Abstract
The development of nitric oxide (NO)- and hydrogen sulfide (H2S)-releasing nonsteroidal anti-inflammatory drugs (NSAIDs) has generated more potent anti-inflammatory drugs with increased safety profiles. A new hybrid molecule incorporating both NO and H2S donors into aspirin (NOSH-aspirin) was recently developed. In the present study, the antinociceptive activity of this novel molecule was compared with aspirin in different models of inflammatory pain. It was found that NOSH-aspirin inhibits acetic acid-induced writhing response and carrageenan (Cg)-induced inflammatory hyperalgesia in a dose-dependent (5–150 μmol/kg, v.o.) manner, which was superior to the effect of the same doses of aspirin. NOSH-aspirin’s antinociceptive effect was also greater and longer compared to aspirin upon complete Freund’s adjuvant (CFA)-induced inflammatory hyperalgesia. Mechanistically, NOSH-aspirin, but not aspirin, was able to reduce the production/release of interleukin-1 beta (IL-1β) during Cg-induced paw inflammation. Furthermore, NOSH-aspirin, but not aspirin, reduced prostaglandin E2-induced hyperalgesia, which was prevented by treatment with a ATP-sensitive potassium channel (KATP) blocker (glibenclamide; glib.). Noteworthy, the antinociceptive effect of NOSH-aspirin was not associated with motor impairment. The present results indicate that NOSH-aspirin seems to present greater potency than aspirin to reduce inflammatory pain in several models. The enhanced effects of NOSH-aspirin seems to be due to its ability to reduce the production of pronociceptive cytokines such as IL-1 β and directly block hyperalgesia caused by a directly acting hyperalgesic mediator in a mechanism dependent on modulation of KATP channels. In conclusion, we would like to suggest that NOSH-aspirin represents a prototype of a new class of analgesic drugs with more potent effects than the traditional NSAID, aspirin.
Keywords: Aspirin and NOSH-aspirin, hyperalgesia, inflammatory pain, prostaglandins
Introduction
Inflammatory pain is one of the most common symptoms of the inflammatory disease and represents an important health problem. It is principally caused by the sensitization of primary nociceptive neurons by the direct action of inflammatory mediators (e.g., prostaglandins). In this context, pharmacologic control of inflammatory pain is mainly based on the use of nonsteroidal anti-inflammatory drugs (NSAIDs; aspirin and aspirin like drugs), which inhibits cyclooxygenase-derived prostaglandin production and, consequently, reduces nociceptor sensitization (Ferreira 1972, 1980; Ferreira and Vane 1974). This effect ultimately prevents the development of hyperalgesia (decrease in nociceptive threshold) in humans and animals. However, NSAIDs are only partially effective and prolonged exposure can cause serious gastrointestinal (GI), renal, and cardiovascular side effects (Kashfi 2009). Thus, there is continuous effort to identify novel therapeutics for pain control that elicits fewer side effects.
A second strategy to control inflammatory pain is through the use of drugs, which are able to directly block ongoing nociceptor sensitization through peripheral actions (Lorenzetti and Ferreira 1985; Ferreira et al. 1991; Duarte et al. 1992). In fact, local administration of opioids and dipyrone reversed already established hyperalgesia induced by prostaglandin E2 (PGE2) (Ferreira 1972; Lorenzetti and Ferreira 1996). Therefore, in contrast to NSAIDs, drugs that act through the prevention of nociceptor sensitization by inhibiting prostaglandin synthesis are able to reverse ongoing inflammatory hyperalgesia.
In order to elucidate the mechanism involved in the directly blockage of ongoing inflammatory hyperalgesia, inhibitors of neuronal nitric oxide (NO) synthase as well as ATP-sensitive potassium channel (KATP) potassium channels blockers prevented peripheral antinociception achieved with opioids and dipyrone (Rodrigues and Duarte 2000; Soares and Duarte 2001; Sachs et al. 2004). Therefore, it was suggested that this antinociceptive effect is mediated by NO and KATP. Corroborating with this fact, the direct blockade of ongoing mechanical hyperalgesia has been observed after administration of NO donors, SNAP and sodium nitroprusside as well as KATP channels openers (Soares et al. 2000). Besides NO donors, it has also been described that hydrogen sulfide (H2S) donors are also able to directly block ongoing inflammatory hyperalgesia through the modulation of KATP currents (Distrutti et al. 2006a; Zanardo et al. 2006; Cunha et al. 2008a). Thus, these types of peripheral analgesics seem to act by restoring the threshold of nociceptors via the increase in KATP permeability promoting the hyperpolarization of primary nociceptive neurons (Distrutti et al. 2006a; Cunha et al. 2008a).
In attempt to enhance the activity of NSAIDs and to minimize their potential side effect, novel NSAIDs hybrids have been developed (Kashfi 2009; Qandil 2012). NO-releasing NSAIDs have been shown to be effective in preclinical models of inflammation with less side effects and they have been evaluated in several clinical trials (Schnitzer et al. 2005; Fiorucci and Distrutti 2011). Similarly, H2S-releasing NSAIDs have been shown to have anti-inflammatory properties (Kashfi and Olson 2013). Based on the scientific evidence that NO and H2S are gaseous mediators with physiological relevance, a new hybrid incorporating these two entities into an aspirin molecule was developed, the so-called NOSH-aspirin (Kodela et al. 2012). NOSH-aspirin was shown to be more efficient and/or powerful than traditional aspirin in several experimental models (Kodela et al. 2012). For instance, NOSH-aspirin was a potent inhibitor of colon cancer cell growth in vitro and in an in vivo model of human colon cancer xenografts in mice (Chattopadhyay et al. 2012). Furthermore, NOSH-aspirin was more potent than aspirin to attenuate microglial and astrocytes activation in an in vitro model of neuroinflammation (Lee et al. 2013). Based on the above evidences, in the present study we investigated the possible analgesic effects of NOSH-aspirin in several inflammatory models of pain, comparing it to aspirin.
