Abstract
Objective
To determine whether primary or mesh herniorrhaphy reverses abdominal wall atrophy and fibrosis associated with hernia formation.
Background
We previously demonstrated that hernia formation is associated with abdominal wall atrophy and fibrosis after 5 weeks in an animal model.
Methods
Arat model of chronic incisional hernia was used. Groups consisted of uninjured control (UC, n = 8), sham repair (SR, n = 8), unrepaired hernia (UR, n = 8), and 2 repair groups: primary repair (PR, n = 8) or tension-free polypropylene mesh repair (MR, n = 8) hernia repair on postoperative day (POD) 35. All rats were killed on POD 70. Intact abdominal wall strips were cut perpendicular to the wound for tensiometric analysis. Internal oblique muscles were harvested for fiber type and size determination.
Results
No hernia recurrences occurred after PR or MR. Unrepaired abdominal walls significantly demonstrated greater stiffness, increased breaking and tensile strengths, yield load and yield energy, a shift to increased type IIa muscle fibers than SR (15.9% vs 9.13%; P < 0.001), and smaller fiber cross-sectional area (CSA, 1792 vs 2669 μm2; P < 0.001). PR failed to reverse any mechanical changes but partially restored type IIa fiber (12.9% vs 9.13% SR; P < 0.001 vs 15.9% UR; P < 0.01) and CSA (2354 vs 2669 μm2 SR;P < 0.001 vs 1792 μm2 UR; P < 0.001). Mesh-repaired abdominal walls demonstrated a trend toward an intermediate mechanical phenotype but fully restored type IIa muscle fiber (9.19% vs 9.13% SR; P > 0.05 vs 15.9% UR; P < 0.001) and nearly restored CSA (2530 vs 2669 μm2 SR; P < 0.05 vs 1792 μm2 UR; P < 0.001).
Conclusions
Mesh herniorrhaphy more completely reverses atrophic abdominal wall changes than primary herniorrhaphy, despite failing to restore normal anatomic muscle position. Techniques for hernia repair and mesh design should take into account abdominal wall muscle length and tension relationships and total abdominal wall compliance.
Keywords: abdominal wall pathology, abdominal muscles, compliance, hernia model, internal oblique muscle, laparotomy, muscle atrophy, ventral hernia
Laparotomy wound failure progressing to incisional hernia formation is the most frequent complication of abdominal surgery, with an overall estimated incidence of 11% to 16%.1–5 Despite many advances in surgical technique and patient care, the rate of primary incisional hernia formation has not changed appreciably in 75 years.6 Rates of hernia recurrence after repair are substantially higher, up to 63% in long-term follow-up.7 Although the use of mesh has significantly improved hernia repair, long-term recurrence rates as high as 32% remain unacceptably high7 and worsen with each subsequent repair.8
Incisional abdominal hernias most frequently develop from early laparotomy wound separation and failure within the first postoperative month, often clinically occult.1,9 The cause of wound failure may be multifactorial—related to closure technique, wound ischemia, infection, excessive straining from coughing or weight lifting, or patient comorbidities such as older age, obesity, malnutrition, diabetes, or chronic steroid therapy.3,10–17 These same factors may prevent reestablishment of fascial healing once separation has occurred and contribute to the increased frequency of recurrence after hernia repair.18 The exact mechanism of progression from wound separation to hernia formation is unknown, and the factors involved in higher recurrence rates are unclear.
The development of a functionally pathological abdominal wall suggests another mechanism for the high recurrence rates after hernia repair. The fascial separation and loss of midline muscle attachment in large ventral hernias lead to abdominal muscle shortening and relative unloading, particularly of the lateral oblique muscles whose insertions are lost.19,20 Animal models of muscle unloading through hindlimb immobilization and tenotomy demonstrate pathological changes associated with disuse atrophy, including a decrease in muscle fiber size, a shift in muscle fiber-type distribution, increased intramuscular collagen deposition, and alterations to muscle mechanical properties.21–25 Our previous work using a rat model of hernia formation confirmed that ventral incisional hernia formation is associated with abdominal wall muscular atrophy and decreased compliance as early as 5 weeks after laparotomy.19 Interestingly, healed laparotomy wounds without development of a fascial defect (sham) were associated with similar pathological changes, although to a lesser extent. It is unknown whether this pathologic state is reversible and how various abdominal wall repair techniques may affect this recovery. We hypothesize that primary repair (PR), by reestablishing normal muscle position and length, will lead to faster recovery than a tension-free mesh repair (MR) that may improve muscle loading forces but will not alter muscle position. The aim of this study is to determine (1) whether the pathologic changes associated with healed laparotomy wounds recover to the normal, uninjured physiology over time and (2) whether the pathological changes associated with hernia formation are reversed after primary or mesh herniorrhaphy by measuring changes in internal oblique muscle mechanical properties and changes in muscle fiber and type and size.
