Abstract
We report a simple and versatile method for grafting polymers on nanoparticles. A procedure was developed for the synthesis and subsequent functionalization of silica nanoparticles with a perfluorophenylazide. Polymers were then grafted by the photochemically induced insertion reactions of the perfluorophenylnitrene. Polystyrene, poly(4-vinylpyridine), and poly(2-ethyl-2-oxazoline) were successfully grafted on silica nanoparticles. Grafting density was studied with regard to polymer concentration and molecular weight.
Introduction
Synthesis of core–shell nanohybrids, consisting of an inorganic nanoparticle core and a polymer shell, is a topic of great interest. Surface coatings such as polymers markedly influence particle behavior and spatial distribution.1 Properties including solubility, dispersibility, corrosion resistance, biocompatibility, and mechanical stability can be conveniently altered by applying surface coatings to nanoparticles. Furthermore, the synergistic interactions enable hybrid nanomaterials to display properties that are not apparent in the constituent materials alone. These nanoparticles have already demonstrated potentials in a wide range of applications including sensors, drug delivery, biomedical devices, optics, electronics, and catalysis.2-9
A number of methods have been employed for coating nanoparticles with polymers. In traditional colloidal chemistry, polymers are often coated on nanoparticles by physisorption, thus providing a passivation layer preventing the nanoparticles from aggregation or degradation.10 A popular deposition procedure employs polyelectrolytes as the coating materials, which relies on the Coulombic attraction between charged nanoparticles and the oppositely charged polyelectrolyte polymer.11-14 After the first layer is coated, additional layers can be deposited by using polyelectrolytes of opposite charges, and therefore the thickness of the coating can be controlled by the number of polyelectrolyte layers used in the deposition. However this approach is limited to polymers that are available in the polyelectrolyte form. More frequently, polymers are attached to nanoparticle surfaces through covalent linkages. The strong covalent bonds often yield markedly improved properties and enhanced performance for the resulting materials. Covalent immobilization generally falls into one of the two categories, the first of which is the graft-from approach where the polymer is produced in situ by way of chain growth polymerization or surface-initiated polymerization. A common practice is to immobilize a polymerization initiator on nanoparticles and subsequently grow polymer chains from the surface by the addition of monomers.15-22 Surface-bound polymers prepared by the graft-from technique have a higher grafting density, forming the so-called polymer brushes. A strong driving force for the brush conformation is the covalent bond formation that compensates for the entropy loss resulting from the polymer chains stretching away from the surface. The second covalent attachment method is the graft-to approach that employs polymers having functional groups that can react with the surface functionalities on the nanoparticles. Frequently the polymers are end-functionalized and are chemisorbed onto the substrate by coupling, for example, thiols/dilfides to metallic surfaces such as gold or silver, silanes to silicon oxide, or phosphates to titanium oxide surfaces.10,19,23-26
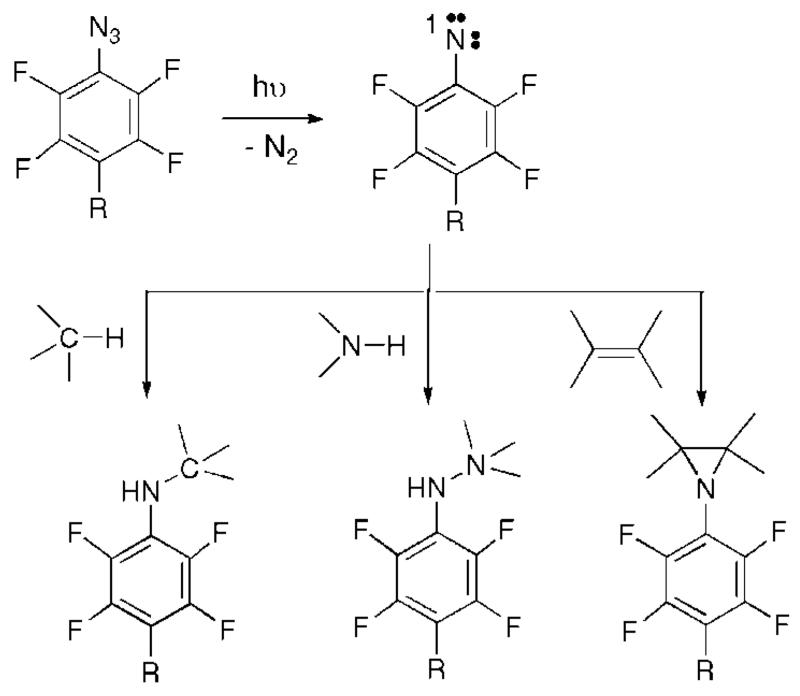