Material and Methods
Animals
The experiments were performed on male Balb/C mice weighing between 20 and 30 g. The mice were housed in the animal care facility of the Ribeirao Preto Medical School and moved to the testing room at least 1 h before the experiments. Food and water were available ad libitum. Behavioral tests were performed on animals in a randomized order in a blind fashion in which the person who performed the treatments was not the same as one who made the behavioral assessment. Animal care and handling procedures were in accordance with the guidelines of the International Association for the Study of Pain (IASP) on the use of animals in pain research, and with the approval of the Ethics Committee of the Ribeirao Preto Medical School, University of São Paulo.
Chemicals and drugs
The following drugs and chemicals were obtained from the sources indicated: PGE2, glib., complete Freund’s adjuvant (CFA), aspirin and diazepam were from Sigma-Aldrich (St. Louis, MO); Cg (FMC Corp., Philadelphia, PA); acetic acid, dimethylsulfoxide (DMSO) and Tween 20 were from Merck (Darmstadt, Germany); NOSH-aspirin (Fig.1) [4-(3-thioxo-3H-1,2-dithiol-5-yl)phenyl 2-((4-(nitrooxy)butanoyl) oxy)benzoate] was synthesized and characterized as previously described (Kodela et al. 2012) and was a gift from Avicenna Pharmaceuticals (New York, NY). The drugs were prepared as follows: glib. was dissolved in 1% Tween 20 in saline; PGE2 was dissolved in 2% ethanol in saline; diazepam was dissolved in saline; aspirin and NOSH-aspirin were dissolved in DMSO/Tween 20 and resuspended in saline. The final concentrations of DMSO and Tween 20 were 5%.
Figure 1.
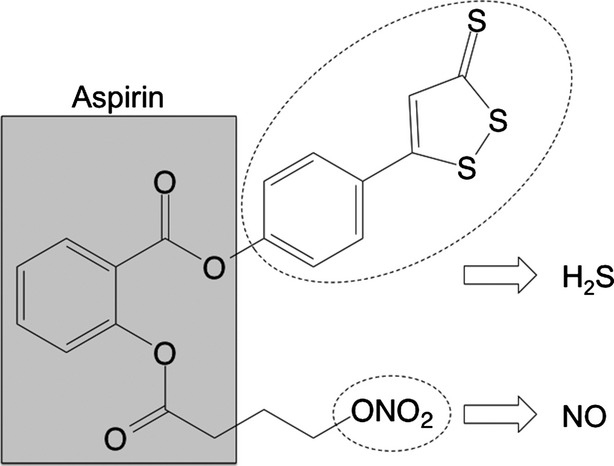
Structural components of NOSH-aspirin. The parent compound aspirin is shown in the shaded box. The parts of the molecule that releases NO and H2S are shown in the dotted ellipses. NO, nitric oxide; H2S, hydrogen sulfide.
Acetic acid-induced writhing test
Mice were treated by gavage (p.o.) with aspirin (5, 15 or 150 μmol/kg), NOSH-aspirin (5, 15, or 150 μmol/kg) or vehicle an hour before testing. Writhing responses were induced by intraperitoneal (i.p.) injection of acetic acid 0.8% (100 μL per 10 g body weight). The numbers of writhing responses were counted during a 20-min period as reported previously (Collier et al. 1968). The writhing response consists of a contraction of the abdominal muscle together with a stretching of hind limbs. The inhibitory effects were calculated based on the effect of the vehicle.
Evaluation of mechanical hyperalgesia: electronic von Frey test
In this study, we have used the term hyperalgesia to describe the decrease in the mechanical nociceptive threshold of mice. Mechanical hyperalgesia was tested as previously reported (Cunha et al. 2004). Briefly, in a quiet room, mice were placed in acrylic cages (12 × 20 × 17 cm) with wire grid floors, 15–30 min before the start of the test. The test consisted of evoking a hind paw flexion reflex with a hand-held force transducer adapted with a 0.5-mm2 polypropylene tip for mice (Electronic von Frey; IITC Life Science, Woodland Hills, CA). A tilted mirror placed under the grid provided a clear view of the mice hind paw. The investigator was trained to apply the tip in between the five distal footpads with a gradual increase in pressure. The stimulus was automatically discontinued and its intensity recorded when the paw was withdrawn. The end-point was characterized by the removal of the paw in a clear flinch response after paw withdrawal. The animals were tested before and after treatments. A different investigator performed each test, as was the case in the preparation of solutions and treatment of the animals. The results are expressed by the Δ mechanical threshold (in grams, g), which was calculated by subtracting the average of the last three measurements after treatments from the average of three measurements before treatments.
Effect of NOSH-aspirin on inflammatory hyperalgesia
In attempt to compare the antinociceptive effects of aspirin and NOSH-aspirin on inflammatory hyperalgesia, initially, we evaluated the dose–response effects of these drugs against Cg-induced acute mechanical inflammatory hyperalgesia. The animals were pretreated (30 min before) with NOSH-aspirin (5, 50 and 150 μmol/kg, p.o.), aspirin (5, 50, and 150 μmol/kg, p.o.), or vehicle (p.o.) followed by intraplantar (i.p.) injection of Cg (100 μg/paw). Mechanical hyperalgesia was determined 3 h after Cg injection using electronic von Frey. This time point corresponds to the maximal hyperalgesia produced by Cg. Furthermore, to evaluate the effect of these drugs on long-lasting mechanical inflammatory hyperalgesia, CFA was injected first (10 μL/paw), after 24 h the animals were treated orally with NOSH-aspirin (150 μmol/kg, p.o.), aspirin (150 μmol/kg, p.o.) or vehicle and then mechanical hyperalgesia was evaluated at 1, 3, 5, 7, and 24 h post drugs treatment as described above. As a control we used i.p. injection of saline.