MATERIALS AND METHODS
Incisional Hernia Model
A total of 40 male Sprague-Dawley rats (Charles River Laboratories International, Inc, MA) aged 9 weeks, weighing approximately 250 g, were acclimated and housed under standard conditions. Animals were provided ad libitum intake of standard rat chow and water throughout the study. All animal care and operative procedures were approved by the University Committee on Use and Care of Animals and in accordance with the guidelines of the Unit for Laboratory Animal Medicine of the University of Michigan. An established rat model of ventral hernia formation was used.26–28 Briefly, a 6 × 3-cm ventral full-thickness skin flap was raised through the avascular prefascial plane, and a 5-cm vertical midline laparotomy incision was made through the linea alba. The fascial edges were approximated with 2 interrupted 5-0 fast-absorbing plain gut sutures. The skin flap was replaced and secured with interrupted 4-0 polypropylene sutures. The unrepaired hernia (UR) group (n = 8) developed large ventral hernias in all animals. Two control groups were used: an uninjured control (UC) group (n = 8) and a sham control (SC) group (n = 8). In the UC group, a skin flap was raised and closed without laparotomy. In the sham group, the skin flap was raised, and a 5-cm laparotomy performed and repaired with a running 4-0 nonabsorbable polypropylene suture, followed by flap closure. Two repair groups were created after hernia formation, using the hernia model on postoperative day (POD) 35 (Fig. 1A): a PR group (n = 8) and an MR group (n = 8). In the PR group, the same skin flap was raised again through the existing scar, fascial hernia defects were repaired using a running 4-0 polypropylene suture, and the skin flap was replaced (Fig. 1B). In the MR group, hernia defects were repaired by the placement of polypropylene mesh, trimmed to match the size of the defect, and sutured in place in an intraperitoneal position in a tension-free manner without approximation of the fascial edges (Fig. 1C). Hernia defect size was measured at 5 weeks for each animal in the PR and MR groups and at 10 weeks in the UR and MR groups, using SPOT Imaging Software Version 4.5 (Diagnostic Instruments, Inc, MI).
FIGURE 1.
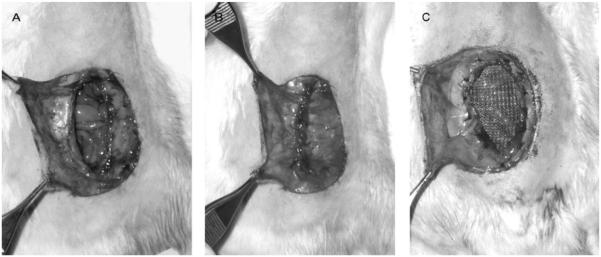
Representative images of hernia formation using the hernia model at 5 weeks. A, Before repair. B, After primary suture repair. C, After tension-free polypropylene MR.
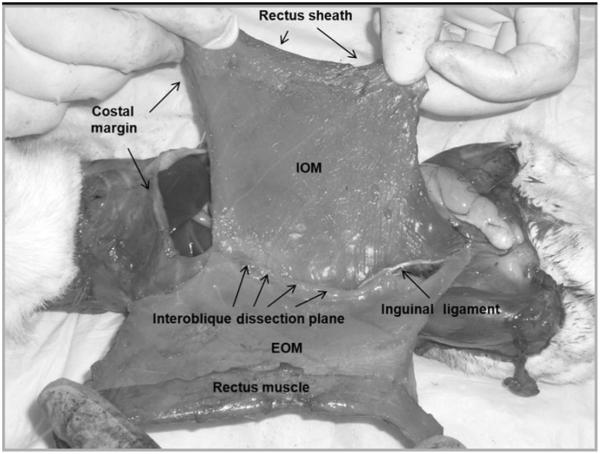
All rats were euthanized on POD 70. The skin was dissected free circumferentially and the abdominal wall circumference measured at the level of the umbilical process from 1 fascial edge moving posteriorly to the other. The external oblique and rectus muscles were then dissected free from the internal oblique muscle by dissection through the interoblique plane as previously described (Fig. 2).19 The composite internal oblique and transversus abdominis muscles were then dissected free from their remaining attachments. The left internal oblique muscle/transversus abdominis muscle sheet was cut into three 10 × 30-mm transverse strips (Fig. 3A) and stored in cold phosphate-buffered saline for tensiometric analysis. The right internal oblique muscle/transversus abdominis muscle sheet was cut into strips approximately 12-mm wide parallel to the internal oblique muscle fibers (Fig. 3B) and snap-frozen in isopentane at −160°C and stored at −70°C for subsequent histological analysis.
FIGURE 2.
Composite internal oblique/transversus abdominis muscle dissection through the interoblique plane.
FIGURE 3.
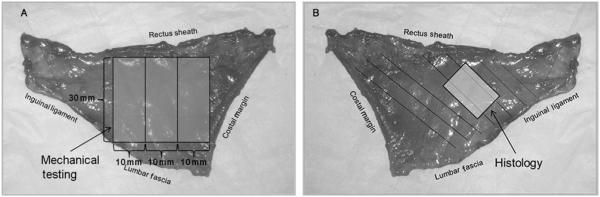
A, Right internal oblique/transversus abdominis muscle composite. Three 10 × 30-mm strips were cut perpendicular to the linea alba for tensiometric analysis. B, Left internal oblique/transversus abdominis muscle composite. Full-thickness samples oriented parallel to the internal oblique muscle fibers were collected for histologic analysis.
Histochemistry
Frozen muscle samples were sectioned (10–12-μm thick) perpendicular to the internal oblique muscle fibers in a Leica CM1850 cryostat (Leica Microsystems, Inc, IL) at −24°C. Fiber type staining was performed using the calcium activated myosin-type adenosine triphosphatase activity method.29–32 Digital microphotographs were taken at 100× magnification using a Nikon Eclipse Ti inverted microscope with a Nikon DS-Fi1 high-resolution digital camera (Nikon Instruments, Inc, NY). A sample of at least 500 internal oblique muscle fibers from each animal was classified as type I, type IIa, or type IIb according to myosine ATPase activity. Muscle fiber cross-sectional area (CSA) was quantified using planimetry software (SPOT Imaging Software Version 4.5). At least 50 internal oblique muscle fibers of each fiber type were quantified for each sample.
