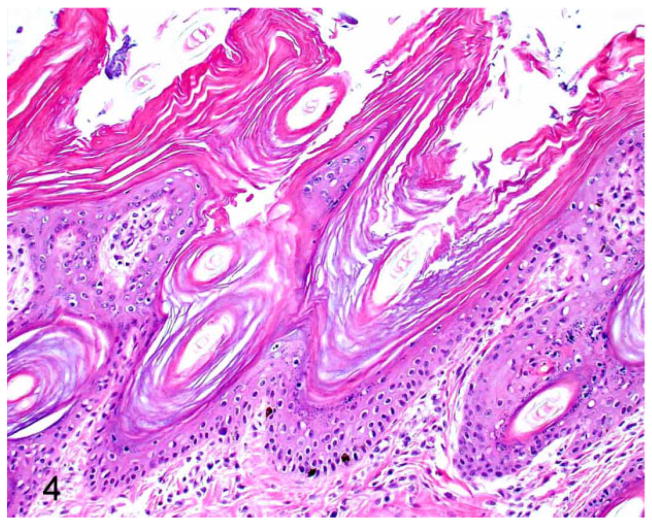
Light microscopy samples were examined from 15 of the 32 affected dogs. All dogs exhibited diffuse laminated to compact orthokeratotic hyperkeratosis with hypergranulosis and mild acanthosis (Figs. 4, 5). In all cases, the epidermis had a prominent granular layer, and multifocal granular layer keratinocytes displayed a perinuclear clear space (Fig. 5). Malassezia could be found within the corneal layer in at least once sample in approximately 60% of cases. The yeast were typically present without an inflammatory response.
Figure 4.
Autosomal recessive ichthyosis (ARCI), 3-week-old American Bulldog puppies. Severe and diffuse laminar orthokeratotic hyperkeratosis with mild acanthosis. Hematoxylin and eosin (HE).
Figure 5.
Autosomal recessive ichthyosis (ARCI), 3-week-old American Bulldog puppies. Perinuclear clearings in granular layer keratinocytes (arrow). HE.
