Abstract
Vacuolar proton pyrophosphatase (V-PPase), an electrogenic proton pump widely distributed in non-mammalian species, is one of the important targets for acidocalcisomes. In this study, a novel method of peptide-based antibody generation was performed to produce monoclonal antibodies (MAbs) against Toxoplasma gondii V-PPase. Three hybridomas were identified and confirmed by ELISA, Western blotting, and immunofluorescence. All of them can react with an 85 kDa band of T. gondii protein in purified acidocalcisomal fraction. The three MAbs were all specific to the synthetic peptide of YTKAADVGADLSGKNEYGMSEDDPRNPAC, corresponding to amino acids at the location of 292aa–320aa of TgVP1 amino acid sequence. These specific MAbs will be valuable tools for further study of T. gondii infection biology, pathogenesis, and host immune response.
Introduction
Toxoplasmosis is a disease caused by infection of Toxoplasma gondii,(1) a protozoan parasite with a worldwide infection rate of more than one third in the global human population.(2) Although felines have been found to be the only definitive host, most warm-blooded animals, including humans, are susceptible to infection by T. gondii.(3) In immunocompetent individuals, infection is typically asymptomatic or causes a mild, flu-like illness. But for those with compromised immune systems, such as pregnant women and those with acquired immunodeficiency syndrome (AIDS), various organs can be seriously affected by this parasite,(4) leading to the development of encephalitis, myocarditis, or pneumonitis which, in turn, can be fatal.(5) The developing fetus is at particular risk because toxoplasmosis can be transmitted from mother to fetus, leading to birth defects(6) and making the baby a lifelong carrier.(7) To protect humans from this threat, in this study we investigated acidocalcisome, a recently discovered organelle, as a novel drug target.
The acidocalcisome is an electron-dense acidic organelle containing a large amount of pyrophosphate and polyphosphates. Pumps and exchangers on its membrane play important roles for the uptake and release of its contents, with vacuolar proton pyrophosphatase being one of them.(8) Vacuolar proton pyrophosphatase (V-PPase) is widely distributed among land plants, but is found much less frequently in algae, protozoa, bacteria, and archaebacteria. There are two types of V-PPases, type I (K+-dependent) and type II (K+-independent); both of these are localized in vacuolar compartments such as the plant vacuole and the acidocalcisomes of bacteria and protists and utilize a simple and low-cost substrate pyrophosphate (PPi) for the active transport of sodium or protons against an electrochemical gradient,(9) a process of great significance to physiological function. T. gondii vacuolar proton pyrophosphatase (TgVP1) consists of a single polypeptide,(10) as well as V-PPases in other species, with a molecular mass of about 85 kDa.
Several antibodies against TgVP1 homologue and polyclonal antibodies against TgVP1 have been developed, but no monoclonal antibody has been generated. This limiting factor in the study of TgVP1 means that specific antibodies against TgVP1 are very much in need. However, the polypeptide of TgVP1 possesses 17 transmembrane helices, which makes the process of prokaryotic and eukaryotic expression difficult. The aim of this study was to produce and characterize a monoclonal antibody against a synthetic peptide outside the transmembrane domains of TgVP1 as a tool for research applications. With the deployment of a novel method of peptide-based antibody generation, we generated three monoclonal antibodies and characterized their properties in laboratory testing.
Materials and Methods
Parasite strains, cells, and growth conditions
T. gondii was maintained in 6-week-old female BALB/c mice, by intraperitoneal inoculation of T. gondii (RH strain, ME49 strain) tachyzoite, 3×104 per mouse. The mice were infected about a week later and received a final intraperitoneal injection of 5 mL of sterile 0.9% normal saline (NS). After gently rubbing the abdomen, the ascitic fluid was collected and centrifuged at 800 rcf at RT for 10 min to remove impurities. The pellet was then re-suspended in sterilizing 0.9% NS after washing three times under aseptic conditions. Monolayers of OFTu cells were infected with treated parasites at a ratio of 10:1 after reaching 80% confluency in T150 tissue culture flasks, using MEM supplemented with 10% FBS, and cultured in 5% CO2 for 1 week at 37°C. The parasites and cells were harvested when 50% of the cells showed CPE.