Tensiometric Analysis
Mechanical testing was performed within 6 hours of necropsy on the 3 composite internal oblique muscle/transversus abdominis muscle abdominal wall strips collected from each animal. Tissue sample width and thickness were measured with Digimatic calipers (Mitutoyo American Corporation, IL). Force extension curves were generated using an Instron tensiometer (model 5542; Instron Corporation, MA) equipped with a 50-N static load cell set at a crosshead speed of 10 mm per minute. Samples were mounted in the load frame, using pneumatic grips, preloaded to 0.1 N, and the gauge length was measured (approximately 10 mm). The load frame applied tensile loads to the samples perpendicular to the abdominal midline until tissue disruption occurred. Data analysis was performed using the Merlin materials testing software package (Version 5.41.00; Instron Corporation) from which the following mechanical properties were determined: breaking strength, the maximum load force at mechanical failure (N); tensile strength, the maximum force per unit sample area (N/mm2); yield load, the force at which irreversible tissue deformity occurs (N); yield energy (mJ); and stiffness, the slope of the linear elastic region of the force-extension curve (N/mm).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism Version 5.00 for Windows (GraphPad Software, CA) and R Version 2.10.1 (R Foundation, www.r-project.org). One-way analysis of variance with Tukey post hoc test was used for all comparisons between multiple groups. Significance level was set at P < 0.05 for all statistical analyses. Results from UC, SC, and UR groups were compared to determine whether repaired laparotomy without hernia formation (SC) is associated with pathological abdominal wall changes at 10 weeks. SC, UR, PR, and MR were compared to assess whether the pathological changes associated with hernia formation were reversed after repair.
RESULTS
All animals gained weight appropriately throughout the experiment, and average weights were similar between all groups at the start of the experiment, at the time of hernia repair (primary and mesh), and at final killing. All rats in the hernia model developed large abdominal wall defects, and there were no significant differences in hernia sizes between groups at the time of repair (5 weeks) or abdominal wall harvest (10 weeks). These data are summarized in Table 1. No fascial defects developed in the SR group or after PR. No wound infections were noted.
TABLE 1.
Comparison of Group Weights and Abdominal Wall Measurements
| Uninjured Control | Sham Control | Unrepaired Hernia | Primary Repair | Mesh Repair | Significance (P)* | |
|---|---|---|---|---|---|---|
| Initial rat weight (g) | 294.4 ± 22.1 | 300.1 ± 22.7 | 288.1 ± 23.0 | 282.3 ± 20.5 | 281 ± 16.5 | 0.336 |
| Weight at hernia repair (g) | — | — | — | 449.9 ± 40.0 | 470.1 ± 46.6 | 0.367 |
| Weight when killed (g) | 538.9 ± 24.5 | 558.4 ± 40.2 | 551.3 ± 41.8 | 549.3 ± 20.3 | 532.8 ± 63.4 | 0.740 |
| Hernia size at repair (cm2) | — | — | — | 3.95 ± 1.49 | 4.12 ± 2.20 | 0.862 |
| Hernia size when killed (cm2) | — | — | 3.90 ± 1.55 | — | 3.99 ± 2.51 | 0.929 |
| Final body wall diameter (cm) | 18.6 ± 1.0 | 18.8 ± 0.9 | 16.8 ± 0.6*,# | 18.8 ± 1.1† | 16.9 ± 1.7*,#,‡ | 0.002 |
Values are shown as mean ± SD.
All-group comparisons performed by 1-way ANOVA; 2 groups compared by t test.
Between-group significance by Tukey post hoc test (P < 0.05) shown compared to *UC, #SC, †UH, and .PR.
Mechanical Testing
A summary of mechanical testing results is shown in Table 2. Consistent with our previous findings at 5 weeks,19 tensiometric analysis at 10 weeks revealed greater internal oblique muscle/transversus abdominis muscle composite stiffness in the hernia group than in the control groups. Breaking strength, tensile strength, yield load, and yield energy were also higher in the hernia group, indicating greater tissue strength and toughness in addition to lower compliance. Unlike our previous findings, however, no differences were found between the uninjured and sham groups for any of these measures at 10 weeks (P = 0.835–0.972). PR did not restore any of these mechanical properties and was not different from UR (P = 0.716–1.000). MR seemed to partially reverse the mechanical changes; however, only the tensile strength was statistically different from hernia (P = 0.030) and PR (P = 0.001). The statistical power was insufficient to differentiate changes in the other mechanical properties after MR from either hernia (P = 0.481–0.747) or sham (P = 0.164–0.998).
TABLE 2.
Tensiometric Analysis of IOM/TAM Composite
| Mechanical Property |
Uninjured Control |
Sham Control |
Unrepaired Hernia |
Significance (P)* | Primary Repair |
Mesh Repair |
Significance (P)** |
|---|---|---|---|---|---|---|---|
| Breaking strength (N) | 5.32 ± 2.05 | 5.58 ± 2.40 | 7.38 ± 1.79*,# | 0.002 | 7.75 ± 2.31# | 6.51 ± 1.58 | 0.002 |
| Tensile strength (N/mm2) | 0.29 ± 0.11 | 0.28 ± 0.10 | 0.36 ± 0.09*,# | 0.010 | 0.40 ± 0.16# | 0.27 ± 0.07† | <0.001 |
| Yield load (N) | 4.18 ± 1.18 | 4.29 ± 1.96 | 5.78 ± 1.67*,# | 0.002 | 5.88 ± 2.46# | 5.06 ± 1.48 | 0.018 |
| Yield energy (mJ) | 16.04 ± 8.36 | 17.96 ± 10.84 | 28.25 ± 14.72*,# | 0.001 | 28.57 ± 14.43# | 22.67 ± 13.55 | 0.022 |
| Stiffness (N/mm) | 0.46 ± 0.12 | 0.44 ± 0.16 | 0.63 ± 0.22*,# | <0.001 | 0.61 ± 0.27# | 0.57 ± 0.16 | 0.012 |
Values are shown as mean ± SD.
