Abstract
The carboxyl-terminal domain (CTD) of the largest subunit of RNA polymerase II (RNAP II) functions at multiple stages of transcription and is involved in the coupling of transcription to pre-mRNA processing. We have used site-specific protein-DNA photocross-linking to determine the position of the CTD along promoter DNA in the transcriptional pre-initiation complex. Comparison of the promoter contacts made by RNAP II with or without the CTD indicate that the CTD approaches promoter DNA downstream of the transcriptional initiation site between positions +16 and +26. Incubation of pre-assembled initiation complexes with antibodies to the CTD prior to UV irradiation led to specific photocross-linking of the IgG heavy chain to nucleotide +17, indicating that the CTD is accessible for protein-protein interactions in a complex containing RNAP II and the general initiation factors. In conjunction with previously published observations, our structural data are fully compatible with the notion that DNA wrapping around RNAP II places the CTD and the RNA exit channel into juxtaposition and provide a rationale for contacts between the SRB-mediator complex and core RNAP II observed in the RNAP II holoenzyme.
The CTD1 of Rpb1, the largest subunit of RNAP II, contains a seven-amino acid motif tandemly repeated 52 times in humans and 26–27 times in yeast (reviewed in Ref. 1). This repeated heptapeptide has the consensus sequence Tyr-Ser-Pro-Thr-Ser-Pro-Ser and is highly conserved among eukaryotic organisms. The CTD is essential for cell viability (2–5), and recent evidence indicates a central role for this domain in the coordination of the various enzymatic activities involved in mRNA formation including transcription, 5′-end capping, 3′-end formation, and pre-mRNA splicing (reviewed in Ref. 6).
Two different forms of RNAP II exist in vivo, namely RNAP IIA and IIO. The IIA form is not phosphorylated on the CTD and preferentially enters the pre-initiation complex, whereas the IIO form is extensively phosphorylated on the CTD and is found in the elongation complex (reviewed in Ref. 7). Conversion of IIA to IIO occurs either concomitant with, or shortly after, the passage from initiation to elongation and involves extensive CTD phosphorylation (8, 9). These observations support the notion that phosphorylation of the CTD regulates the conversion of RNAP II from the form involved in promoter recognition to that associated with the elongation complex. Consistent with a role of the unphosphorylated CTD in preinitiation complex formation, direct protein-protein interactions of the hypophosphorylated CTD with general initiation factors TBP (10), RAP74 (11), and TFIIE34 (11) have been reported. However, a form of RNAP II lacking the CTD (form IIB) is capable of transcriptional initiation at TATA box-containing promoters in vitro, but not at TATA-less promoters (12, 13). A number of kinases, including the general initiation factor TFIIH, and one phosphatase that can regulate CTD phosphorylation have been identified (reviewed in Refs. 7 and 14).
Although the CTD is essential for cell viability, partial truncation of the yeast Rpb1 CTD can lead to a range of conditional growth phenotypes (15, 16). This property was exploited by Young and collaborators to isolate various dominant extragenic suppressors in yeast strains bearing a partially deleted CTD (15). This approach resulted in the identification of the SRB genes, which encode some of the components of the SRB-mediator complex required for transcriptional activation (reviewed in Refs. 17–19), and suggested that the CTD plays a role in transcriptional activation. Consistent with this notion, direct binding of the CTD to components of the SRB-mediator complex has been reported (20, 21). The SRBs have been found in association with large multisubunit complexes, called RNAP II holoenzymes, containing core RNAP II, a number of general initiation factors and additional polypeptides (20–22). Roles for the CTD in transcriptional elongation and in transactivation by the HIV-1 tat protein have also been documented (23, 24).
