Abstract
We previously reported that adding freeze-dried tomato powder from transgenic plants expressing the apolipoprotein A-I mimetic peptide 6F at 2.2% by weight to a Western diet (WD) ameliorated dyslipidemia and atherosclerosis in mice. The same dose in a human would require three cups of tomato powder three times daily. To reduce the volume, we sought a method to concentrate 6F. Remarkably, extracting the transgenic freeze-dried tomato overnight in ethyl acetate with 5% acetic acid resulted in a 37-fold reduction in the amount of transgenic tomato needed for biologic activity. In a mouse model of dyslipidemia, adding 0.06% by weight of the tomato concentrate expressing the 6F peptide (Tg6F) to a WD significantly reduced plasma total cholesterol and triglycerides (P < 0.0065). In a mouse model of colon cancer metastatic to the lungs, adding 0.06% of Tg6F, but not a control tomato concentrate (EV), to standard mouse chow reduced tumor-associated neutrophils by 94 ± 1.1% (P = 0.0052), and reduced tumor burden by two-thirds (P = 0.0371). Adding 0.06% of either EV or Tg6F by weight to standard mouse chow significantly reduced tumor burden in a mouse model of ovarian cancer; however, Tg6F was significantly more effective (35% reduction for EV vs. 53% reduction for Tg6F; P = 0.0069). Providing the same dose of tomato concentrate to humans would require only two tablespoons three times daily making this a practical approach for testing oral apoA-I mimetic therapy in the treatment of dyslipidemia and cancer.
Keywords: ApoA-I mimetic peptides, cancer, drug delivery, lipids, transgenic tomatoes
Introduction
Recent studies suggest that apolipoprotein (apo) A-I and apoA-I mimetic peptides may have a role in the treatment of a number of malignancies. HDL-associated proteins apoA-I, transthyretin, and transferrin have been identified as biomarkers of ovarian cancer (Kozak et al. 2003, 2005; Nossov et al. 2008, 2009). In a mouse model of ovarian cancer in which the mice have a normal immune system, it was found that transgenic expression of human apoA-I significantly decreased tumor burden and extended survival of the mice (Su et al. 2010). Subsequently, Zamanian-Daryoush et al. (2013) reported that apoA-I potently suppressed tumor growth and metastasis in multiple animal tumor models through both innate and adaptive immune processes.
Su et al. (2010) demonstrated that the apoA-I mimetic peptides 4F and 5F administered either orally or by injection decreased tumor growth, and decreased levels of a known tumor growth promoter, lysophosphatidic acid (LPA). Mechanisms deduced to play a role in the efficacy of apoA-I mimetic peptides included decreased tumor angiogenesis (Gao et al. 2011).
In other studies, it was found that the 4F peptide upregulated the antioxidant enzyme manganese superoxide dismutase (MnSOD) and inhibited proliferation and the ability to form tumors of epithelial ovarian cancer cells (Ganapathy et al. 2012).
The risk for endometrial (Cust et al. 2007) and colon cancer (van Duijnhoven et al. 2011) is inversely correlated with HDL-cholesterol levels. Adding the 4F peptide to mouse chow decreased tumor burden, tumor angiogenesis, and plasma LPA levels in a mouse model of colon cancer (Su et al. 2012). In a mouse model of familial adenomatous polyposis, administering 4F in mouse chow also reduced the number and size of tumors in the intestinal tract and decreased LPA levels in C57BL/6J-ApcMin/+ mice (Su et al. 2012).
Hypoxia-inducible factor-1α (HIF-1α) was induced in vitro by LPA in human ovarian cancer cell lines, and was decreased by addition of the 4F peptide (Gao et al. 2012). Moreover, administration of the 4F peptide markedly decreased the expression of HIF-1α in mouse ovarian tumor tissues (Gao et al. 2012).
Inflammation (Mantovani et al. 2008) and tumor-associated macrophages play an important role in the progression and metastasis of cancers (Qian and Pollard 2010; Zamanian-Daryoush et al. 2013). LPA has been reported to increase the expression of scavenger receptor A (SR-A) on macrophages (Chang et al. 2008). SR-A expression on macrophages has been shown to be necessary and sufficient to promote tumor invasiveness (Neyen et al. 2013a). The 4F peptide was reported to be a potent inhibitor of SR-A (Neyen et al. 2009); administration of the 4F peptide inhibited tumor invasiveness (Neyen et al. 2013a,b). Thus, there is evidence in animal models that apoA-I and apoA-I mimetic peptides may be potential therapeutic agents for the amelioration of cancer.
We recently reported a novel means of administering apoA-I mimetic peptides in mouse models of atherosclerosis (Chattopadhyay et al. 2013; Navab et al. 2013, 2015). We showed that the apoA-I mimetic peptide 6F could be expressed in transgenic tomato plants. When the tomatoes were freeze-dried and fed to LDLR null mice in a Western diet (WD), they ameliorated dyslipidemia and atherosclerosis (Chattopadhyay et al. 2013).
The transgenic tomatoes expressing the 6F peptide (Tg6F) also ameliorated dyslipidemia and atherosclerosis induced by adding unsaturated LPA to standard mouse chow (Navab et al. 2015). In the mouse studies (Chattopadhyay et al. 2013; Navab et al. 2013, 2015) the freeze-dried, ground tomato powder was added to mouse diets at 2.2% by weight. Laboratory mice eat a single diet making it easy to mix in the freeze-dried tomato powder. In contrast, human diets are much more complicated, and it would be a challenge to use freeze-dried tomato powder as a dietary supplement because of the amount of powder required to achieve the same doses of the peptide as were achieved in mice; three cups of powder three times per day would be required. It was felt that this volume would be impractical for widespread use. Therefore, we sought a simple and economical method to concentrate the 6F peptide from freeze-dried tomatoes in order to decrease the volume required to achieve therapeutic doses. We now report that concentrates of Tg6F can easily be prepared, such that the required doses could be administered to humans using no more than two tablespoons of concentrate three times daily. We also present evidence that these concentrates are effective in mouse models of dyslipidemia, and in mouse models of cancer.
Materials and Methods
Materials
Chemical reagents
Ethanol (catalog no. BP2818-100), ethyl acetate (HPLC grade; catalog no. E195-4), and glacial acetic acid (HPLC grade; catalog no. A35-500) were purchased from Fisher Scientific (Pittsburgh, PA, USA).
Tumor cells
The ID8 cell line (a mouse ovarian epithelial papillary serous adenocarcinoma cell line) was a generous gift from K. F. Roby (Center for Reproductive Sciences, University of Kansas Medical Center, Kansas City, KS). The CT26 cell line derived from N-nitroso-N-methyl urethane-induced mouse colon carcinoma of BALB/c origin was purchased from the American Type Culture Collection (ATCC).
Mouse apoA-I ELISA kit was from USCN Life Science, Inc. Wuhan, China (catalogue no. SEA519Mu). All other materials were from sources described previously (Chattopadhyay et al. 2013; Navab et al. 2013, 2015) or as specifically stated below.
