Abstract
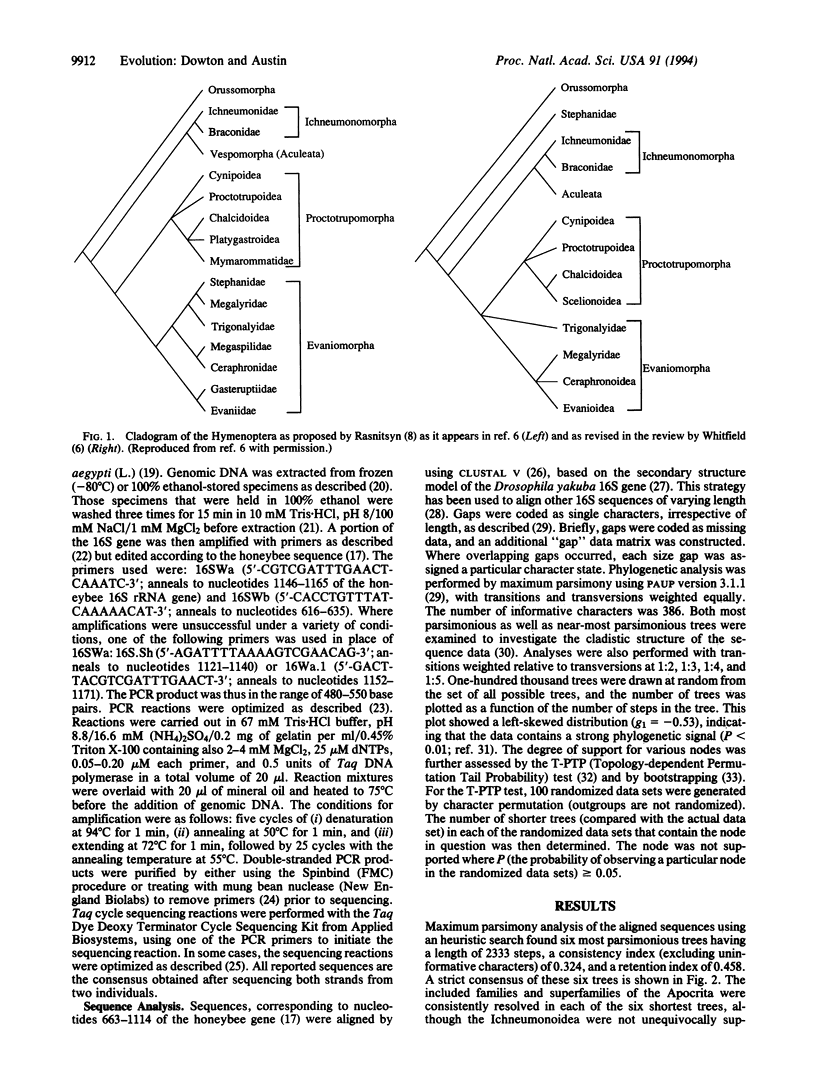
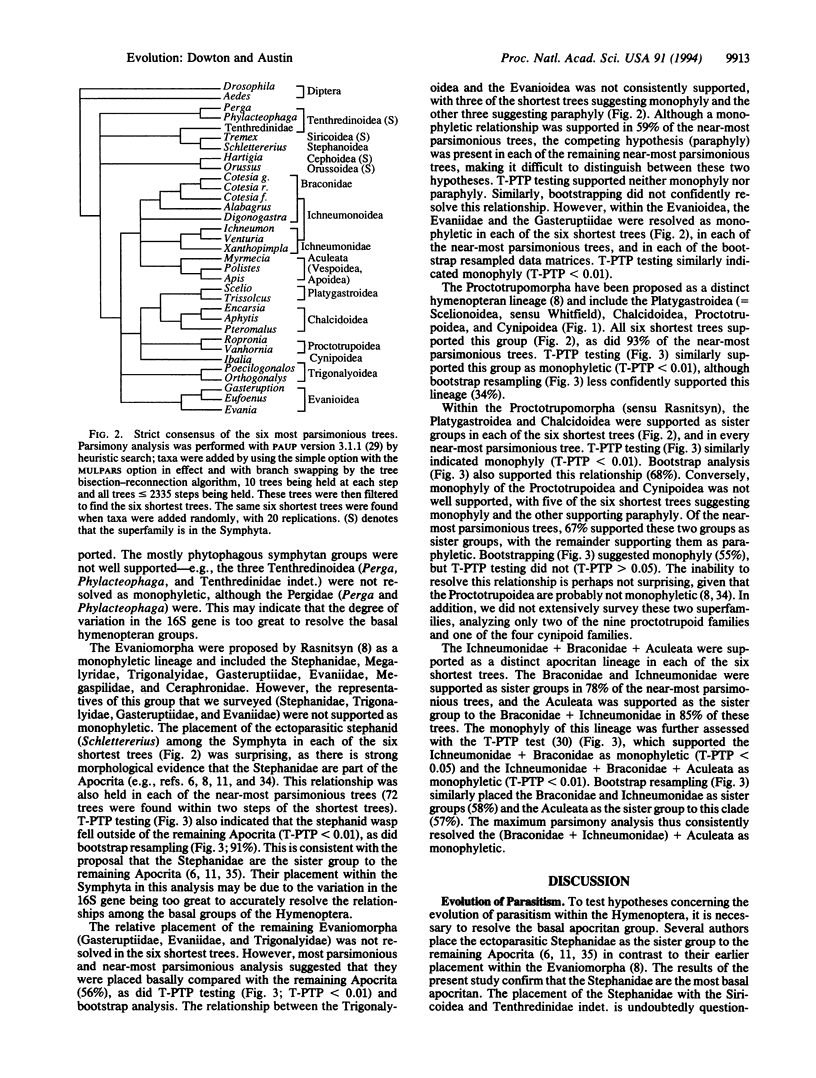
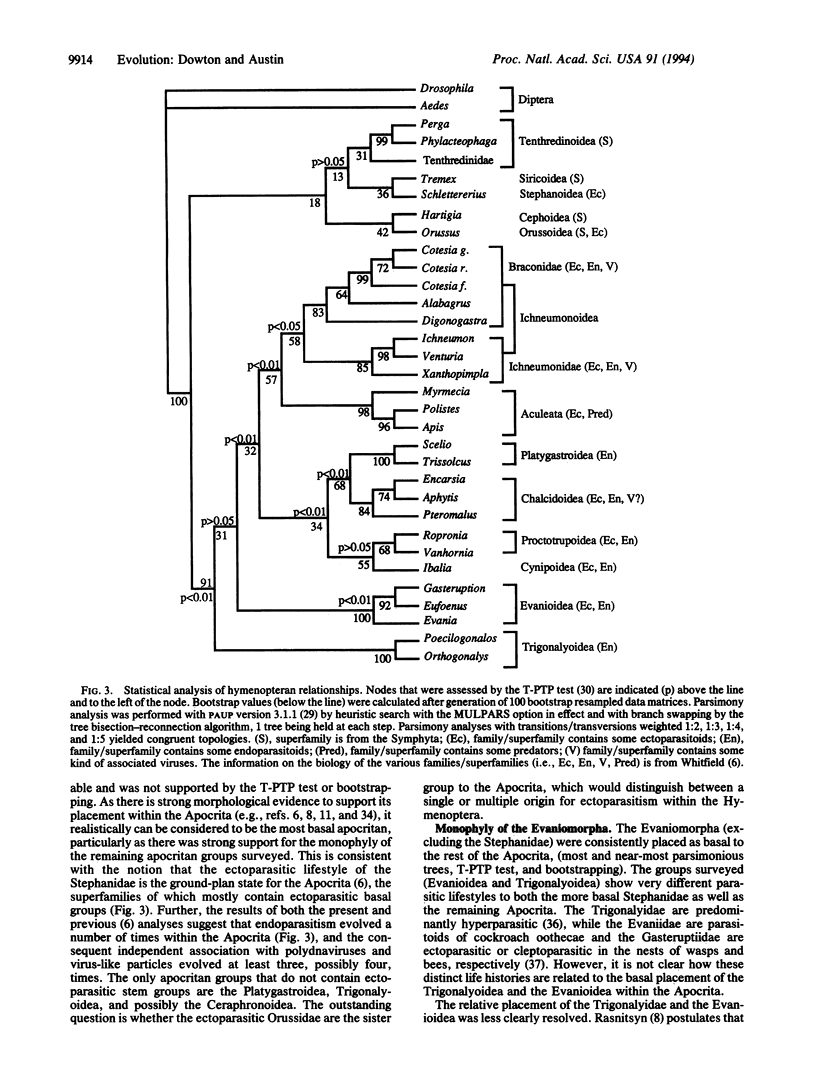
Phylogenetic relationships among the major groups of hymenopteran insects were investigated by using comparative sequence information from the mitochondrial 16S rRNA gene. The placement of the ectoparasitic Stephanidae as the sister group to the remaining Apocrita confirmed ectoparasitism as the ground plan biology for the Apocrita. Endoparasitism evolved at least eight times within the Apocrita, and the consequent association with polydnaviruses and virus-like particles evolved at least three times. The Evaniomorpha were consistently placed as basal to the remaining Apocrita but were not resolved as monophyletic. The Gasteruptiidae were resolved as the sister group to the Evaniidae, but the relationship between the Trigonalyoidea and the Evanioidea was unclear. The Proctotrupomorpha (sensu Rasnitsyn) was resolved by topology-dependent permutation tail probability (T-PTP) testing as monophyletic, with strong evidence for a sister group relationship between the Platygastroidea and the Chalcidoidea. Strong evidence was found for the monophyly of the Ichneumonomorpha (Ichneumonidae + Braconidae) and the sister-group relationship between the Aculeata (Vespomorpha) and the Ichneumonomorpha.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cameron J. S. A clinician's view of the classification of glomerulonephritis. Perspect Nephrol Hypertens. 1973;1(Pt 1):63–79. [PubMed] [Google Scholar]