Recent evidence from a number of laboratories indicates that the CTD is also involved in pre-mRNA processing (reviewed in Refs. 6 and 25). Splicing is inhibited both in vivo and in vitro by polypeptides containing CTD repeats and by antibodies directed against the CTD (26, 27). In addition, proteins that may physically link the spliceosome to RNAP II through the CTD have been identified (27–30). The CTD has also been implicated in both the 5′-end capping and the 3′-end formation of mRNA (reviewed in Refs. 6 and 25). The 5′-end capping enzymes and the 3′-end cleavage and polyadenylation factors have been shown to associate with the CTD (31–34). These results support a model in which the hyperphosphorylated, negatively charged CTD of RNAP IIO facilitates interactions with positively charged domains of mRNA processing factors, providing an interface for the recruitment of the enzymes required for pre-mRNA maturation.
NMR and circular dichroism studies have suggested that the CTD has an extended conformation in solution (35). Kornberg and co-workers (36) have used electron crystallography of two-dimensional microcrystalline arrays formed on mica sheets and stained with heavy metals to determine the three-dimensional structure of RNAP II. Difference maps between the wild-type enzyme and that lacking the CTD have resulted in its localization. The CTD appears as a mobile region, and its site of attachment to Rpb1 is opposite the 25-Å channel containing the catalytic center of the enzyme. These findings led to the prediction that the CTD is located near the region where TBP and TFIIB bind to the TATA box in the pre-initiation complex (37).
The structure of the CTD in a pre-initiation complex containing the general initiation factors and RNAP II remains elusive. Here, we report on the use of site-specific protein-DNA photocross-linking to localize the CTD along promoter DNA within the RNAP II pre-initiation complex. Our results provide a structural basis for the roles of the CTD in both transcription and pre-mRNA processing.
EXPERIMENTAL PROCEDURES
Protein Factors
Recombinant TBP, TFIIB, RAP74, RAP30, TFIIE34, TFIIE56, and RNAP IIB were prepared as described previously (38–40). RNAP IIA was purified from calf thymus extracts by affinity chromatography using an antibody directed against the CTD repeat (49).
N3R Photocross-linking
The synthesis of the photoreactive nucleotide N3R-dUMP, the preparation of the photoprobes, and the conditions for binding reactions were as described (38–41). For each photoprobe, the concentration of poly(dI-dC) in the binding reactions was optimized so as to favor specific over nonspecific binding. A typical reaction with all factors contained 200 ng each of TBP, TFIIB, RAP30, RAP74, TFIIE34, TFIIE56, and purified RNAP IIA or IIB as specified in the legends to Figs. 1 and 2. UV irradiation, nuclease treatment, and SDS-PAGE analysis of radiolabeled photocross-linking products were as described (38–41).
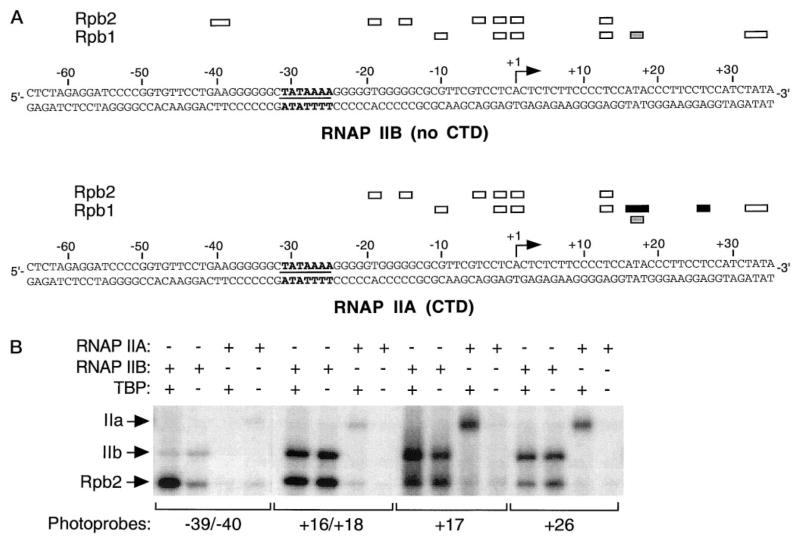
Fig. 1. Photocross-linking of RNA polymerases IIA and IIB along promoter DNA.