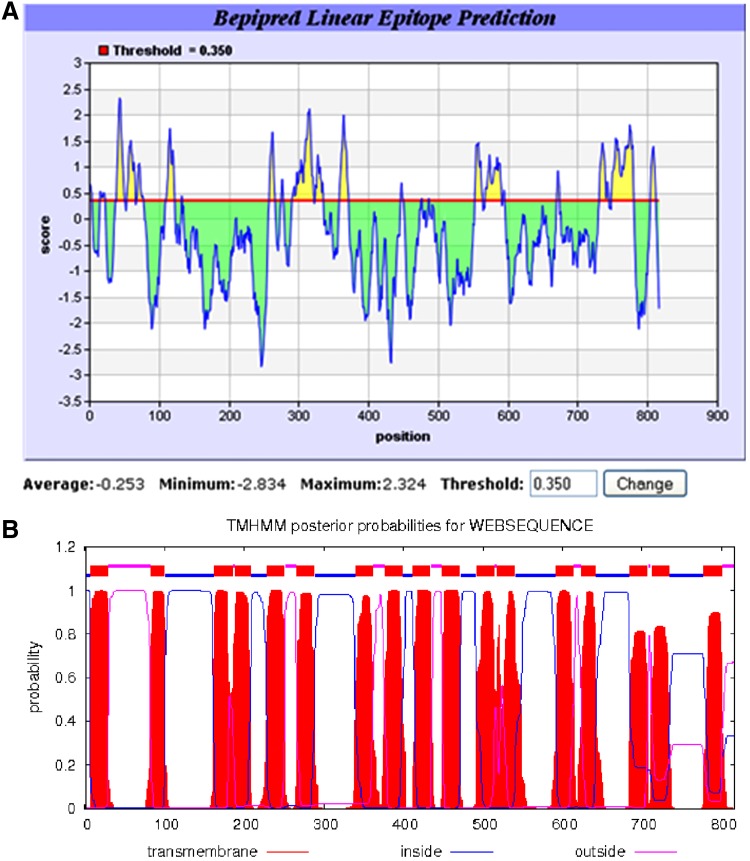
Antibody generation against T. gondii V-PPase
A systematic approach was employed for the prediction of potential B-cell epitopes in T. gondii V-PPase. Analysis resource was used to determine overall antigenicity of TgVP1 determined by the Immune Epitope Database (IEDB) with a threshold of 0.350, using the bepipred linear epitope prediction method. The IEDB (www.iedb.org) is a dataset of information about immune epitopes published or submitted by researchers.(11) The bepipred linear epitope prediction method is an integrated algorithm of hydrophilicity prediction, flexibility prediction, and surface accessibility prediction. Greater understanding about this database is detailed in the work of Vita and colleagues.(11) After the primary selection by IEDB, topology analysis of TgVP1 with TMHMM Server v 2.0 was performed to eradicate the transmembrane domains. TMHMM is a software based on a hidden Markov model (HMM), which is used to predict transmembrane helices (TMHs) within a given amino acid sequence after analyzing it with relevant statistics and indices.(12) Further understanding about this software was introduced through the research of Krogh and colleagues.(12) The predicted epitopes with higher antigenicity scores were chosen as haptens to cross-link to carrier proteins as described below.
A synthetic N-terminal peptide, TgVP1-1, corresponding to amino acids 292–320 of TgVP1 (NH2-YTKAADVGADLSGKNEYGMSEDDPRNPAC-COOH), was synthesized. This peptide, located outside transmembrane domains and without post-translational modification sites, was used to immunize mice to obtain monoclonal antibodies.
For conjugation, the Imject Immunogen Kit with Maleimide-activated mcKLH and BSA (Pierce, Rockford, IL) was used according to the manufacturer's instructions. Efficiency of conjugation was tested by 10% SDS-PAGE electrophoresis. The gel was stained with Coomassie Brilliant Blue R-250. KLH-peptides were used for producing murine monoclonal antibodies while BSA peptides were used for screening.
Six- to 8-week-old female BALB/c mice were given intraperitoneal injections comprised of 50 μg of KLH conjugated with TgVP1-1, emulsified with an equal amount of Incomplete Freund's adjuvant (IFA). Four weeks after priming, the mice were sensitized intraperitoneally four times at two-week intervals with 50 μg of TgVP1-1 plus IFA per mouse. Splenocytes were isolated from the immunized mice until the tail blood titer rose to 1:10,000. Three days prior to the fusion, the mice were boosted intraperitoneally with 50 μg antigen. Then, after washing twice with PBS, 1×108 splenocytes were fused with 2∼5×107 SP2/0 mouse myeloma cells using 50% PEG 3500. RPMI 1640 medium was used to stop the fusion and the treated cells were re-suspended in HAT media, supplemented to select hybridomas. After plating into 96-well tissue culture plates (1.2×105 cells/well, 200 μL), the cells were grown at 37°C in a humidified incubator plus 5% CO2. After 1 week, indirect ELISA and Western blotting were used to screen the supernatants and monitor the immune responses of the injected mice with the aim of determining the presence of antibodies against the peptide. By sub-cloning the positive clones three times, hybridomas 1D7-H11, 3A6-E4, and 3G6-F9 showed high specificity not only to BSA-synthetic peptide, but also to purified T. gondii protein. These hybridomas were cultured and diluted to 1×106 cells/0.5 mL PBS before being separately amplified in mice that had been intraperitoneally sensitized by IFA a week prior to injection. A week later, ascitic fluid was collected and centrifuged (10,000 rcf, 4°C, 10 min) to remove tissue and cells. The supernatants were affinity purified by Protein G Sepharose 4 Fast Flow (Invitrogen, Carlsbad, CA) according to the manufacturer's guidelines.
All animal experiments were carried out under the supervision of the Southern Medical University Animal Care and Use Committee in accordance with guidelines for the ethical treatment of animals.
Indirect ELISA
Indirect ELISA was performed in a 96-well Microlon ELISA plate (Corning, Corning, NY). Wells were coated overnight with 10 ng BSA-synthetic peptides in 100 μL of coating buffer (CBS) per well at 4°C, and then blocked with protein-free blocking solution at 37°C for 2 h. After washing three times with PBS, 100 μL per well of culture supernatants or MAbs were added and incubated for 1 h at 37°C. Meanwhile, 100 μL normal serum and RPMI 1640 medium supplemented with 10% FBS were used as negative and blank controls. The plates were then washed three times with PBS and loaded with 1:10,000 diluted goat anti-mouse antibodies labeled with horseradish peroxidase (HRP) following incubation for 30 min at 37°C. After a final three washes with PBS, the wells were incubated with TMB substrate, and the reaction was terminated after 15 min by adding 2 M H2SO4. Absorbance was read at 450nm.