Mice
Female wild-type C57BL/6J and BALB/c mice were purchased from Jackson Laboratory and maintained on standard mouse chow (Teklad, Harlan, catlog no. T7013M15, Indianapolis, Indiana, USA). LDLR null mice on a C57BL/6J background were purchased from Jackson Laboratory and bred in the Division of Laboratory and Animal Medicine at UCLA. All LDLR null mice were female. All mice were maintained on standard mouse chow (Ralston Purina). The LDLR null mice were switched to a WD (Teklad, Harlan, catalog no. TD88137) (Indianapolis, Indiana, USA) for the period stated in the figure legend. The ages of the mice are stated in the figure legends. All mouse experiments were approved by the Animal Research Committee at UCLA.
Methods
Preparation of tomato concentrates
Transgenic tomato plants expressing the marker protein β-glucuronidase (EV), or expressing the 6F peptide (Tg6F) were constructed and grown at the Donald Danforth Plant Science Center in St. Louis, MO as previously described (Chattopadhyay et al. 2013). The tomatoes were rendered free of seeds, rapidly frozen, and shipped frozen by overnight courier to UCLA where they were freeze-dried (VirTis lyophilizer, Gardner, NY) to obtain dried tomato fruit tissue (pulp plus skin), which was stored at −80°C as previously described (Chattopadhyay et al. 2013).
The freeze-dried EV and Tg6F tomato fruit tissue was thoroughly mixed in either aqueous ethanol (ethanol/water, 60/40; v/v), or ethyl acetate containing 5% glacial acetic acid at a ratio of 1/25, w/v (gm/mL), and extracted for 24 h at room temperature. After 24 h the liquid phase was clearly separated from the solid phase in both the ethanol/water and ethyl acetate/acetic acid extractions. The liquid phase was collected and the ethanol/water extract was dried under vacuum; the ethyl acetate/acetic acid extract was dried under a stream of argon. The remaining solids were redissolved in distilled water to 10% of the original volume and then lyophilized. The final concentrates were weighed and stored at −20°C until use.
Analysis of tomato concentrates
100 or 200 μg of tomato concentrate protein, or 5–20 μg of authentic 6F peptide produced by solid-phase synthesis as previously described (Chattopadhyay et al. 2013) was added to lanes of 18% SDS PAGE gels that were run overnight. The gels were fixed in water/methanol/acetic acid (50/50/7, v/v/v, 30 min at room temperature), stained overnight with Sypro Ruby (s12000 kit, Life Technologies, NY, Grand Island, NY, USA), and de-stained with water/methanol/acetic acid (9/10/7, v/v/v, 30 min at room temperature). Densitometry images of the destained gels were recorded using image J software as previously described (Chattopadhyay et al. 2013). To confirm the identity of 6F, bands of interest were manually excised and treated with trypsin (10 μL, 100 ng/mL 50 mmol/L ammonium bicarbonate, Trypsin Gold, V5280; Promega, Madison, WI, USA) overnight, at 37°C. The treated gel slices were eluted with water/acetonitrile/TFA (1 mL, 50/50/0.1, v/v/v) and the eluates were dried in a vacuum centrifuge and re-dissolved in 10 μL of water/acetonitrile/TFA and injected onto a reverse-phase LC column (Agilent PLRP-S (Santa Clara, CA, USA), 5 micron particle size, 300 Å pore diameter, 150 × 2 mm) equilibrated in 95% Solvent A (water/formic acid, 100/0.1, v/v) and 5% Solvent B (acetonitrile/formic acid, 100/0.1, v/v) and eluted at constant flow (40°C, 200 μL/min) with an increasing proportion of Solvent B (%B/min: 0/5, 5/5, 45/100, 50/5, 60/5). The effluent from the column was directed to an electrospray ion source connected to a quadrupole ion trap mass spectrometer (Thermo LCQ Deca XP Plus, West Palm Beach, FL, USA) scanning in the positive ion mode (m/z 50–2000, 3 microscans were averaged, 50 msec maximum inject time). Data were processed in Thermo Xcalibre™ software.
Preparation of diets
The tomato concentrates were taken from the freezer and added to standard mouse chow or to WD in an industrial mixer and thoroughly mixed for 30 min as previously described (Chattopadhyay et al. 2013; Navab et al. 2013, 2015) to yield a final diet containing 0.015%, 0.03% or 0.06% by weight of each tomato concentrate. In some experiments, the starting material (i.e., the freeze-dried transgenic tomatoes from which the concentrates were made) was added to standard mouse chow at 2.2%, or 1.1% or 0.55% by weight as described previously (Chattopadhyay et al. 2013; Navab et al. 2013, 2015) and used as controls. The diets were packaged into 16 g portions in aluminum foil and kept at −80°C until use. Addition of 0.06% by weight provided the mice with a daily dose of ∼120 mg/kg/day per mouse of tomato concentrate, which provided ∼7 mg/kg per day per mouse of the 6F peptide. In the cancer studies administration of the tomato concentrates began the day after the cancer cells were injected.
Metastatic colon cancer studies
Female BALB/c mice 6 weeks of age were administered 2 × 104 CT26 cells in 100 μL of PBS via tail vein injection as described previously (Su et al. 2012). After injection the mice were maintained on either standard mouse chow or standard mouse chow containing 0.06% by weight of EV, or 0.06% by weight of Tg6F. After 4 weeks the mice were subjected to a terminal bleed, and after sacrifice the lungs were harvested, weighed, and fixed with Bouin’s solution (Sigma, St.Louis, MO, USA), and the number of tumor nodules on the surface of the lungs was determined as previously described (Su et al. 2012). For determination of tumor-associated neutrophils, sections were prepared of randomly selected tumors from the lungs of three mice in each treatment group. The slides were placed in xylene to remove paraffin and then processed through a series of ethanol rinses. After a wash in tap water, the slides were incubated in 3% hydrogen peroxide/methanol solution for 10 min. After a wash in distilled water, the slides were incubated for 2 min in EDTA solution pH 8.0 (Invitrogen, catalog no. 005501) at 95°C using a pressure cooker. The slides were brought to room temperature, rinsed in phosphate-buffered saline containing Tween-20 (PBST), and then incubated at room temperature for 1 h with anti-Ly6G antibody (BD Pharmingen (San Jose, CA, USA), catalog number 551459) at a dilution of 1:1500. The slides were rinsed with PBST and then incubated with polyclonal rabbit anti-rat immunoglobulins/HRP (Dako (Carpentaria, CA, USA), catalog number P0450) at a dilution of 1:200 at room temperature for 30 min. The slides were rinsed with PBST, and incubated with Dako EnVision + System-HRP labeled polymer anti-rabbit antibody (Dako, catalog number K4003) at room temperature for 30 min. After a rinse with PBST, the slides were incubated with 3,3′-Diaminobenzidine (DAB) for visualization. Subsequently, the slides were washed in tap water, counterstained with Harris’ Hematoxylin, dehydrated in ethanol, and mounted with media. Controls consisted of sections exposed to secondary-only antibodies. Photomicrographs were captured using an Olympus BX51 microscope and the application Q Capture 7.0 (Q Imaging Inc., Surrey, BC, Canada). Randomly selected fields were quantified for each sample and the ratio of the stain signal to the tumor surface area was determined using Image Pro Plus 7 (Media Cybernetics, Rockvillie, MD, USA).