A, a summary of the photocross-linking data obtained using RNAP IIA (carrying the CTD) or IIB (lacking the CTD) is presented. The cross-links of Rpb1 and Rpb2 are represented by open boxes. The two additional cross-links of Rpb1 in reactions containing RNAP IIA are indicated by black boxes. The specific cross-linking of Rpb1 at position +17, which is increased with RNAP IIA, is represented by shaded boxes. The positions of the TATA element and the initiation site are indicated. B, cross-linking experiments using photoprobes −39/−40, +16/+18, +17, and +26 are shown for RNAP IIA and IIB. The positions of subunits IIa, IIb, and Rpb2 are shown. The specificity of the cross-linking experiments was assessed by comparing reactions performed in the presence or the absence of TBP (38–41).
Fig. 2. Direct contact of the CTD to position +17 of promoter DNA.

A, subunit IIa that had been affinity labeled using either photo-probe +1 or +17 was treated with chymotrypsin and analyzed by SDS-PAGE. The positions of subunits IIa, IIb, and Rpb2 are shown. B, initiation complexes assembled on either photoprobe +1 or +17 were incubated with an antibody directed against the CTD prior to UV irradiation. Photocross-linking reactions were then conducted as described. The position of the IgG heavy chain (H) is shown.
Protease Digestion of Affinity-labeled Polypeptides
The photocross-linking procedure was as described above. The affinity-labeled polypeptides were treated with α-chymotrypsin (0.04 μg/ml) for 40 min. at 25 °C in the presence of 50 mM Tris-HCl, pH 7.8; 0,1 mM EDTA; 25% glycerol (v/v); and, 0.5 mM dithiothreitol prior to the denaturation steps of the cross-linking procedure. Chymotrypsin reactions were terminated by boiling for 5 min. The digested products were resolved using SDS-PAGE.
Antibody Treatment
Pre-initiation complexes were assembled in a total volume of 25 μl using RNAP IIA and the general initiation factors prior to incubation with 1 μl (15 mg/ml) of antibody directed against the CTD of RNAP IIA (antibody 8WG16) (49). The anti-CTD-treated complex was irradiated with UV and processed as in a standard photocross-linking reaction.
RESULTS
Comparison of Promoter Contacts by RNA Polymerase IIA and IIB
To determine the position of the CTD in the preinitiation complex, we have used site-specific protein-DNA photocross-linking to compare the promoter contacts by RNAP II either carrying (form IIA) or lacking (form IIB) the CTD. As we have previously described, the photocross-linking reactions were performed in both the presence and the absence of TBP to assess the specificity of the cross-linking signals (38–41). Photocross-linking signals that are significantly weaker in the absence of TBP are considered as specific because the omission of TBP from the reactions always had the same effect as using a photoprobe with a mutated TATA element. As shown in Fig. 1, the cross-linking of RNAP IIA is strikingly similar to that of RNAP IIB but includes four notable differences: first, Rpb2, the second largest RNAP II subunit, which cross-linked upstream of the TATA box to positions −39/−40 when we used RNAP IIB, did not cross-link to this position with RNAP IIA; second, Rpb1 specifically cross-linked to the two additional photo-probes +16/+18 and +26 in experiments using RNAP IIA; and third, the cross-linking of Rpb1 to photoprobe +17, which is only slightly above the nonspecific signal using RNAP IIB, is increased in reactions using RNAP IIA. The presence of both TFIIH and ATP in our experiments did not modify the cross-linking sites of RNAP II subunits in the +16/+26 region (data not shown). These results indicate that the role of the CTD in enzyme-promoter interactions is modest and suggest that the CTD approaches promoter DNA in the region between +16 and +26.
