Abstract
Pregabalin is an anticonvulsant that successfully treats many neuropathic pain syndromes, although the mechanism of its antihyperalgesic action remains elusive. This study aims to help delineate pregabalin’s antihyperalgesic mechanisms. We assessed the effectiveness of pregabalin at decreasing mechanical and cold hypersensitivity induced in a rat model of neuropathic pain. Thus, we compared the effectiveness of pre- or post-treatment with systemic or intrathecal (i.t.) pregabalin at reducing the development and maintenance of the neuropathic pain symptoms. Pregabalin successfully decreased mechanical and cold hypersensitivity, as a pretreatment, but was less effective at suppressing cold hypersensitivity when administered as a post-treatment. Furthermore, both i.t. and systemic administration of pregabalin were effective in reducing the behavioral hypersensitivity, with the exception of systemic post-treatment on cold hypersensitivity. We also examined pregabalin’s effects at inhibiting hind paw formalin-induced nociception in naïve rats and formalin-induced release of excitatory amino acids in the spinal cord dorsal horn (SCDH) both in naïve rats and in rats with neuropathic pain. Pregabalin dose-dependently reduced nociceptive scores in the formalin test. We also present the first evidence that pregabalin reduces the formalin-induced release of glutamate in SCDH. Furthermore, i.t. pregabalin reduces the enhanced noxious stimulus-induced spinal release of glutamate seen in neuropathic rats. These data suggest that pregabalin reduces neuropathic pain symptoms by inhibiting the release of glutamate in the SCDH.
Keywords: nociception, hyperalgesia, neuropathy, chronic constriction injury, formalin test, in vivo microdialysis
INTRODUCTION
Recently, pregabalin has received considerable attention as an analgesic for neuropathic pain (see Sonnett et al., 2006; Baron et al., 2008). With a superior pharmacokinetic profile to it’s analogue gabapentin (Guay, 2005), pregabalin has quickly become a first line treatment for neuropathic pain (Moulin et al., 2007). Like gabapentin, pregabalin binds to the α2δ-subunit (type 1) of voltage-gated calcium (Ca2+) channels (Gee et al., 1996; Bian et al., 2006) at which binding attenuates neuronal calcium influx (Fink et al., 2002; McClelland et al., 2004), likely through actions at P/Q-type Ca2+ channels (Dooley et al., 2002). This reduction in calcium influx results in an inhibition of the release of neurotransmitters, including norepinephrine (Dooley et al., 2000a, 2002; Fink et al., 2002), substance P (Maneuf et al., 2001) and glutamate (Cunningham et al., 2004). Importantly, an upregulation of α2δ Ca2+ channel subunits is found in the dorsal root ganglion (DRG) of rats after spinal nerve ligation (Luo et al., 2001; 2002).
Pregabalin’s actions may target Ca2+ in peripheral nerve, since α2δ Ca2+ channel subunits are upregulated in the DRG after sciatic nerve crush, but not after rhizotomy (Luo et al., 2001), and upregulation is less in the spinal cord (Luo et al., 2001). Pregabalin has also been found to suppress ectopic activity in a ligated sciatic nerve (Chen & Pan, 2001). Alternatively, a spinal action of pregabalin is suggested since mutations of α2δ1 Ca2+ that reduce spinal pregabalin binding reduces the drug’s ability to reduce murine neuropathic pain (Bian et al., 2006), while rats with a chronic constriction injury of the sciatic nerve have increased [3H]-pregabalin binding in the spinal dorsal horn ipsilateral to the injury (Melrose et al., 2007). Furthermore, transgenic mice that overexpress α2δ1 Ca2+ subunits have an enhanced amplitude of calcium currents in dorsal root ganglion neurons, enhanced frequency of miniature excitatory post-synaptic currents in deep spinal cord dorsal neurons (suggestive of enhanced spinal glutamate release), and exhibit allodynia and exaggeration dorsal horn neuronal responses to cutaneous sensory stimuli that is attenuated by intrathecal administration of AMPA/kainite or NMDA antagonists (Li et al., 2006; Nguyen et al., 2009). Recent evidence suggests there is also increased trafficking of α2δ Ca2+ channel subunits to presynaptic terminals in the spinal cord of neuropathic rats, a pathology that is inhibited by chronic pregabalin treatment (Bauer et al., 2009). Furthermore, pregabalin inhibits the release of substance P and calcitonin-gene related peptide (CGRP) from spinal cord slices taken from rats with inflamed hind paws (Fehrenbacher et al., 2003). While both systemic (Field et al., 1999; Nozaki-Taguchi et al., 2001; Wallin et al., 2002; Han et al., 2007) and spinal pregabalin (Wallin et al., 2002; Han et al., 2007) have been shown to reduce neuropathic pain, these studies employed different nerve injury models and nociceptive tests.
Luo et al. (2001, 2002) demonstrated that upregulation of α2δ Ca2+ channel subunits does not reach a peak until 7–14 days after nerve injury, when neuropathic pain behaviors are well established. Thus, pregabalin treatment may be more effective after the neuropathy is well established. Conversely, if actions of pregabalin on glutamate neurotransmission are more important, early treatment with gabapentin may be more effective, since NMDA antagonists work best pre-emptively in neuropathic pain models (Coderre, 1993). Here we assess both pre- and post-treatment with either systemic or spinal pregabalin in the same neuropathic pain model.
There is growing evidence that gabapentin (Bayer et al., 2004; Dooley et al., 2000b; Maneuf & McKnight 2001; Maneuf et al. 2004) and pregabalin (Cunningham et al., 2004) inhibit the release of glutamate in brain or spinal cord slices in vitro. However, only two studies have assessed the effects of gabapentin (Feng et al., 2003; Coderre et al., 2005), and none have examined the effects of pregabalin, on glutamate release from spinal cord dorsal horn in vivo. Thus, we examined the ability of pregabalin to inhibit formalin-induced release of excitatory amino acids (EAAs) in the SCDH, in both naïve and neuropathic rats, using in vivo microdialysis. The present study provides the first in vivo evidence that pregabalin reduces noxious stimulus-induced EAA release in naïve rats, as well as the enhanced release of glutamate in neuropathic rats.
MATERIALS AND METHODS
Animals
The present studies employed male Long Evans hooded rats (250–300 grams, Charles River, PQ). Rats were housed in groups of 3–4, with food and water available ad libitum, on a 12:12 hour light: dark cycle. All surgical and testing procedures were approved by the Animal Care Committee at McGill University, and conformed to the ethical guidelines of the Canadian Council on Animal Care and the Society for Neuroscience.
Chronic Constriction Injury (CCI)
Chronic constriction injury (CCI) was induced to the left sciatic nerve according to the method of Bennett and Xie (1988). Under sodium pentobarbital anesthesia (50 mg/ kg, i.p.) supplemented with isofluorane anesthetic (2% in 95% O2, 5% CO2) when necessary, the sciatic nerve was exposed by blunt dissection at mid-thigh level, and the adhering tissue was dissected for an approximately 10 mm section of the nerve. Four ligatures (4.0 chromic gut, Ethicon) were loosely tied around the nerve at 2 mm spacing, to produce a loose constriction of the nerve. Wounds were closed with one or two sutures to the dissected muscle, followed by suturing of the skin incision and treatment with Furacin topical antibacterial ointment (0.2% nitrofurazone). Sham-operated animals had the sciatic nerve exposed but not manipulated in any way.