Herein we report a general approach to the covalent attachment of polymers to nanoparticles. This approach relies on the photochemistry of perfluorophenylazides (PFPAs). Irradiation of PFPAs generates highly reactive singlet perfluorophenylnitrenes that can subsequently undergo C–H, N–H insertion and C=C addition reactions with the neighboring molecules (Scheme 1).27,28 The functional group R serves as the anchoring point to the nanoparticle, whereas the azido groups form covalent bonds to the polymer upon UV activation. The functionalized PFPA therefore acts as a coupling agent attaching polymers to nanoparticles surfaces. The F atoms on the phenyl group increase the lifetime of the perfluorophenyl nitrene that subsequently undergoes insertion reactions.29 Without F, the photogenerated singlet nitrene quickly rearranges to the corresponding seven-membered ketenimine, which reacts with amines to give azepinamines, or produces polymer tars in the absence of a nucleophile. This immobilization technique can be considered as a graft-to approach, although it differs from the conventional protocol that the polymer is attached to the substrate by insertion reactions that would unlikely occur at the chain ends because of the low statistical probability. A more likely scenario is the bonding of the polymer via the train segments of the polymer. An advantage of this technique is the possibility to immobilize a wide variety of molecules because no special functional groups are necessary for the attachment. In addition, the R group can be selected depending on the chemical composition of the nanoparticle. This approach is therefore general with respect to both the molecule to be attached and the type of the nanoparticle material.
Scheme 1. Simplified Schematic of the Photochemical Reactions of Perfluorophenylazides.
Experimental Section
Materials
3-Aminopropyltrimethoxysilane (United Chemical Technologies, Bristol, PA), methyl pentafluorobenzoate (99%, Aldrich), sodium azide (99%, Aldrich), dicyclohexylcarbodiimide (99%, Aldrich), n-octadecyltrimethoxysilane (Gelest, Morrisville, PA), tetraethyl orthosilicate (99%, Aldrich), ammonium hydroxide (30%, J.T. Baker), ethanol (200 proof, Aaper), CHCl3 and CH2C12 (Fischer) were used as received. Polystyrene (PS, average molecular weight 280 000), poly(2-ethyl-2-oxazoline) (PEOX) (average molecular weight 500 000, 200 000, and 50 000), and poly(4-vinyl pyridine) (P4VP) (average molecular weight 160 000) were purchased from Aldrich and were used as received. CDCl3 used in the NMR analysis was purchased from Cambridge Isotope Laboratories (Andover, MA). The 280-nm long pass optical filter was purchased from Schott Glass Technologies, Inc. (Fullerton, CA).
Instruments
Photolysis was carried out with a medium pressure Hg lamp (450 W, Hanovia). The lamp reached its full power after an ~2 min warm up to an intensity of 5 mW/cm2 as measured by a power meter using a 365 nm sensor. IR spectra were obtained on a Perkin-Elmer Spectrum RX I model 1600. The 1H NMR spectroscopy was performed on a Nicolet NT-500 MHz FT-NMR. To prepare samples for the NMR experiments, polymer-coated nanoparticles were first dried in an oven at 75 °C overnight, then 10.0 mg of the dry particles was dispersed in 0.5 mL of CDCl3 in the NMR tube; particle dispersion was achieved by sonication. All spectra were acquired at 64 scans. Scanning electron microscopy (SEM) analysis was performed on an FEI Siron XL30 SEM. Samples were prepared by placing a drop of the nanoparticle suspension onto a silica wafer and letting the solvent evaporate. Thermogravimetric analysis (TGA) was carried out on a Mettler Toledo thermogravimetric analyzer, Model TGA/SDTA851e. The samples were prepared by centrifugation and removal of the supernatant followed by drying for several days in an oven at 80 °C. TGA experiments were carried out by heating the sample in air to 1000 °C at a rate of 10 °C/min. Elemental analyses were performed by Quantitative Technologies, Inc. (Whitehouse, NJ).