- Cameron S. A. Multiple origins of advanced eusociality in bees inferred from mitochondrial DNA sequences. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8687–8691. doi: 10.1073/pnas.90.18.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmean D., Kimsey L. S., Berbee M. L. 18S rDNA sequences and the holometabolous insects. Mol Phylogenet Evol. 1992 Dec;1(4):270–278. doi: 10.1016/1055-7903(92)90002-x. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The ribosomal RNA genes of Drosophila mitochondrial DNA. Nucleic Acids Res. 1985 Jun 11;13(11):4029–4045. doi: 10.1093/nar/13.11.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C. W., Blackstone N. W., Buss L. W. Evolution of king crabs from hermit crab ancestors. Nature. 1992 Feb 6;355(6360):539–542. doi: 10.1038/355539a0. [DOI] [PubMed] [Google Scholar]
- Derr J. N., Davis S. K., Woolley J. B., Wharton R. A. Reassessment of the 16S rRNA nucleotide sequence from members of the parasitic Hymenoptera. Mol Phylogenet Evol. 1992 Dec;1(4):338–341. doi: 10.1016/1055-7903(92)90008-5. [DOI] [PubMed] [Google Scholar]
- Derr J. N., Davis S. K., Woolley J. B., Wharton R. A. Variation and the phylogenetic utility of the large ribosomal subunit of mitochondrial DNA from the insect order Hymenoptera. Mol Phylogenet Evol. 1992 Jun;1(2):136–147. doi: 10.1016/1055-7903(92)90025-c. [DOI] [PubMed] [Google Scholar]
- Dowton M., Austin A. D. A simple method for finding optimal conditions for the direct sequencing of PCR products. Biotechniques. 1994 May;16(5):816–817. [PubMed] [Google Scholar]