Direct Photocross-linking of the CTD to Position +17
To determine whether the CTD directly approaches promoter DNA in the +16/+26 region, we performed two other experiments. First, we submitted affinity-labeled subunit IIa (Rpb1 subunits derived from the IIA and IIB forms of RNAP are referred to as subunits IIa and IIb, respectively) to a mild digestion with chymotrypsin. Limiting concentrations of chymotrypsin selectively removed the CTD from Rpb1, transforming IIa to IIb (Ref. 42 and data not shown). Chymotrypsin treatment of IIa that had been affinity labeled using protoprobe +1 led to the formation of affinity-labeled IIb (Fig. 2A), indicating that the photolabel is linked to an amino-terminal part of the polypeptide which is not degraded by chymotrypsin. In contrast, a similar treatment of IIa that had been affinity labeled using photoprobe +17 failed to produce affinity-labeled IIb (Fig. 2A), indicating that the photolabel is directly attached to the CTD which is completely proteolyzed in these experiments. These results indicate that the promoter contact at +17, but not that at +1, is directly to the CTD of Rpb1. Second, we incubated initiation complexes that had been pre-assembled onto promoter DNA with an antibody raised against the CTD. The reactions were then irradiated with UV and processed as in standard cross-linking conditions. When the complexes were formed onto photoprobe +17, we obtained cross-linking of the IgG heavy (H) chain (Fig. 2B). No cross-linking of the antibody was obtained using photoprobe +1. Incubation with a purified control antibody did not produce affinity labeling of the antibody (data not shown). Altogether, these data demonstrate that the CTD of Rpb1 is close to our photosensitive nucleotide at position +17. In addition, and interestingly, they indicate that the CTD is accessible for protein-protein interactions in the context of a TBP-TFIIB-TFIIF-RNAP II-TFIIE-promoter complex.
DISCUSSION
We have mapped the position of the CTD of the largest RNAP II subunit downstream of the transcriptional initiation site between nucleotides +16 and +26. This conclusion is supported by three distinct observations. First, comparison of the photocross-linking of RNAP II with and without the CTD revealed that subunit IIa (e.g. Rpb1 with the CTD) makes additional promoter contacts in the +16/+26 region but not in other regions of promoter DNA. In addition, the specific cross-linking of Rpb1 to position +17 is increased with RNAP IIA. Second, treatment of affinity-labeled Rpb1 using conditions that specifically degrade the CTD indicates that the photolabel is attached directly to the CTD when the photolabeling is performed using photoprobe +17, whereas it is attached to a amino-terminal part of Rpb1 when we used photoprobe +1. Third, treatment of a pre-assembled initiation complex with an anti-CTD antibody led to the photocross-linking of the IgG heavy chain to photoprobe +17, but not to other photoprobes, indicating that the CTD recruits the antibody to this specific promoter region. Importantly, the incubation of RNAP IIA with the anti-CTD antibody prior to initiation complex assembly reduced the cross-linking of Rpb1 to position +17 (data not shown), further indicating that at least part of the CTD must be accessible for its cross-linking to the +16/+26 region.
Using electron crystallography, Kornberg and co-workers determined the structure of free RNAP II both with and without the CTD (36). Their results indicate that the attachment point of the CTD to the body of the enzyme is opposite to the 25-Å channel that contains the catalytic center (see Fig. 3A for a schematic representation), thereby placing the CTD in close proximity to the site of TFIIB binding to RNAP II (36). This would imply that the CTD is close to the position where TBP binds to the TATA box in the initiation complex (37). In support of this prediction, direct binding of the CTD to TBP has been reported (10). We have recently provided compelling evidence indicating that promoter DNA is wrapped around RNAP II in the pre-initiation complex (40). DNA wrapping around RNA polymerase may be a fundamental mechanism that facilitates initiation, elongation, and activation of transcription (43). DNA wrapping places the portion of the promoter region centered at −40 into juxtaposition with that centered at +20. This implies that the 5′-border of the space occupied by TBP/TFIIB, which is approximately at position −40, is also juxtaposed to the region where the CTD has been found to cross-link in the +16/+26 region (see Fig. 3B). Promoter contacts by RAP74 and TFIIE34 have also been reported in the +16/+26 region (40), fully supporting the notion that TBP, RAP74, and TFIIE34 can all interact with the hypophosphorylated CTD (10, 11). In conclusion, our results indicate that DNA wrapping around RNAP II allows direct promoter contacts by the CTD downstream of the initiation site.