Synthesis of Silica Nanoparticles
Silica particles were synthesized following the Stöber procedure.30 TEOS (2.8 mL) was added to 200 proof EtOH (34 mL) followed by the addition of NH4OH (2.8 mL). The reaction was allowed to proceed at room temperature for at least 8 h with vigorous stirring. This recipe resulted in a suspension that was 2.7% solids by weight as determined by gravimetric analysis. The particle diameters were determined using SEM by measuring the diameters of ~100 particles at random and taking the average.
Functionalization of Silica Nanoparticles
PFPA–silane was synthesized following a previously reported procedure.31 Briefly, a solution of N-succinimidyl 4-azidotetrafluorobenzoate, 3-aminopropyltimethoxysilane in CH2Cl2 was stirred in argon at room temperature for 5 h. The product was purified by column chromatography on silica gel using 1:3 v/v CHCl3/hexane containing 2% methanol. To functionalize silica nanoparticles, a 3.5-fold excess of PFPA–silane, (110.0 mg, 0.277 mmol), or, in the case of the control, octadecyltrimethoxysilane (104.0 mg, 0.277 mmol), was added directly to the Stöber solution, and the mixture was stirred at room temperature overnight. The stoichiometry was calculated from the total surface area of the nanoparticles assuming PFPA–silane reacts with the surface silanol group, and each silanol group occupies a surface area of 0.6 nm2.32 The next day the mixture was brought to reflux while continuing stirring for 1 h at ~78 °C to facilitate covalent bonding of the silane to the silica. Functionalized silica nanoparticle suspension (9.0 mL) was centrifuged (10 min, 10 000 rpm) and was redispersed in the fresh solvent by sonication. This centrifugation/redispersion procedure was repeated five times, with the first three rinses in EtOH and the last two in CHCl3. After the last centrifugation, the nanoparticles were redispersed in 5 mL of CHCl3 and were transferred into a 50-mL beaker.
Attachment of Polymer to Nanoparticles
PEOX, PS, or P4VP (1.0, 0.5, or 0.25 g) was added to the suspension of PFPA-functionalized silica nanoparticles in CHCl3 while stirring until the polymer was completely dissolved to give either 200, 100, or 50 mg/mL polymer solution. A 280-nm optical filter was then placed over the beaker, and the mixture was irradiated for 15 min with a medium pressure Hg lamp while stirring was continued. The unattached polymer was removed from the diluted solution by way of five centrifugation/redispersion cycles in CHCl3.
Results and Discussion
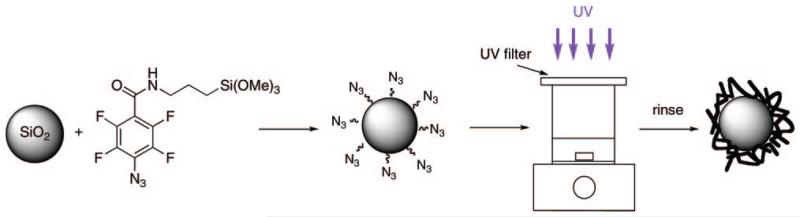
In this article we demonstrate the successful immobilization of polymers on silica nanoparticles using PFPA as the coupling agent. The key step is the preparation of PFPA-functionalized nanoparticles that can be used to attach a variety of molecules and materials. To achieve this, a simple one-pot procedure was developed for the synthesis and the subsequent functionalization of silica nanoparticles. The widely adopted Stöber method was employed to prepare monodisperse silica nanoparticles from Si(OEt)4 by way of a hydrolysis and condensation reaction.30 The particle size was measured to be 93 ± 14 nm in diameter by SEM. These particles were not as monodisperse, which is often the case for Stöber nanoparticles smaller than 100 nm. The size of these nanoparticles could be conveniently controlled by adjusting the mole ratio of the reactants used in the synthesis. The prepared nanoparticles were then functionalized in situ by subsequently adding PFPA-silane directly into the Stöber solution, reacting at room temperature overnight followed by refluxing for 1 h (Scheme 2). The IR spectrum of obtained nanoparticles showed the typical absorption of the azido group at 2126 cm−1, indicating the successful functionalization of the nanoparticles with PFPA.
Scheme 2. Functionalization of Silica Nanoparticles with PFPA–Silane and Subsequent Polymer Immobilization.