Effect of NOSH-aspirin on hyperalgesia produced by a directly acting hyperalgesic mediator: involvement of KATP
In attempting to verify whether NOSH-aspirin was able to affect hyperalgesia produced by a directly acting mediator, its effect on PGE2-induced hyperalgesia was evaluated. Mice were pretreated 50 min before with aspirin (150 μmol/kg), NOSH-aspirin (150 μmol/kg) or vehicle orally followed by i.p. injection of PGE2 (100 ng/paw). Mechanical hyperalgesia was evaluated 60 min after injection of PGE2, which correspond to the maximal hyperalgesic response produced by PGE2 (Cunha et al. 2008b). To ascertain whether the actions of NOSH-aspirin were mediated through KATP, mice were pretreated with a KATP blocker, glib. (10 mg/kg, i.p.) and after 30 min, they received vehicle or NOSH-aspirin (150 μmol/kg) orally. After 50 min all groups received i.p. injection of PGE2. Mechanical hyperalgesia was evaluated 60 min after injection of PGE2.
Determination of neutrophil accumulation in the inflammatory site
Myeloperoxidase (MPO) activity was used as an index of neutrophil accumulation in the mice’s plantar tissues, based on a kinetic-colorimetric assay as described previously (Bradley et al. 1982). Mice were pretreated with NOSH-aspirin (150 μmol/kg, v.o.), aspirin (150 μmol/kg, v.o.) or vehicle followed by i.p. injection of Cg (100 μg/paw). Plantar tissue was harvested from the injected and control paws (saline). Samples were collected in 50 mmol/L K2HPO4 buffer (pH 6.0) containing 0.5% hexadecyl trimethylammonium bromide and kept at −80°C. Just before the assay, the tissue was homogenized using a Polytron (PT3100) and centrifuged at 13,000 g for 4 min. In our experimental condition (low pH), the MPO assay did not detect the activity of this enzyme in mononuclear cells. To prepare the solution for the analysis, 5 μL of the supernatant was mixed with 200 μL of phosphate buffer (50 mmol/L, pH 6.0), containing 0.167 mg/mL O-dianisidine dihydrochloride and 0.0005% hydrogen peroxide. The solution was analyzed by spectrophotometry for MPO activity determination at 450 nm (Spectra max, Molecular Devices, Sunnyvale CA, USA). The MPO activity was compared with a standard curve of neutrophils obtained from mice blood. The results were presented as number of neutrophils ×106/mg tissue.
Cytokine measurements
Mice were pretreated with NOSH-aspirin (150 μmol/kg, v.o.), aspirin (150 μmol/kg, v.o.) or vehicle orally followed by i.p. injection of Cg (100 μg/paw). Plantar tissue was harvested from the injected and control paws (saline) 3 h after Cg injection. The time for cytokine measurement was defined based on the peak of the production of the analyzed cytokines (Cunha et al. 2005). The samples were triturated and homogenized in 300 μL of the appropriate buffer containing protease inhibitors. The concentrations of the cytokines tumor necrosis factor-α (TNF-α), interleukin-1 beta (IL-1β), and keratinocyte-derived chemokine (KC/CXCL1) were determined as previously described by ELISA (Cunha et al. 2005) using paired antibodies (R&D Systems, Minneapolis, MN, USA). The results are expressed as pg/paw of each cytokine.
Measurement of motor performance
In order to discard possible nonspecific muscle relaxant or sedative effects of NOSH-aspirin, mice motor performance was evaluated on the rota-rod test (Rosland et al. 1990). The apparatus consisted of a bar with a diameter of 2.5 cm, subdivided into six compartments by disks 25 cm in diameter (Model 7600; Ugo Basile, Varese, Italy). The bar rotated at a constant speed of 22 rotations per minute. The animals were selected 24 h previously by eliminating those mice that did not remain on the bar for two consecutive periods of 120 sec. Animals were treated orally with vehicle or NOSH-aspirin (150 μmol/kg, v.o.). Diazepam (5 mg/kg, i.p.) was used as a positive control. The animal’s latency to fall off was measured automatically by a mechanical sensor located in the floor of the apparatus. The cut-off time used was 120 sec.
Statistical analysis
Data are reported as the means ± SEM and are representative of two separate experiments. The letter n in the legends refers to the number of mice used in the experimental group of each experiment. The differences between the experimental groups were compared by one-way analysis of variance (ANOVA), and individual comparisons were subsequently made with Tukey’s post hoc test. Two-way ANOVA was used to compare the groups when the hypernociceptive responses were measured at different times after the stimulus injection. A value of P < 0.05 was considered significant.
Results
NOSH-aspirin reduced acetic acid-induced writhing responses in mice: Comparison with aspirin
Intraperitoneal injection of acetic acid-induced writhing response has been extensively used in the screening of novel analgesic drugs (Collier et al. 1968). Thus, this nociceptive behavior test was initially used to evaluate the possible antinociceptive effect of NOSH-aspirin in comparison to that of aspirin. NOSH-aspirin and aspirin were both effective in inhibiting acetic acid-induced writhing response in a dose-dependent manner (Fig.2). However, whereas only the highest dose of aspirin presented a significant effect, the low and intermediary doses of NOSH-aspirin were significantly effective in inhibiting the writhing responses (Fig.2). Regarding efficacy, at the highest dose tested (450 μmol/kg), inhibition due to aspirin was ∼49%, while in the NOSH-aspirin-treated animals this effect reached ∼59% (Fig.2). Although NOSH-aspirin’s effect was higher than aspirin at this dose no statistical difference (P = 0.089) was observed.
Figure 2.
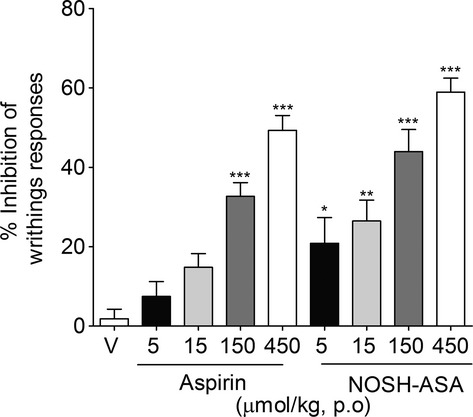
Effect of NOSH-aspirin (NOSH-ASA) and aspirin on acetic acid-induced writhing responses in mice. Animals were pretreated orally (p.o.) with aspirin (5, 15, 150 μmol/kg), NOSH-ASA (5, 15, 150, and 450 μmol/kg) or vehicle (v), 50 min before the intraperitoneal administration of acetic acid (0.8%, i.p.). Writhing responses was assessed during 20 min after acetic acid injection. The graphic represents the percentage of inhibition relative to vehicle. Data are the means ± SEM (n = 7). *P < 0.05, **P < 0.01, and ***P < 0.001 versus vehicle group.