Microdialysis and intrathecal (i.t.) catheter
Rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and a microdialysis (hollow) fiber was implanted according to the method described by Zahn et al. (2002). After shaving the fur and sterilizing the skin on the back, the T13 thoracic vertebra was exposed, and a small hole was made on each side of the vertebra at the posterior pedicle. A single dialysis fiber (200 μm outer diameter, 45,000 mol. wt. cut-off, Hospal AN69-HF) was passed transversely through the spinal cord. The dialysis fiber was coated with epoxy except for a 2 mm active zone, which was positioned within the grey matter of the spinal cord (approximately lamina III–VI). Each end of the fiber was connected to PE-20 tubing with Super glue® and fixed to the vertebral bone with the dental cement (Lang Dental Mfg Co, Wheeling, IL). The inlet and outlet tubing was tunneled under the skin and externalized at the back of the neck. Only rats with normal behavior and no paralysis of hind limbs after the surgery were included in the microdialysis studies.
During the same surgery, some rats were also implanted with a chronic indwelling i.t. catheter, which was inserted between the L5 and L6 vertebrae during a lumbar puncture according to the methods of Pogatzki et al. (2000). The lumbar puncture was performed using a 23 gauge needle, and PE-32 polyurethane tubing was pushed 3 cm through the needle to the lumbar enlargement, before the needle was removed. The tubing was then anchored to the back muscles using 3-0 silk sutures. This procedure was performed following placement of the microdialysis catheter, while the rats were still anesthetized as described above, and the i.t. catheter was placed only in CCI rats between 7 and 14 days post-CCI surgery, when neuropathic symptoms (i.e., allodynia) were confirmed by mechanical sensitivity testing as described below. Only rats with normal behavior and no paralysis of hind limbs after the surgery were included in the microdialysis studies.
Pharmacological Administration
Pregabalin was obtained as a donation from Pfizer/Warner Lambert, Ann Arbor, MN, and mixed in saline for i.p. injections and artificial cerebrospinal fluid (aSCF, see below) for i.t. injections.
Intrathecal Administration
In behavioral studies, separate groups of CCI rats were administered either 0 (aCSF vehicle), 10, 30 or 100 μg pregabalin i.t. by lumbar puncture, in a volume of 20 μl, between the L4 and L5 vertebrae, while under brief halothane anesthesia. These doses were administered repeatedly either: 15 min prior to surgery and then every 12 hours from postoperative (PO) day 0 to 4; or every 12 hrs from PO day 8 to 12. In microdialysis studies in neuropathic and sham rats, a single injection of 100 μg pregabalin was adminstered through a chronic i.t. catheter, with a 20 μl injection volume pushed with 5 μl saline to flush the catheter.
Systemic Administration
Separate groups of rats were injected with saline or pregabalin (10, 30, 100 mg/kg, i.p.) 15 min prior to, and every 12 hours from post-operative (PO) day 0 to 4; or every 12 hrs from PO day 8 to 12. Separate groups of naïve rats received a single injection of either 10, 30 or 100 mg/kg of i.p. pregabalin 30 min prior to the formalin test.
Assessing Mechanical and Cold Sensitivity
Mechanical and cold sensitivity testing was performed 1–2 days prior to surgery to obtain baseline values, and then on PO days 4, 8 and 12 for the pretreatment groups and days 4, 8, 12 and 16 for the post-treatment groups.
Mechanical sensitivity testing
For baseline and PO testing, each rat was placed in a testing box (27 × 16 × 21 cm) with a 2 × 2 mm wire-mesh grid floor. A series of von Frey hairs (0.41, 0.70, 1.20, 2.00, 3.63, 5.50, 8.50, and 15.10 g) were applied through to the grid floor to the ventral surface of the hind paw of the operated hind limb of each rat. Each hair was applied for a 7 second period or until the animal withdrew their affected hind paw without ambulating. During each testing trial, the series of hairs were presented following an up-down procedure, described and validated by Chaplan et al. (1994), and the 50% response threshold was calculated for each rat.
Cold sensitivity testing
For baseline and PO testing, each rat was placed in a 1 cm deep, 1°C water bath, with a metal floor, for 75 sec. During the testing period, a response was counted each time the rat licked, shook, or showed an extended lift of the hind paw of the affected limb when not ambulating.
Formalin Test
For chemical nociception, naïve rats were given a subcutaneous injection of 50 μl of 2.5% formalin into the plantar surface of one hind paw. For nociceptive testing, animals were placed in a 30 × 30 × 30 cm Plexiglas box with a mirror below the surface at 45° angle to allow an unobstructed view of the paws. Scoring of nociceptive behaviours began immediately after formalin injection and continued for 60 min. A nociceptive score was determined for each 5 min time block by measuring the amount of time spent in each of the following behavioral categories : 0, the injected paw is not favoured ; 1, the injected paw has little or no weight placed on it ; 2, the injected paw is elevated and is not in contact with any surface; and 3, the injected paw is licked, bitten or shaken. A weighted average nociceptive score, ranging from 0 to 3, was calculated by multiplying the time spent in each category by the category weight, summing these products, and then dividing by the total time for each 5 min time block. All the rats were acclimatized to the test chamber before testing began.
In vivo microdialysis
After recovery from surgery, rats with microdialysis catheters were placed in a Raturn® Interactive System (Bioanalytic Systems, Inc. West Lafayette, IN) with a tethering system which allowed tubing from the microdialysis catheter to be connected to a syringe pump on one end and to a refrigerated fraction collector on the other. Following 12 hrs flow of artificial CSF (0.1 μl/min), samples were collected every 5 min at a flow rate of 5 μl/min during a one hour equilibration period. The aCSF contained (in mM) 154.7 Na+, 2.9 K+, 1.1Ca2+, 0.82 Mg2+, 132.49 Cl−, and was bubbled with 95% oxygen and 5% CO2 at the start of the experiment to adjust the pH to 7.4. In microdialysis experiments for naïve rats, we collected the following samples: 3 basal, 6 after pretreatment with pregabalin (100 mg/kg, i.p.) or vehicle (5 ml/kg, i.p.), and 12 following subcutaneous plantar hind paw injection of 50 μl of 5.0% formalin into one hind paw. The first microdialysis study in rat spinal cord (Skilling et al., 1988) demonstrated EAA release following hind paw injection of this same concentration of formalin, and this higher concentration (compared to 2.5% in behavioural studies) is required to produce robust spinal glutamate release in naïve rats. For neuropathic and sham surgery rats, microdialysis experiments were performed between 7 and 12 days after CCI or sham surgery, as described above. In these groups, we collected the following samples: 3 basal, 3 after pretreatment with pregabalin (100 μg, i.t.) or vehicle (20 μl, i.t.), and 12 following subcutaneous plantar hind paw injection of 50 μl of 1.0% formalin into one hind paw. We have previously shown that this lower concentration of formalin produce robust spinal glutamate release in CCI rats (Coderre et al., 2005). Samples were stored at −80°C until assayed for glutamate by HPLC.
Following the collection of samples, a solution of methylene blue was administered for 10 min through the filament. Staining in the tissue indicated the location of the permeable portion of the fiber. At the end of the experiment, rats were killed with an overdose of chloral hydrate (600 mg/kg, i.p.) and the lumbar spinal cord was removed. The tissue was post-fixed with formaldehyde, cryoprotected in a 30% sucrose solution and sectioned (50 μm). The sections were slide mounted and cover-slipped, and the location of the dye mark was determined when viewing with a light microscope. An investigator who was blind to the experimental condition determined the spinal cord level of the dialysis fiber in the dorsal horn. Samples were included in the analysis only if the microdialysis catheter was in the dorsal horn (i.e., the gray matter of the spinal cord above the central canal).