One-way analysis of variance comparing UC, SC and UR.
One-way analysis of variance comparing SC, UR, PR, and MR.
Between-group significance by Tukey post hoc test (P < .05) shown compared to *UC, #SC, and †UR.
Muscle Fiber Analysis
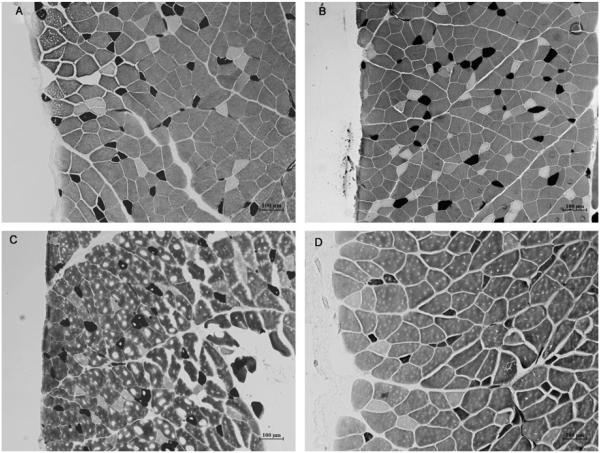
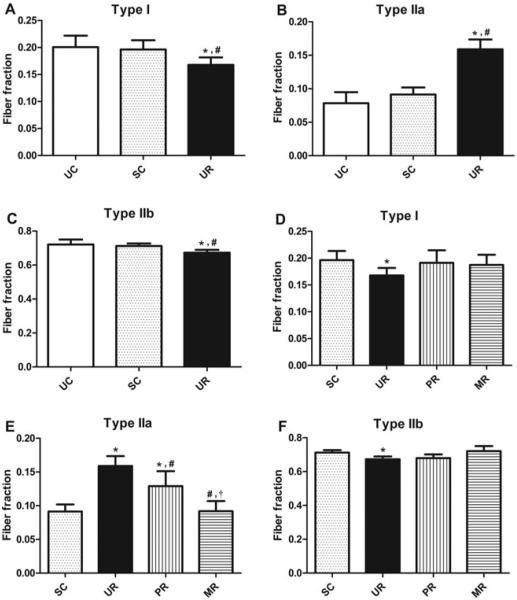
Myofibrillar ATPase staining classified muscle samples into type I (slow-twitch), type IIa (intermediate fast-twitch), and type IIb (fast-twitch) fibers (Figs. 4A–D). Fiber-type distribution for uninjured controls was similar to that previously reported in normal rat internal oblique muscles.33 At 10 weeks, internal oblique muscle fibers in the UR group demonstrated a fiber-type shift (Figs. 5A–C) with an increase in type IIa fibers (15.9%) compared to UC (7.8%; P<0.001) versus SC (9.1%; P < 0.001), and a relative decrease in type I (16.8% vs 20.1%; P = 0.003; and 16.8% vs 19.6%; P < 0.05, respectively) and IIb fibers (6.7% vs 7.2%; P < 0.001; and 6.7% vs 7.1%; P = 0.004, respectively). This similar pattern of fiber-type shift, but to a lesser degree, previously identified in SCs versus uninjured controls at 5 weeks was not seen at 10 weeks (P = 0.870, 0.189, and 0.712 for type I, IIa, and IIb fibers, respectively) and normal composition seems to have been restored. PR and MR had no clear effect on type I or type IIb fiber composition (Figs. 5D and F). Both repairs led to a decrease in type IIa fibers (Fig. 5E); however, PR resulted in only partial restoration (12.9% vs 15.9% UR; P = 0.005; and 12.9% vs 9.1% SC; P<0.001), and to a lesser extent than MR (12.9% vs 9.2%; P < 0.001). MR fully reversed the Type IIa increase (9.2% vs 15.9% UR; P < 0.001; and 9.2% vs 9.1% SC; P = 1.000).
FIGURE 4.
Representative photomicrographs of internal oblique myofibrillar ATPase staining. A, SR. B, UR. C, PR. D, MR. The smaller, dark-staining fibers are classified as type I, the intermediate size, pale-staining fibers are classified as type IIa, and the larger, intermediate-staining fibers are classified as type IIb. Note the greater number of type IIa fibers and overall smaller fiber size in the UR and PR groups, consistent with muscle atrophy.
FIGURE 5.
Average muscle fiber fraction by fiber type: A and D, type I. B and E, type IIa. C and F, Type IIb. Error bars shown as ±standard deviation. Statistical significance (P < 0.05) determined by 1-way analysis of variance with Tukey post hoc test: for A–C, compared to *UC and #SC; for D–F, compared to *SC, #UR, and †PR.