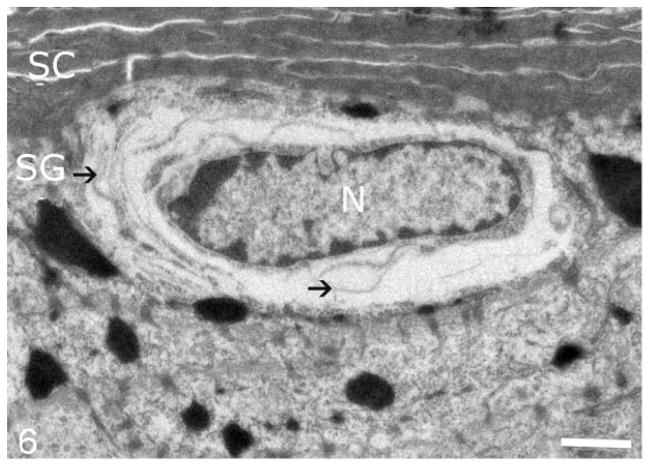
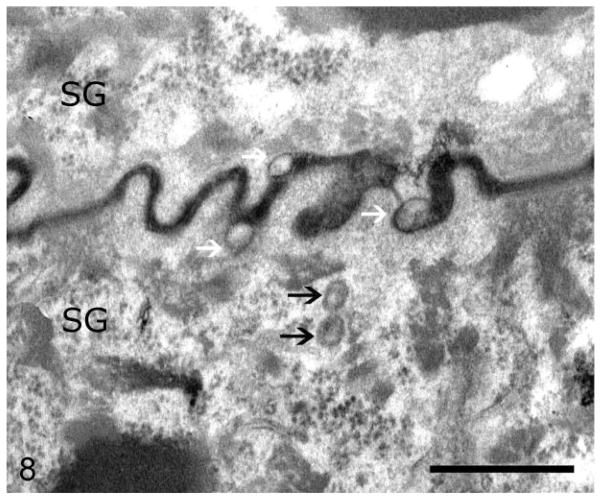
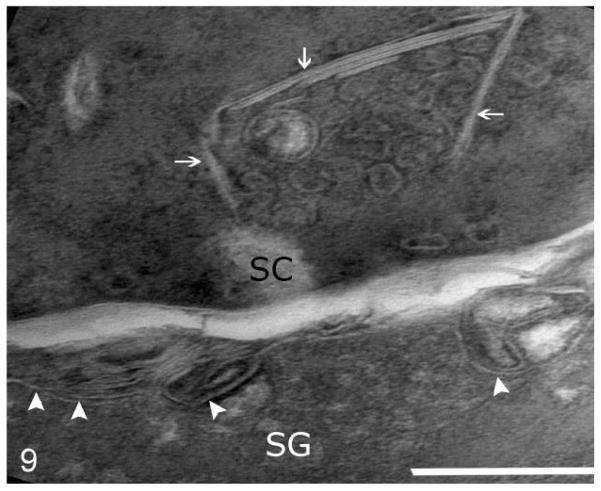
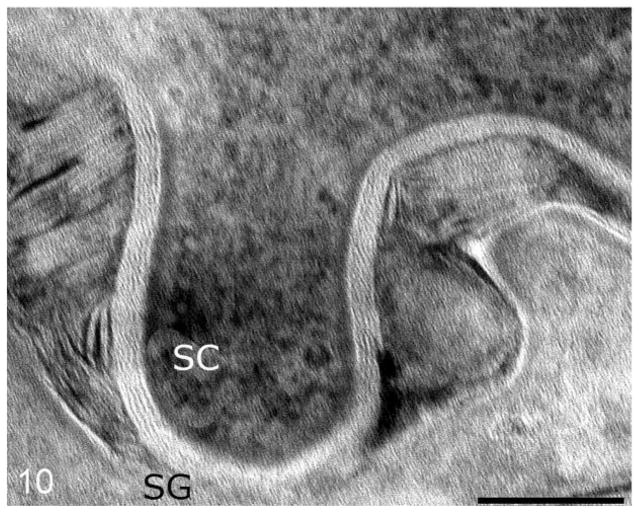
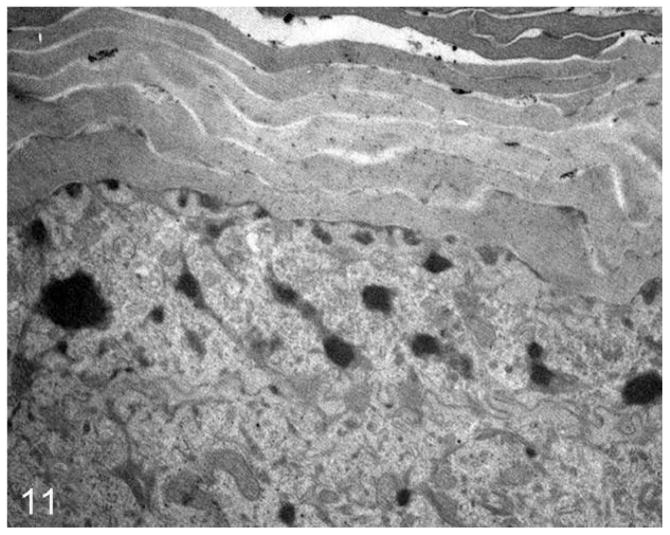
Routine electron microscopy revealed that curvilinear membranous material was scattered within the granular layer cytosol and within the perinuclear swellings seen on light microscopy (Figs. 6, 7). The lamellar body density was normal, but some organelles displayed decreased lipid arrays or electron lucent microvesicles (Fig. 8). The stratum corneum contained haphazardly arranged layers of variably sized, often enlarged corneocytes (Fig. 7). Ruthenium tetroxide postfixation showed discontinuous lipid bilayers and retained lipid within corneocytes (Fig. 9) as compared to the organized lamellar arrangement in age-matched skin from an unaffected dog (Figs. 10 and 11).