HPLC Assay
Aspartate and glutamate content in each sample was determined using reverse phase HPLC with fluorescent detection as described by Böttcher et al. (2003). In brief, pre-column derivatization of amino acids in samples were carried out in the autoinjector of the HPLC system (Waters Alliance 2690). Samples of 10 μl microdialysis perfusate were incubated with 10 μl of derivatizing reagent consisting of o-phthalaldehyde (incomplete reagent) and 2-mercaptoethanol 0.4% (v/v) (both Sigma, St. Louis, MO) for 1.5 minutes and then injected into an HPLC system. The system was fitted with a C18 ODS Supelco column (15 cm × 4.6 mm, 5 μm, Supelco, Bellefonte, PA). The derivatized samples were eluted with a mobile phase at a flow rate of 1 ml/min. The mobile consisted of 70 mM sodium acetate pH-6.95, 1.5 % of tetrahydrofuran and 11 % methanol. An isocratic elution was maintained for 8.5 min to get clear separation of glutamate. The temperature of column heater and sample chamber was kept at 37°C and 5°C, respectively. The eluted derivatives were detected with a fluorescent detector (Waters 474) at excitation and emission wavelength 330 nm and 450 nm, respectively. Peak area was used to calculate the concentration of each analyte, and quantification of glutamate was determined from a standard curve derived using external standards. The data were expressed in percentage of baseline concentration for each animal for ease of calculation, and since this is a standard method of presentation. Baseline was obtained by averaging the three basal samples collected before administering pregabalin or vehicle.
Statistical Analyses
The effect of i.t. or i.p. treatment with pregabalin (0, 10, 30 or 100 μg) or (0, 30, 100 or 300 mg/kg), respectively, on mechanical or cold sensitivity was assessed by a Kruskal-Wallis ANOVA by ranks for multiple independent groups and followed by subsequent post hoc comparisons (Dunn’s multiple comparisons for ranked data). Effects of i.t. pregabalin on hind paw formalin-evoked release of glutamate and i.p. pregabalin in the formalin test, were assessed with two-way repeated measures ANOVA followed by Fisher’s least squared difference post hoc comparisons.
RESULTS
There were consistent decreases in mechanical withdrawal thresholds and increases in cold water response frequencies on days 4, 8 and 12 after injury in vehicle-treated CCI rats, as depicted in Figs. 1, 2, 3 and 4. These vehicle-treated rats were compared with separate groups of CCI rats that received repeated dosing (8×) of varying doses of i.t. or systemic pregabalin at 12 hr intervals.
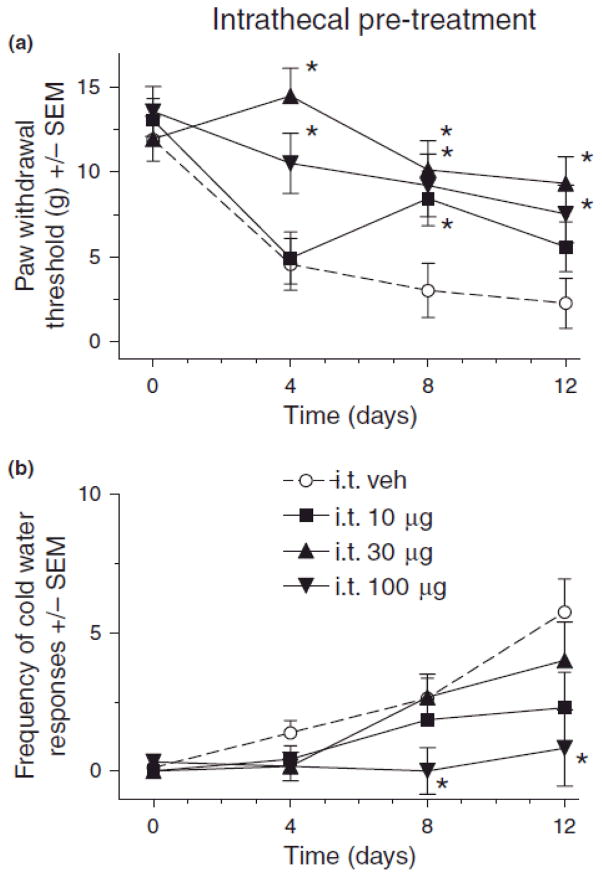
Fig. 1.
Effect of intrathecal (i.t.) pregabalin pre-treatment on mechanical (A) and cold (B) sensitivity in rats after chronic constriction injury (CCI) of sciatic nerve. For von Frey thresholds, ANOVA indicated significant main effects of i.t. pregabalin pretreatment (F(3,24) = 10.0, p > 0.001), time (F(3,72) = 14.4, p < 0.001, and a significant treatment X time interaction (F(9,72) = 2.53, p < 0.05). For cold water frequencies, ANOVA indicated a significant main effects of i.t. pregabalin pretreatment (F(3,24) = 4.39, p < 0.05) and time (F(3,72) = 13.9, p < 0.001, and a non-significant treatment X time interaction (F(9,72) = 1.80, p > 0.05). Post hoc comparisons revealed that compared to vehicle (n=8) there was a significant reduction in mechanical hypersensitivity (A) with 30 (n=6) and 100 (n=6) μg i.t. pregabalin pretreatment on days 4, 8 and 12, and 10 μg (n=7) on day 8 after CCI (*p < 0.05), and cold hypersensitivity (B) was significantly reduced by 100 μg pregabalin on days 8 and 12 after CCI (*p < 0.05) as compared to vehicle (n’s same as in A).
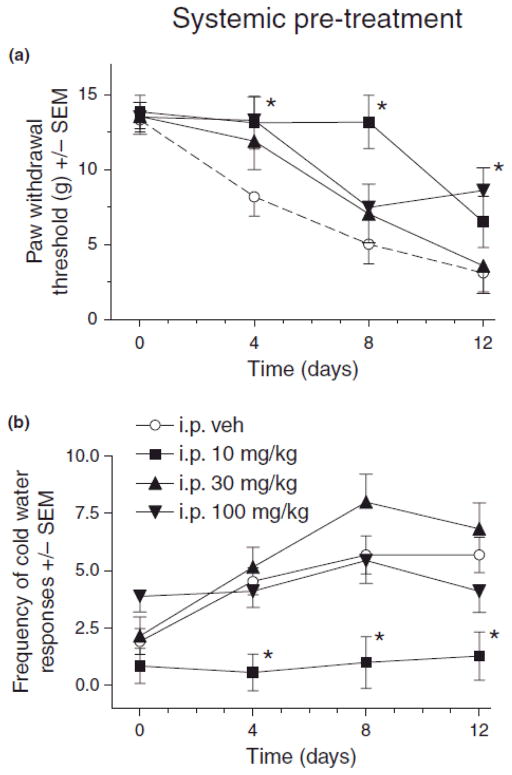
Fig. 2.
Effect of systemic (i.p.) pregabalin pretreatment on mechanical (A) and cold (B) sensitivity in rats after chronic constriction injury (CCI) of sciatic nerve. For von Frey thresholds, ANOVA indicated a significant main effects of i.p. pregabalin pretreatment (F(3,31) = 4.17, p < 0.05) and time (F(3,93) = 31.6, p < 0.001, as well as a significant treatment X time interaction (F(9,93) = 2.25, p < 0.05). For cold water frequencies, ANOVA indicated a significant main effects of i.p. pregabalin pretreatment (F(3,31) = 13.4, p < 0.001) and time (F(3,93) = 8.70, p < 0.001, but a non-significant treatment X time interaction (F(9,93) = 1.83, p > 0.05). Post hoc comparisons revealed that compared to vehicle (n=13), 10 (n=7) and 100 (n=9) mg/kg pregabalin pretreatment on day 4 and 10 mg/kg on day 8 significantly reduced mechanical hypersensitivity (A) after CCI (*p < 0.05, n=6 for 30 mg/kg), and cold hypersensitivity (B) was significantly reduced by 10 mg/kg pregabalin on day 4, 8 and 12 after CCI (*p < 0.05) as compared to vehicle (n’s same as in A).