SDS electrophoresis and preparation of Western blots
Purified parasites were separately harvested and lysed with RIPA lysis buffer (CWBiotech, Beijing, China) supplemented with PMSF (Thermo, Waltham, MA; 20 min on ice) to prepare T. gondii total protein. After centrifugation (12,000 rpm, 4°C, 10 min), the supernatant was collected and diluted 1:1 with 2×SDS loading buffer, boiled for 10 min, and centrifuged at 10000 rpm for 10 min. A 20 μL aliquot of the supernatant with approximately 30 μg total protein was subjected to 10% SDS-PAGE. Separated proteins were then transferred to the polyvinylidene fluoride (PVDF) membranes and blocked with 5% non-fat milk /TBST overnight at 4°C.After rinsing three times with TBST, the membranes were incubated with the cultured medium from the hybridomas or purified MAbs (1 mg/mL) diluted 1:1000 with TBST for 1.5 h at RT. After further washing, the membranes were incubated with 1:10,000 diluted secondary HRP-goat anti-mouse IgG antibody for another 1 h at RT. After a final wash, the immunoreactive bands were developed using enhanced chemiluminescence (ECL) substrate. Purified total protein of P. falciparum and P. berghei were performed using the same procedure to verify the conservation of protein VP1 among apicomplexan parasites.
Immunofluorescence microscopy
For immunofluorescence, purified T. gondii were placed into glass-bottom cell culture dishes (Corning) and covered with 4% paraformaldehyde for 15 min. They were then permeabilized with 0.25% Triton X-100/PBS for 10 min at RT. The dishes were washed three times with PBS and blocked with 1% BSA/PBST for 2 h at RT. Dishes were rinsed again with PBS, then incubated with purified MAbs diluted 1:500 with PBST for 1.5 h at RT. After unbound antibodies were washed away, samples were incubated with diluted (1:1000) anti-mouse IgG Alexa Fluor 546 (red) for 1 h at RT, then washed and stained with 40,60-diamidino-2-phenyl-indole (DAPI) at 1:1000 for 10 min. Meanwhile, 1:100 diluted immunized serum and normal serum were treated under the same procedure for use as positive and negative controls.
Sandwich ELISA
Antibodies from hybridomas 1D7-H11, 3A6-E4, and 3G6-F9, along with a clone of rabbit polyclonal antibody (RPA), were set in pairs as capture and detector antibodies for the development of a pre-test sandwich ELISA for synthetic peptide TgVP1-1. A 96-well Microlon ELISA plate was coated with 100 ng capture antibodies in 100 μL coating buffer (CBS) per well and incubated overnight at 4°C. After coating, the plates were blocked with 5% non-fat milk/PBS at 37°C for 2 h. After washing three times with PBST, multiple dilutions (from 200–3.125 ng/mL) of synthetic peptide TgVP1-1 in 100 μL PBS were added to each well and the plates incubated at 37°C for 1 h. Two wells of 100 μL PBS blank controls were performed in duplicate. Each well was loaded with 1:2000 diluted biotinylated detection antibodies (1 mg/mL), incubated for 1 h at 37°C, and washed three times. The diluted (1:200,000) streptavidin-HRP conjugate was added individually and incubated at 37°C for 30 min. The subsequent steps were the same as those with indirect ELISA described above. All data were averages of initial duplicate data. Eight pairs of data with levels of OD450 (0.700) greater than others have been combined to draw a portrait of OD450 along the natural logarithm of antigen concentration (ng/mL).
Results
Antibody generation against T. gondii V-PPase
A peptide-based antibody generation producing monoclonal antibody against T. gondii V-PPase was performed in this study. B-cell linear epitopes were predicted with IEDB analysis and 23 epitopes were predicted using the method of bepipred linear epitope prediction with the threshold of 0.350; the higher the score, the greater antigenicity epitopes might have (Fig. 1A). With the help of membrane topology analysis performed by TMHMM Server v 2.0, 17 transmembrane helices were detected and epitopes located in transmembrane domains were eradicated (Fig. 1B). A peptide as above was finally selected. To make up the lack of immunogenicity, KLH was conjugated with the synthetic peptide using the Imject Immunogen Kit with Maleimide-activated mcKLH and BSA. The conjugated protein was subjected to SDS-PAGE electrophoresis. The gel was stained with Coomassie Brilliant Blue R-250, and the change in mobility shift of peptide-carrier protein could be used to assess the efficiency of conjugation.
FIG. 1.
Amino acid sequence analysis of TgVP1. (A) IEDB Analysis Resource is used for B-cell linear epitope prediction. The method of bepipred linear epitope prediction is performed. Peptides of scores greater than 0.350 are determined to be potential epitopes––the higher the better. (B) Topology analysis of TgVP1 with TMHMM Server v 2.0. The transmembrane domains are shown in red while regions inside membrane in blue and outside in pink.
Indirect ELISA
For the indirect ELISA assay, BSA peptides were used for specificity testing of the antibody against KLH peptide. After examining, 43 wells containing positive clones in reaction with BSA-TgVP1-1 were grown. Nine wells of hybridomas, comprising highly reactive 2A3, 3A6, 1B9, 2C3, 1D7, 3G6, 3G7, 3H1, and 3H7 were selected. These positive clones were then cultured and transferred to a 24-well plate, one cell per well, for sub-cloning. Six antibody-producing cultures, 2A3-F8, 1B9-A2, 1D7-H11, 3A6-E4, 3G6-F9, and 3H7-C6, were screened out after three repetitions of the sub-cloning process.
SDS electrophoresis and preparation of Western blots
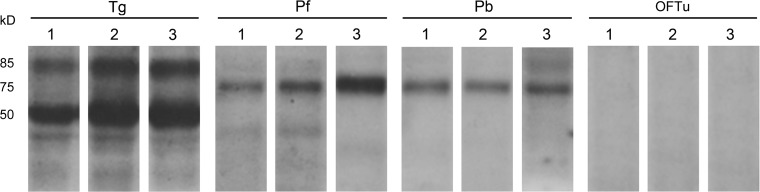
Western blotting results revealed that only 1D7-H11, 3A6-E4, and 3G6-F9 reacted with the purified 85 kDa T. gondii protein, which is predicted as T. gondii V-PPase, and that they also detected a T. gondii protein band of 50 kDa. In addition, these three MAbs can bind to both purified proteins of Plasmodium falciparum and Plasmodium berghei with a size of 76 kDa (Fig. 2). These three hybridomas were harvested and frozen with RPMI 1640 medium supplemented with 40% FBS and 10% DMSO in liquid nitrogen.