Fig. 3.
Shown are integrative molecular models of RNAP IIA in solution (A) and in the pre-initiation complex (B). C, a tentative model showing the close proximity of the CTD to the RNA exit channel. Two different views are shown (Back and Right) and refer to directions used in Robert et al. (40). The attachment point of the CTD to RNAP II and the location of the RNA exit channel of E. coli RNA polymerase are from Meredith et al. (36) and Nudler et al. (47), respectively. The general initiation factors have been omitted to simplify the models. D, the position of the CTD and the path of the promoter DNA (dashed line) have been superimposed on a schematic representation of the structure of core RNAP II bound to the SRB-mediator complex (see Fig. 3 of Ref. 44). Putative sites of interactions of the mediator with both the CTD of RNAP II (white arrows) and transcriptional activators located in upstream promoter regions (black arrow) are indicated.
Incubation of an anti-CTD antibody with pre-assembled initiation complexes resulted in the cross-linking of the IgG heavy chain to the promoter region contacted by the CTD (see Fig. 2B). This result further supports the conclusion that the CTD is located in the +16/+26 region and indicates that it is accessible for binding to additional factors in the context of a pre-initiation complex assembled with purified general initiation factors and core RNAP II. In the RNAP II holoenzyme, the CTD is expected to form protein-protein interactions with the SRB-mediator complex (21). Although our data do not address putative CTD-promoter contacts in the context of the holoenzyme, they nevertheless allow us to speculate that the mediator, or at least part of it, may co-localize with the CTD in the preinitiation complex. Recently, Asturias et al. (44) used electron microscopy to analyze the structure of particles containing both the SRB-mediator complex and core RNAP II. Their results indicate that the SRB-mediator complex makes apparent contacts with RNAP II near the point of attachment of the CTD, and also at a number of sites between this point and the 25-Å channel on one face of the enzyme. Fig. 3D shows a schematic representation of the structure of the mediator-RNAP II complex described by Kornberg and co-workers on which we have positioned the CTD according to the results described here. This composite model provides a structural basis for the interactions between core RNAP II and the SRB-mediator complex through direct contacts with the CTD. The model also indicates that the tail of the SRB-mediator complex is located near upstream promoter sequences where it could be a direct target for protein-protein interactions with DNA-bound transcriptional activators. Recently, Woychik and co-workers demonstrated that the Rpb5 subunit of yeast RNAP II has a role very similar to that of the Rpb1-CTD in transcriptional activation (45). The cross-linking data indicate that the CTD (this paper) and Rpb5 (46)2 occupy adjacent spaces between +13 and +16/+26. We propose that Rpb5 either interacts physically with the SRB-mediator complex or regulates the interaction of the CTD with this complex.
The exact time at which the CTD is phosphorylated during the passage from initiation to elongation remains elusive. Inclusion of both TFIIH and ATP, but not other nucleotides, in our experiments, which resulted in the association of TFIIH with the promoter and open complex formation as judged by striking modifications in enzyme-promoter interactions immediately upstream of the transcriptional initiation site, did not cause the conversion of subunit IIa to IIo (data not shown). This finding suggests that extensive phosphorylation of the CTD occurs only after the formation of the first phosphodiester bond.