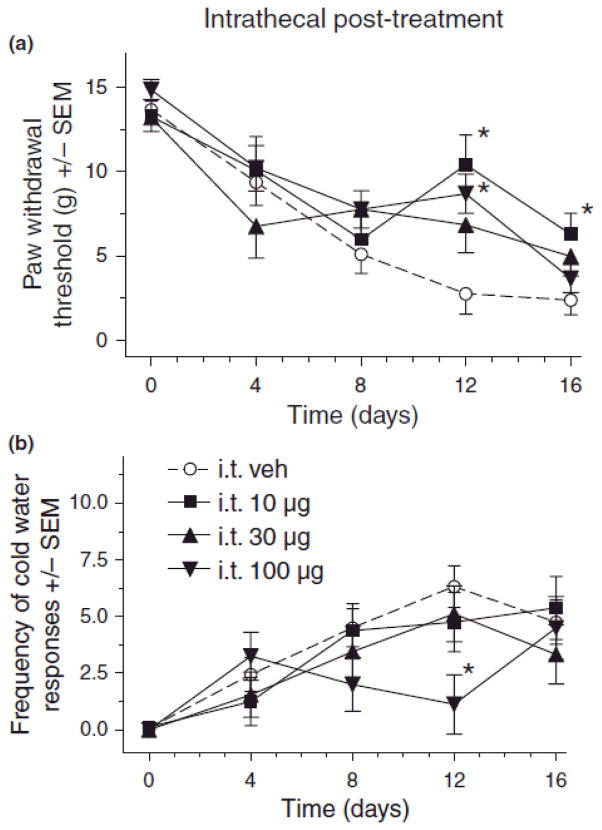
Fig. 3.
Effect of intrathecal (i.t.) pregabalin post-treatment on mechanical (A) and cold (B) sensitivity in rats after chronic constriction injury (CCI) of sciatic nerve. For von Frey thresholds, ANOVA indicated a significant main effects of i.t. pregabalin post-treatment (F(3,48) = 2.97, p < 0.05) and time (F(4,192) = 36.1, p < 0.001, as well as a significant treatment X time interaction (F(12,192) = 2.19, p < 0.05). For cold water frequencies, ANOVA indicated a non-significant main effect of i.t. pregabalin post-treatment (F(3,47) = 0.61, p > 0.05), but a significant main effect of time (F(4,92) = 26.9, p < 0.001, and a significant treatment X time interaction (F(12,92) = 2.68, p < 0.01). Post hoc comparisons revealed that compared to vehicle (n=17) there was a significant reduction in mechanical hypersensitivity (A) with 10 (n=8) and 100 (n=18) μg i.t. pregabalin pretreatment on day 12, and 10 μg on day 16 after CCI (*p < 0.05, n=9 for 30 μg), and cold hypersensitivity (B) was significantly reduced by 100 μg pregabalin on day 12 after CCI (*p < 0.05) as compared to vehicle (n’s same as in a, except vehicle n=16).
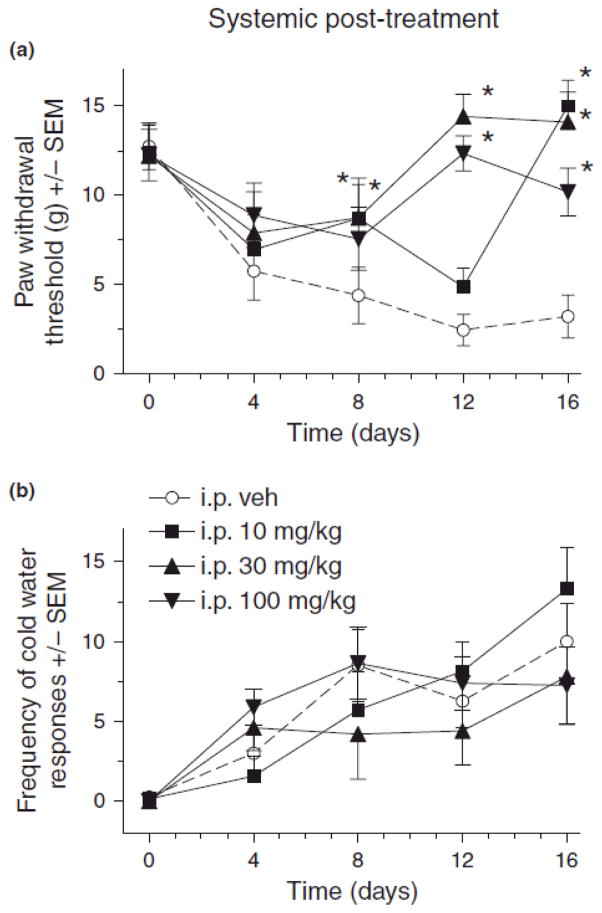
Fig. 4.
Effect of systemic (i.p.) pregabalin post-treatment on mechanical (A) and cold (B) sensitivity in rats after chronic constriction injury (CCI) of sciatic nerve. For von Frey thresholds, ANOVA indicated a significant main effects of i.p. pregabalin post-treatment (F(3,26) = 16.9, p < 0.001) and time (F(4,104) = 7.29, p < 0.001, as well as a significant treatment X time interaction (F(12,104) = 3.73, p < 0.001). For cold water frequencies, ANOVA indicated a non-significant main effects of i.p. pregabalin post-treatment (F(3,24) = 0.31, p > 0.05), but a significant main effect of time (F(4,96) = 20.8, p < 0.001, and a non-significant treatment X time interaction (F(12,96) = 1.61, p > 0.05). Post hoc comparisons revealed that compared to vehicle (n=13), 30 (n=6) and 100 (n=9) mg/kg pregabalin post-treatment (n=8) on days 8, 12 and 16 and 10 (n=7) mg/kg on day 16 significantly reduced mechanical hypersensitivity (A) after CCI (*p < 0.05), and cold hypersensitivity (B) was not significantly reduced by any pregabalin post-treatment on any day after CCI as compared to vehicle (n’s same as in a).
Spinal pregabalin pre-treatment effects on neuropathic pain
Results indicated that i.t. pregabalin pretreatment attenuated mechanical and cold allodynia at various times after CCI. As compared to vehicle treatment, 30 μg i.t. doses of pregabalin significantly attenuated the CCI-induced decrease in von Frey thresholds on day 4, 8 and 12, while 10 and 100 μg i.t. pregabalin significantly attenuated mechanical allodynia on days 8 and 4 & 12, respectively (Fig. 1A, p < 0.05). Conversely, only 100 μg of i.t. pregabalin pretreatment significantly attenuated CCI-induced increases in cold water response frequencies (Fig. 1B) (*p < 0.05).
Systemic pregabalin pre-treatment effects on neuropathic pain
Results indicated that systemic pregabalin pretreatment attenuated mechanical and cold allodynia at various times after CCI. As compared to vehicle treatment, rats pretreated with 10 mg/kg i.p. pregabalin significantly attenuated the CCI-induced decrease in von Frey thresholds on days 4 and 8 (Fig 2A, *p < 0.05) and significantly attenuated CCI-induced increases in cold water response frequencies on days 4, 8 and 12 (Fig. 2B, *p < 0.05). Rats pretreated with the 100 mg/kg dose also had significantly reduced allodynia on day 4 (Fig. 2A, p < 0.05). No significant change in the cold water withdrawal frequencies on days 4, 8 and 12 was observed with 30 and 100 mg/kg of pregabalin (Fig. 2B).
Spinal pregabalin post-treatment effects on neuropathic pain
Results showed that intrathecal pregabalin post-treatment attenuated mechanical and cold allodynia at various times after CCI.. As compared to vehicle treatment, 10 and 100 μg of i.t. pregabalin post-treatment significantly attenuated CCI-induced decreases in mechanical thresholds on day 12 & 16 and day 12, respectively (Fig. 3A, *p < 0.05). A post-treatment dose of 100 μg i.t. pregabalin also attenuated CCI-induced increases in cold water response frequency on day 12 (Fig. 3B, *p < 0.05).