NOSH-aspirin did not produce significant impairment in mice motor coordinance
It is important to note that sedative agents may produce false-positive effects on the acetic acid-induced writhing response test (Lopes et al. 2013). In this context, we used the rota-rod test to evaluate whether the antinociceptive effect of NOSH-aspirin upon acetic acid-induced writhing response might be due to an unspecific effect on motor coordinance. Importantly, NOSH-aspirin (150 μmol/kg, p.o.) did not change locomotor activity in all evaluated times. As a positive control, Diazepam produced significant motor impairment (Fig.3).
Figure 3.

Effect of NOSH-aspirin (NOSH-ASA) on motor coordinance. Mice were treated orally with NOSH-ASA (150 μmol/kg, v.o.), diazepam (5 mg/kg, i.p.) (positive control) or vehicle and were subjected to Rota-rod test. Sections were performed before and 1, 3, and 5 h after treatments. Data are the means ± SEM (n = 6). *P < 0.05, **P < 0.01, and ***P < 0.001 versus vehicle group.
NOSH-aspirin inhibits Cg- and CFA-induced inflammatory hyperalgesia: comparison with aspirin
Further investigating the potential antinociceptive effect of NOSH-aspirin, we next evaluated its effect against Cg-induced acute inflammatory hyperalgesia (Joris et al. 1987). Aspirin and NOSH-aspirin treatments were also able to inhibit Cg-induced inflammatory hyperalgesia (Fig.4A and B). Similar to that observed in the acetic acid model, whereas the intermediary dose of NOSH-aspirin (50 μmol/kg) produced a significant effect, only the higher dose of aspirin was able to reduce inflammatory hyperalgesia (Fig.4C). In terms of efficacy, at 50 μmol/kg, hyperalgesic inhibition due to aspirin was ∼25%; whereas at the same dose, NOSH-aspirin caused a 50% inhibition (Fig.4C). At the highest dose tested (150 μmol/kg), inhibition due to aspirin was ∼52%, while in the NOSH-aspirin-treated animals this effect reached ∼72% (Fig.4C).
Figure 4.
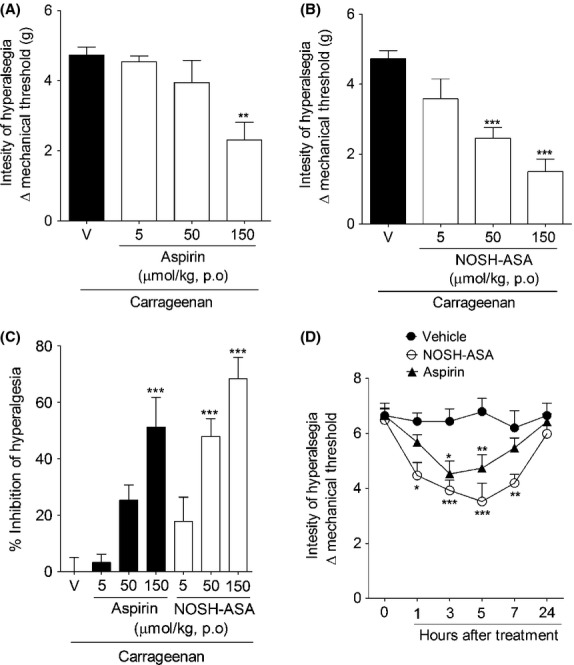
Effect of NOSH-aspirin (NOSH-ASA) and aspirin on carrageenan- and CFA-induced inflammatory hyperalgesia. Mice were pretreated with (A) aspirin (5, 50 and 150 μmol/kg), (B) NOSH-ASA (5, 50, and 150 μmol/kg) or vehicle (v) orally (p.o.) 30 min before the intraplantar injection of carrageenan (Cg; 100 μg/paw). The hypernociceptive responses were evaluated 3 h after carrageenan injection. (C) Percentage of inhibition caused by aspirin and NOSH-ASA (150 μmol/kg) upon carrageenan-induced hyperalgesia. (D) Animals received an injection of 10 μL of CFA in the hind paw. At 24 h after, mechanical nociceptive threshold was evaluated followed by the treated with NOSH-ASA (150 μmol/kg), aspirin (150 μmol/kg) or vehicle (p.o.). Mechanical hyperalgesia was evaluated 1, 3, 5, 7, and 24 h after treatment. Data are the means ± SEM (n = 6). *P < 0.05, **P < 0.01, and ***P < 0.001 versus vehicle-treated group. CFA, complete Freund’s adjuvant.
In a therapeutic perspective, next we evaluated the posttreatment effect of NOSH-aspirin against CFA-induced inflammatory hyperalgesia. At 24 h after i.p. injection of CFA, mechanical hyperalgesia was measured followed by the treatment with vehicle, aspirin and NOSH-aspirin (150 μmol/kg; Fig.4D). CFA-induced inflammatory hyperalgesia was reduced by the treatment with aspirin and NOSH-aspirin. However, the effect produced by NOSH-aspirin was initiated earlier, was greater in magnitude, and long lasting (Fig.4D).
Effect of NOSH-aspirin on Cg-induced neutrophil migration and pronociceptive cytokines production
In order to assess the mechanisms by which NOSH-aspirin further inhibits inflammatory hyperalgesia compared to aspirin, we next compared the effects of NOSH-aspirin and aspirin on Cg-induced neutrophil migration and pronociceptive cytokines production in the plantar tissue. Pretreatment with aspirin or NOSH-aspirin did not affect Cg-induced neutrophil migration in mice (Fig.5A). Although pretreatment with aspirin or NOSH-aspirin did not change the production of TNF-α (Fig.5B) or KC/CXCL1 (Fig.5C), NOSH-aspirin but not aspirin significantly reduced the production of IL-1β (Fig.5D) when compared to the vehicle-treated group. Thus, the enhanced antinociceptive effect promoted by NOSH-aspirin when compared with aspirin may in part be due to its ability to reduce IL-1-β production.