Recently, a number of laboratories have provided evidence that the CTD of RNAP II is involved in the coupling of mRNA processing to transcription, probably by promoting the recruitment of processing factors to nascent RNA attached to elongating polymerase. If this notion is correct, the conformation of RNAP II on the DNA of an elongation complex must allow the juxtaposition of the CTD and the RNA exit channel at the surface of the enzyme. Recently, Goldfarb and co-workers (47) have determined the spatial organization of the transcriptional elongation complex in E. coli. These authors concluded that the RNA exit channel co-localizes with the point where DNA enters the complex. This striking observation indicates that the nascent transcript exits the elongation complex in a direction oriented toward the downstream end of the DNA relative to the catalytic center (a tentative model is presented in Fig. 3C). Our observation that the CTD makes promoter contacts in the +16/+26 region indicates that there is a close juxtaposition of the CTD and the RNA exit channel in the initiation complex. This conclusion fully supports a role for the CTD in the recruitment of mRNA processing factors to the vicinity of the nascent transcript during transcription. The accessibility of the CTD to IgG observed in our experiments is also consistent with this conclusion. The CTD must be accessible not only to the SRB-mediator complex during assembly of the pre-initiation complex and to pre-mRNA processing factors during elongation but also to kinases and phosphatases that have been shown to modulate its phosphorylation during the transcription cycle. Our results are fully compatible with this idea.
Whether or not the phosphorylation of the CTD induces a conformational change that repositions the CTD of elongating RNAP II relative to the DNA cannot be resolved by our experiments. Because the juxtaposition of the CTD with the RNA exit channel is likely to be required for mRNA processing, we speculate that the promoter contact by the CTD approximately 20 base pairs downstream of the catalytic center would be maintained during elongation. Laybourn and Dahmus (48) have shown that elongating RNAP II is more sensitive to proteolytic cleavage of its CTD than is the free enzyme. We speculate that interaction of the CTD with general initiation factors or DNA near position +17 may increase the exposure to protease of the site where the CTD attaches to the body of the enzyme (see Fig. 3B), consequently facilitating proteolytic cleavage.
Acknowledgments
We thank members of our laboratory for valuable discussions, Pierre Vandenberghe for the molecular models, and Will Home for critical reading of the manuscript.
Footnotes
This work was supported by grants from the Medical Research Council of Canada (to B. C. and J. G.) and the Cancer Research Society Inc. (to B. C.).
The abbreviations used are: CTD, carboxyl-terminal domain of Rpb1; N3R-dUMP, 5-[N-(p-azidobenzoyl)-3-aminoallyl]-deoxyuridine monophosphate; RAP, RNA polymerase II-associated protein; RNAP II, RNA polymerase II; PAGE, polyacrylamide gel electrophoresis; SRB, suppressor of RNA polymerase B; TBP, TATA box-binding protein; TF, transcription factor.
M. Douziech, D. Forget, J. Greenblatt, and B. Coulombe, unpublished data.
References
- 1.Young RA. Annu Rev Biochem. 1991;60:689–715. doi: 10.1146/annurev.bi.60.070191.003353. [DOI] [PubMed] [Google Scholar]