Ovarian cancer studies
Female C57BL/6J mice 9 weeks of age were given an intraperitoneal injection of 8 × 106 ID8 cells in a total volume of 0.8 mL of Dulbecco’s Modified Eagle Medium (DMEM without supplements). Following injection, the mice were maintained on standard mouse chow or standard mouse chow containing 0.06% by weight of EV, or 0.06% by weight of Tg6F. After 12 weeks the mice were subjected to a terminal bleed, and after sacrifice the number of tumor nodules on the peritoneal surfaces and on the surface of abdominal organs was determined as previously described (Su et al. 2010).
Plasma assays
Plasma total cholesterol, triglycerides, HDL-cholesterol, paraoxonase-1 (PON) activity, serum amyloid A (SAA), and apoA-I were assayed using kits and procedures outlined by the kit manufacturers as previously described (Chattopadhyay et al. 2013; Navab et al. 2013, 2015). Species of LPA were determined by LC-MS/MS/MRM as previously described (Chattopadhyay et al. 2013; Navab et al. 2013, 2015), and Fast Performance Liquid Chromatography (FPLC) was performed as previously described (Chattopadhyay et al. 2013; Navab et al. 2013).
Statistical methods
Statistical analyses were performed by analysis of variance (ANOVA) and by unpaired two-tailed t-test, or by linear regression using GraphPad Prism version 5.03 (GraphPad Software, San Diego, CA). Statistical significance was considered achieved if P < 0.05.
Results
Ethyl acetate with 5% acetic acid (but not 60% ethanol) efficiently concentrates the 6F peptide produced in transgenic tomatoes-
Twenty-three micrograms of 6F were found in 100 μg of tomato concentrate protein prepared with ethyl acetate with 5% acetic acid (Fig.1A). In contrast, concentrates prepared with 60% ethanol did not contain detectable amounts of 6F (Fig.1B). The presence of the 6F peptide was confirmed in the Tg6F concentrate (but not in the EV concentrate) by reverse-phase LC and mass spectrometry (Fig.1C). Based on these results, all subsequent experiments utilized tomato concentrates prepared with ethyl acetate with 5% acetic acid.
Figure 1.
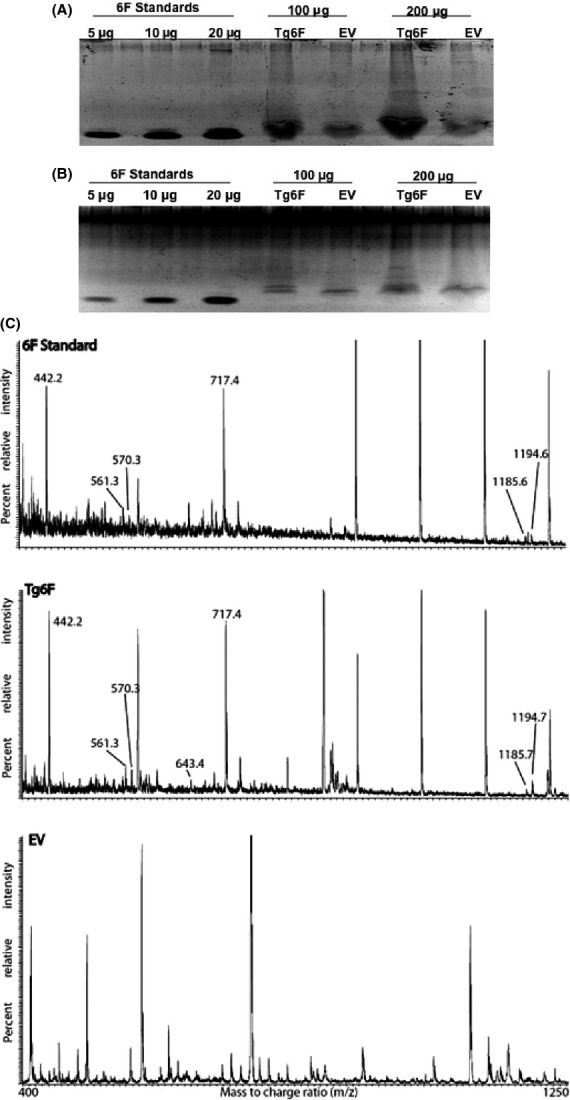
Ethyl acetate with 5% acetic acid (but not 60% ethanol) efficiently concentrates the 6F peptide produced in transgenic tomatoes. Freeze-dried transgenic tomatoes expressing β-glucuronidase (EV), or expressing the 6F peptide (Tg6F) were extracted overnight at room temperature in either ethyl acetate/acetic acid, or in aqueous ethanol as described in Materials and Methods. The ethyl acetate/acetic acid extract was dried under argon; the aqueous ethanol extract was dried under vacuum as described in Materials and Methods. The remaining solids were suspended in water and analyzed on 18% SDS PAGE gels as described in Materials and Methods. (A) Image of the Sypro Ruby-stained gel of the tomato concentrates prepared with ethyl acetate/acetic acid (100 μg or 200 μg of protein per lane). (B) Image of the Sypro Ruby-stained gel of tomato concentrates prepared with aqueous ethanol (100 μg protein per lane). Authentic chemically synthesized 6F peptide at 5, 10, or 20 μg per lane was included as standards in (A) and (B). (C) Summary of the results of LC/MS analysis of the trypsin digest of the authentic 6F standard (6F Standard, top of panel C, 5 μg), the trypsin digest of the corresponding band from the Tg6F lane (Tg6F, middle of panel C, 100 μg), and the trypsin digest of the corresponding band from the EV lane (EV, bottom of C, 100 μg) performed as described in Materials and Methods. To simplify data presentation, the spectra collected between 13 and 25 min from all three chromatograms were averaged. All the tryptic peptides eluted within this window. The arrows point to signals identified on the basis of molecular weight concordance as being derived from 6F peptide: EFF (residues 16–18), found m/z 442.2 (detected in both 6F Standard and Tg6F, but not in EV), calculated for MH+ 442.197 Da (monoisotopic, mi); DWLK (residues 1–4), found m/z 561.3 (detected in both 6F Standard and Tg6F, but not in EV), calculated for MH+ 561.303 Da (mi); FFEK (residues 10–13), found m/z 570.3 (detected in both 6F Standard and Tg6F, but not in EV), calculated for MH+ 570.292 Da (mi); AFYDK (residues 5–9), found m/z 643.3 (detected in Tg6F, but not in 6F Standard or EV), calculated for MH+ 643.308 Da (mi); FKEFF (residues 14–18), found m/z 717.4 (detected in both 6F Standard and Tg6F, but not in EV), calculated for MH+ 717.361 Da (mi); DWLKAFYDK (residues 1–9), found m/z 1185.6 and 1185.7 (detected in 6F Standard and Tg6F, respectively, but not in EV), calculated for MH+ 1185.594 Da (mi); AFYDKFFEK (residues 5–13), found m/z 1194.6 and 1194.7 (detected in 6F Standard and Tg6F, respectively, but not in EV), calculated for MH+ 1194.583 Da (mi). These partially overlapping tryptic peptides provided 100% sequence coverage for both the 6F standard and the Tg6F sample. These results are representative of two experiments.