Elemental analysis of the nanoparticles after surface functionalization gave 0.54% F. Assuming that PFPA–silane has reacted with all of the available surface silanol groups (Si–OH) on the nanoparticle, and each silanol group occupies a surface area of 0.6 nm2,32 the theoretical maximal amount of %F can be calculated from the following equation:
| (1) |
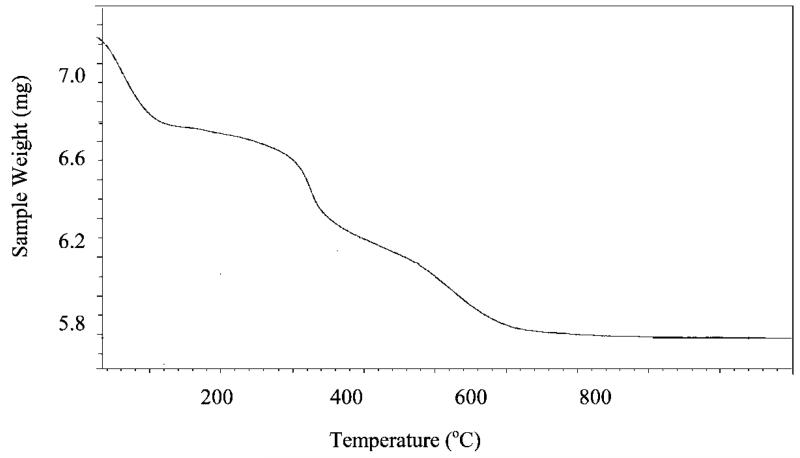
where r is the radius of the silica nanoparticles (averaged at 46.5 nm measured by SEM), NA is the Avogadro number, d is the density of silica (2.0 g/cm3or 2.0 × 10−21 g/nm3),33 FWF and FWPFPA are the formula weights of F and PFPA, respectively. The calculated value, 0.64% F, is slightly higher than the result from the elemental analysis of 0.54%. The lower experimental value might have resulted from a lower silanization yield, incomplete hydrolysis of ethoxy groups on TEOX thus reducing the amount of available Si-OH groups,34 or the solvent trapped inside the nanoparticles, which would lead to increased sample weight and thus a lower F% value. The presence of solvents or other volatile components in the nanoparticles is supported by the TGA. Upon heating, PFPA-coated silica nanoparticles exhibited a weight loss of ca. 5% in the temperature range of 25–120 °C. In fact, weight losses of a similar magnitude in this temperature range were observed for all nanoparticle samples subsequently immobilized with polymers (Figure 1) as well as unfunctionalized Stöber nanoparticles. The mole ratio of F to N calculated from the elemental analysis was found to be 1.01 for the PFPA-functionalized nanoparticles (Table 1). This is in excellent agreement with the structure of PFPA–silane, further confirming the successful attachment of PFPA on the silica nanoparticles.
Figure 1.
Typical TGA graph of polymer-coated silica nanoparticles. The polymer used in this case was P4VP immobilized at a concentration of 200 mg/mL in chloroform.
Table 1. Elemental Analysis of PFPA-Functionalized Silica Nanoparticles.
| wt% N | wt% F | mole ratio F:N |
|---|---|---|
| 0.38 | 0.51 | 1.01 |
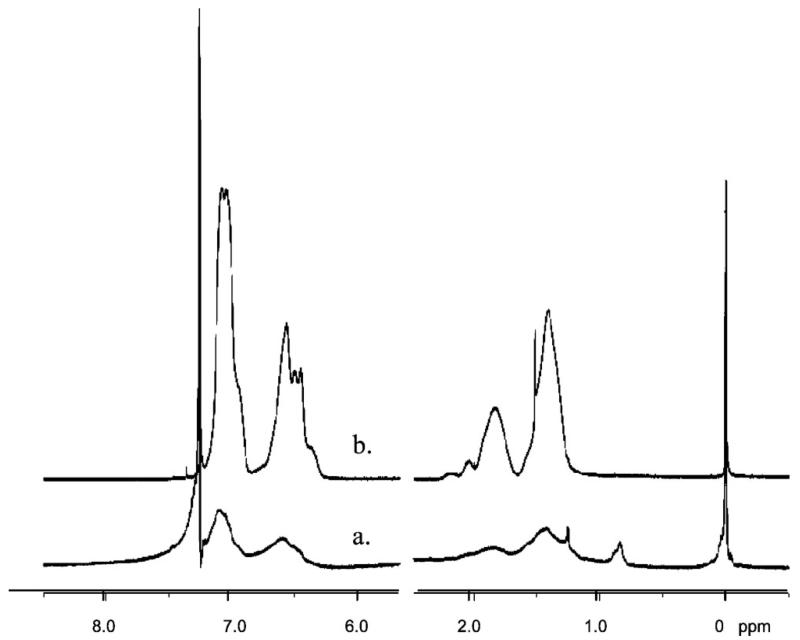
Polymer immobilization was carried out by irradiating PFPA-functionalized nanoparticles suspended in the polymer solution while stirring (Scheme 2). Chloroform was used as the solvent because of its excellent solubilizing ability and the increased lifetime of the singlet perfluorophenylnitrene in this solvent, which should result in an enhanced insertion/addition reaction yield (Scheme 1).35 Excess polymer was removed by repetitive extraction of the nanoparticles with the solvent. The presence of polymers attached to SiO2 nanoparticles was confirmed by 1H NMR. Nanoparticles coated with polystyrene (Figure 2b) showed peaks that corresponded to those of the polymer itself (Figure 2a). This immobilization procedure was also applied to PEOX, a hydrophilic polymer, and P4VP, a basic polymer. 1H NMR spectra confirmed that both polymers were successfully attached to the PFPA-functionalized nanoparticles.