FIG. 2.
Western blot analysis of antigen specificities of selected monoclonal antibodies. Lane 1 is incubated with 1D7-H11; lane 2, 3A6-E4; lane 3, 3G6-F9. Among all hybridomas, only 1D7-H11, 3A6-E4, and 3G6-F9 can react with purified Toxoplasma gondii protein of 85 kDa, predicted as TgVP1, and 76 kDa as PfVP1 and PbVP1. All three hybridoma supernatants can detect another protein of approximately 50 kDa within purified T. gondii total protein. None of the three reacted with purified OFTu total protein.
Immunofluorescence microscopy
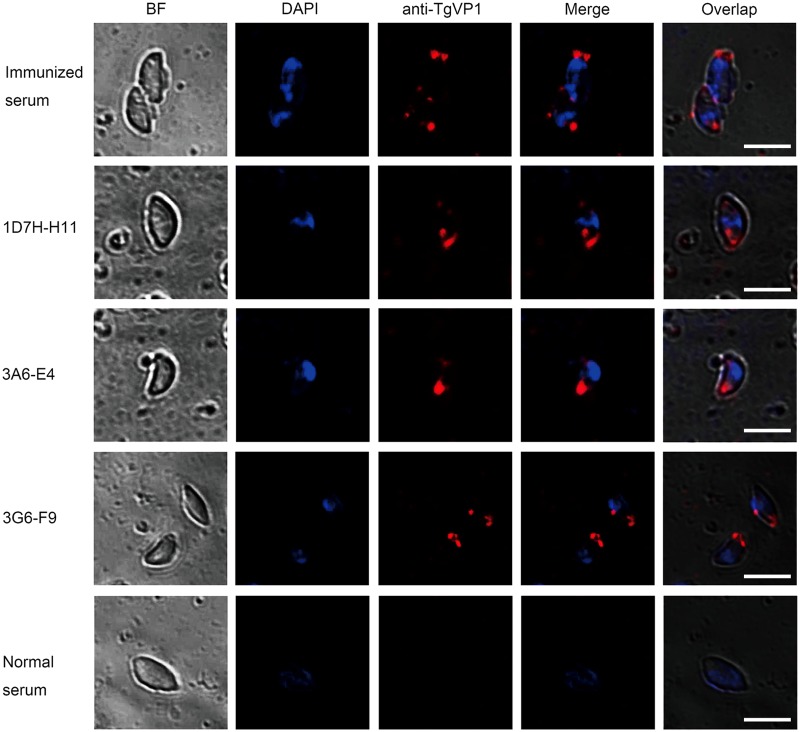
To visualize the cell nucleus and virus factories, the cells were counterstained with DAPI (blue). As seen in Figure 3, all three MAbs were reactive in the cytoplasm of T. gondii tachyzoite, and no associated fluorescence signal (red) was observed in the nuclei (blue). Image magnification was obtained with a 100×oil immersion objective of a laser scanning confocal microscope.
FIG. 3.
Immunofluorescence images of TgVP1 in T. gondiitachyzoites. The location of TgVP1 in T. gondii was probed with purified MAbs and labeled with anti-mouse IgG Alexa Fluor 546 (red) and the nucleus was stained with DAPI (blue). The results show that TgVP1, probed with 1D7-H11, 3A6-E4, 3G6-F9, and immunized mice serum, as positive control, is distributed in cytoplasma, and no red fluorescence is observed in nucleus. Parasites incubated with normal mice serum indicate no red fluorescence as negative control. As a point of reference, the bright field images (BF) of the parasites are also shown. Bar, 10 μm.
Sandwich ELISA
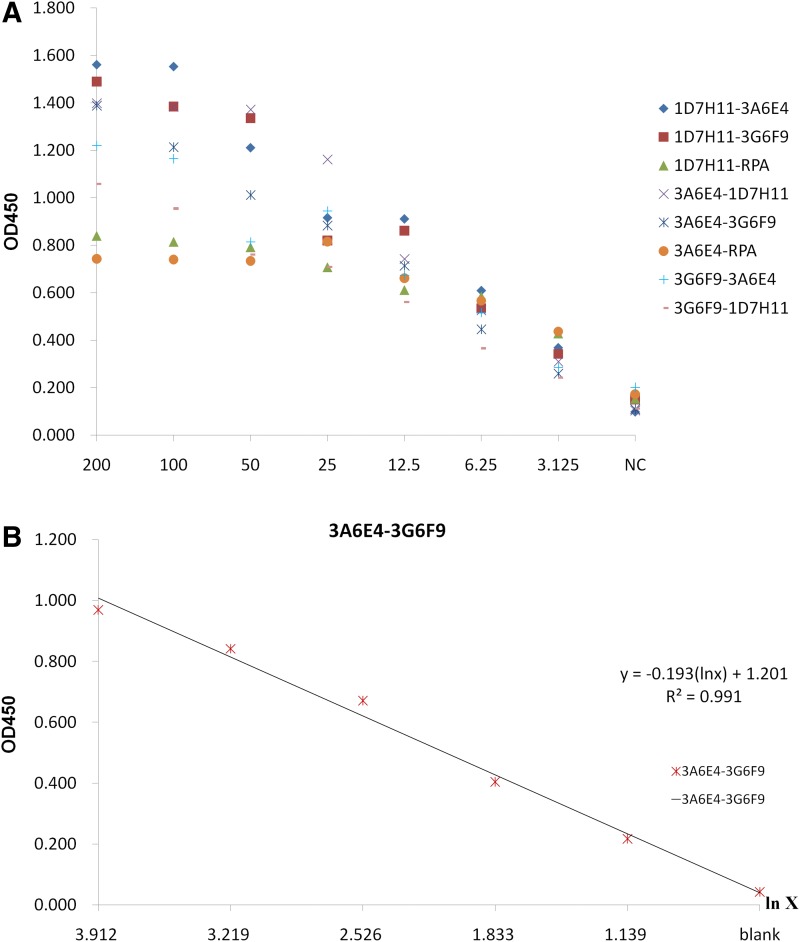
A sandwich ELISA assay was performed to investigate whether the MAbs reacted with the same epitope. A spreadsheet (Table 1) of OD450 data was presented with the following parameters (Fig. 4). Analysis by establishing the standard curve indicated that, for each pair, OD450 dropped with decreasing antigen concentration, and the correlation coefficient between OD450 and the natural logarithm of antigen concentration was higher than 0.99 in only one pair.