- 2.Nonet M, Sweetser D, Young RA. Cell. 1987;50:909–915. doi: 10.1016/0092-8674(87)90517-4. [DOI] [PubMed] [Google Scholar]
- 3.Allison LA, Wong JKC, Fitzpatrick VD, Moyle M, Ingles CJ. Mol Cell Biol. 1988;8:321–329. doi: 10.1128/mcb.8.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartolomei MS, Halden NF, Cullen CR, Corden JL. Mol Cell Biol. 1988;8:330–339. doi: 10.1128/mcb.8.1.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zehring WA, Lee JM, Weeks JR, Jokerst RS, Greenleaf AL. Proc Natl Acad Sci U S A. 1988;85:3698–3702. doi: 10.1073/pnas.85.11.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinmetz EJ. Cell. 1997;89:491–494. doi: 10.1016/s0092-8674(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 7.Dahmus ME. J Biol Chem. 1996;271:19009–19012. doi: 10.1074/jbc.271.32.19009. [DOI] [PubMed] [Google Scholar]
- 8.Lu H, Flores O, Weinmann R, Reinberg D. Proc Natl Acad Sci U S A. 1991;88:10004–10008. doi: 10.1073/pnas.88.22.10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien T, Hardin S, Greenleaf A, Lis JT. Nature. 1994;370:75–77. doi: 10.1038/370075a0. [DOI] [PubMed] [Google Scholar]
- 10.Usheva A, Maldonado E, Goldring A, Lu H, Houbavi C, Reinberg D, Aloni Y. Cell. 1992;69:871–881. doi: 10.1016/0092-8674(92)90297-p. [DOI] [PubMed] [Google Scholar]
- 11.Kang ME, Dahmus ME. J Biol Chem. 1995;270:23390–23397. doi: 10.1074/jbc.270.40.23390. [DOI] [PubMed] [Google Scholar]
- 12.Akoulitchev S, Makela TP, Weinberg RA, Reinberg D. Nature. 1995;377:557–560. doi: 10.1038/377557a0. [DOI] [PubMed] [Google Scholar]
- 13.Buermeyer AB, Strasheim LA, McMahon SL, Farnham PJ. J Biol Chem. 1995;270:6798–6807. doi: 10.1074/jbc.270.12.6798. [DOI] [PubMed] [Google Scholar]
- 14.Hampsey M. Microbiol Mol Biol Rev. 1998;62:465–503. doi: 10.1128/mmbr.62.2.465-503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nonet ML, Young RA. Genetics. 1989;123:715–724. doi: 10.1093/genetics/123.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West ML, Corden JL. Genetics. 1995;140:1223–1233. doi: 10.1093/genetics/140.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emili A, Ingles CJ. Curr Opin Genet Dev. 1995;5:204–209. doi: 10.1016/0959-437x(95)80009-3. [DOI] [PubMed] [Google Scholar]
- 18.Björklund S, Kim YJ. Trends Biochem Sci. 1996;21:335–337. doi: 10.1016/s0968-0004(96)10051-7. [DOI] [PubMed] [Google Scholar]
- 19.Carlson M. Annu Rev Cell Dev Biol. 1997;13:1–23. doi: 10.1146/annurev.cellbio.13.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Kim YJ, Björklund S, Li Y, Sayre MH, Kornberg RD. Cell. 1994;77:599–608. doi: 10.1016/0092-8674(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 21.Greenblatt J. Curr Opin Cell Biol. 1997;9:310–319. doi: 10.1016/s0955-0674(97)80002-6. [DOI] [PubMed] [Google Scholar]
- 22.Koleske AJ, Young RA. Nature. 1994;368:466–469. doi: 10.1038/368466a0. [DOI] [PubMed] [Google Scholar]
- 23.Shilatifard A, Conaway JW, Conaway CR. Curr Opin Genet Dev. 1997;7:199–204. doi: 10.1016/s0959-437x(97)80129-3. [DOI] [PubMed] [Google Scholar]
- 24.Yankulov K, Bentley D. Curr Biol. 1998;8:447–449. doi: 10.1016/s0960-9822(98)70289-1. [DOI] [PubMed] [Google Scholar]
- 25.Greenleaf AL. Trends Biochem Sci. 1993;18:117–119. doi: 10.1016/0968-0004(93)90016-g. [DOI] [PubMed] [Google Scholar]