Figure 2.
1H NMR spectra of (a) PS-coated silica nanoparticles, and (b) PS in CDCl3.
To establish that the polymer was indeed covalently bonded to the surface of the silica nanoparticles rather than just physisorbed, control experiments were carried out in which the Stöber nanoparticles were functionalized with octadecyltrimethoxysilane (ODTMS) instead of PFPA–silane. The ODTMS-coated nanoparticles were subjected to the same procedure as PFPA-coated nanoparticles to attach PS, PEOX, or P4VP. In all cases, the 1H NMR spectrum of the resulting nanoparticles did not show peaks in common with the respective polymer but instead contained only peaks corresponding to ODTMS. In another experiment, freshly prepared Stöber nanoparticles were irradiated in the presence of a solution of polystyrene in chloroform. After the resulting nanoparticles were washed with the solvent, no polystyrene molecules were detected in 1H NMR. These results confirmed that the surface azido groups were responsible for the successful attachment of polymers.
The amount of polymers immobilized on the nanoparticles was determined thermogravimetrically using TGA. Figure 1 is a typical TGA graph showing the weight loss of the polymer-functionalized nanoparticles upon heating. The weight losses in the temperature range of 120–940 °C were considered, eliminating the contribution from the volatile components trapped inside nanoparticles (below 120 °C).34 The weight of the coupling agent PFPA on the nanoparticles is subtracted from the total weight loss to obtain the weigh of the immobilized polymer only. The polymer grafting density per unit area (mg/m2) was calculated using the following equation:
| (2) |
where ΔWp is the change in the sample weight upon heating from 120 to 940 °C, ΔWPFPA is the weight change of PFPA-functionalized nanoparticles at 120–940 °C, Wp and WPFPA are the weights of the polymer-coated and PFPA-functionalized nanoparticles at 940 °C, respectively, d is the density of the nanoparticles, which is 2.0 g/cm3or 2.0 × 109 mg/m3,33 and r is the radius of the nanoparticles (averaged at 46.5 nm measured by SEM).
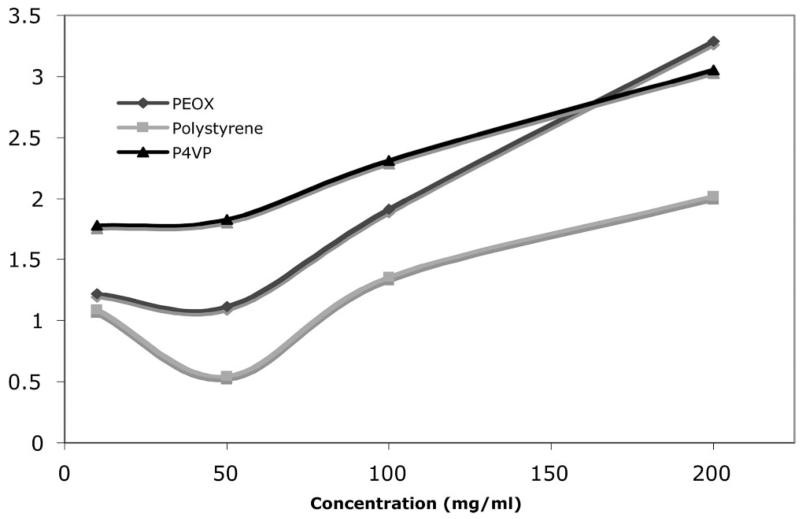