- Dowton M., Austin A. D. Direct sequencing of double-stranded PCR products without intermediate fragment purification; digestion with mung bean nuclease. Nucleic Acids Res. 1993 Jul 25;21(15):3599–3600. doi: 10.1093/nar/21.15.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H. G., Muralidharan K. Evidence from mitochondrial DNA that African honey bees spread as continuous maternal lineages. Nature. 1989 May 18;339(6221):211–213. doi: 10.1038/339211a0. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Bleasby A. J., Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992 Apr;8(2):189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- Hillis D. M., Huelsenbeck J. P. Signal, noise, and reliability in molecular phylogenetic analyses. J Hered. 1992 May-Jun;83(3):189–195. doi: 10.1093/oxfordjournals.jhered.a111190. [DOI] [PubMed] [Google Scholar]
- HsuChen C. C., Kotin R. M., Dubin D. T. Sequences of the coding and flanking regions of the large ribosomal subunit RNA gene of mosquito mitochondria. Nucleic Acids Res. 1984 Oct 25;12(20):7771–7785. doi: 10.1093/nar/12.20.7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milinkovitch M. C., Ortí G., Meyer A. Revised phylogeny of whales suggested by mitochondrial ribosomal DNA sequences. Nature. 1993 Jan 28;361(6410):346–348. doi: 10.1038/361346a0. [DOI] [PubMed] [Google Scholar]
- Vlasak I., Burgschwaiger S., Kreil G. Nucleotide sequence of the large ribosomal RNA of honeybee mitochondria. Nucleic Acids Res. 1987 Mar 11;15(5):2388–2388. doi: 10.1093/nar/15.5.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]