Figure 6.
Skin; epidermis at junction of stratum granulosum (SG) and stratum corneum (SC); American Bulldog with autosomal recessive congenital ichthyosis. A granular layer keratinocyte has a perinuclear clear space that contains curvilinear membranous structures (black arrows). N, nucleus. Bar = 2 μm.
Figure 7.
Skin; epidermis at junction of stratum granulosum (SG) and stratum corneum (SC); American Bulldog with autosomal recessive congenital ichthyosis. Retained lipid within corneocytes (white arrows) and curvilinear membranous structures (black arrows). Bar = 1 μm.
Figure 8.
Skin; epidermis at junction of stratum granulosum (SG) and stratum corneum (SC); American Bulldog with autosomal recessive congenital ichthyosis. Lamellar bodies with cleared contents in SG (black arrows) and at the SC/SG junction (white arrows). Bar = 1 μm.
Figure 9.
Skin; epidermis at junction of stratum granulosum (SG) and stratum corneum (SC); American Bulldog with autosomal recessive congenital ichthyosis. Abnormal contents of lamellar bodies at SG/SC fusion (white arrowheads) and retained lamellar arrays in corneocytes (white arrows). Bar = 0.5 μm.
Figure 10.
Normal dog. The neat arrangement of lipid lamellae within the stratum corneum (SC)/stratum granulosum (SG) junction and the lipid arrays within the lamellar bodies at the SC fusion. Bar = 0.5 μm.
Figure 11.
Normal dog. A low-magnification survey showing the orderly arrangement of SC and SG.
Sample Collection
For the initial linkage analysis, DNA from 3 families (A, B, and C) of American Bulldogs was used. Two families (B and C) were closely related, whereas the extended pedigree (beyond 6 generations) was not available for family A. In all 3 families, the parents were closely related. Litter A consisted 8 puppies (4 affected), litter B of 8 puppies (4 affected), and litter C of 6 puppies (3 affected). Sexes were not available for litter C, but for litters A and B, 3 of 7 males and 5 of 9 females were affected with ARCI. After linkage was established and a marker discovered, 545 DNA samples from clinically normal and 32 clinically affected American Bulldogs were obtained from referring veterinarians and breeders, and DNA was extracted. Fifteen skin biopsy samples from affected dogs were available and reviewed. Most samples were received from North America and Germany, with a smaller number from other European countries, Russia, and Australia.
Linkage Analysis and Sequencing
Initial marker analysis indicated that the disease seen in the American Bulldogs was linked to NIPAL4 (data not shown). Therefore, the entire genomic sequence of NIPAL4 was sequenced, but no mutation was found within the gene (Suppl. Table S1). However, an additional 338-bp sequence was discovered in affected dogs 5781 bp upstream of the start codon in a highly conserved region (Suppl. Table S1). The difference in the PCR product sizes was used to genotype the submitted samples (Fig. 1). A total of 545 DNA samples from American Bulldogs, including 1 mixed breed dog, were examined, and 32 dogs (17 females, 15 males) were homozygous for the larger PCR fragment. All of these dogs were clinically affected. The canine genome was searched for the presence of the sequence of the 338-bp insert, and it was present on all chromosomes multiple times, suggesting that it was a small interspersed element (SINE). However, the 338-bp insertion was not present 5781 bp upstream of NIPAL4 in the published canine genome sequences.