The grafting densities, in the range of several milligrams per square meter, are comparable to those reported in the literature for polymers attached to nanoparticles by the graft-to approach, demonstrating good coupling efficiencies under the current experimental conditions. For the immobilization to take place, polymer molecules must be in close proximity with the surface azido groups. In our studies, the coupling reaction was carried out where the PFPA-functionalized nanoparticles were suspended in the polymer solution. The immobilization was likely achieved where the polymer molecules were first adsorbed from solution to the nanoparticle surface and were then covalently attached upon UV activation of the azido groups. For homopolymers, the amount of polymers adsorbed depends on several parameters including polymer concentration, solvency, and molecular weight. Higher solution concentrations usually give increased amounts of adsorbed polymers, thus higher grafting density. This was observed in the present studies in which the grafting density increased with increasing polymer concentration (Figure 3). In addition, higher grafting densities were observed for PEOX and P4VP, whereas PS gave lower values. This could be attributed to the solvent effect where chloroform is a better solvent for PS than for PEOX or P4VP. Generally, in the absence of strong solvent-substrate interactions, more polymer will be adsorbed to substrates from poor solvents than from good solvents.36-38 In a good solvent, the polymer is well solvated. The repulsion between segments of adsorbed polymers is greater, leading to a lower amount of surface-adsorbed polymer. Polymer adsorption generally increases in a poor or the theta solvent.
Figure 3.
Grafting density of polymer on silica nanoparticles at various polymer concentrations. The lines were drawn to aid the eyes.
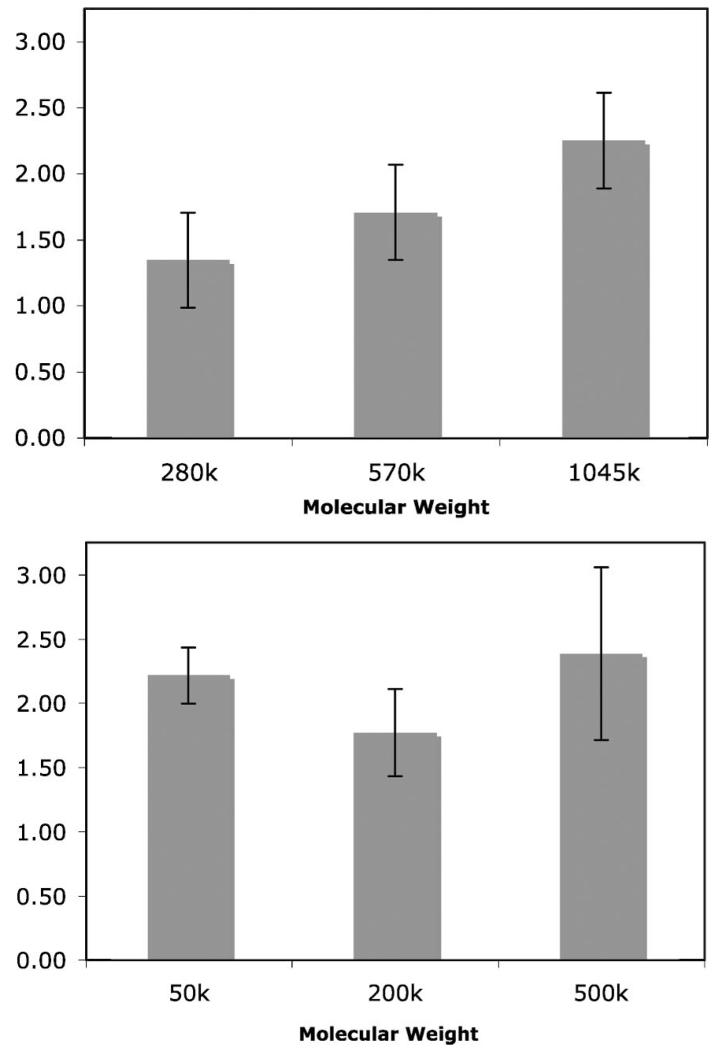
The effect of molecular weight on the polymer grafting density was investigated using PS and PEOX of different molecular weights. For PS, the grafting density increased with increasing molecular weight (Figure 4). Assuming that a polymer adsorption process precedes the photochemical immobilization, this result implies that more polymers were adsorbed on the functionalized nanoparticles as the size of the polymer increased. For PEOX, however, the grafting density was more or less similar for all three molecular weights (Figure 4). The result can be explained by assuming that the surface was fully covered with PEOX molecules. In this case, a unit surface area can accommodate a smaller number of larger polymers and a higher number of smaller polymers. The size and the number of polymers offset each other, yielding similar amounts of polymer on the surface.