Dose response of tomato concentrates
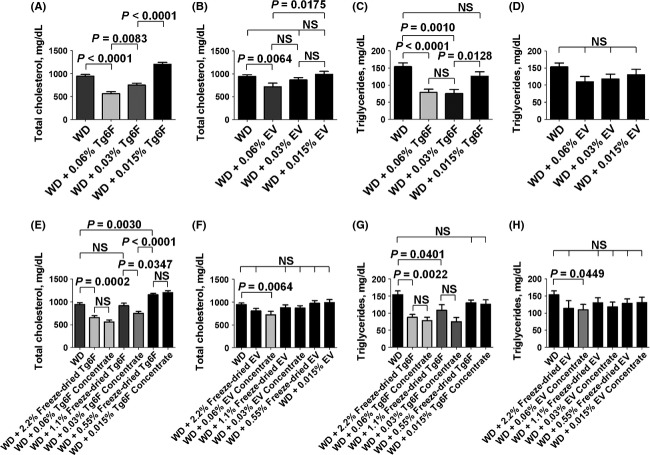
To determine the dose response of the tomato concentrates, we used a mouse model of dyslipidemia as shown in Figure2. The data in Figure2 demonstrate that the tomato concentrates were at least as effective in improving plasma lipids as was the starting material (i.e., the freeze-dried transgenic tomatoes from which the concentrates were made). Based on these results we used a dose of 0.06% by weight of the tomato concentrates in all subsequent experiments.
Figure 2.
Dose response of tomato concentrates in a mouse model of dyslipidemia. Female LDLR null mice 4–5 months of age (n = 8–24 mice per group) were fed a Western diet (WD) or WD supplemented with 0.015%, 0.03% or 0.06% tomato concentrate by weight or the mice were fed the freeze-dried transgenic tomatoes from which the concentrates were made added to the WD at 0.55%, 1.1% or 2.2% by weight as described in Materials and Methods. After 2 weeks the mice were fasted overnight and plasma total cholesterol and triglycerides were determined as described in Materials and Methods. (A and B) Plasma total cholesterol levels for mice receiving Tg6F or EV tomato concentrates, respectively. (C and D) Plasma triglyceride levels for mice receiving Tg6F or EV tomato concentrates, respectively. (E and F) Plasma total cholesterol levels for the mice in (A and B), respectively, compared to mice that received the freeze-dried transgenic tomatoes from which the concentrates were made. (G and H) Plasma triglyceride levels for the mice in (C and D), respectively, compared to mice that received the freeze-dried transgenic tomatoes from which the concentrates were made. The data shown are mean ± SEM. NS, not significant; Tg6F, tomato concentrate from transgenic tomatoes expressing the 6F peptide; EV, tomato concentrate from transgenic tomatoes expressing the marker protein, β-glucuronidase. The experiment was done once.
Administration of tomato concentrate containing the 6F peptide significantly reduced metastasis of colon cancer cells to the lungs
The weight of the lungs after intravenous administration of the colon cancer cells was significantly less after administration of Tg6F compared to mice not receiving any tomato concentrate, or compared to mice receiving EV (Fig.3A). The number of tumor nodules on the surface of the lungs was less after administration of EV, but this did not reach statistical significance (Fig.3B). In contrast, there was a significant and remarkable two-thirds reduction in the number of tumor nodules on the surface of the lungs after administration of Tg6F (Fig.3B). Consistent with our previous report on the efficacy of the 4F peptide in this model (Su et al. 2012), the plasma levels of LPA 20:4 were significantly reduced with administration of Tg6F, but plasma LPA 20:4 levels were not decreased with administration of EV (Fig.3C). The plasma levels of other LPA species including LPA 18:2, LPA 18:1, and LPA 18:0 were not significantly reduced (data not shown).
Figure 3.
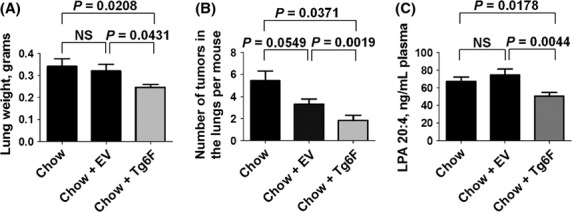
Tomato concentrate containing the 6F peptide (Tg6F), but not control tomato concentrate (EV) reduced metastasis of colon cancer cells to the lungs. Female BALB/c mice 6 weeks of age (n = 12 per group) were injected with 2 × 104 CT26 cells in 100 μL of PBS via tail vein as described in Materials and Methods. After injection, the mice were maintained on either standard mouse chow (Chow), or standard mouse chow containing 0.06% by weight of the control transgenic tomato concentrate (EV), or standard mouse chow containing 0.06% by weight of the transgenic 6F tomato concentrate (Tg6F), which provided the mice with a dose of tomato concentrate of ∼120 mg/kg per day per mouse, which provided the mice with a dose of the 6F peptide of ∼7 mg/kg per day. After 4 weeks the mice were subjected to a terminal bleed, and after sacrifice the lungs were harvested, weighed, and fixed with Bouin’s solution, and the number of tumor nodules on the surface of the lungs was determined as described in Materials and Methods. (A) Weight of the lungs in grams. (B) Number of tumor nodules on the surface of the lungs. (C) Plasma levels of lysophosphatidic acid 20:4 (LPA 20:4). The data shown are mean ± SEM; NS, not significant. These results are representative of two of two experiments.
As early as 1995 evidence was accumulating that granulocytes could promote tumor growth (Pekarek et al. 1995). Tumor growth in the lungs was later shown to be promoted by neutrophil elastase (Houghton et al. 2010). Therefore, we determined the content of tumor-associated neutrophils in this mouse model of colon cancer cells metastasizing to the lungs. A representative photomicrograph of the tumor-associated neutrophils in each of the treatment groups is shown in Figure4A and the data are shown in Figure4B. Remarkably, addition of 0.06% by weight of Tg6F to the standard mouse chow reduced tumor associated neutrophils by 94 ± 1.1% (P = 0.0052).
Figure 4.