Figure 5.
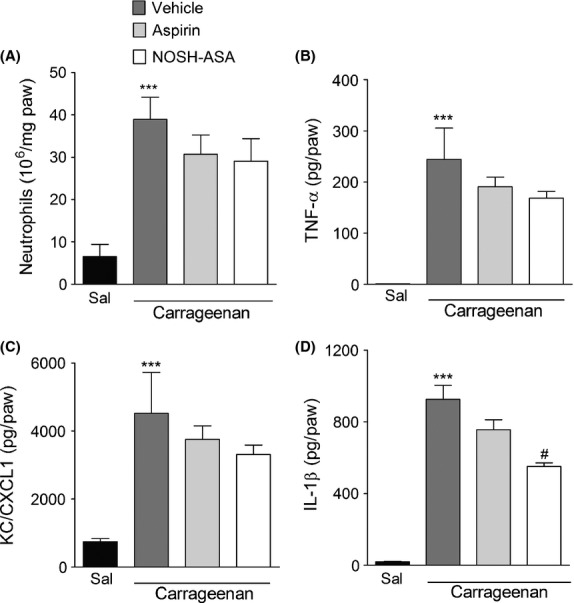
Effect of NOSH-aspirin (NOSH-ASA) and aspirin on carrageenan-induced neutrophil migration and local production of pronociceptive cytokines. Mice were pretreated with aspirin (150 μmol/kg), NOSH-ASA (150 μmol/kg) or vehicle orally 30 min before the intraplantar injection of carrageenan (Cg; 100 μg/paw) or saline. At 3 h after Cg injection plantar tissue were collected for measurement of (A) neutrophil migration (MPO activity assay) and the levels of (B) TNF-α, (C) KC/CXCL1, and (D) IL1-β by ELISA. Data are the means ± SEM (n = 6). ***P < 0.001 versus saline group. #P < 0.05 versus vehicle-treated group. MPO, myeloperoxidase; TNF-α, tumor necrosis factor-α; KC/CXCL1, keratinocyte-derived chemokine.
NOSH-aspirin directly blocks PGE2-induced hyperalgesia: Involvement of KATP channels
NO and H2S are able to reduced inflammatory hyperalgesia by acting directly on neuronal excitability through modulation of KATP channels (Soares et al. 2000; Cunha et al. 2008a). Therefore, in the next step we evaluated whether NOSH-aspirin was able to reduce hyperalgesia produced by a directly acting hyperalgesic mediator, PGE2, and whether this effect was dependent on KATP channels modulation. First, it was observed that pretreatment with NOSH-aspirin, but not with aspirin, was able to reduce PGE2-induced mechanical hyperalgesia (Fig.6A). Furthermore, the antinociceptive effect of NOSH-aspirin upon PGE2-induced hyperalgesia was prevented when mice were treated with a KATP channels blocker (glib.) (Fig.6B). As a control, glibenclaminde alone caused no change in PGE2-induced hyperalgesia (Fig.6B). Therefore, these results further indicate that the additive effects of NOSH-aspirin over aspirin might be partially due to its direct effect on inflammatory hyperalgesia through up modulation of KATP currents.
Figure 6.
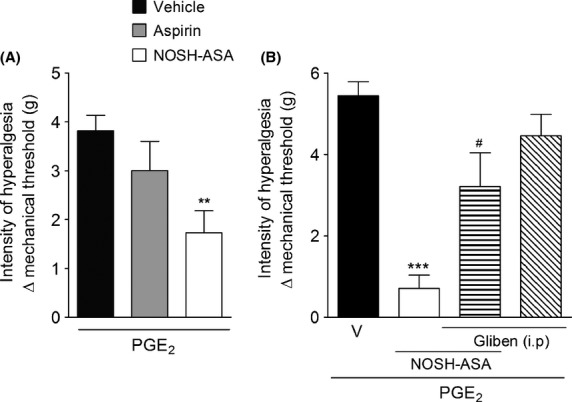
Effect of NOSH-aspirin (NOSH-ASA) and aspirin on PGE2-induced hyperalgesia: involvement of KATP. (A) Mice were pretreated 50 min before with aspirin (150 μmol/kg), NOSH-ASA (150 μmol/kg) or vehicle (v) orally followed by intraplantar (i.p.) injection of PGE2. Mechanical hyperalgesia was evaluated 60 min after PGE2 injection. (B) Mice were pretreated with KATP blocker (glibenclamide; 10 mg/kg, i.p.) and after 30 min, they received vehicle or NOSH-ASA (150 μmol/kg), and after 50 min all groups received i.p. injection of PGE2. Mechanical hyperalgesia was evaluated 60 min after PGE2 injection. Data are the means ± SEM (n = 6). **P < 0.01 and ***P < 0.001 versus vehicle group, #P < 0.001 versus NOSH-ASA-treated group. KATP, ATP-sensitive potassium channel; PGE2, prostaglandin E2.
Discussion
NSAIDs are used to relive the most common forms of pain and in the United States over 30 million people use NSAIDs on a daily basis (American-Gastroenterological-Association 2005). However, their effective pain relief may at times be limited and their long-term use may lead to severe side effects, including GI, cardiovascular, and renal disorders (Ejaz et al. 2004; Patricio et al. 2013). Thus, developing NSAIDs that offer effective pain control with fewer or less serious adverse effects would be a valuable medical advance (Nalamachu et al. 2014). To address these potentially harmful effects, NOSH-aspirin (NBS-1120), a NO- and H2S-releasing hybrid was developed (Kodela et al. 2012). The rational for developing NOSH-aspirin was based on the observations that NO (Wallace and Miller 2000) and H2S (Fiorucci 2009) have some of the same properties as PGs within the gastric mucosa, thus modulating some components of the mucosal defense systems. NOSH-aspirin was shown to have potent anti-inflammatory properties (Kodela et al. 2012) and be devoid of any GI side effects (Nia et al. 2013). Herein, we provide evidence that NOSH-aspirin is a novel therapeutic agent for controlling inflammatory pain. It was more potent than aspirin, its parent compound and presents long-lasting effect, in producing analgesia in different experimental models of inflammatory pain. Moreover, it seems that NOSH-aspirin’s-enhanced antinociceptive effect might be due to its ability to reduce the production of IL-1β and restore neuronal sensitization caused by PGE2 through upregulation of KATP channels, which was not observed with aspirin.