Systemic pregabalin post-treatment effect on neuropathic pain
Results indicated that systemic pregabalin post-treatment attenuated mechanical and cold allodynia at various times after CCI. As compared to vehicle controls, rats treated with 30 and 100 mg/kg pregabalin on days 8, 12 and 16, and 10 mg/kg pregabalin on day 16, had significantly attenuated CCI-induced decreases in von Frey thresholds (Fig 4A, *p < 0.05). No effect of the pregabalin treatment was observed on cold water response frequency on all days (Fig. 4B).
Effect of systemic pregabalin on nociceptive scores in formalin test
An injection of 2.5% formalin, in a hind paw on ipsilateral side, in naive rats showed a typical biphasic nociceptive response. Compared to vehicle saline treatment, systemic pretreatment with 30 and 100 mg/kg pregabalin (i.p.) significantly decreased nociceptive scores at various time points after the intraplantar formalin injection. (Fig. 5, *p < 0.05).
Fig. 5.
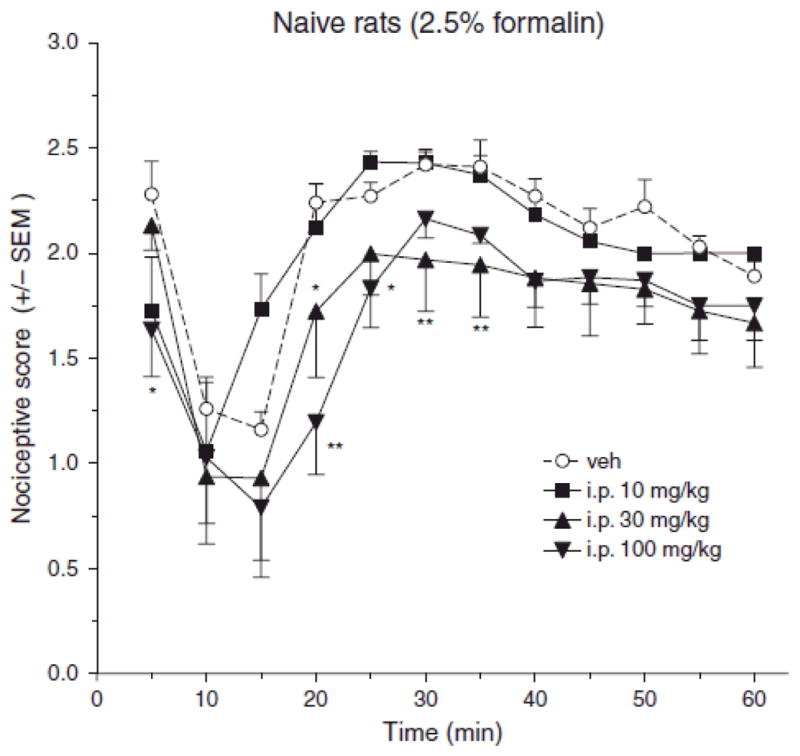
Effect of systemic (i.p.) pregabalin pretreatment on behavioral nociceptive scores in the formalin test. Repeated measures ANOVA reveals a significant main effects of i.p. pregabalin dose (F(3,22) = 3.21, p < 0.05) and time (F(11,242) = 22.5, p < 0.001), but a non-significant dose X time interaction (F(33,242) = 1.27 p > 0.05). Post hoc comparison revealed that compared to vehicle (n=6), formalin test nociceptive scores were significantly reduced by 100 mg/kg pregabalin (n=8) at 5, and 20–35 min post-formalin, and by 30 mg/kg pregabalin (n=6) at only 20 min post-formalin, while 10 mg/kg (n=6) was without effect.
Pregabalin effects formalin-induced release of excitatory amino acids in dorsal horn of spinal cord
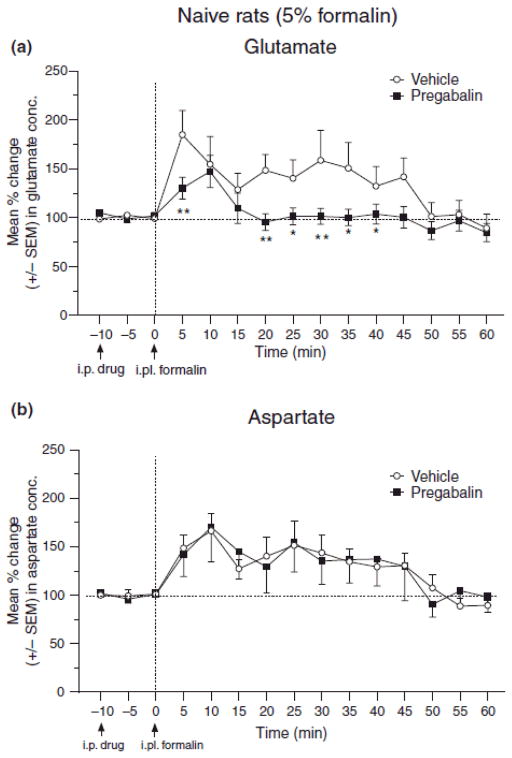
Injection of 5% formalin in ipsilateral hind paw of naive rats increased glutamate concentration in microdialysis samples of SCDH. 100 mg/kg i.p. pregabalin significantly attenuated the formalin-induced release of glutamate in naive rats (Fig. 6A, *p < 0.05), but did not influence spinal aspartate concentrations (Fig. 6B).
Fig. 6.
Effect of systemic pregabalin pretreatment (100 mg/kg, i.p.) on the glutamate (A) and aspartate (B) concentration in samples from spinal cord dorsal horn of naïve rats given a hind paw injection of 5.0% formalin. The line graph shows post-pregabalin/vehicle treatment, and post-formalin injection time points for group of rats treated with pregabalin (n=10 for glutamate, n=9 for aspartate) or vehicle (n=12 for both glutamate & aspartate). Arrows indicate timing of i.p. pregabalin/vehicle and intraplantar (i.pl.) formalin injections at −10 and 0 min, respectively. ANOVA reveals significant main effects of drug (F(1,20) = 5.10, p < 0.05) and time (F(14,280) = 6.58, p < 0.001), and a significant drug–time interaction (F(14,280) = 1.91, p < 0.05) in the assessment for glutamate (A). ANOVA reveals a non-significant main effects of drug (F(1,19) = 0.04, p > 0.05), but a significant main effect of time (F(14,266) = 4.05, p < 0.001), and a non-significant drug–time interaction (F(14,266) = 0.65, p > 0.05) in the assessment for aspartate (A). Post hoc comparisons (Fisher’s) indicated that pregabalin reduced glutamate levels at the 5 and 20–40 min time points after formalin (A), and did not significantly affect aspartate levels at any point after formalin (B) (*p < 0.05, **p < 0.01).
Pregabalin effects formalin-induced release of excitatory amino acids in dorsal horn of spinal cord in CCI rats
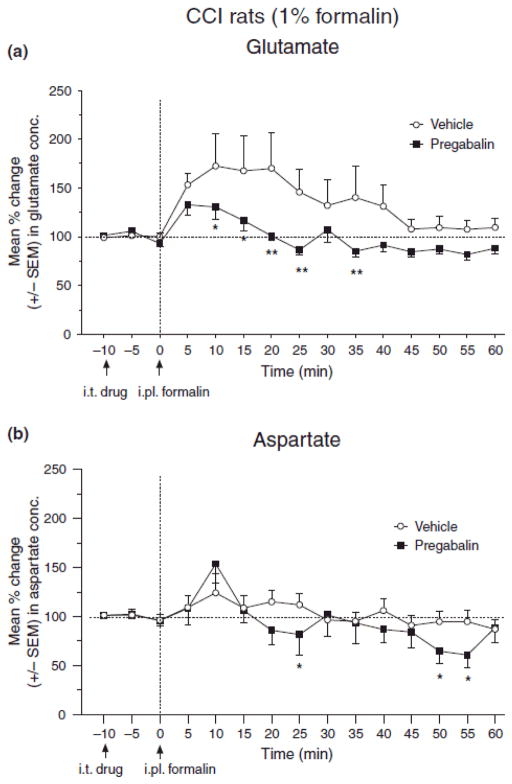
Intraplantar injection of 1% formalin in ipsilateral hind paw of CCI rats increased glutamate concentration in microdialysis samples of SCDH of neuropathic rats; however, the same concentration of formalin did not show increase in sham rats (data not shown). A dose of 100 μg i.t. pregabalin significantly attenuated the formalin-induced release of glutamate in the SCDH of neuropathic rats, between 10 and 35 min after intraplantar formalin injection (Fig. 7A, p < 0.05), and aspartate at 25, 50 and 55 min (Fig. 7B).