Immunolabeling
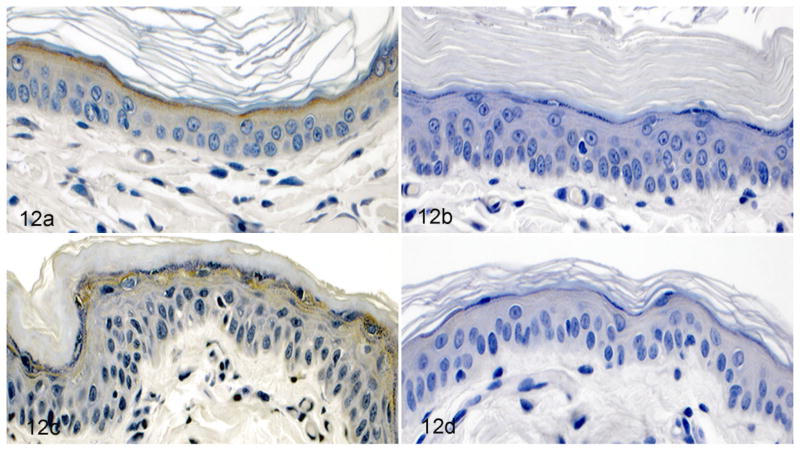
An absence of ichthyin was seen with both immunoperoxidase and immunofluorescence staining. Normal dogs showed fine granular staining of ichthyin within the cytosol of granular layer keratinocytes (Fig. 12a). Similarly, dogs with Golden Retriever ichthyosis had positive staining in the granular layer (Fig. 12c). In contrast, affected dogs had an absence of positive staining by both immunoperoxidase (Fig. 12b) and immuno-fluorescence (Fig. 13b).
Figure 12.
Skin; dog. Immunohistochemistry for ichthyin. (a) Normal dog. Fine brown granular staining in the granular cell layer. (b) Autosomal recessive congenital ichthyosis (ARCI) in the American Bulldog. Absence of staining in the granular cell layer. (c) ARCI in the Golden Retriever (PNPLA1 mutation). Positive stain uptake in the granular cell layer resembles the normal dog. (d) Normal dog. Negative control. Immunohisto-chemistry.
Figure 13.
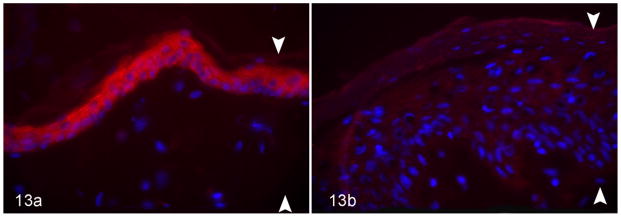
Skin; dog. Arrowheads delineate epidermal thickness. Anti-ichthyin immunofluorescence. (a) Normal dog. Strong positive (red) staining in the granular cell layer. (b) Autosomal recessive congenital ichthyosis in the American Bulldog. Absence of staining in the epidermis. Anti-ichthyin immunofluorescence.
Discussion
This study was sparked by a series of routine skin biopsies from young American Bulldogs that had features of nonepidermolytic ichthyosis. In veterinary medicine, the term ichthyosis has been limited to rare congenital or hereditary disorders believed to be due to primary defects in the formation of the stratum corneum. The most straightforward way to approach a case of suspected ichthyosis is to determine if the changes are epidermolytic or nonepidermolytic on light microscopy.23 Epidermolytic ichthyosis (aka epidermolytic hyperkeratosis) is very rare but has unique histopathologic features (namely, hyperkeratosis and epidermal hyperplasia with hypergranulosis and granular cell vacuolation/degeneration) that specifically correlate to mutations in epidermal keratins.4 On the contrary, nonepidermolytic ichthyosis is more common and has a broad mutational spectrum. The Bulldogs had features of nonepidermolytic ichthyosis: minimal to mild epidermal hyperplasia, sparse dermal inflammation, and laminar orthokeratotic hyperkeratosis. Specifically, the keratin bars in the stratum corneum were closely apposed as compared with the open basket-weave arrangement seen in normal skin. Another feature seen in the American Bulldogs, as well as ARCI in Golden Retrievers, is perinuclear clearings in granular layer keratinocytes.23
Our investigations specifically linked the nonepidermolytic ichthyosis in American Bulldogs to NIPAL-4. Several mutations in NIPAL4 have been described in humans with ARCI.6,7,10,20,29 Mutations have been found in highly conserved regions of exons 2, 4, 5, and 6, including a mutation in the slice acceptor site of exon 3, as well as the slice donor site in exon 5. Interestingly, mutations in humans have not been shown to lead to decreased or absent expression but rather the presence of the protein in the granular layer, and cDNA levels of NIPAL4 were similar in affected patients compared with normal controls.6,10,20,29 The 338-bp insert found upstream of NIPAL4 in a highly conserved region suggests a mutation in a promoter or enhancer region.