Initially, the acetic acid-induced writhing test was used to investigate the in vivo antinociceptive activity of NOSH-aspirin. This test is a visceral pain model that is widely used to evaluate antinociceptive activity of novel compounds (Morucci et al. 2012). In this model, nociceptive behaviors are generated through direct action of acetic acid on sensory neurons. However, endogenous mediators such as bradykinin, prostaglandins, and cytokines (TNF-α and IL-1β), may indirectly also cause sensory neurons sensitization and activation (Martinez et al. 1999). Oral administration of NOSH-aspirin reduced the number of writhes and its effect was more potent than aspirin. It is important to mention that in high doses there is only a tendency of NOSH-aspirin to be more efficacious that aspirin. The effect could be ascribed to the inhibition of prostaglandins production as previously shown (Chattopadhyay et al. 2012; Kodela et al. 2012). Moreover, NOSH-aspirin-derived NO could also be modulating pain sensitivity. In fact, the antinociceptive effects of several drugs in the acetic acid pain model are mediated thought endogenous production of NO (Staurengo-Ferrari et al. 2013).
In certain situations the analgesic effects of some drugs upon acetic acid-induced writhing can be confused with motor impairment or myorelaxant activities (Lopes et al. 2013). For NOSH-aspirin this is not the case, since it did not cause any alterations in the rota-rod test, which is a classical model to evaluate these unspecific effects (Rosland et al. 1990). Therefore, the antinociception effects of NOSH-aspirin do not appear to be through peripheral neuromuscular blockade or induction of sedation.
We also used Cg- and CFA-induced inflammatory hyperalgesia to assess the antinociceptive effects of NOSH-aspirin. Whereas the paw injection of Cg promotes acute inflammatory hyperalgesia which are very useful for mechanism of action studies, the CFA model is clinically more relevant due to its chronic character (Colpaert 1987). In fact, CFA-induced paw inflammation is accompanied by hyperalgesia, which is robust over several days (Ma and Woolf 1996). Furthermore, CFA-induced inflammatory hyperalgesia may be used to evaluate the pharmacokinetic profile of antinociceptive drugs. In this context, using the CFA-induced hyperalgesia it was possible to ascertain that NOSH-aspirin had an effect when given 24 h after stimulus injection, suggesting a therapeutic effect. In this model the antinociceptive effect of NOSH-aspirin seems to be longer lasting than aspirin.
The mechanisms involved in the sensitization of the primary sensory neurons, and consequently in the establishment of inflammatory hypernociception, may be divided into two phases. The first involves the nonneuronal events: the resident and migratory immune cells produce a vast number of hypernociceptive inflammatory mediators including pronociceptive cytokines TNF-α, IL-1β, and chemokines, nerve growth factor, and kinins, which trigger the release of directly acting hyperalgesic mediators (Verri et al. 2006). These mediators are considered direct acting because they activate their specific receptors on the membrane of primary nociceptive neurons (Cunha et al. 2005). Prostaglandins are known to be direct-acting hyperalgesic mediators. The second phase includes neuronal events: activation of the receptors on primary nociceptive neurons by direct-acting mediators leading to enhanced neuron excitability (Aley and Levine 1999). In order to elucidate the possible mechanisms by which NOSH-aspirin is superior to aspirin in inhibiting inflammatory hyperalgesia, initially the impact of NOSH-aspirin on the production of pronociceptive cytokines and neutrophil migration was determined. Firstly, NOSH-aspirin’s-enhanced antinociceptive effect was not associated with reduction in neutrophil migration. These results might reflect the fact that NSAIDs do not interfere with leukocyte migration, but inhibit inflammatory hyperalgesia (Moncada et al. 1973; Lukkarinen et al. 2006). Furthermore, whereas NO is an important inhibitor of neutrophil migration to inflammatory site, H2S donors seems to enhance neutrophil migration in Cg-induced paw inflammation (Dal Secco et al. 2003; Bhatia et al. 2005; Cunha and Verri 2007). Thus, with NOSH-aspirin there appears to be a balance between pro- and antineutrophil migration factors and in the last instance neutrophil migration is not altered. Nevertheless, it is noteworthy that the effects of NO and H2S upon neutrophil migration is concentration dependent (Bhatia et al. 2005; Distrutti et al. 2006b; Li et al. 2006; Kawabata et al. 2007; Cunha et al. 2008a).
Inflammatory hyperalgesia is mediated by the production of a cascade of pronociceptive cytokines/chemokines, including TNF-α, IL-β, and KC/CXCCL1 (Cunha et al. 2005, 2008b). Further evaluating the mechanisms by which NOSH-aspirin is different to aspirin, their effect upon Cg-induced pronociceptive cytokines release was determined. Interestingly, whereas both did not alter the in vivo production of TNF-α and KC/CXCCL1, NOSH-aspirin, but not aspirin, was able to reduce the production/release of IL-1β. Although, we have previously shown that NaHS, another H2S donor, did not reduce the production of any of these cytokines in the mice paw during Cg-induced paw inflammation (Cunha et al. 2008a), the literature suggests that H2S can modulate pronociceptive cytokines production (Li et al. 2008; Lee et al. 2013). In addition, there is evidence that drugs, which promote peripheral antinociceptive action through increases in NO, modulate the production of IL-1β (Zarpelon et al. 2013). Moreover, NO-releasing NSAIDs reduce the activation of NF-κB, which is an important transcription factor involved in the production of IL-1β (Chattopadhyay et al. 2010; Lee et al. 2013). Thus, the effect of NOSH-aspirin on IL-1β production could be ascribed to the released NO, or even by an additive (or synergistic) effect of both.