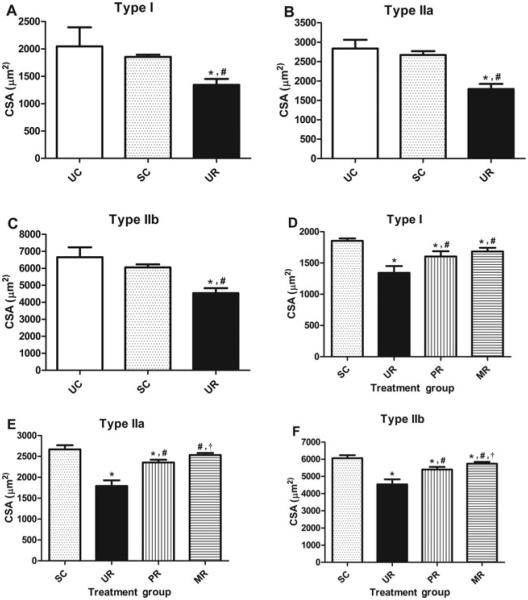
Figures 6A–C compare the average muscle fiber CSAs for hernia and control groups by fiber type. Average fiber size for uninjured controls was similar to that previously reported in normal rat internal oblique muscles.33 By 10 weeks, the previously identified differences at 5 weeks between uninjured and sham were not seen in any fiber type. Hernia formation was once again associated with a decrease in all 3 fiber types compared to uninjured (P < 0.001 for all fiber types) and sham (P < 0.001 for all fiber types) controls. Muscle fiber sizes for the sham, UR, PR, and MR groups are shown in Figures 6D–F. PR partially restored fiber size compared to sham (P < 0.001 for all fiber types) and hernia (P < 0.001 for all fiber types) groups. MR fully restored type IIa, and partially restored I and IIb fiber sizes, and was associated with significantly larger type IIa and type IIb fibers compared to PR (P < 0.01 for both fiber types).
FIGURE 6.
Average muscle fiber size by fiber type: A and D, type I. B and E, type IIa. C and F, Type IIb. Error bars shown as ±SD. Statisti- cal significance (P < .05) determined by 1-way ANOVA with Tukey post hoc test: for A–C, compared to *UC and #SC; for D–F, compared to *SC, #UR, and †PR.
DISCUSSION
We have demonstrated that hernia formation is associated with internal oblique muscle myopathic changes consistent with disuse atrophy and fibrosis. PR partially reversed the fiber-type changes associated with muscle atrophy but failed to improve the mechanical properties of the stiff, tough abdominal wall. MR, without restoring internal oblique muscle length, more completely reversed muscle atrophy; however, the effects on the mechanical properties of the abdominal wall were indeterminate.
Previously, we identified myopathic changes in SR compared with uninjured controls at 5 weeks, indicating that laparotomy alone with intact fascial repair results in similar changes as seen in the hernia group, but to a lesser degree.19 This suggests that other biomechanical changes occur after laparotomy and hernia formation in addition to muscle shortening. These factors may be related to pain and decreased mobility leading to relative muscle disuse or direct sequelae of the fascial injury itself. In this study, we found no differences between SC and UC at 10 weeks, suggesting that the changes attributed to these additional factors are reversible at later time points. Therefore, observed differences between uninjured and hernia groups from this point onward are likely due to the mechanical effects of hernia formation alone.
Other models of muscle unloading, including suspension, immobilization, and tenotomy, consistently demonstrate myopathic changes of disuse atrophy. Within 2 days histological evidence of muscle degeneration can be seen,34 and muscle atrophy with a reduction in fiber diameter begins within 3 to 5 days.21,23,35 A fiber-type shift from slow (type I) to fast (type II) occurs, particularly with an increase in type IIa fibers.19,22,23,36,37 Increased collagen deposition in the perimysium and endomysium can be identified within 1 week, ultimately resulting in fibrosis with disruption of normal collagen fiber orientation and increased connective tissue density.24,25 These pathological changes associated with muscle atrophy seem to peak by 3 to 5 weeks.22,24,38 Slow twitch predominant muscles, such as the soleus and medial gastrocnemius, are more susceptible to disuse atrophy than fast twitch muscles, such as the plantaris, tibialis anterior, and extensor digitorum longus.21,22,39–41 The muscles of the abdominal wall, including the internal and external obliques, transversus abdominis, and rectus are composed primarily of slow twitch fibers33 and therefore would be expected to develop disuse atrophy, as we have redemonstrated. Degenerative muscle changes and alterations to the intramuscular connective tissue, including increased and disordered collagen production, likely account for the pathological mechanical properties we observed in the herniated abdominal wall.
Many studies have described atrophic muscle recovery with reloading. Although there is variation between animal species and muscles studied, reloaded muscle generally recovers to its normal or near normal state in terms of fiber-type distribution and size. In some studies, muscle unloaded for 14 to 28 days recovered normal or close to normal fiber size and distribution after 5 to 28 days of reloading, with the length of recovery somewhat approximating the length of prior unloading.23,37,42,43 A study of 5 week hindlimb suspension in rats found complete restoration of muscle fiber and CSA after 8 weeks’ recovery.36 On the basis of these studies, our selected time points using a 5 week hernia model with abdominal wall harvest an additional 5 weeks after repair should allow adequate time for changes associated with disuse atrophy to occur, and sufficient time to assess for muscle recovery after reloading.