Typical for a SINE, it is less than 500 bp long and followed by approximately 30 adenosine nucleotides that are interspersed with thymine nucleotides. SINE elements upstream of genes in potential regulatory regions have been shown to be associated with disease in some species and color traits in dogs.9,27,30 All affected dogs had the insertion at the same location—namely, 5781 bp upstream of NIPAL4. The PCR results in Fig. 1 demonstrate that each affected allele is larger (ie, containing the insertion) and each normal allele is smaller (ie, lacking the insertion). The PCR primers were designed to only amplify the region of interest as described. Therefore, only the region that may contain the SINE insertion was amplified. More studies will need to be performed to determine how and if the insertion leads to loss of ichthyin expression.
Inheritance of ichthyosis in the American Bulldog fulfills all of the features of an autosomal recessive trait: both females and males were equally affected, and their parents were clinically normal. Segregation analysis performed in those litters with complete family information and obligate carrier parents demonstrated 24.2% affected dogs, which is very close to the expected 25% in autosomal recessive Mendelian traits. The frequency of the diseased allele in the population of American Bulldogs was calculated as 23.2%. To avoid bias toward affected dogs, we used only the general screening population for the calculations. The results suggest that 35.6% of American bulldogs are heterozygote (carriers) for the disease and 5.4% are affected with ichthyosis. In fact, in our studies, 5.9% of the dogs (32 of 545) were affected, which is close to the expected number. The high frequency of this gene in the canine population highlights the importance of screening for the deployment of appropriate breeding strategies. If one were to remove the gene from the population all at once through testing, roughly 40% of the population would be eliminated. Thus, breeders are advised to test all dogs, use those with high-quality attributes for breeding, and, if they are carriers, breed only to noncarriers to retain highly desirable genes but not produce affected dogs. Over time, the deleterious gene will be eliminated without losing desirable traits.
As expected, electron microscopy revealed normal keratin fibrils, keratohyalin granules, and cornified envelopes, further ruling out defects in structural proteins as a potential cause. During cornification, cytosolic lipid is delivered to lamellar bodies (LBs) by means of a transmembrane transporter (eg, ABCA12). Once inside the LBs, the lipid is arranged in lamellar arrays. At the junction of the stratum granulosum (SG) and stratum corneum (SC), LBs fuse with the plasma membrane and rapidly disgorge their lipid contents into the SC interstices. After secretion, the lipid is further processed into mature lamellar bilayers. The LBs in our ichthyin-deficient dogs were adequate in number, but some contained microvesicles with an absence of lamellar arrays, an abnormality that remained evident at the SG/SC interface. The lipid bilayers in the stratum corneum, as seen with ruthenium fixation, were discontinuous and uneven. Furthermore, retained lipid material was present within the corneocytes. The corneocyte layers were unevenly stacked and shaped, which may be due, in part, to the structurally flawed intervening lipid bilayers. Together, these results suggest that the mutation alters lipid metabolism, with subsequent downstream consequences for epidermal barrier function.