The main mechanism by which NSAIDs reduce inflammatory hyperalgesia is inhibiting the production of inflammatory mediators (Ferreira 1972), and preventing the sensitization of primary nociceptive neurons (Ferreira 1972; Moncada et al. 1975). However, there are some analgesics that act on the periphery, thus directly blocking nociceptive neurons sensitization (Ferreira et al. 1991; Duarte et al. 1992). These analgesics have the characteristics of inhibiting PGE2-induced hyperalgesia, suggesting they might be restoring neuronal excitability (Alves et al. 2004; Cunha et al. 2010). This appears to be the case for NOSH-aspirin, because it reduced the production of pronociceptive cytokines and inhibited mechanical hyperalgesia triggered by PGE2. In agreement, NO- and H2S-donors unlike NSAIDs are able to reduce PGE2-induced hyperalgesia (Cunha et al. 2008a, 2010).
The mechanism by which NOSH-aspirin and other H2S-donors inhibit PGE2-induced hyperalgesia seems to be dependent on upregulation of KATP currents in the primary nociceptive neurons (Distrutti et al. 2006a; Cunha et al. 2008a, 2010), leading to the hyperpolarization of primary nociceptive neurons (Cunha et al. 2010). There are also data showing that H2S hyperpolarizes dorsal raphe neurons by activating the ATP-sensitive KATP channels (Moore et al. 2003). Thus, the potent and efficacious effect of NOSH-aspirin against inflammatory pain is due to its ability to prevent and restore nociceptive neurons sensitization. Although our main hypothesis is that NOSH-aspirin is acting peripherally to reduce inflammatory pain, we must also acknowledge that it could be promoting analgesia by acting centrally. In this context, we like to point out a recent study by Distrutti et al. (2010) suggesting that the antinociception effects of H2S in a rodent model of visceral pain was modulated by the transactivation of mu opioid receptors.
From a therapeutic point of view, it should be stressed that NSAIDs can have serious side effects including GI, cardiovascular, and renal (Kashfi 2009). Several studies have shown that H2S and NO are important mediators of gastric mucosal protection (Phillipson et al. 2003; Fiorucci et al. 2006). In agreement with these findings, NOSH-aspirin which releases both NO and H2S (Chattopadhyay et al. 2012; Kodela et al. 2012) has been shown to be safe to the stomach (Nia et al. 2013). It addition to its GI safety, NOSH-aspirin might also prove to have enhanced cardiovascular and renal safety profiles. This is because NO and H2S have protective roles in the cardiovascular and renal system (Wallace et al. 2002; Huledal et al. 2005; Rossoni et al. 2010).
In summary, the present results indicate that NOSH-aspirin offers greater potency than aspirin in reducing inflammatory pain in several clinically relevant models. The enhanced antinociceptive effect of NOSH-aspirin appears to be due to its ability to reduce the production of pronociceptive cytokines such as IL-1β. Furthermore, NOSH-aspirin is also able to reduce hyperalgesia, caused by a directly acting hyperalgesic mediator in a mechanism dependent on modulation of KATP channels. In conclusion, we would like to suggest that NOSH-aspirin represents a prototype of a new class of analgesic drugs with more potent effects than the traditional NSAID, aspirin.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Ieda R. dos Santos, Ana Kátia dos Santos, Giuliana Bertozi, and Sergio R. Rosa.
Glossary
- ANOVA
analysis of variance
- CFA
complete Freund’s adjuvant
- H2S
hydrogen sulfide
- IL-1β
interleukin-1 beta
- KATP
ATP-sensitive potassium channel
- KC/CXCL1
keratinocyte-derived chemokine
- MPO
myeloperoxidase
- NO
nitric oxide
- NSAIDs
nonsteroidal anti-inflammatory drugs
- PGE2
prostaglandin E2
- TNF-α
tumor necrosis factor
Author Contributions
M. D. F., F. Q. C., K. K., and T. M. C. participated in research design. M. D. F. conducted experiments. K. K. contributed new reagents or analytic tools. M. D. F., F. Q. C., and T. M. C. performed data analysis. M. D. F., F. Q. C., K. K., and T. M. C. wrote or contributed to the writing of the manuscript.
Disclosure
The authors have nothing to disclose except for K. K., who has an equity position in Avicenna Pharmaceuticals, Inc. the supplier of NOSH-aspirin (NBS-1120) used in these studies.