Table 1.
OD450 Data of Sandwich Elisa for TgVP1-1 Detection
| Concentration of antigen (ng/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cat. # of coated Abs | 1D7H11 | 1D7H11 | 1D7H11 | 3A6E4 | 3A6E4 | 3A6E4 | 3G6F9 | 3G6F9 | 3G6F9 | rp1 | rp1 | rp1 |
| 200 | 1.561 | 1.489 | 0.839 | 1.398 | 1.388 | 0.743 | 1.219 | 1.058 | 0.66 | 0.274 | 0.309 | 0.278 |
| 100 | 1.553 | 1.384 | 0.814 | 1.382 | 1.213 | 0.74 | 1.164 | 0.955 | 0.623 | 0.206 | 0.291 | 0.264 |
| 50 | 1.211 | 1.335 | 0.791 | 1.371 | 1.011 | 0.734 | 0.813 | 0.761 | 0.564 | 0.193 | 0.246 | 0.249 |
| 25 | 0.916 | 0.819 | 0.707 | 1.161 | 0.883 | 0.816 | 0.944 | 0.709 | 0.521 | 0.168 | 0.214 | 0.192 |
| 12.5 | 0.911 | 0.86 | 0.611 | 0.742 | 0.713 | 0.662 | 0.673 | 0.561 | 0.576 | 0.187 | 0.173 | 0.181 |
| 6.25 | 0.609 | 0.533 | 0.584 | 0.525 | 0.446 | 0.567 | 0.516 | 0.366 | 0.511 | 0.143 | 0.148 | 0.185 |
| 3.125 | 0.368 | 0.341 | 0.428 | 0.309 | 0.259 | 0.437 | 0.285 | 0.242 | 0.433 | 0.173 | 0.127 | 0.158 |
| blank | 0.052 | 0.049 | 0.048 | 0.042 | 0.042 | 0.044 | 0.042 | 0.043 | 0.043 | 0.044 | 0.044 | 0.05 |
| Detection Ab | 3A6E4 | 3G6F9 | rp1 | 1D7H11 | 3G6F9 | rp1 | 3A6E4 | 3G6F9 | rp1 | 1D7H11 | 3A6E4 | 3G6F9 |
FIG. 4.
Statistical graph of data from sandwich ELISA. (A) Scatter diagram of data without four antibody pairs with lower level of OD450 (<0.7). (B) Standard curve of OD450 along natural logarithm of antigen concentration is represented by a solid line for 3A6E4-3G6F9; regression equation is shown above the line as well. R2=0.991.
Discussion
T. gondii is an opportunistic parasite that represents a potentially serious threat to immunocompromised persons. Up to now, more than three quarters of the world population of warm-blooded animals have become toxoplasmosis patients or carriers, according to the World Health Organization. However, therapies are limited and there is an urgent need to develop and exploit novel drugs. The acidocalcisome, a recently discovered organelle with a key role in metabolism and featuring numerous pumps and exchangers on its membrane, may be a viable drug target.(13) Recent research on vacuolar proton pyrophosphatase of T. gondii (TgVP1), a kind of pump located in the membrane of acidocalcisome, indicates that a lack of TgVP1 decreases the virulence and extracellular survival of the parasite.(14) To further help with the study of TgVP1, MAbs with high specificity to vacuolar proton pyrophosphatase were required. This is due to the fact that, although several antibodies against TgVP1 homologues and polyclonal antibodies against TgVP1 have been developed, no monoclonal antibody against TgVP1 has been generated. In other studies, researchers use polyclonal antibodies (PABHK and PABTK) against plant V-PPase(15) to study not only TgVP1, but also V-PPase of other parasites.(14,16–19) This reduces the utility of further study of TgVP1.