The ultrastructural findings in the American Bulldogs with ACRI resemble those reported in human patients with NIPAL-4 mutations. This includes decreased lamellar body contents as well as electron lucent perinuclear regions in granular layer that contain linear membranous structures.6 However, in the American Bulldogs, the linear membranes were not strictly localized to cells with perinuclear clearings as they could be found throughout the granular layer. It should be noted that these membranous structures are not specific for ichthyin deficiency but are seen in other disorders of lipid metabolism, including PNPLA1–deficient ACRI in Golden Retrievers and ichthyosis prematurity syndrome in humans.3,15
These findings, taken together, further support ichthyin’s role in epidermal lipid biology and perhaps in the assembly and secretion of LBs. LB lipids serve as the precursor for the lipid bilayers. The LBs contain pro-barrier (polar) lipids (phospholipids, glucosylceramides, and cholesterol) with respective lipid-processing enzymes as well as a number of proteases and protease inhibitors. After the lipid is expulsed from the apical stratum granulosum, the lipid is modified into barrier (hydrophobic) lipids such that the normal stratum corneum is a mixture of free fatty acids, cholesterol, and ceramides. The defective lipid, when delivered to the stratum corneum, causes structural deficits in the stratum corneum, which adversely affects the permeability barrier. The hyperkeratosis is a response to increase lipid in an attempt to repair the barrier defect.11
Clinically, American Bulldogs have striking Malassezia overgrowth, which is uncommon in PNPLA1–deficient Golden Retrievers,15,16,23 and this finding is further supported by the authors’ clinical experience. TGM1-deficient lamellar ichthyosis in Jack Russell Terriers is also characterized by Malassezia overgrowth.5 Malassezia are commensal yeast in the skin of dogs, as well as many other species. In dogs, Malassezia become opportunistic pathogens in the presence of predisposing factors such as allergic skin disease. In tissue sections, Malassezia may be lost during processing due to the superficial location in the stratum corneum. However, the yeast are easily detected in routine sections of skin from American Bulldogs with ARCI. Most Malassezia sp require very long-chain fatty acids for growth in culture. During cornification, short-chain fatty acids are replaced by long-chain species.19,26 In addition, antimicrobial enzymes (cathelicidins, β-defensins) are a component of the lamellar body contents, and altered LB content may directly alter the skin innate immunity.22,26 Cathelicidins, in particular, are known to provide innate immunity to the epidermis and combat fungal growth.22 Thus, excessive or abnormal lipid profiles along with decreased antimicrobial proteins within the SC may facilitate yeast growth in the ichthyotic skin of Bulldogs.
Further work is needed to document and assess the physical barrier abnormality in the affected dogs (eg, pH, hydration, transepidermal water loss) and to determine the lipid content of the stratum corneum. Increased cutaneous pH is known to promote serine protease activity, which drives epidermal hyperplasia and inflammation.8,13 To date, tremendous progress has been made in the molecular characterization and pathogenesis of ichthyoses; however, the treatment has remained largely unchanged. A recent study showed that pathogenesis-related therapy was successful in the treatment of CHILD syndrome (congenital hemidysplasia ichthyosiform erythroderma with limb defects)—an X-linked syndromic disorder of lipid metabolism. In CHILD syndrome, the defect lies in distal cholesterol metabolism with decreased cholesterol in the skin and the generation of toxic metabolites. Topical treatment with lovastatin and cholesterol was shown to reverse skin lesions in 3 months.25
Herein, the first spontaneous animal model of ichthyin-deficient ACRI is described. The cutaneous phenotype overlaps with that of lamellar ichthyosis and congenital ichthyosiform erythroderma. The phenotype is nonsyndromic, is consistent in all affected dogs, and is first manifest shortly after birth. This phenotype is unique in the lack of ichthyin expression, which undoubtedly makes the dog highly suitable to investigate the function of ichthyin in epidermal lipid metabolism as well as to pursue a pathogenesis-directed treatment.
Supplementary Material
Acknowledgments
The authors would like to thank Peter Elias, MD for review of this manuscript as well as Debra Crumrine for assistance with electron microscopy (University of California San Francisco VA Medical Center).
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: International Society for Veterinary Dermatopathology, National Institutes of Health (RR02152).