- 26.Yuryev A, Patturajan M, Litingtung Y, Joshi RV, Gentile C, Gebara M, Corden JL. Proc Natl Acad Sci U S A. 1996;93:6975–6980. doi: 10.1073/pnas.93.14.6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du L, Warren SL. J Cell Biol. 1997;136:5–18. doi: 10.1083/jcb.136.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chabot B, Bisotto S, Vincent M. Nucleic Acids Res. 1995;23:3206–3213. doi: 10.1093/nar/23.16.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinmetz EJ, Brow DA. Mol Cell Biol. 1996;16:6993–7003. doi: 10.1128/mcb.16.12.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent M, Lauriault P, Dubois MF, Lavoie S, Bensaude O, Chabot B. Nucleic Acids Res. 1996;24:4649–4652. doi: 10.1093/nar/24.23.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho EJ, Takagi T, Moore CR, Buratowski S. Genes Dev. 1997;11:3319–3326. doi: 10.1101/gad.11.24.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCracken S, Fong N, Rosonina E, Yankulov K, Brothers G, Siderovski D, Hessel A, Foster S, Shuman S, Bentley DL. Genes Dev. 1997;11:3306–3318. doi: 10.1101/gad.11.24.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCracken S, Fong N, Yankulov K, Ballantyne S, Pan GH, Greenblatt J, Patterson SD, Wickens M, Bentley DL. Nature. 1997;385:357–361. doi: 10.1038/385357a0. [DOI] [PubMed] [Google Scholar]
- 34.Yue Z, Maldonado E, Pillutla R, Cho H, Reinberg D, Shatkin AJ. ) Proc Natl Acad Sci U S A. 1997;94:12898–12903. doi: 10.1073/pnas.94.24.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cagas PM, Corden JL. Proteins Struct Funct Genet. 1995;21:149–160. doi: 10.1002/prot.340210209. [DOI] [PubMed] [Google Scholar]
- 36.Meredith GD, Chang WH, Li Y, Bushnell DA, Darst SA, Kornberg R. J Mol Biol. 1996;258:413–419. doi: 10.1006/jmbi.1996.0258. [DOI] [PubMed] [Google Scholar]
- 37.Kornberg RD. Trends Biochem Sci. 1996;21:325–326. [PubMed] [Google Scholar]
- 38.Robert F, Forget D, Li J, Greenblatt J, Coulombe B. J Biol Chem. 1996;271:8517–8520. doi: 10.1074/jbc.271.15.8517. [DOI] [PubMed] [Google Scholar]
- 39.Forget D, Robert F, Grondin G, Burton ZF, Greenblatt J, Coulombe B. Proc Natl Acad Sci U S A. 1997;94:7150–7155. doi: 10.1073/pnas.94.14.7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robert F, Douziech M, Forget D, Egly JM, Greenblatt J, Burton ZF, Coulombe B. Mol Cell. 1998;2:341–351. doi: 10.1016/s1097-2765(00)80278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robert F, Coulombe B. In: Methods in Molecular Biology; Protein-DNA Interaction Protocols. Moss T, editor. Humana Press Inc; Totowa: 1999. in press. [Google Scholar]
- 42.Buratowski S, Sharp PA. Mol Cell Biol. 1990;10:5562–5564. doi: 10.1128/mcb.10.10.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coulombe B, Burton ZF. Microbiol Mol Biol Rev. 1999;63:457–478. doi: 10.1128/mmbr.63.2.457-478.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asturias FJ, Jiang YW, Myers LC, Gustafsson CM, Kornberg RD. Science. 1999;283:985–987. doi: 10.1126/science.283.5404.985. [DOI] [PubMed] [Google Scholar]
- 45.Miyao T, Woychik NA. Proc Natl Acad Sci U S A. 1998;95:15281–15286. doi: 10.1073/pnas.95.26.15281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim TK, Lagrange T, Wang YH, Griffith JD, Reinberg D, Ebright RH. Proc Natl Acad Sci U S A. 1997;94:12268–12273. doi: 10.1073/pnas.94.23.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nudler E, Gusarov I, Avetissova E, Kozlov M, Goldfarb A. Science. 1998;281:424–428. doi: 10.1126/science.281.5375.424. [DOI] [PubMed] [Google Scholar]
- 48.Laybourn PJ, Dahmus ME. J Biol Chem. 1989;264:6693–6698. [PubMed] [Google Scholar]
- 49.Thompson NE, Steinberg TH, Aronson DB, Burgess RR. J Biol Chem. 1989;264:11511–11520. [PubMed] [Google Scholar]