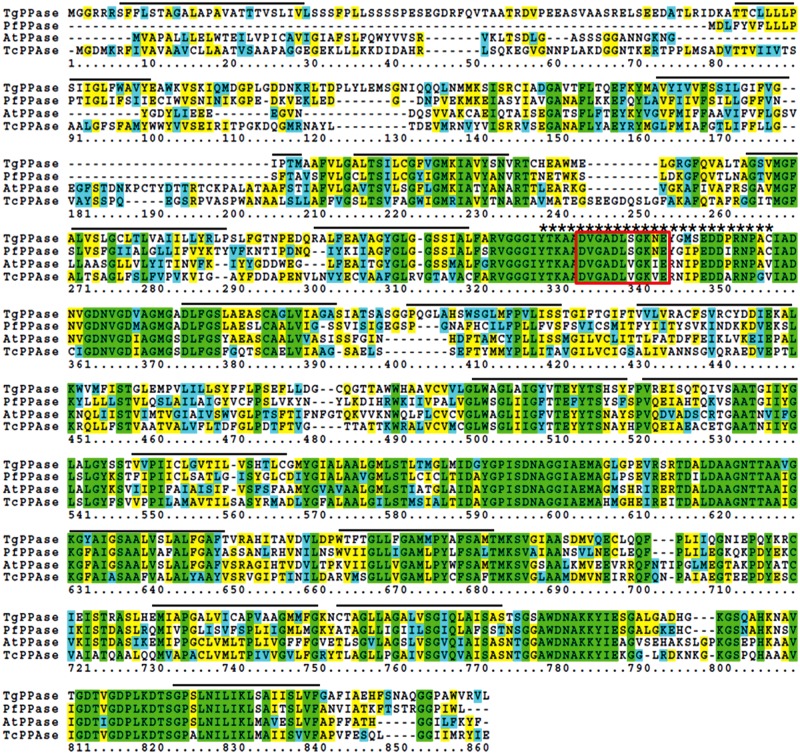
The aim of this study was to produce and characterize monoclonal antibodies against TgVP1 as a tool for research applications. First we obtained the amino acid sequence of TgVP1 (gi|527308392|gb|EPT25031.1) from Genebank (www.ncbi.nlm.nih.gov/protein/527308392/) by Blast. Amino acid sequences of vacuolar proton pyrophosphatase from P. falciparum and Trypanosoma cruzi were obtained in the same way. Thereafter, a brief homologous comparison of these three V-PPases was performed by SDSC Biology Workbench using a Boxshade algorithm. The amino acid sequence of TgVP1 is 66% similar to the corresponding region of the Arabidopsis V-H+-PPase sequence, 72% similar to P. falciparum, and 63% similar to Trypanosoma cruzi (Fig. 5). This result indicates that the vacuolar proton pyrophosphatase 1 species is relatively conserved. The putative PPi binding domain (DX7KXE motif) within the catalytic domain is located in a conservative domain, and also found in both soluble and membrane-associated V-PPases.(20) The IEDB used in this work to determine antigenicity of TgVP1 was widely used because of its timeliness and accuracy.(11) Compared with MacVector, another mainstream sequence analysis application,(21) the IEDB was much more convenient for its independence of Mac OS system and free license. And it was a more professional amino acid sequence analysis application to compare with DNA star, a free bioinformatics software package, and easier to perform. The structure determination was significant for a transmembrane protein. Most mainstream prediction methods were based on HMM,(22) among which TMHMM was the most accurate when used alone, especially with limited experimental information.(23) Topological analysis by TMHMM Server v2.0 revealed as many as 17 transmembrane domains, which makes the prokaryotic and eukaryotic expression of TgVP1 difficult. Therefore, we chose to initiate the process of MAb production with a novel method of peptide-based antibody generation. Although new, this approach to generate specific immunological antibodies for some purposes has become a versatile tool in the molecular analysis of proteins, and has been used more and more frequently in recent years for its advantages.(24) First, it is easier and faster to get high purity antigens by passing the complicated expression and purification steps. Second, it is possible to select a particular antigen epitope to generate a high affinity antibody fit for purpose. Finally, the positive clones reacting to the protein or epitope of interest are easy to screen. However, there are still some significant problems in the design of peptides for the production of protein-reactive anti-peptide antibodies, such as quantifying the epitope scores of the peptides, isolating the location of the selected peptides (whether inside or outside the membrane or in the transmembrane domains), how long the peptides should be, etc.(25)
FIG. 5.
Alignment comparison of V-H+-PPases from T. gondii, P. falciparum, A. thaliana, and T. cruzi. Same residues are in green while similar ones are in blue and yellow. Amino acid residues not present within other sequences are denoted by dashes. The 17 transmembranes predicted by TMHMM Server v2.0 are indicated by lines above and the selected epitope is denoted by asterisks above, which is conserved among apicomplexan parasites and plants. The putative PPi-binding domain within catalytic domain is boxed.
The rest of the steps are the same as the common procedures of monoclonal antibody generation after the selected peptides were conjugated to KLH as carrier protein. In this study, we succeeded in developing three high-affinity monoclonal antibodies against TgVP1 with this method and have ascertained their characterization with laboratory tests. At first, nine positive clones with high absorbance were selected by indirect ELISA. Using Western blot analysis after sub-cloning, only three sub-clones could react with 85 kDa purified parasite proteins, as the molecular mass predicted from the nucleotide sequence of gene TgVP1, thus the other clones might be false positive. However, it should be mentioned that in other research, the molecular mass of TgVP1 estimated from PAGE is about 76 kDa. Additionally, PfVP1 is predicted to be 76 kDa in this study but 67 kDa in others (data not shown).(26) These differences are common to highly hydrophobic proteins and are likely caused by SDS when incompletely saturating highly hydrophobic polypeptides under unstable electrophoresis conditions.(27) All three MAbs were applicable in Western blot with the specimens, indicating that the selected peptide was conservative among T. gondii, P. falciparum, and P. berghei, and may be among the whole apicomplexan parasites, which implied that these three MAbs could provide help for the study of PfVP1 and PbVP1. However, these three MAbs were found to react with not only 85 kDa TgVP1, and 76 kDa PfVP1, or PbVP1, but also detected a 50 kDa cross-reacting band only in purified total proteins of T. gondii. We found that this band could be observed after the transferred PVDF membranes were incubated with secondary HRP-goat anti-mouse IgG antibody only, but the 50 kDa proteins could not be detected when rabbit polyclonal antibody against TgVP1-1 and secondary HRP-goat anti-rabbit IgG antibody were used for Western blotting (Fig. 6). This implies that the secondary HRP-goat anti-mouse IgG antibody we used could detect some 50 kDa proteins of T. gondii. In IFA, red fluorescence labeled mouse TgVP1 monoclonal antibodies randomly spread throughout the cell body did not merge with blue fluorescence in nucleus, implying that TgVP1 was distributed in cytoplasm, as acidocalsisome generally are.(8) All three MAbs labeled with anti-mouse IgG Alexa Fluor 546 (red) could bind well with the native protein of TgVP1.