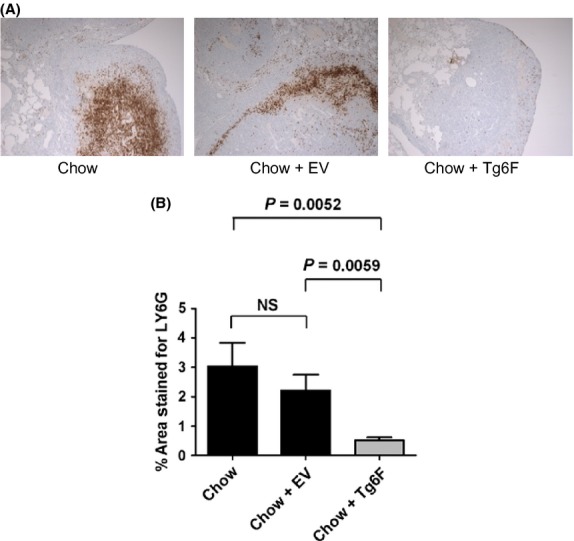
Tumor-associated neutrophils are dramatically reduced by tomato concentrate containing the 6F peptide (Tg6F), but not control tomato concentrate (EV) in a mouse model of colon cancer cells metastasizing to the lungs. Tumor-associated neutrophils in the lungs of the mice described in Figure3 were determined as described in Materials and Methods. (A) Representative examples from each treatment group. (B) The % area stained for Ly6G (mean ± SEM). NS, not significant. The experiment was done once.
Administration of tomato concentrate containing the 6F peptide was significantly more effective than the control tomato concentrate in reducing ovarian cancer cell tumor burden
In a mouse model of ovarian cancer in which mouse ovarian cancer cells were injected into the peritoneum of female mice with normal immune function, the total number of tumor nodules in the abdomen was significantly reduced by approximately 35% in mice receiving EV (P = 0.0013) compared to mice receiving standard mouse chow alone (Fig.5A). In the mice receiving Tg6F, the total number of tumor nodules in the abdomen was reduced by approximately 53% (P < 0.0001) compared to mice receiving standard mouse chow alone (Fig.5A). The difference between the total number of tumor nodules in the abdomen of mice receiving EV compared to those receiving Tg6F was highly significant (P = 0.0069) (Fig.5A).
Figure 5.
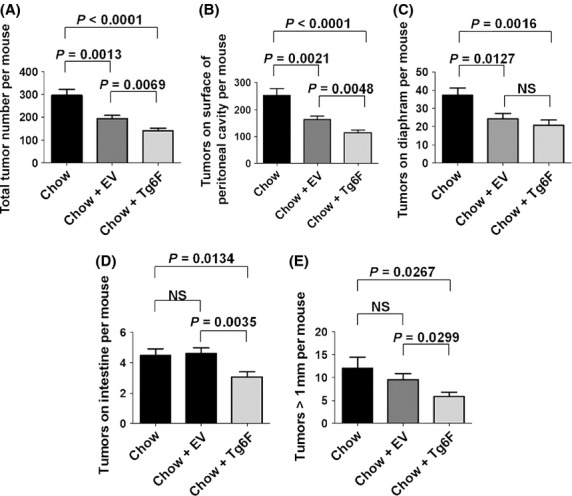
Both the control tomato concentrate (EV) and the tomato concentrate containing the 6F peptide (Tg6F) significantly reduced ovarian cancer cell tumor burden, but the tomato concentrate containing the 6F peptide was significantly more effective. Female C57BL/6J mice at age 9 weeks (n = 24 per group) were given an intraperitoneal injection containing 8 × 106 ID8 cells in a total volume of 0.8 mL of DMEM (without supplements). Following injection of the ID8 cells the mice were maintained on either standard mouse chow (Chow), or standard mouse chow containing 0.06% by weight of the control transgenic tomato concentrate (EV), or containing 0.06% by weight of the transgenic 6F tomato concentrate (Tg6F), which provided the mice with a dose of the 6F peptide of ∼7 mg/kg per day. After 12 weeks the mice were subjected to a terminal bleed, and after sacrifice the number of tumor nodules on peritoneal surfaces and on the surface of abdominal organs was determined as described in Materials and Methods. (A) The total number of tumor nodules in the abdomen. (B) The number of tumor nodules on the surface of the peritoneal cavity. (C) Number of tumor nodules on the diaphragm. (D) The number of tumor nodules on the surface of the intestine. (E) The number of tumor nodules in the abdomen that were greater than 1 mm in size. The data shown are mean ± SEM; NS, not significant. These results are representative of two of two experiments.
The number of tumor nodules on the surface of the peritoneal cavity significantly decreased by approximately 35% in mice receiving EV (P = 0.0021) compared to standard mouse chow alone (Fig.5B). The decrease in the mice receiving Tg6F was approximately 55% (P < 0.0001) compared to standard mouse chow alone (Fig.5B). The difference in the number of tumor nodules on the surface of the peritoneal cavity in mice receiving EV compared to mice receiving Tg6F was highly significant (P = 0.0048) (Fig.5B).
The number of tumor nodules on the surface of the diaphragm was significantly reduced by approximately 35% in mice receiving EV (P = 0.0127) compared to standard mouse chow alone (Fig.5C). The number of tumor nodules on the surface of the diaphragm was significantly reduced in mice receiving Tg6F by approximately 45% (P = 0.0016) compared to standard mouse chow alone (Fig.5C). The difference between the mice receiving EV and Tg6F was not significant (Fig.5C).
The number of tumor nodules on the surface of the intestine was not significantly changed in mice receiving EV compared to standard mouse chow alone, but there was a significant decrease in the mice receiving Tg6F (P = 0.0134) compared to mice receiving standard mouse chow alone (Fig.5D). The difference between the number of tumor nodules on the surface of the intestine in mice receiving EV compared to the mice receiving Tg6F was highly significant (P = 0.0035) (Fig.5D).
The number of tumor nodules in the abdomen that measured >1 mm in size was not significantly decreased by EV compared to mice receiving standard mouse chow alone, but there was a significant 52% decrease in the number of tumor nodules in the abdomen measuring greater than 1 mm in size in the mice receiving Tg6F (P = 0.0267) compared to mice receiving standard mouse chow alone (Fig.5E). The difference between the mice receiving EV compared to the mice receiving Tg6F was also significant (P = 0.0267) (Fig.5E).