Figure 4.
Grafting density versus molecular weight for PS (top) and PEOX (bottom). The immobilization was carried out by irradiating a suspension of PFPA-functionalized nanoparticles in 100 mg/mL solution of the polymer in CHCl3. All data is the average of three samples.
Conclusion
In summary, we have developed a simple procedure for covalently grafting polymers on silica nanoparticles. The nanoparticles were first surface functionalized with PFPA introducing the photoactive group and at the same time rendering them stable from agglomeration. Polymers were then covalently attached to these nanoparticles by a photochemically initiated insertion reaction. The advantage of the developed technique is its simplicity and versatility. PFPA-functionalized nanoparticles can be considered as universal functional surfaces facilitating the immobilization of a wide range of molecules and materials regardless of their structures or molecular architecture. Especially beneficial are molecules that are difficult to immobilize by the conventional graft-to or graft-from approaches as a result of a lack of functional groups or the inability to surface polymerize in situ. This approach employs an external light-induced process that is fast, tunable, and efficient. The protocols developed can be readily applied to other nanomaterials. We have successfully prepared PFPA-functionalized Au and Ag nanoparticles for nanocomposites39 and sensing.
Acknowledgment
This material is based on research sponsored by the Air Force Research Laboratory under agreement number FA8650-05-1-5041. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of the Air Force Research Laboratory or the U.S. Government. Additional financial support was provided by NIH/NIGMS through an AREA Award GM066279. The authors thank Dr. Fabien Choffat, ETH Zurich, for his assistance in TGA experiments, and Professor Raymond Bard, University of Portland, and Dr. Maged Osman, ETH Zurich, for their helpful discussions.
References
- (1).Gravano SM, Patten TE. Macromol. Eng. 2007;2:1179. [Google Scholar]
- (2).Balazs AC, Emrick T, Russell TP. Science. 2006;314:1107. doi: 10.1126/science.1130557. [DOI] [PubMed] [Google Scholar]
- (3).Yarin AL, Zussman E, Wendorff JH, Greiner A. J. Mater. Chem. 2007;17:2585. [Google Scholar]
- (4).Schmidt AM. Colloid Polym. Sci. 2007;285:953. [Google Scholar]
- (5).Bhavsar MD, Amiji MM. Expert Opin. Drug Delivery. 2007;4:197. doi: 10.1517/17425247.4.3.197. [DOI] [PubMed] [Google Scholar]
- (6).Choi Y, Baker JF., Jr. Nanoparticles in medical diagnostics and therapeutics. In: Vo-Dinh T, editor. Nanotechnology in Biology and Medicine. CRC Press, LLC; Boca Raton, FL: 2007. [Google Scholar]
- (7).Gupta S, Zhang Q, Emrick T, Balazs AC, Russell TP. Nat. Mater. 2006;5:229. [Google Scholar]
- (8).Sinha R, Kim GJ, Nie S, Shin DM. Mol. Cancer Ther. 2006;5:1909. doi: 10.1158/1535-7163.MCT-06-0141. [DOI] [PubMed] [Google Scholar]
- (9).Howard KA, Dong M, Oupicky D, Bisht HS, Buss C, Besenbacher F, Kjems J. Small. 2007;3:54. doi: 10.1002/smll.200600328. [DOI] [PubMed] [Google Scholar]
- (10).Glogowski E, Tangirala R, Russell TP, Emrick T. J. Polym. Sci. Part A: Polym. Chem. 2006;44:5076. [Google Scholar]