FIG. 6.
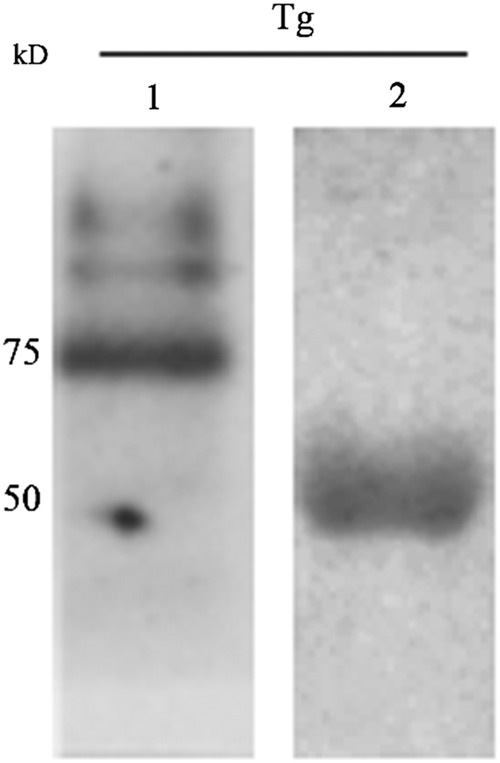
Western blot analysis of rabbit polyclonal antibody against TgVP1-1. Purified T. gondii total protein was subjected to 10% SDS-PAGE under the same condition as described above. Lane 1 was incubated with RPA as primary antibody and goat anti-rabbit antibody as secondary antibody, no 50 kDa protein band was detected. Lane 2 was incubated with goat anti-mouse secondary antibody only; a protein band of 50 kDa was observed.
In order to investigate the further application of the three MAbs described above, a sandwich ELISA was performed as a preliminary experiment. In this experiment, each MAb, along with a clone of rabbit polyclonal antibody against TgVP1-1, was used as capture and biotinylated detector to form 12 pairs of antibody combinations. The results from checkerboard experiments indicate that all pairs of antibody combinations are capable of capturing TgVP1-1 in sandwich assays, but only eight pairs give high signals. The statistical graph shows a strong linear relationship between OD450 and the natural logarithm of antigen concentration in the range of 3.125–200 ng/mL using the combination of 3A6E4 as capture and 3G6F9 as biotinylated detector, R2=0.991. We defined the positive cut-off value as 2.1 times higher than OD450 of the blank control, so the lower limit of detection in this combination could reach 3.125 ng/mL. Thus, it can be seen that i) there are at least two different epitopes contained in peptide TgVP1-1; ii) MAb 3A6-E4 and 3G6-F9 against the same peptide bound with two different epitopes respectively just as two different MAbs; and iii) these two MAbs set in pairs should be the best combination to detect TgVP1, but methods and conditions of the experiment need to be further refined (e.g., in terms of the best concentration of capture and detector antibody, the best concentration of purified total T. gondii protein, the treatment of patient specimens, and so on). This in turn would be a step forward in the study of T. gondii, and might be of some assistance in developing a new kit deploying the ELISA method for detection of T. gondii based on the MAbs described here.
Because the clinical signs of toxoplasmosis are non-specific and not characteristic enough for a definite diagnosis, this disease is usually diagnosed by serological, biological, histological, or molecular methods.(2) Otherwise, the treatment of toxoplasmosis has been restricted to a combined therapy of pyrimethamine, sulfadiazine with clindamycin, or spiramycin,(28) as novel drug targets have not been discovered. These three MAbs might be very valuable for the development of novel therapeutics and efficient subunit vaccine, since V-PPase and acidocalsisome are both potential targets. In a word, the TgVP1 monoclonal antibodies generated in this study will provide new tools for further study of the survival mechanisms of T. gondii, and may also provide some possibilities for the development of new diagnostic and therapeutic drugs.
Acknowledgments
This work was supported by a grant (no. 81171608 to H.W.) from the National Natural Science Foundation of China and by grants from the State Key Development Program of Basic Research of China (2007CB513101). We thank Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, for providing us with purified total proteins of P. falciparum and P. berghei. We also acknowledge RayBiotech (Guangzhou, China) for their support in peptide synthesis.
Author Disclosure Statement
The authors have no financial interests to disclose.