In contrast to the case in the metastatic colon cancer model (Fig.3C), and in contrast to our previous studies with the 4F peptide (Su et al. 2010, 2012) there was no significant change in the levels of plasma LPA species (data not shown). Similarly, there was no significant difference in total plasma cholesterol levels in mice receiving EV compared to Tg6F (Fig.6A), and plasma total cholesterol levels did not correlate with the total number of tumor nodules (Fig.6B). However, plasma triglyceride levels were significantly reduced in mice receiving either tomato concentrate compared to mice receiving standard mouse chow alone, and there was no significant difference in plasma triglyceride levels in mice receiving EV compared to Tg6F (Fig.6C). The total number of tumor nodules was significantly and positively correlated with plasma triglyceride levels (r2 = 0.1375; P = 0.0022) (Fig.6D). Plasma HDL-cholesterol levels were significantly higher in mice receiving either tomato concentrate compared to mice receiving standard mouse chow alone, and there was no significant difference in the HDL-cholesterol levels in mice receiving EV compared to Tg6F (Fig.6E). HDL-cholesterol levels were not significantly correlated with the total number of tumor nodules (Fig.6F). Plasma apoA-I levels were significantly higher in mice receiving either tomato concentrate compared to mice receiving chow alone, and there was no significant difference in the apoA-I levels in mice receiving EV compared to Tg6F (Fig.6G). Consistent with our previous data (Su et al. 2010) and that of Zamanian-Daryoush et al. (2013), plasma apoA-I levels were inversely and significantly correlated with the total number of tumor nodules (r2 = 0.1096; P = 0.0062) (Fig.6H). Plasma SAA levels were significantly lower in mice receiving either tomato concentrate compared to mice receiving chow alone, and there was no significant difference in SAA levels in mice receiving EV compared to Tg6F (Fig.6I). Plasma SAA levels were weakly but significantly correlated with the total number of tumor nodules (r2 = 0.06950; P = 0.0325) (Fig.6J).
Figure 6.
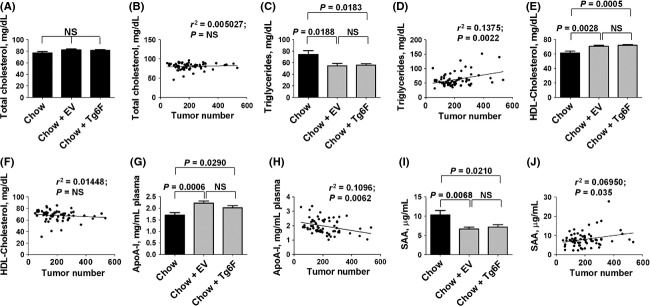
Plasma lipids and plasma serum amyloid A (SAA) levels in mice after intraperitoneal injection of ovarian cancer cells. Plasma lipid levels and SAA levels were measured in the mice described in Figure5. (A) Plasma total cholesterol levels. (B) The linear correlation between plasma total cholesterol levels and the total number of tumors in the abdomen. (C) Plasma triglyceride levels. (D) The linear correlation between plasma triglyceride levels and the total number of tumors in the abdomen. (E) Plasma HDL-cholesterol levels. (F) The linear correlation between plasma HDL-cholesterol levels and the total number of tumors in the abdomen. (G) Plasma apoA-I levels. (H) The linear correlation between plasma apoA-I levels and the total number of tumors in the abdomen. (I) plasma serum amyloid A (SAA) levels. (J) The linear correlation between plasma SAA levels and the total number of tumors in the abdomen. The data shown in the bar graphs are mean ± SEM; NS, not significant. This experiment was done once.
Discussion
The results reported here establish a novel way to concentrate apoA-I mimetic peptides produced in transgenic plants. Tomatoes are known to be rich in antioxidant polyphenols (Minoggio et al. 2003). Both ethanol and ethyl acetate concentrate polyphenols, with ethanol being superior at high concentration (Gadkari et al. 2014), however, the 6F peptide was found in abundance in ethyl acetate, but not in aqueous ethanol concentrates (Fig.1). The ability to concentrate the 6F peptide in ethyl acetate was enhanced by the addition of small amounts of acetic acid and the maximum effect was seen at 5% acetic acid (data not shown). The extraction efficiency of this solvent is likely due to the high content of phenylalanine (6 of the 18 amino acid residues are phenylalanine) and 8 of 10 hydrophobic residues are aromatic in 6F. The extraction efficiency resulted in 23 μg of 6F peptide being found in the band from lanes in which 100 μg of Tg6 protein were loaded on to the gels (Fig.1A). The explanation as to why seven of nine predicted 6F fragments (http://web.expasy.org/peptide_mass/; maximum 1 missed cleavage) were detected in the trypsin digests from Tg6F, but not from the 6F standard where six of the predicted fragments in the trypsin digests were detected, likely was due to the fact that 23 μg of 6F were subjected to trypsin treatment followed by reverse-phase LC and mass spectrometry in the case of Tg6F, while in the case of the 6F standard we chose to analyze the 5 μg band (Fig.1A and C).
The concentration process resulted in a 37-fold reduction in the weight of transgenic tomato powder required for biological activity (Fig.2). Perhaps more importantly, the volume of transgenic freeze-dried tomato powder required to deliver doses to humans similar to those used in the mouse studies would be reduced from three cups of freeze-dried tomato powder three times daily to two tablespoons three times daily. This dramatic reduction in the volume required makes this approach much more likely to be acceptable as a treatment modality.
We previously reported that the 6F peptide content of the freeze-dried transgenic tomatoes expressing 6F accounted for 0.6–1.0% of the weight of the freeze-dried tomato powder (Chattopadhyay et al. 2013). Thus, 1 mg of the freeze-dried tomato powder would contain 0.006–0.01 mg of 6F peptide. The data in Figure1 indicate that the 6F peptide in the tomato concentrate from the Tg6F constituted 23% of the weight of the proteins in the concentrate. The protein content of the tomato concentrates was ∼25% (data not shown). Thus, 1 mg of the tomato concentrate would contain 0.0575 mg of 6F. This represents approximately a 6–10-fold concentration of the 6F peptide compared to the starting freeze-dried tomato powder. However, there was a 37-fold reduction in the weight of the Tg6F concentrate required to achieve the same biologic activity as was achieved with the starting Tg6F freeze-dried tomato powder (Fig.2E–H). The explanation for the disproportionate gain in biologic activity might be due to the concomitant concentration of tomato anti-oxidants and/or a change in the conformation of the 6F peptide in the concentrate that favors interaction with receptors compared to the freeze-dried starting material.
It is interesting that ingestion of a tea rich in catechins for 12 weeks led to a significant reduction in body fat that was correlated with a change in the concentrations of malondialdehyde-modified LDL in humans (Nagao et al. 2005). Both ethanol and ethyl acetate efficiently concentrate catechins (Gadkari et al. 2014). Ethyl acetate is naturally found in some fruits and vegetables, and is used commercially to decaffeinate coffee and tea (Ramalakshmi and Raghavan 1999) suggesting that its use to prepare tomato concentrates is likely to be safe for humans.
It has been reported that dietary lycopene and tomato extract supplementations inhibited nonalcoholic steatohepatitis-promoted hepatocarcinogenesis in rodents by a process that appears to involve reduced oxidative stress (Wang et al. 2009). Ethyl acetate extracts of tea that were rich in polyphenols and antioxidants were shown to reduce LDL and triglyceride levels in rats and induced human hepatoma cell growth arrest by inducing p53 expression and upregulating p21 expression (Way et al. 2009). Thus, it seems likely that some of the benefits seen in our studies are due to naturally occurring tomato antioxidants that have been extracted into the concentrates.