- (11).Ostrander JW, Mamedov AA, Kotov NA. J. Am. Chem. Soc. 2001;123:1101. doi: 10.1021/ja0029578. [DOI] [PubMed] [Google Scholar]
- (12).Wang D, Caruso F. Chem. Mater. 2002;14:1909. [Google Scholar]
- (13).Kim JS, Rieter WJ, Taylor KML, An H, Lin W, Lin W. J. Am. Chem. Soc. 2007;129:8962. doi: 10.1021/ja073062z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Dotzauer DM, Dai J, Sun L, Bruening ML. Nano Lett. 2006;6:2268. doi: 10.1021/nl061700q. [DOI] [PubMed] [Google Scholar]
- (15).Pyun J, Matyjaszewski K. Chem. Mater. 2001;13:3436. [Google Scholar]
- (16).Li D, Sheng X, Zhao B. J. Am. Chem. Soc. 2005;127:6248. doi: 10.1021/ja0422561. [DOI] [PubMed] [Google Scholar]
- (17).Bartholome C, Beyou E, Bourgeat-Lami E, Chaumont P, Lefebvre F, Zydowicz N. Macromolecules. 2005;38:1099. [Google Scholar]
- (18).Yokoyama R, Suzuki S, Shirai K, Yamauchi T, Tsubokawa N, Tsuchimochi M. Eur. Polym. J. 2006;42:3221. [Google Scholar]
- (19).Li ZF, Ruckenstein E. Nano Lett. 2004;4:1463. [Google Scholar]
- (20).Savin DA, Pyun J, Patterson GD, Kowalewski T, Matyjaszewski K. J. Polym. Sci., Part B: Polym. Phys. 2002;40:2667. [Google Scholar]
- (21).von Werne T, Patten TE. J. Am. Chem. Soc. 2001;123:7497. doi: 10.1021/ja010235q. [DOI] [PubMed] [Google Scholar]
- (22).Gravano SM, Dumas R, Liu K, Patten TE. J. Polym. Sci., Part A: Polym. Chem. 2005;43:3675. [Google Scholar]
- (23).Bockstaller MR, Thomas EL. Phys. Rev. Lett. 2004;93:166106. doi: 10.1103/PhysRevLett.93.166106. [DOI] [PubMed] [Google Scholar]
- (24).Liu J, Tanaka T, Sivula K, Alivisatos AP, Fréchet JMJ. J. Am. Chem. Soc. 2004;126:6550. doi: 10.1021/ja0489184. [DOI] [PubMed] [Google Scholar]
- (25).Mangeney C, Ferrage F, Aujard I, Marchi-Artzner V, Jullien L, Ouari O, Rekai ED, Laschewsky A, Vikholm I, Sadowski JW. J. Am. Chem. Soc. 2002;124:5811. doi: 10.1021/ja010796h. [DOI] [PubMed] [Google Scholar]
- (26).Pathmananoharan C. Colloids Surf. 1990;50:1. [Google Scholar]
- (27).Scriven EFU. Azides and Nitrenes. Academic Press; New York: 1984. [Google Scholar]
- (28).Gritsan NP, Zhu Z, Hadad CM, Platz MS. J. Am. Chem. Soc. 1999;121:1202. doi: 10.1021/ja9944305. [DOI] [PubMed] [Google Scholar]
- (29).Platz MS. Acc. Chem. Res. 1995;28:487. [Google Scholar]
- (30).Stober W, Fink A. J. Colloid Interface Sci. 1968;26:62. [Google Scholar]
- (31).Yan M, Ren J. Chem. Mater. 2004;16:1627. [Google Scholar]
- (32).Waddell TG, Leyden DE, DeBello MT. J. Am. Chem. Soc. 1981;103:5303. [Google Scholar]
- (33).Westcott SL, Oldenburg SJ, Lee TR, Halas NJ. Langmuir. 1998;14:5396. doi: 10.1021/la980870i. [DOI] [PubMed] [Google Scholar]
- (34).Suratwala TI, Hanna ML, Miller EL, Whitman PK, Thomas IM, Ehrmann PR, Maxwell RS, Burnham AK. J. Non-Cryst. Solids. 2003;316:349. [Google Scholar]
- (35).Gritsan NP, Zhai HB, Yuzawa T, Karweik D, Brooke J, Platz MS. J. Phys. Chem. 1997;101:2833. [Google Scholar]
- (36).Fleer GJ, Stuart MAC, Scheutjens JMHM, Cosgrove T, Vincent B. Polymers at Interfaces. Springer; New York: 1993. p. 502. [Google Scholar]
- (37).Cosgrove T. Polymers at interfaces. In: Cosgrove T, editor. Colloid Science. Blackwell Publishing; Oxford: 2005. p. 113. [Google Scholar]
- (38).Netz RR, Andelman D. Phys. Rep. 2003;380:1. [Google Scholar]
- (39).Schmid D, Handge U, Gann JP, Yan M, Caseri W. Macromol. Sci. Eng. 2008. in press. [Google Scholar]