Fig. 7.
Effect of intrathecal pregabalin pretreatment (100 μg, i.t.) on the glutamate (A) and aspartate (B) concentration in samples from spinal cord dorsal horn of CCI rats given a hind paw injection of 1.0% formalin. The line graph shows post-pregabalin/vehicle treatment, and post-formalin injection time points for group of rats treated with pregabalin (n=9) or vehicle (n=11). Arrows indicate timing of i.p. pregabalin/vehicle and intraplantar (i.pl.) formalin injections at −10 and 0 min, respectively. ANOVA reveals significant main effects of drug (F(1,20) = 4.15, p < 0.05) and time (F(14,266) = 3.54, p < 0.001), and a non-significant drug–time interaction (F(14,266) = 1.13, p > 0.05) in the assessment for glutamate (A). ANOVA reveals a non-significant main effects of drug (F(1,25) = 1.54, p > 0.05), but a significant main effect of time (F(14,350) = 5.76, p < 0.001), and a non-significant drug–time interaction (F(14,350) = 1.44, p > 0.05) in the assessment for aspartate (A). Post hoc comparisons (Fisher’s) indicated that pregabalin reduced glutamate levels at the 10–25 and 35 min time points after formalin (A), and aspartate levels at the 25 and 50–55 min time points after formalin (B) (*p < 0.05, **p < 0.01).
DISCUSSION
Effect of spinal and systemic pregabalin on neuropathic and formalin pain
Generally, the results suggest that pregabalin attenuates mechanical and cold hypersensitivity in rats with CCI of the sciatic nerve. However, pregabalin attenuated mechanical allodynia to a much greater extent than cold allodynia. Thus, pregabalin anti-allodynic effects on mechanical allodynia occurred with more doses, were greater in magnitude and lasted longer than its effects on cold allodynia. Furthermore, while i.t. pretreatment with pregabalin tended to be more effective against mechanical allodynia than systemic pretreatment, systemic post-treatment tended to be more effective than i.t. post-treatment. In addition, the anti-allodynic effects of either i.t. or systemic pregabalin were not particularly dose-dependent, with the middle dose often producing greater effects than the highest dose, and occasionally the lowest dose producing the greatest effects. This pattern suggests that pregabalin may be producing an inverted-U or bell shaped dose response curve, as has been noted for other analgesics (Cowan et al., 2003; Xu et al., 1994). Importantly, gabapentin’s effects on [3H]GABA release from neostriatal slices has been reported to have a bell shaped dose response curve (Gotz et al., 1993).
The anti-allodynic effects of pregabalin support the previous studies which indicate that pregabalin attenuates tactile allodynia administered either i.t. or systemically (Wallin et al 2002; Takeuchi et al., 2007). Similarly, Han et al. (2007 have shown that i.t. or systemic (i.p.) post-treatment with pregabalin attenuated mechanical- and cold-allodynia in neuropathic rats. The dose of i.t. pregabalin was 900 times less than i.p. dose, suggesting that the anti-allodynic effect of pregabalin is mainly at spinal cord. Both studies indicated that the attenuation of allodynia was stronger when pregabalin was given i.t., and the maximum suppressive effects on allodynia occurred earlier after i.t. than systemic treatment. In our study, the i.t. dose was 1000 times less than the i.p. dose.
Our results suggest that pregabalin pretreatment is more effective than post-treatment at attenuating cold allodynia in CCI rats, despite the finding that pregabalin is less effective for cold allodynia than mechanical allodynia. Although, Tanimoto-Mori et al. (2008) and Gustafsson and Sandin (2009) have recently shown that cold allodynia in neuropathic rats is alleviated by systemic pregabalin post-treatment, no earlier animal studies assessed the effects of pretreatment of pregabalin on cold allodynia. Han et al. (2007) have shown that pregabalin post-treatment attenuates both cold allodynia and mechanical allodynia in CCI rats, and the i.t. route is more effective than systemic route. Similar results have also been shown with gabapentin, which reduces cold allodynia after i.t. pre or post-treatment, but only after systemic pretreatment (Coderre at al 2005).
A predominant peripheral action of pregabalin in neuropathic pain, may be suggested by the up-regulation of α2δ Ca2+ channel subunits in the DRG after peripheral nerve injury (Luo et al., 2001), and the ability of pregabalin to reduce the ectopic discharges from injured afferents in neuropathic rats (Chen & Pan, 2001). However, the upregulation of α2δ Ca2+ channel subunits does not reach a peak until 7–14 days after nerve injury (Luo et al., 2001, 2002), when neuropathic pain behaviors are well established. We speculate that pregabalin may initially be most effective spinally prior to the upregulation of α2δ Ca2+ channel subunits, and later may be more effective systemically following the upregulation. This conclusion is consistent with the most effective treatments observed here, which were i.t. pregabalin pretreatment and systemic pregabalin post-treatment.
Pregabalin’s effectiveness as an i.t. pretreatment suggests that pregabalin may have significant effects on spinal glutamatergic neurotransmission. Given as a pretreatment, pregabalin may be acting pre-emptively to prevent central sensitization, an effect that has been shown often for NMDA antagonists. Interestingly, NMDA antagonists (Coderre, 1993), i.t. gabapentin (Shimoyama et al. 1997, Coderre at al 2005), and pregabalin (Chesler et al 2003) more effectively block the second phase of the formalin test than the first, stressing their role as treatments that prevent central sensitization. Furthermore, systemic and i.t. pregabalin also relieve pain or hyperalgesia in animal models of chemically-induced or inflammatory pain such after formalin or zymosan (Chesler et al., 2003) injections. Since these models reflect nociception lasting between 30 min and 24 h, it shows that pregabalin can act rapidly on pain that is relatively acute, and that its actions occur at times when up-regulation of the α2δ-calcium channel subunit probably has not occurred. However, systemic pregabalin may have effects other than at the presynaptic central terminals of primary afferent nerves, since pregabalin has also been found to reduce formalin-induced nociceptive scores after local administration (Carlton & Zhuo, 1998).
In the present study, systemic administration of pregabalin dose-dependently attenuated tonic formalin-induced nociceptive scores in naive rats, results which support the previous studies in rats and mice (Chesler et al 2003, Field et al., 1997, 2006). This antinociceptive effect of the pregabalin in rodents is likely due the inhibition of formalin-induced glutamate release in the SCDH that we have shown here in naïve rats. In naïve rats, pregabalin did not influence formalin-induced increases in aspartate concentrations. However, in our hands spinal aspartate concentrations in microdialysis samples are very close to the detection limit, and group differences are consequently often difficult to obtain. Nonetheless the present results support previous results that gabapentin reduces spinal glutamate release induced by formalin (Coderre et al 2005), as well as following intraperitoneal injection of acetic acid (Feng et al. 2003). A direct presynaptic effect of pregabalin on glutamatergic neurons is suggested by findings that pregabalin (Maneuf et al., 2001), like gabapentin (Maneuf & McKnight 2001; Maneuf et al. 2004), is capable of blocking the substance P and protein kinase C-induced facilitation of K+-evoked glutamate release in trigeminal dorsal horn slices. These researchers argue that pregabalin acts presynaptically, in this area, to mitigate changes in glutamate neurotransmission that are induced by elevated levels of protein kinase C. Previous studies indicate that pregabalin binds potently to the α2δ subunit of voltage-gated calcium channels, modulates calcium influx at nerve terminals (Fink et al., 2002), and reduces the release of many neurotransmitters, including glutamate, noradrenaline, serotonin, dopamine, and substance P (Dooley et al. 2000a,b; Fink et al. 2002; Maneuf et al. 2001; Maneuf & McKnight, 2001; Errante & Petroff, 2003; Cunningham et al. 2004). Our finding that pregabalin is capable of reducing the enhancement of spinal glutamate and asparatate release in the SCDH of CCI rats given a hind paw injection of formalin supports our previous similar results with gabapentin, and further suggested that these agents reduce neuropathic pain by inhibiting enhanced spinal EAA release.