The reduction in LPA 20:4 levels in the metastatic colon cancer model by Tg6F, but not by EV (Fig.3C) is consistent with LPA playing a role in this model. However, the mechanism of action of Tg6F in reducing tumor burden in the ovarian cancer model must be more complicated since Tg6F did not reduce LPA levels. These results are in contrast to the case for 4F (Su et al. 2010, 2012). Despite the lack of superiority compared to EV in altering plasma lipids, apoA-I and SAA levels in the mouse model of ovarian cancer, Tg6F was clearly superior in reducing tumor burden in the mouse model of ovarian cancer compared to EV (Fig.5). Thus, the mechanism(s) of action of Tg6F in reducing tumor burden in the mouse model of ovarian cancer remains unknown at this time.
In considering mechanisms which account for the superiority of Tg6F compared to EV, it should be remembered that 2 h after feeding Tg6F to mice we found intact 6F peptide in the lumen of the small intestine, but not in the plasma (Chattopadhyay et al. 2013). Subsequently, another group using a completely different apoA-I mimetic peptide (Zhao et al. 2014) found that oral administration of their apoA-I mimetic peptide was as effective as when the same dose was administered by injection. However, when the peptide was administered by injection high plasma peptide levels were found, and when administered orally, the peptide was not detected in the plasma (Zhao et al. 2014). Thus, our work and that of Zhao et al. (2014) is consistent with a primary mechanism of action for these apoA-I mimetic peptides in the intestine; not in the plasma.
What mechanisms might be involved? In the case of the mouse model of metastatic colon cancer, and in the mouse model of ovarian cancer, the treatments began the day after the tumor cells were injected, therefore it seems unlikely that they prevented tumor uptake. We think the most likely mechanism of action for Tg6F in reducing tumor burden is by modulating the immune cells that interact with the tumor as shown in Figure4.
There are at least two receptors known to be important in the small intestine that have been shown to interact with apoA-I mimetic peptides resulting in modulation of the biologic activity of these receptors; CD36 (Baranova et al. 2008) and class B scavenger receptor types I and II (Leelahavanichkul et al. 2012). CD36 is known to be important for fatty acid and cholesterol uptake by the proximal, but not the distal small intestine (Nassir et al. 2007). CD36 is also known to be important in modulating the interaction of gut bacteria with the small intestine (Baranova et al. 2008). Thus, it is possible that Tg6F interacts with CD36 in the proximal small intestine resulting in an alteration in the immune cells in the lamina propria of the intestinal villi. The intestine contains the largest number of immune cells in the body (Mowat and Agace 2014). It has been proposed that there may be different types of neutrophils similar to the case for protumorigenic and antitumorigenic macrophages (Fridlender and Albelda 2012). Perhaps the striking results seen in Figure4 with regard to tumor-associated neutrophils are due to a change in myeloid differentiation induced by interaction of Tg6F with CD36 in the proximal small intestine. Of course this is just speculation as our data do not directly address these points. Future research is needed to determine the actual mechanisms involved.
Based on the data presented here and our previously published data, regardless of the mechanisms involved, the use of Tg6F for cancer prevention or treatment appears promising. The oral route of administration is convenient, the preparation of a tomato concentrate using the simple methods described here is likely to be economical, and a tomato concentrate containing an 18 amino acid residue that appears to be degraded in the process of digestion (i.e., intact peptide was found in the small intestine 2 h after feeding, but the peptide was never detected in plasma) (Chattopadhyay et al. 2013) is likely to be safe.
In summary, these studies (i) provide a simple and economical means of dramatically concentrating an apoA-I mimetic peptide produced in transgenic tomatoes that makes testing oral apoA-I therapy in humans a feasible strategy; and (ii) they provide further evidence that oral apoA-I mimetic peptides are a novel means of reducing tumor burden in mouse models, but by mechanisms that are likely to be more complicated than previously thought.
Disclosures
M. N., S. T. R, G. M. A, and A. M. F. are principals in Bruin Pharma and A. M. F. is an officer in Bruin Pharma.
Glossary
- 4F
the peptide Ac-D-W-F-K-A-F-Y-D-K-V-A-E-K-F-K-E-A-F-NH2
- 5F
the peptide Ac-D-W-L-K-A-F-Y-D-K-V-F-E-K-F-K-E-F-F-NH2
- 6F
the peptide D-W-L-K-A-F-Y-D-K-F-F-E-K-F-K-E-F-F without blocked end groups
- ApoA-I
apolipoprotein A-I
- EV
transgenic tomatoes expressing a control marker protein, β-glucuronidase
- FPLC
Fast Performance Liquid Chromatography
- LDLR
low-density lipoprotein receptor
- LPA
lysophosphatidic acid
- SAA
serum amyloid A
- Tg6F
transgenic tomatoes expressing the 6F peptide
- WD
Western diet
References
- Baranova IN, Kurlander R, Bocharov AV, Vishnyakova TG, Chen Z, Remaley A, et al. Role of CD36 in bacterial recognition, phagocytosis, and pathogen-induced JNK-mediated signaling. J Immunol. 2008;181:7147–7156. doi: 10.4049/jimmunol.181.10.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-L, Hsu H-Y, Lin H-Y, Chiang W, Lee H. Lysophosphatidic acid-induced oxidized low-density lipoprotein uptake is class A scavenger receptor-dependent in macrophages. Prostaglandins Other Lipid Mediat. 2008;87:20–25. doi: 10.1016/j.prostaglandins.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A, Navab M, Hough G, Gao F, Meriwether D, Grijalva V, et al. A novel approach to oral apoA-I mimetic therapy. J Lipid Res. 2013;54:995–1010. doi: 10.1194/jlr.M033555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cust AE, Kaaks R, Friedenreich C, Bonnet F, Laville M, Tjonneland A, et al. Metabolic syndrome, plasma lipid, lipoprotein and glucose levels, and endometrial cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Endocr Relat Cancer. 2007;14:755–767. doi: 10.1677/ERC-07-0132. [DOI] [PubMed] [Google Scholar]
- van Duijnhoven FJ, Bueno-De-Mesquita HB, Calligaro M, Jenab M, Pischon T, Jansen EH, et al. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Gut. 2011;60:1094–1102. doi: 10.1136/gut.2010.225011. [DOI] [PubMed] [Google Scholar]
- Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- Gadkari PV, Kadimi US, Balaraman M. Catechin concentrates of garden tea leaves (Camellia sinensis L.): extraction/isolation and evaluation of chemical composition. J Sci Food Agric. 2014;94:2921–2928. doi: 10.1002/jsfa.6633. [DOI] [PubMed] [Google Scholar]