Footnotes
Supplemental material for this article is available on the Veterinary Pathology website at http://vet.sagepub.com/supplemental.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Anton R, Camacho M, Puig L, et al. Hepoxilin B3 and its enzymatically formed derivative trioxilin B3 are incorporated into phospholipids in psoriatic lesions. J Invest Dermatol. 2002;118:139–146. doi: 10.1046/j.0022-202x.2001.01593.x. [DOI] [PubMed] [Google Scholar]
- 2.Anton R, Vila L. Stereoselective biosynthesis of hepoxilin B3 in human epidermis. J Invest Dermatol. 2000;114:554–559. doi: 10.1046/j.1523-1747.2000.00903.x. [DOI] [PubMed] [Google Scholar]
- 3.Anton-Lamprecht I. Diagnostic ultrastructure of nonneoplastic diseases. In: Papadimitriou JSD, editor. The Skin. Edinburgh, UK: Churchill Livingstone; 1992. pp. 459–550. [Google Scholar]
- 4.Credille KM, Barnhart KF, Minor JS, et al. Mild recessive epidermolytic hyperkeratosis associated with a novel keratin 10 donor splice-site mutation in a family of Norfolk Terrier dogs. Br J Dermatol. 2005;153:51–58. doi: 10.1111/j.1365-2133.2005.06735.x. [DOI] [PubMed] [Google Scholar]
- 5.Credille KM, Minor JS, Barnhart KF, et al. Transglutaminase 1–deficient recessive lamellar ichthyosis associated with a LINE-1 insertion in Jack Russell Terrier dogs. Br J Dermatol. 2009;161:265–272. doi: 10.1111/j.1365-2133.2009.09161.x. [DOI] [PubMed] [Google Scholar]
- 6.Dahlqvist J, Klar J, Hausser I, et al. Congenital ichthyosis: mutations in ichthyin are associated with specific structural abnormalities in the granular layer of epidermis. J Med Genet. 2007;44:615–620. doi: 10.1136/jmg.2007.050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahlqvist J, Westermark GT, Vahlquist A, et al. Ichthyin/NIPAL4 localizes to keratins and desmosomes in epidermis and ichthyin mutations affect epidermal lipid metabolism. Arch Dermatol Res. 2012;304:377–386. doi: 10.1007/s00403-012-1207-7. [DOI] [PubMed] [Google Scholar]
- 8.Demerjian M, Hachem JP, Tschachler E, et al. Acute modulations in permeability barrier function regulate epidermal cornification: role of caspase-14 and the protease-activated receptor type 2. Am J Pathol. 2008;172:86–97. doi: 10.2353/ajpath.2008.070161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dreger DL, Schmutz SM. A new mutation in MC1R explains a coat color phenotype in 2 “old” breeds: Saluki and Afghan hound. J Hered. 2010;101:644–649. doi: 10.1093/jhered/esq061. [DOI] [PubMed] [Google Scholar]
- 10.Dreger DL, Schmutz SM. A SINE insertion causes the black-and-tan and saddle tan phenotypes in domestic dogs. J Hered. 2011;102(suppl 1):S11–S18. doi: 10.1093/jhered/esr042. [DOI] [PubMed] [Google Scholar]
- 11.Elias PM. Structure and function of the stratum corneum extracellular matrix. J Invest Dermatol. 2012;132:2131–2133. doi: 10.1038/jid.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elias PM, Williams ML, Holleran WM, et al. Pathogenesis of permeability barrier abnormalities in the ichthyoses: inherited disorders of lipid metabolism. J Lipid Res. 2008;49:697–714. doi: 10.1194/jlr.R800002-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feingold KR, Schmuth M, Elias PM. The regulation of permeability barrier homeostasis. J Invest Dermatol. 2007;127:1574–1576. doi: 10.1038/sj.jid.5700774. [DOI] [PubMed] [Google Scholar]
- 14.Fischer J. Autosomal recessive congenital ichthyosis. J Invest Dermatol. 2009;129:1319–1321. doi: 10.1038/jid.2009.57. [DOI] [PubMed] [Google Scholar]
- 15.Grall A, Guaguere E, Planchais S, et al. PNPLA1 mutations cause autosomal recessive congenital ichthyosis in golden retriever dogs and humans. Nat Genet. 2012;44:140–147. doi: 10.1038/ng.1056. [DOI] [PubMed] [Google Scholar]