Unexpectedly, PR was less effective than MR in reversing internal oblique muscle atrophy and failed to restore mechanical compliance despite reestablishing midline fascial attachment and abdominal wall muscle length. Compared to muscle suspension or immobilization, tenotomy creates the additional insult of shortening the affected muscle. Myofibrillar remodeling restores the initially kinked sarcomeres to normal length by approximately 4 to 6 weeks with a reduction in sarcomere number to accommodate the new muscle position and restore optimal length for active force production.34,44 The muscle shortening that accompanies fascial separation and hernia formation requires sarcomere remodeling to adapt to the new position. During repair, significant tension was required to reapproximate the fascial edges and restore the abdominal wall muscles to their preoperative positions. This may have led to overstretching of the chronically shortened sarcomeres. Remodeling must occur once again to add more sarcomeres.45 Compared to tension-free MR, which restores some degree of midline muscle attachment and force without altering muscle length, the process of sarcomere remodeling after PR may delay recovery from atrophy.
Mesh repair resulted in almost full atrophy reversal at 5 weeks, whereas PR resulted in partial reversal. Given sufficient time, the muscle atrophy would likely be completely or nearly restored to normal by PR. An important question, therefore, is whether the findings of delayed recovery from atrophy in the primary compared with the mesh groups is significant, given that complete or near complete restoration will occur at a later time. At 5 weeks, however, early injury to the healing laparotomy repair may have already begun. Previous studies have established that primary ventral hernias most commonly develop from early laparotomy wound separation and failure within the first postoperative month.1,9 Although this has not been established for hernia recurrences, it is likely that similar early wound failures lead to recurrence at least in a significant portion of cases, if not most. After hernia repair, the stiff, atrophic musculature would less effectively absorb abdominal wall forces, transferring them to the weakest point–the vulnerable healing repair wound. Wound strength only recovers to approximately 20% of normal in the first 3 weeks after injury,46 achieving its maximum strength after approximately 12 weeks.47 Hernia repair in this setting creates an impedance mismatch at the wound healing interface between the noncompliant abdominal wall muscles and the relatively weak repair wound, potentially resulting in mechanical wound failure and an increased risk of recurrent hernia formation. Hernia repair techniques that protect the fascial closure, such as with the use of reinforcing mesh, may provide ben- efit by protecting the wound until adequate strength has developed and/or abdominal wall atrophy and compliance have recovered.
Our finding of improved recovery from atrophy and mechanical stiffness with tension-free MR compared with PR has other potential implications for optimizing hernia repair technique, particularly in cases of large ventral hernias. Given that excessive tension may delay muscle recovery, there may be an optimal range of muscle length and tension during repair that results in sufficient reloading forces, while avoiding excessive tension that may delay recovery. Techniques such as component separation,48 which allows medial mobilization of the rectus, internal oblique and transversus abdominis muscles through myofascial release of the external oblique muscle, may provide the benefit of reestablishing normal midline insertion of these muscles while reducing muscle strain. A technique of progressive fascial closure49,50 or other staged procedures51 may provide the benefit of partial abdominal wall muscle reloading while avoiding excessive tension and more closely matching the rate of sarcomere synthesis with increasing muscle length to achieve fascial closure. Some of these techniques may not be feasible in the elective setting, however, and it remains to be studied whether these techniques allow for faster recovery from muscle atrophy and restoration of abdominal wall compliance. It is also possible that the elastic properties of the mesh itself may contribute to the rate of the abdominal wall recovery. Mechanical characteristics vary significantly between mesh constructed of different materials, thickness, and filament diameter.52,53 These properties and resorption characteristics of absorbable and biological meshes may influence recovery as well.
In summary, this study finds that tension-free MR more completely reverses the myopathic changes associated with hernia formation than PR in a rat model of chronic incisional hernia. This finding has potential implications for designing and evaluating hernia repair techniques and potentially for engineering mesh materials, which should be taken into account for abdominal wall muscle length, tension relationships and total abdominal wall compliance.
Acknowledgments
Disclosure: This study was supported by NIH Institute of General Medical Sciences Grant Numbers 5R01GM0782880 (M.G.F.) and 5F32GM08887302 (E.J.C.).
REFERENCES
- 1.Pollock AV, Evans M. Early prediction of late incisional hernias. Br J Surg. 1989;76:953–954. doi: 10.1002/bjs.1800760926. [DOI] [PubMed] [Google Scholar]