- Ganapathy E, Su F, Meriwhether D, Devarajan A, Grijalva V, Gao F, et al. D-4F, an apoA-I mimetic peptide, inhibits proliferation and tumorigenicity of epithelial ovarian cancer cells by upregulating the antioxidant enzyme MnSOD. Int J Cancer. 2012;130:1071–1081. doi: 10.1002/ijc.26079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Vasquez SX, Su F, Roberts S, Shah N, Grijalva V, et al. L-5F, an apolipoprotein A-I mimetic, inhibits tumor angiogenesis by suppressing VEGF/basic FGF signaling pathways. Integr Biol. 2011;3:479–489. doi: 10.1039/c0ib00147c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Chattopadhyay A, Navab M, Grijalva V, Su F, Fogelman AM, et al. Apolipoprotein A-I mimetic peptides inhibit expression and activity of Hypoxia-inducible factor-1α in human ovarian cancer cell lines and a mouse ovarian cancer model. J Pharmacol Exp Ther. 2012;342:255–262. doi: 10.1124/jpet.112.191544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16:219–223. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak KR, Amneus MW, Puseyu SM, Su F, Luong MN, Luong SA, et al. Identification of biomarkers for ovarian cancer using strong anion-exchange ProteinChips: potential use in diagnosis and prognosis. Proc Natl Acad Sci USA. 2003;100:12343–12348. doi: 10.1073/pnas.2033602100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak KR, Su F, Whitelegge JP, Faull K, Reddy S, Farias-Eisner R. Characterization of serum biomarkers for detection of early stage ovarian cancer. Proteomics. 2005;5:4589–4596. doi: 10.1002/pmic.200500093. [DOI] [PubMed] [Google Scholar]
- Leelahavanichkul A, Bocharov AV, Kurlander R, Baranova IN, Vishnyakova TG, Souza CP, et al. Class B scavenger receptor types I and II and CD36 targeting improves sepsis survival and acute outcomes in mice. J Immunol. 2012;188:2749–2758. doi: 10.4049/jimmunol.1003445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Minoggio M, Bramati L, Simonetti P, Gardana C, Iemoli L, Santangelo E, et al. Polyphenol pattern and antioxidant activity of different tomato lines and cultivars. Ann Nutr Metab. 2003;47:64–69. doi: 10.1159/000069277. [DOI] [PubMed] [Google Scholar]
- Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14:667–685. doi: 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- Nagao T, Komine Y, Soga S, Meguro S, Hase T, Tanaka Y, et al. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am J Clin Nutr. 2005;81:122–129. doi: 10.1093/ajcn/81.1.122. [DOI] [PubMed] [Google Scholar]
- Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not the distal intestine. J Biol Chem. 2007;282:19493–19501. doi: 10.1074/jbc.M703330200. [DOI] [PubMed] [Google Scholar]
- Navab M, Hough G, Buga GM, Su F, Wagner AC, Meriwether D, et al. Transgenic 6F tomatoes act on the small intestine to prevent systemic inflammation and dyslipidemia caused by Western diet and intestinally derived lysophosphatidic acid. J Lipid Res. 2013;54:3403–3418. doi: 10.1194/jlr.M042051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M, Chattopadhyay A, Hough G, Meriwether D, Fogelman SI, Wagner AC. Source and role of intestinally-derived lysophosphatidic acid in dyslipidemia and atherosclerosis. J Lipid Res. 2015;56:871–887. doi: 10.1194/jlr.M056614. , et al. ( [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyen C, Pluddemann A, Roversi P, Thomas B, Cai L, van der Westhuyzen DR, et al. Macrophage scavenger receptor A mediates adhesion to apolipoproteins A-I and E. Biochemistry. 2009;48:11858–11871. doi: 10.1021/bi9013769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyen C, Pluddemann A, Mukhopadhyay S, Maniati E, Bossard M, Gordon S, et al. Macrophage scavenger receptor A promotes tumor progression in murine models of ovarian and pancreatic cancer. J. Immunol. 2013a;190:3798–3805. doi: 10.4049/jimmunol.1203194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyen C, Mukhopadhyay S, Gordon S, Hagemann T. An apolipoprotein A-I mimetic targets scavenger receptor A on tumor-associated macrophages. A prospective treatment? Oncoimmunology. 2013b;2:e24461. doi: 10.4161/onci.24461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossov V, Amneus M, Su F, Lang J, Janco JM, Reddy ST, et al. The early detection of ovarian cancer from traditional methods to proteomics. Can we really do better than serum CA-125? Am J Obstet Gynecol. 2008;199:215–223. doi: 10.1016/j.ajog.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Nossov V, Su F, Amneus M, Birrer M, Robbins T, Kotlerman J, et al. Validation of serum biomarkers for detection of early-stage ovarian cancer. Am J Obstet Gynecol. 2009;200:639.e1–639.e5. doi: 10.1016/j.ajog.2008.12.042. [DOI] [PubMed] [Google Scholar]
- Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181:435–440. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian B-Z, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalakshmi K, Raghavan B. Caffeine in coffee: Its removal. Why and how? Crit Rev Food Sci Nutr. 1999;39:441–456. doi: 10.1080/10408699991279231. [DOI] [PubMed] [Google Scholar]
- Su F, Kozak KR, Imaizumi S, Gao F, Amneus MW, Grijalva V, et al. Apolipoprotein A-I (apoA-I) and apoA-I mimetic peptides inhibit tumor development in a mouse model of ovarian cancer. PNAS. 2010;107:19997–20002. doi: 10.1073/pnas.1009010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su F, Grijalva V, Navab K, Ganapathy E, Meriwether D, Imaizumi S, et al. HDL mimetics inhibit tumor development in both induced and spontaneous mouse models of colon cancer. Mol Cancer Ther. 2012;11:1311–1319. doi: 10.1158/1535-7163.MCT-11-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ausman LM, Greenberg AS, Russell RM, Wang X-D. Dietary lycopene and tomato extract supplementations inhibit nonalcoholic steatohepatitis-promoted hepatocarcinogenesis in rats. Int J Cancer. 2009;126:1788–1796. doi: 10.1002/ijc.24689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way T-D, Lin H-Y, Kuo D-H, Tsai S-J, Shieh J-C, Wu J-C, et al. Pu-erh tea attenuates hyperlipogenesis and induces hepatoma cells growth arrest through activating AMP-activated protein kinase (AMPK) in human HepG2 cells. J Agric Food Chem. 2009;57:5257–5264. doi: 10.1021/jf900730e. [DOI] [PubMed] [Google Scholar]
- Zamanian-Daryoush M, Linder D, Tallant TC, Wang Z, Buffa J, Klipfell E, et al. The cardioprotective protein apolipoprotein A1 promotes potent anti-tumorigenic effects. J Biol Chem. 2013;288:21237–21252. doi: 10.1074/jbc.M113.468967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Black AS, Bonnet DJ, Maryanoff BE, Curtiss LK, Leman LJ, et al. In vivo efficacy of HDL-like nanolipid particles containing multivalent peptide mimetics of apolipoprotein A-I. J Lipid Res. 2014;55:2053–2063. doi: 10.1194/jlr.M049262. [DOI] [PMC free article] [PubMed] [Google Scholar]