Conclusions
We conclude based on the present results that pregabalin’s actions on neuropathic pain after CCI injury depend significantly on effects in the spinal cord. It is expected that pregabalin may act at presynaptic α2δ Ca2+ channel subunits in SCDH to inhibit glutamate release. Our current in vivo microdialysis studies demonstrating that pregabalin reduced the enhanced noxious stimulus-induced spinal release of glutamate observed in neuropathic rats, makes a strong case that pregabalin influences pain perception by affecting presynaptic glutamatergic SCDH neurons. Pretreatment with pregabalin may help prevent the development of central sensitization of glutamate neurotransmission in SCDH and thereby reduce symptoms of neuropathic pain.
Footnotes
Competing Interests
This work was supported by a contract from Pfizer Corporation the manufacturer of pregabalin and TJC has received consulting funds from Pfizer Corporation.
References
- Baron R, Brunnmuller U, Brasser M, May M, Binder A. Efficacy and safety of pregabalin in patients with diabetic peripheral neuropathy or postherpetic neuralgia: Open-label, non-comparative, flexible-dose study. Eur J Pain. 2008;12:850–858. doi: 10.1016/j.ejpain.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Bauer CS, Nieto-Rostro M, Rahman W, Tran-Van-Minh A, Ferron L, Douglas L, Kadurin I, Sri Ranjan Y, Fernandez-Alacid L, Millar NS, Dickenson AH, Lujan R, Dolphin AC. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J Neurosci. 2009;29:4076–4088. doi: 10.1523/JNEUROSCI.0356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer K, Ahmadi S, Zeilhofer HU. Gabapentin may inhibit synaptic transmission in the mouse spinal cord dorsal horn through a preferential block of P/Q-type Ca2+ channels. Neuropharmacology. 2004;46:743–749. doi: 10.1016/j.neuropharm.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bian F, Li Z, Offord J, Davis MD, McCormick J, Taylor CP, Walker LC. Calcium channel alpha2-delta type 1 subunit is the major binding protein for pregabalin in neocortex, hippocampus, amygdala, and spinal cord: an ex vivo autoradiographic study in alpha2-delta type 1 genetically modified mice. Brain Res. 2006;1075:68–80. doi: 10.1016/j.brainres.2005.12.084. [DOI] [PubMed] [Google Scholar]
- Böttcher T, Goiny M, Bering J, Domhof S, Nau R, Ungerstedt U. Regional differences in glutamine synthetase inhibition by L-methionine sulfoximine: a microdialysis study in the rabbit brain. Exp Brain Res. 2003;50:194–200. doi: 10.1007/s00221-003-1401-0. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Zhou S. Attenuation of formalin-induced nociceptive behaviors following local peripheral injection of gabapentin. Pain. 1998;76:201–207. doi: 10.1016/s0304-3959(98)00043-8. [DOI] [PubMed] [Google Scholar]
- Chen SR, Xu Z, Pan HL. Stereospecific effect of pregabalin on ectopic afferent discharges and neuropathic pain induced by sciatic nerve ligation in rats. Anesthesiology. 2001;95:1473–1479. doi: 10.1097/00000542-200112000-00029. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Ritchie J, Kokayeff A, Lariviere WR, Wilson SG, Mogil JS. Genotype-dependence of gabapentin and pregabalin sensitivity: the pharmacogenetic mediation of analgesia is specific to the type of pain being inhibited. Pain. 2003;106:325–335. doi: 10.1016/S0304-3959(03)00330-0. [DOI] [PubMed] [Google Scholar]
- Coderre TJ. Non-competitive NMDA receptor antagonists, central sensitization and persistent pain and hyperalgesia. Pain. 1993;55:126–128. [Google Scholar]
- Coderre TJ, Kumar N, Lefebvre CD, Yu JS. Evidence that gabapentin reduces neuropathic pain by inhibiting the spinal release of glutamate. J Neurochem. 2005;94:1131–1139. doi: 10.1111/j.1471-4159.2005.03263.x. [DOI] [PubMed] [Google Scholar]
- Cowan A. Buprenorphine: new pharmacological aspects. Int J Clin Pract Suppl. 2003;133:3–8. [PubMed] [Google Scholar]
- Cunningham M, Woodhall G, Thompson S, Dooley D, Jones R. Dual effects of gabapentin and pregabalin on glutamate release at rat entorhinal synapses in vitro. Eur J Neurosci. 2004;20:1566–1576. doi: 10.1111/j.1460-9568.2004.03625.x. [DOI] [PubMed] [Google Scholar]
- Dooley DJ, Donovan C, Pugsley T. Stimulus-dependent modulation of [3H]norepinephrine release from rat neocortical slices by gabapentin and pregabalin. J Pharmacol Exp Ther. 2000a;295:1086–1093. [PubMed] [Google Scholar]
- Dooley DJ, Mieske CA, Borosky SA. Inhibition of K(+)-evoked glutamate release from rat neocortical and hippocampal slices by gabapentin. Neurosci Lett. 2000b;280:107–110. doi: 10.1016/s0304-3940(00)00769-2. [DOI] [PubMed] [Google Scholar]
- Dooley DJ, Donovan CM, Meder WP, Whetzel SZ. Preferential action of gabapentin and pregabalin at P/Q-type voltage-sensitive calcium channels: inhibition of K+evoked [3H]-norepinephrine release from rat neocortical slices. Synapse. 2002;45:171–190. doi: 10.1002/syn.10094. [DOI] [PubMed] [Google Scholar]
- Errante L, Petroff OAC. Acute effects of gabapentin and pregabalin on rat forebrain cellular GABA, glutamate, and glutamine concentrations. Seizure. 2003;12:300–306. doi: 10.1016/s1059-1311(02)00295-9. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain. 2003;105:133–141. doi: 10.1016/s0304-3959(03)00173-8. [DOI] [PubMed] [Google Scholar]
- Feng Y, Cui M, Willis WD. Gabapentin markedly reduces acetic acid-induced visceral nociception. Anesthesiology. 2003;98:729–733. doi: 10.1097/00000542-200303000-00023. [DOI] [PubMed] [Google Scholar]
- Field MJ, McCleary S, Hughes J, Singh L. Gabapentin and pregabalin, but not morphine and amitriptyline, block both static and dynamic components of mechanical allodynia induced by streptozocin in the rat. Pain. 1999;80:391–398. doi: 10.1016/s0304-3959(98)00239-5. [DOI] [PubMed] [Google Scholar]
- Field MJ, Cox PJ, Stott E, Melrose H, Offord J, Su TZ, Bramwell S, Corradini L, England S, Winks J, Kinloch RA, Hendrich J, Dolphin AC, Webb T, Williams D. Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci USA. 2006;103:7537–7542. doi: 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MJ, Oles RJ, Lewis AS, McCleary S, Hughes J, Singh L. Gabapentin (neurontin) and S-(+)-3-isobutylgaba represent a novel class of selective antihyperalgesic agents. Br J Pharmacol. 1997;121:1513–1522. doi: 10.1038/sj.bjp.0701320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink K, Dooley DJ, Meder WP, Suman-Chauhan N, Duffy S, Clusmann H, Gothert M. Inhibition of neuronal Ca2+ influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42:229–236. doi: 10.1016/s0028-3908(01)00172-1. [DOI] [PubMed] [Google Scholar]
- Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin(Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- Götz E, Feuerstein TJ, Lais A, Meyer DK. Effects of gabapentin on release of gamma-aminobutyric acid from slices of rat neostriatum. Arzneimittelforschung. 1993;43:636–638. [PubMed] [Google Scholar]
- Guay DR. Pregabalin in neuropathic pain: A More “pharmaceutically elegant” gabapentin? Am J Geriatr Pharmacother. 2005;3:274–287. [PubMed] [Google Scholar]
- Gustafsson H, Sandin J. Oral pregabalin reverses cold allodynia in two distinct models of peripheral neuropathic pain. Eur J Pharmacol. 2009;605:103–108. doi: 10.1016/j.ejphar.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Han DW, Kweon TD, Lee JS, Lee YW. Antiallodynic effect of pregabalin in rat models of sympathetically maintained and sympathetic independent neuropathic pain. Yonsei Med J. 2007;48:41–47. doi: 10.3349/ymj.2007.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Zhang XL, Matthews EA, Li KW, Kurwa A, Boroujerdi A, Gross J, Gold MS, Dickenson AH, Feng G, Luo ZD. Calcium channel alpha2delta1 subunit mediates spinal hyperexcitability in pain modulation. Pain. 2006;125:20–34. doi: 10.1016/j.pain.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZD, Chaplan SR, Higuera ES, Sorkin LS, Stauderman KA, Williams ME, Yaksh TL. Upregulation of dorsal root ganglion (alpha)2(delta) calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J Neurosci. 2001;21:1868–1875. doi: 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZD, Calcutt NA, Higuera ES, Valder CR, Song YH, Svensson CI, Myers RR. Injury type-specific calcium channel alpha 2 delta-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J Pharmacol Exp Ther. 2002;303:1199–1205. doi: 10.1124/jpet.102.041574. [DOI] [PubMed] [Google Scholar]
- Maneuf YP, Hughes J, McKnight AT. Gabapentin inhibits the substance P-facilitated K(++)evoked release of [(3)H]glutamate from rat caudal trigeminal nucleus slices. Pain. 2001;93:191–196. doi: 10.1016/S0304-3959(01)00316-5. [DOI] [PubMed] [Google Scholar]
- Maneuf YP, McKnight AT. Block by gabapentin of the facilitation of glutamate release from rat trigeminal nucleus following activation of protein kinase C or adenylyl cyclase. Br J Pharmacol. 2001;134:237–240. doi: 10.1038/sj.bjp.0704227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneuf YP, Blake R, Andrews NA, McKnight AT. Reduction by gabapentin of K(+) evoked release of [3H]-glutamate from the caudal trigeminal nucleus of the streptozotocin-treated rat. Br J Pharmacol. 2004;141:574–579. doi: 10.1038/sj.bjp.0705579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland D, Evans RM, Barkworth L, Martin DJ, Scott RH. A study comparing the actions of gabapentin and pregabalin on the electrophysiological properties of cultured DRG neurones from neonatal rats. BMC Pharmacol. 2004;14:1–26. doi: 10.1186/1471-2210-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose HL, Kinloch RA, Cox PJ, Field MJ, Collins D, Williams D. [3H] pregabalin binding is increased in ipsilateral dorsal horn following chronic constriction injury. Neurosci Lett. 2007;417:187–192. doi: 10.1016/j.neulet.2007.02.068. [DOI] [PubMed] [Google Scholar]
- Moulin DE, Clark AJ, Gilron I, Ware MA, Watson CP, Sessle BJ, Coderre TJ, Morley-Forster PK, Stinson J, Boulanger A, Peng P, Finley GA, Taenzer P, Squire P, Dion D, Cholkan A, Gilani A, Gordon A, Henry J, Jovey R, Lynch M, Mailis-Gagnon A, Panju A, Rollman GB, Velly A. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12:13–21. doi: 10.1155/2007/730785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki-Taguchi N, Chaplan SR, Higuera ES, Ajakwe RC, Yaksh TL. Vincristine-induced allodynia in the rat. Pain. 2001;93:69–76. doi: 10.1016/S0304-3959(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Nguyen D, Deng P, Matthews EA, Kim DS, Feng G, Dickenson AH, Xu ZC, Luo ZD. Enhanced pre-synaptic glutamate release in deep-dorsal horn contributes to calcium channel alpha-2-delta-1 protein-mediated spinal sensitization and behavioral hypersensitivity. Mol Pain. 2009;5:6. doi: 10.1186/1744-8069-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogatzki EM, Zahn PK, Brennan TJ. Lumbar catheterization of the subarachnoid space with a 32-gauge polyurethane catheter in the rat. Eur J Pain. 2000;4:111–113. doi: 10.1053/eujp.1999.0157. [DOI] [PubMed] [Google Scholar]
- Shi L, Smolders I, Sarre S, Michotte Y, Zizi M, Camu F. Formalin-induced spinal glutamate release in freely moving rats: comparison of two spinal microdialysis approaches. Acta Anaesthesiol Belg. 2004;55:43–48. [PubMed] [Google Scholar]
- Shimoyama N, Shimoyama M, Davis AM, Inturrisi CE, Elliott KJ. Spinal gabapentin is antinociceptive in the rat formalin test. Neurosci Lett. 1997;222:65–67. doi: 10.1016/s0304-3940(97)13331-6. [DOI] [PubMed] [Google Scholar]
- Skilling SR, Smullin DH, Beitz AJ, Larson AA. Extracellular amino acid concentrations in the dorsal spinal cord of freely moving rats following veratridine and ociceptive stimulation. J Neurochem. 1988;51:127–132. doi: 10.1111/j.1471-4159.1988.tb04845.x. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Westlund KN. An experimental arthritis in rats: dorsal horn aspartate and glutamate increases. Neurosci Lett. 1992;145:141–144. doi: 10.1016/0304-3940(92)90006-s. [DOI] [PubMed] [Google Scholar]
- Sonnett TE, Setter SM, Campbell RK. Pregabalin for the treatment of painful neuropathy. Expert Rev Neurother. 2006;6:1629–1635. doi: 10.1586/14737175.6.11.1629. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Takasu K, Ono H, Tanabe M. Pregabalin, S-(+)-3-isobutylgaba, activates the descending noradrenergic system to alleviate neuropathic pain in the mouse partial sciatic nerve ligation model. Neuropharmacology. 2007;53:842–853. doi: 10.1016/j.neuropharm.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Tanimoto-Mori S, Nakazato-Imasato E, Toide K, Kita Y. Pharmacologic investigation of the mechanism underlying cold allodynia using a new cold plate procedure in rats with chronic constriction injuries. Behav Pharmacol. 2008;19:85–90. doi: 10.1097/FBP.0b013e3282f3d0a3. [DOI] [PubMed] [Google Scholar]
- Wallin J, Cui JG, Yakhnitsa V, Schechtmann G, Meyerson BA, Linderoth B. Gabapentin and pregabalin suppress tactile allodynia and potentiate spinal cord stimulation in a model of neuropathy. Eur J Pain. 2002;6:261–272. doi: 10.1053/eujp.2002.0329. [DOI] [PubMed] [Google Scholar]
- Xu W, Qiu XC, Han JS. Serotonin receptor subtypes in spinal antinociception in the rat. J Pharmacol Exp Ther. 1994;269:1182–1189. [PubMed] [Google Scholar]
- Zahn PK, Sluka KA, Brennan TJ. Excitatory amino acid release in the spinal cord caused by plantar incision in the rat. Pain. 2002;100:65–76. doi: 10.1016/s0304-3959(02)00241-5. [DOI] [PubMed] [Google Scholar]