- 2.Seiler CM, Bruckner T, Diener MK, et al. Interrupted or continuous slowly absorbable sutures for closure of primary elective midline abdominal incisions: a multicenter randomized trial (INSECT: ISRCTN24023541) Ann Surg. 2009;249:576–582. doi: 10.1097/SLA.0b013e31819ec6c8. [DOI] [PubMed] [Google Scholar]
- 3.Hoer J, Lawong G, Klinge U, et al. Factors influencing the development of incisional hernia. A retrospective study of 2,983 laparotomy patients over a period of 10 years [in German] Chirurg. 2002;73:474–480. doi: 10.1007/s00104-002-0425-5. [DOI] [PubMed] [Google Scholar]
- 4.Mudge M, Hughes LE. Incisional hernia: a 10 year prospective study of incidence and attitudes. Br J Surg. 1985;72:70–71. doi: 10.1002/bjs.1800720127. [DOI] [PubMed] [Google Scholar]
- 5.Israelsson LA, Jonsson T. Incisional hernia after midline laparotomy: a prospective study. Eur J Surg. 1996;162:125–129. [PubMed] [Google Scholar]
- 6.Carlson MA. Acute wound failure. Surg Clin North Am. 1997;77:607–636. doi: 10.1016/s0039-6109(05)70571-5. [DOI] [PubMed] [Google Scholar]
- 7.Burger JW, Luijendijk RW, Hop WC, et al. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004;240:578–583. doi: 10.1097/01.sla.0000141193.08524.e7. discussion 583–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flum DR, Horvath K, Koepsell T. Have outcomes of incisional hernia repair improved with time? A population-based analysis. Ann Surg. 2003;237:129–135. doi: 10.1097/00000658-200301000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burger JW, Lange JF, Halm JA, et al. Incisional hernia: early complication of abdominal surgery. World J Surg. 2005;29:1608–1613. doi: 10.1007/s00268-005-7929-3. [DOI] [PubMed] [Google Scholar]
- 10.McNeil PM, Sugerman HJ. Continuous absorbable vs interrupted nonabsorbable fascial closure. A prospective, randomized comparison. Arch Surg. 1986;121:821–823. doi: 10.1001/archsurg.1986.01400070091019. [DOI] [PubMed] [Google Scholar]
- 11.Wissing J, van Vroonhoven TJ, Schattenkerk ME, et al. Fascia closure after midline laparotomy: results of a randomized trial. Br J Surg. 1987;74:738–741. doi: 10.1002/bjs.1800740831. [DOI] [PubMed] [Google Scholar]
- 12.Kendall SW, Brennan TG, Guillou PJ. Suture length to wound length ratio and the integrity of midline and lateral paramedian incisions. Br J Surg. 1991;78:705–707. doi: 10.1002/bjs.1800780623. [DOI] [PubMed] [Google Scholar]
- 13.Regnard JF, Hay JM, Rea S, et al. Ventral incisional hernias: incidence, date of recurrence, localization and risk factors. Ital J Surg Sci. 1988;18:259–265. [PubMed] [Google Scholar]
- 14.da Silva AL, Petroianu A. Incisional hernias: factors influencing development. South Med J. 1991;84:1500–1504. doi: 10.1097/00007611-199112000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Bucknall TE, Cox PJ, Ellis H. Burst abdomen and incisional hernia: a prospective study of 1129 major laparotomies. Br Med J (Clin Res Ed) 1982;284:931–933. doi: 10.1136/bmj.284.6320.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugerman HJ, Kellum JM, Jr, Reines HD, et al. Greater risk of incisional hernia with morbidly obese than steroid-dependent patients and low recurrence with prefascial polypropylene mesh. Am J Surg. 1996;171:80–84. doi: 10.1016/S0002-9610(99)80078-6. [DOI] [PubMed] [Google Scholar]
- 17.Sauerland S, Korenkov M, Kleinen T, et al. Obesity is a risk factor for recurrence after incisional hernia repair. Hernia. 2004;8:42–46. doi: 10.1007/s10029-003-0161-x. [DOI] [PubMed] [Google Scholar]
- 18.Franz MG. The biology of hernias and the abdominal wall. Hernia. 2006;10:462–471. doi: 10.1007/s10029-006-0144-9. [DOI] [PubMed] [Google Scholar]
- 19.DuBay DA, Choi W, Urbanchek MG, et al. Incisional herniation induces decreased abdominal wall compliance via oblique muscle atrophy and fibrosis. Ann Surg. 2007;245:140–146. doi: 10.1097/01.sla.0000251267.11012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franz MG. The biology of hernia formation. Surg Clin North Am. 2008;88:1–15. doi: 10.1016/j.suc.2007.10.007. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol. 1990;68:1–12. doi: 10.1152/jappl.1990.68.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Desplanches D, Mayet MH, Sempore B, et al. Structural and functional responses to prolonged hindlimb suspension in rat muscle. J Appl Physiol. 1987;63:558–563. doi: 10.1152/jappl.1987.63.2.558. [DOI] [PubMed] [Google Scholar]
- 23.Oishi Y, Ogata T, Yamamoto KI, et al. Cellular adaptations in soleus muscle during recovery after hindlimb unloading. Acta Physiol (Oxf) 2008;192:381–395. doi: 10.1111/j.1748-1716.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- 24.Jarvinen TA, Jozsa L, Kannus P, et al. Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscles. An immunohistochemical, polarization and scanning electron microscopic study. J Muscle Res Cell Motil. 2002;23:245–254. doi: 10.1023/a:1020904518336. [DOI] [PubMed] [Google Scholar]
- 25.Jozsa L, Kannus P, Thoring J, et al. The effect of tenotomy and immobilisation on intramuscular connective tissue. A morphometric and microscopic study in rat calf muscles. J Bone Joint Surg Br. 1990;72:293–297. doi: 10.1302/0301-620X.72B2.2312572. [DOI] [PubMed] [Google Scholar]
- 26.Franz MG, Kuhn MA, Nguyen K, et al. Early biomechanical wound failure: a mechanism for ventral incisional hernia formation. Wound Repair Regen. 2001;9:140–141. [Google Scholar]