References
- 1.Dardé MAD, and Smith J: Population structure and epidemiology of toxoplasma gondii. Toxoplasma gondii: the model apicomplexan. Persp Methods 2011:49–80 [Google Scholar]
- 2.Hill D, and Dubey JP: Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect 2002;8:634–640 [DOI] [PubMed] [Google Scholar]
- 3.Torda A: Toxoplasmosis. Are cats really the source? Aus Fam Phys 2001;30:743–747 [PubMed] [Google Scholar]
- 4.Dupont CD, Christian DA, and Hunter CA: Immune response and immunopathology during toxoplasmosis. Sem Immunopath 2012;34:793–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bin Dajem SM, and Almushait MA: Detection of Toxoplasma gondii DNA by PCR in blood samples collected from pregnant Saudi women from the Aseer region, Saudi Arabia. Ann Saudi Med 2012;32:507–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sterkers Y, Ribot J, Albaba S, Issert E, Bastien P, and Pratlong F: Diagnosis of congenital toxoplasmosis by polymerase chain reaction on neonatal peripheral blood. Diagn Microbio Infec Dis 2011;71:174–176 [DOI] [PubMed] [Google Scholar]
- 7.Jones JL, Kruszon-Moran D, Wilson M, McQuillan G, Navin T, and McAuley JB: Toxoplasma gondii infection in the United States: seroprevalence and risk factors. Am J Epidemiol 2001;154:357–365 [DOI] [PubMed] [Google Scholar]
- 8.Docampo R, and Moreno SN: Acidocalcisomes. Cell Calc 2011;50:113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooperman BS, Baykov AA, and Lahti R: Evolutionary conservation of the active site of soluble inorganic pyrophosphatase. Trends Biochem Sci 1992;17:262–266 [DOI] [PubMed] [Google Scholar]
- 10.Marchesini N, Luo S, Rodrigues CO, Moreno SN, and Docampo R: Acidocalcisomes and a vacuolar H+-pyrophosphatase in malaria parasites. Biochem J 2000;347 Pt 1:243–253 [PMC free article] [PubMed] [Google Scholar]
- 11.Vita R, Overton JA, Greenbaum JA, Ponomarenko J, Clark JD, Cantrell JR, Wheeler DK, Gabbard JL, Hix D, Sette A, and Peters B: The immune epitope database (IEDB) 3.0. Nucleic acids Res 2015;43:D405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krogh A, Larsson B, von Heijne G, and Sonnhammer EL: Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 2001;305:567–580 [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues CO, Scott DA, Bailey BN, De Souza W, Benchimol M, Moreno B, Urbina JA, Oldfield E, and Moreno SN: Vacuolar proton pyrophosphatase activity and pyrophosphate (PPi) in Toxoplasma gondii as possible chemotherapeutic targets. Biochem J 2000;349 Pt 3:737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Pace D, Dou Z, King TP, Guidot D, Li ZH, Carruthers VB, and Moreno SN: A vacuolar-H(+) -pyrophosphatase (TgVP1) is required for microneme secretion, host cell invasion, and extracellular survival of Toxoplasma gondii. Mol Microbiol 2014;93:698–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim EJ, Zhen RG, and Rea PA: Heterologous expression of plant vacuolar pyrophosphatase in yeast demonstrates sufficiency of the substrate-binding subunit for proton transport. Proc Natl Acad Sci USA 1994;91:6128–6132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McIntosh MT, Drozdowicz YM, Laroiya K, Rea PA, and Vaidya AB: Two classes of plant-like vacuolar-type H(+)-pyrophosphatases in malaria parasites. Mol Biochem Parasitol 2001;114:183–195 [DOI] [PubMed] [Google Scholar]
- 17.Hill JE, Scott DA, Luo S, and Docampo R: Cloning and functional expression of a gene encoding a vacuolar-type proton-translocating pyrophosphatase from Trypanosoma cruzi. Biochem J 2000;351:281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo S, Vieira M, Graves J, Zhong L, and Moreno SN: A plasma membrane-type Ca(2+)-ATPase co-localizes with a vacuolar H(+)-pyrophosphatase to acidocalcisomes of Toxoplasma gondii. EMBO J 2001;20:55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montalvetti A, Rohloff P, and Docampo R: A functional aquaporin co-localizes with the vacuolar proton pyrophosphatase to acidocalcisomes and the contractile vacuole complex of Trypanosoma cruzi. J Biol Chem 2004;279:38673–38682 [DOI] [PubMed] [Google Scholar]
- 20.Rea PA, Kim Y, Sarafian V, Poole RJ, Davies JM, and Sanders D: Vacuolar H(+)-translocating pyrophosphatases: a new category of ion translocase. Trends Biochem Sci 1992;17:348–353 [DOI] [PubMed] [Google Scholar]
- 21.Olson SA: MacVector: an integrated sequence analysis program for the Macintosh. Methods Mol Biol 1994;25:195–201 [DOI] [PubMed] [Google Scholar]
- 22.Zou L, Wang Z, Wang Y, and Hu F: Combined prediction of transmembrane topology and signal peptide of beta-barrel proteins: using a hidden Markov model and genetic algorithms. Comput Biol Med 2010;40:621–628 [DOI] [PubMed] [Google Scholar]
- 23.Melen K, Krogh A, and von Heijne G: Reliability measures for membrane protein topology prediction algorithms. J Mol Biol 2003;327:735–744 [DOI] [PubMed] [Google Scholar]
- 24.Tsurumi Y, Hayakawa M, Shibata Y, and Abiko Y: Production of antibody against a synthetic peptide of Porphyromonas gingivalis 40-kDa outer membrane protein. J Oral Sci 2003;45:111–116 [DOI] [PubMed] [Google Scholar]
- 25.Moniaux N, Varshney GC, Chauhan SC, Copin MC, Jain M, Wittel UA, Andrianifahanana M, Aubert JP, and Batra SK: Generation and characterization of anti-MUC4 monoclonal antibodies reactive with normal and cancer cells in humans. J Histochem Cytochem 2004;52:253–261 [DOI] [PubMed] [Google Scholar]
- 26.Zhen EJK, and Rea PA: The molecular and biochemical basis of pyrophosphate-energized proton translocation at the vacuolar membrane. Adv Botan Res 1997;25:297–337 [Google Scholar]
- 27.Maddy AH: A critical evaluation of the analysis of membrane proteins by polyacrylamide gel electrophoresis in the presence of dodecyl sulphate. J Theor Biol 1976;62:315–326 [DOI] [PubMed] [Google Scholar]
- 28.Djurkovic-Djakovic O, Milenkovic V, Nikolic A, Bobic B, and Grujic J: Efficacy of atovaquone combined with clindamycin against murine infection with a cystogenic (Me49) strain of Toxoplasma gondii. J Antimicrob Chem 2002;50:981–987 [DOI] [PubMed] [Google Scholar]