- 16.Guaguere E, Bensignor E, Kury S, et al. Clinical, histopathological and genetic data of ichthyosis in the Golden Retriever: a prospective study. J Small Anim Pract. 2009;50:227–235. doi: 10.1111/j.1748-5827.2009.00730.x. [DOI] [PubMed] [Google Scholar]
- 17.Hou SY, Mitra AK, White SH, et al. Membrane structures in normal and essential fatty acid–deficient stratum corneum: characterization by ruthenium tetroxide staining and x-ray diffraction. J Invest Dermatol. 1991;96:215–223. doi: 10.1111/1523-1747.ep12461361. [DOI] [PubMed] [Google Scholar]
- 18.Israeli S, Khamaysi Z, Fuchs-Telem D, et al. A mutation in LIPN, encoding epidermal lipase N, causes a late-onset form of autosomal-recessive congenital ichthyosis. Am J Hum Genet. 2011;88:482–487. doi: 10.1016/j.ajhg.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khnykin D, Miner JH, Jahnsen F. Role of fatty acid transporters in epidermis: Implications for health and disease. Dermatoendocrinology. 2011;3:53–61. doi: 10.4161/derm.3.2.14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lefevre C, Bouadjar B, Ferrand V, et al. Mutations in a new cyto-chrome P450 gene in lamellar ichthyosis type 3. Hum Mol Genet. 2006;15:767–776. doi: 10.1093/hmg/ddi491. [DOI] [PubMed] [Google Scholar]
- 21.Lefevre C, Bouadjar B, Karaduman A, et al. Mutations in ichthyin a new gene on chromosome 5q33 in a new form of autosomal recessive congenital ichthyosis. Hum Mol Genet. 2004;13:2473–2482. doi: 10.1093/hmg/ddh263. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Garcia B, Lee PH, Gallo RL. Expression and potential function of cathelicidin antimicrobial peptides in dermatophytosis and tinea versicolor. J Antimicrob Chemother. 2006;57:877–882. doi: 10.1093/jac/dkl078. [DOI] [PubMed] [Google Scholar]
- 23.Mauldin EA, Credille KM, Dunstan RW, et al. The clinical and morphologic features of nonepidermolytic ichthyosis in the Golden Retriever. Vet Pathol. 2008;45:174–180. doi: 10.1354/vp.45-2-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oji V, Tadini G, Akiyama M, et al. Revised nomenclature and classification of inherited ichthyoses: results of the First Ichthyosis Consensus Conference in Soreze 2009. J Am Acad Dermatol. 2010;63:607–641. doi: 10.1016/j.jaad.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Paller AS, van Steensel MA, Rodriguez-Martin M, et al. Pathogenesis-based therapy reverses cutaneous abnormalities in an inherited disorder of distal cholesterol metabolism. J Invest Dermatol. 2011;131:2242–2248. doi: 10.1038/jid.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Martin M, Martin-Ezquerra G, Man MQ, et al. Expression of epidermal CAMP changes in parallel with permeability barrier status. J Invest Dermatol. 2011;131:2263–2270. doi: 10.1038/jid.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmuth SM, Berryere TG, Dreger DL. MITF and white spotting in dogs: a population study. J Hered. 2009;100(suppl):S66–S74. [Google Scholar]
- 28.Traupe H, Fischer J, Oji V. Nonsyndromic types of ichthyoses—an update. J Dtsch Dermatol Ges. 2014;12:109–121. doi: 10.1111/ddg.12229. [DOI] [PubMed] [Google Scholar]
- 29.Wajid M, Kurban M, Shimomura Y, et al. NIPAL4/ichthyin is expressed in the granular layer of human epidermis and mutated in two Pakistani families with autosomal recessive ichthyosis. Dermatology. 2010;220:8–14. doi: 10.1159/000265757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W, Kirkness EF. Short interspersed elements (SINEs) are a major source of canine genomic diversity. Genome Res. 2005;15:1798–1808. doi: 10.1101/gr.3765505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams ML, Elias PM. Heterogeneity in autosomal recessive ichthyosis: clinical and biochemical differentiation of lamellar ichthyosis and nonbullous congenital ichthyosiform erythroderma. Arch Dermatol. 1985;121:477–488. doi: 10.1001/archderm.121.4.477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.