- 27.DuBay DA, Wang X, Adamson B, et al. Progressive fascial wound failure impairs subsequent abdominal wall repairs: a new animal model of incisional hernia formation. Surgery. 2005;137:463–471. doi: 10.1016/j.surg.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Dubay DA, Wang X, Kuhn MA, et al. The prevention of incisional hernia formation using a delayed-release polymer of basic fibroblast growth factor. Ann Surg. 2004;240:179–186. doi: 10.1097/01.sla.0000131576.12153.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooke MH, Kaiser KK. Muscle fiber types: how many and what kind? Arch Neurol. 1970;23:369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- 30.Brooke MH, Kaiser KK. Three “myosin adenosine triphosphatase” systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970;18:670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- 31.Dubowitz V, Brooke MH. Muscle Biopsy: A Modern Approach. W. B. Saunders; London, United Kingdom: 1973. [Google Scholar]
- 32.Sheehan DC, Hrapchak BB. Theory and Practice of Histotechnology. 2nd Battelle Press; Columbus, OH: 1987. [Google Scholar]
- 33.Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- 34.Baker JH, Matsumoto DE. Adaptation of skeletal muscle to immobilization in a shortened position. Muscle Nerve. 1988;11:231–244. doi: 10.1002/mus.880110308. [DOI] [PubMed] [Google Scholar]
- 35.Rantanen J, Hurme T, Kalimo H. Calf muscle atrophy and Achilles tendon healing following experimental tendon division and surgery in rats. Comparison of postoperative immobilization of the muscle-tendon complex in relaxed and tensioned positions. Scand J Med Sci Sports. 1999;9:57–61. doi: 10.1111/j.1600-0838.1999.tb00208.x. [DOI] [PubMed] [Google Scholar]
- 36.Desplanches D, Mayet MH, Sempore B, et al. Effect of spontaneous recovery or retraining after hindlimb suspension on aerobic capacity. J Appl Physiol. 1987;63:1739–1743. doi: 10.1152/jappl.1987.63.5.1739. [DOI] [PubMed] [Google Scholar]
- 37.Kasper CE, White TP, Maxwell LC. Running during recovery from hindlimb suspension induces transient muscle injury. J Appl Physiol. 1990;68:533–539. doi: 10.1152/jappl.1990.68.2.533. [DOI] [PubMed] [Google Scholar]
- 38.Tomanek RJ, Lund DD. Degeneration of different types of skeletal muscle fibres. II. Immobilization. J Anat. 1974;118:531–541. [PMC free article] [PubMed] [Google Scholar]
- 39.Shaw SR, Zernicke RF, Vailas AC, et al. Mechanical, morphological and biochemical adaptations of bone and muscle to hindlimb suspension and exercise. J Biomech. 1987;20:225–234. doi: 10.1016/0021-9290(87)90289-2. [DOI] [PubMed] [Google Scholar]
- 40.Roy RR, Bello MA, Bouissou P, et al. Size and metabolic properties of fibers in rat fast-twitch muscles after hindlimb suspension. J Appl Physiol. 1987;62:2348–2357. doi: 10.1152/jappl.1987.62.6.2348. [DOI] [PubMed] [Google Scholar]
- 41.Flynn DE, Max SR. Effects of suspension hypokinesia/hypodynamia on rat skeletal muscle. Aviat Space Environ Med. 1985;56:1065–1069. [PubMed] [Google Scholar]
- 42.Fluck M, Schmutz S, Wittwer M, et al. Transcriptional reprogramming during reloading of atrophied rat soleus muscle. Am J Physiol Regul Integr Comp Physiol. 2005;289:R4–R14. doi: 10.1152/ajpregu.00833.2004. [DOI] [PubMed] [Google Scholar]
- 43.Warren GL, Stallone JL, Allen MR, et al. Functional recovery of the plan-tarflexor muscle group after hindlimb unloading in the rat. Eur J Appl Physiol. 2004;93:130–138. doi: 10.1007/s00421-004-1185-3. [DOI] [PubMed] [Google Scholar]
- 44.Baker JH, Hall-Craggs EC. Recovery from central core degeneration of the tenotomized rat soleus muscle. Muscle Nerve. 1980;3:151–159. doi: 10.1002/mus.880030208. [DOI] [PubMed] [Google Scholar]
- 45.Tabary JC, Tabary C, Tardieu C, et al. Physiological and structural changes in the cat’s soleus muscle due to immobilization at different lengths by plaster casts. J Physiol. 1972;224:231–244. doi: 10.1113/jphysiol.1972.sp009891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clark RA. Biology of dermal wound repair. Dermatol Clin. 1993;11:647–666. [PubMed] [Google Scholar]
- 47.Burke M. Scars. Can they be minimised? Aust Fam Physician. 1998;27:275–278. [PubMed] [Google Scholar]
- 48.Ramirez OM, Ruas E, Dellon AL. “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg. 1990;86:519–526. doi: 10.1097/00006534-199009000-00023. [DOI] [PubMed] [Google Scholar]
- 49.Tieu BH, Cho SD, Luem N, et al. The use of the Wittmann Patch facilitates a high rate of fascial closure in severely injured trauma patients and critically ill emergency surgery patients. J Trauma. 2008;65:865–870. doi: 10.1097/TA.0b013e31818481f1. [DOI] [PubMed] [Google Scholar]
- 50.Garner GB, Ware DN, Cocanour CS, et al. Vacuum-assisted wound closure provides early fascial reapproximation in trauma patients with open abdomens. Am J Surg. 2001;182:630–638. doi: 10.1016/s0002-9610(01)00786-3. [DOI] [PubMed] [Google Scholar]
- 51.Fabian TC, Croce MA, Pritchard FE, et al. Planned ventral hernia. Staged management for acute abdominal wall defects. Ann Surg. 1994;219:643–650. doi: 10.1097/00000658-199406000-00007. discussion 651–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deeken CR, Abdo MS, Frisella MM, et al. Physicomechanical evaluation of polypropylene, polyester, and polytetrafluoroethylene meshes for inguinal hernia repair. J Am Coll Surg. 2011;212:68–79. doi: 10.1016/j.jamcollsurg.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 53.Deeken CR, Abdo MS, Frisella MM, et al. Physicomechanical evaluation of absorbable and nonabsorbable barrier composite meshes for laparoscopic ventral hernia repair. Surg Endosc. 2011;25:1541–1552. doi: 10.1007/s00464-010-1432-0. [DOI] [PubMed] [Google Scholar]