References
- Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci. 1999;19:2181–2186. doi: 10.1523/JNEUROSCI.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves DP, Tatsuo MA, Leite R, Duarte ID. Diclofenac-induced peripheral antinociception is associated with ATP-sensitive K+ channels activation. Life Sci. 2004;74:2577–2591. doi: 10.1016/j.lfs.2003.10.012. [DOI] [PubMed] [Google Scholar]
- American Gastroenterological Association. Study Shows Long-term Use Of NSAIDs Causes Severe Intestinal Damage. ScienceDaily. 2005 , January 16). Available at http://www.sciencedaily.com/releases/2005/01/050111123706.htm (accessed September 01, 2014) [Google Scholar]
- Bhatia M, Sidhapuriwala J, Moochhala SM, Moore PK. Hydrogen sulphide is a mediator of carrageenan-induced hindpaw oedema in the rat. Br J Pharmacol. 2005;145:141–144. doi: 10.1038/sj.bjp.0706186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay M, Goswami S, Rodes DB, Kodela R, Velazquez CA, Boring D, et al. NO-releasing NSAIDs suppress NF-kappaB signaling in vitro and in vivo through S-nitrosylation. Cancer Lett. 2010;298:204–211. doi: 10.1016/j.canlet.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay M, Kodela R, Olson KR, Kashfi K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid is a potent inhibitor of colon cancer cell growth in vitro and in a xenograft mouse model. Biochem Biophys Res Commun. 2012;419:523–528. doi: 10.1016/j.bbrc.2012.02.051. [DOI] [PubMed] [Google Scholar]
- Collier HO, Dinneen LC, Johnson CA, Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother. 1968;32:295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpaert FC. Evidence that adjuvant arthritis in the rat is associated with chronic pain. Pain. 1987;28:201–222. doi: 10.1016/0304-3959(87)90117-5. [DOI] [PubMed] [Google Scholar]
- Cunha TM, Verri WA., Jr Hydrogen sulfide, is it a promise analgesic drug or another inflammatory pain mediator? Pain. 2007;130:300–302. doi: 10.1016/j.pain.2007.04.036. ; author reply 302–303. [DOI] [PubMed] [Google Scholar]
- Cunha TM, Verri WA, Jr, Vivancos GG, Moreira IF, Reis S, Parada CA, et al. An electronic pressure-meter nociception paw test for mice. Braz J Med Biol Res. 2004;37:401–407. doi: 10.1590/s0100-879x2004000300018. [DOI] [PubMed] [Google Scholar]
- Cunha TM, Verri WA, Jr, Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci USA. 2005;102:1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha TM, Dal-Secco D, Verri WA, Jr, Guerrero AT, Souza GR, Vieira SM, et al. Dual role of hydrogen sulfide in mechanical inflammatory hypernociception. Eur J Pharmacol. 2008a;590:127–135. doi: 10.1016/j.ejphar.2008.05.048. [DOI] [PubMed] [Google Scholar]
- Cunha TM, Verri WA, Jr, Schivo IR, Napimoga MH, Parada CA, Poole S, et al. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol. 2008b;83:824–832. doi: 10.1189/jlb.0907654. [DOI] [PubMed] [Google Scholar]
- Cunha TM, Roman-Campos D, Lotufo CM, Duarte HL, Souza GR, Verri WA, Jr, et al. Morphine peripheral analgesia depends on activation of the PI3Kgamma/AKT/nNOS/NO/KATP signaling pathway. Proc Natl Acad Sci USA. 2010;107:4442–4447. doi: 10.1073/pnas.0914733107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Secco D, Paron JA, de Oliveira SH, Ferreira SH, Silva JS, Cunha Fde Q. Neutrophil migration in inflammation: nitric oxide inhibits rolling, adhesion and induces apoptosis. Nitric Oxide. 2003;9:153–164. doi: 10.1016/j.niox.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Antonelli E, et al. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J Pharmacol Exp Ther. 2006a;316:325–335. doi: 10.1124/jpet.105.091595. [DOI] [PubMed] [Google Scholar]
- Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Russo G, et al. 5-Amino-2-hydroxybenzoic acid 4-(5-thioxo-5H-[1,2]dithiol-3yl)-phenyl ester (ATB-429), a hydrogen sulfide-releasing derivative of mesalamine, exerts antinociceptive effects in a model of postinflammatory hypersensitivity. J Pharmacol Exp Ther. 2006b;319:447–458. doi: 10.1124/jpet.106.106435. [DOI] [PubMed] [Google Scholar]
- Distrutti E, Cipriani S, Renga B, Mencarelli A, Migliorati M, Cianetti S, et al. Hydrogen sulphide induces micro opioid receptor-dependent analgesia in a rodent model of visceral pain. Mol Pain. 2010;6:36. doi: 10.1186/1744-8069-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte ID, dos Santos IR, Lorenzetti BB, Ferreira SH. Analgesia by direct antagonism of nociceptor sensitization involves the arginine-nitric oxide-cGMP pathway. Eur J Pharmacol. 1992;217:225–227. doi: 10.1016/0014-2999(92)90881-4. [DOI] [PubMed] [Google Scholar]
- Ejaz P, Bhojani K, Joshi VR. NSAIDs and kidney. J Assoc Physicians India. 2004;52:632–640. [PubMed] [Google Scholar]
- Ferreira SH. Prostaglandins, aspirin-like drugs and analgesia. Nat New Biol. 1972;240:200–203. doi: 10.1038/newbio240200a0. [DOI] [PubMed] [Google Scholar]
- Ferreira SH. Peripheral analgesia: mechanism of the analgesic action of aspirin-like drugs and opiate-antagonists. Br J Clin Pharmacol. 1980;10(Suppl. 2):237S–245S. doi: 10.1111/j.1365-2125.1980.tb01806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SH, Vane JR. New aspects of the mode of action of nonsteroid anti-inflammatory drugs. Ann Rev Pharmacol. 1974;14:57–73. [Google Scholar]
- Ferreira SH, Duarte ID, Lorenzetti BB. The molecular mechanism of action of peripheral morphine analgesia: stimulation of the cGMP system via nitric oxide release. Eur J Pharmacol. 1991;201:121–122. doi: 10.1016/0014-2999(91)90333-l. [DOI] [PubMed] [Google Scholar]
- Fiorucci S. Prevention of nonsteroidal anti-inflammatory drug-induced ulcer: looking to the future. Gastroenterol Clin North Am. 2009;38:315–332. doi: 10.1016/j.gtc.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Distrutti E. COXIBs, CINODs and H(2)S-releasing NSAIDs: current perspectives in the development of safer non steroidal anti-inflammatory drugs. Curr Med Chem. 2011;18:3494–3505. doi: 10.2174/092986711796642508. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Distrutti E, Cirino G, Wallace JL. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology. 2006;131:259–271. doi: 10.1053/j.gastro.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Huledal G, Jonzon B, Malmenas M, Hedman A, Andersson LI, Odlind B, et al. Renal effects of the cyclooxygenase-inhibiting nitric oxide donator AZD3582 compared with rofecoxib and naproxen during normal and low sodium intake. Clin Pharmacol Ther. 2005;77:437–450. doi: 10.1016/j.clpt.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Joris JL, Dubner R, Hargreaves KM. Opioid analgesia at peripheral sites: a target for opioids released during stress and inflammation? Anesth Analg. 1987;66:1277–1281. [PubMed] [Google Scholar]
- Kashfi K. Anti-inflammatory agents as cancer therapeutics. Adv Pharmacol. 2009;57:31–89. doi: 10.1016/S1054-3589(08)57002-5. [DOI] [PubMed] [Google Scholar]
- Kashfi K, Olson KR. Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras. Biochem Pharmacol. 2013;85:689–703. doi: 10.1016/j.bcp.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata A, Ishiki T, Nagasawa K, Yoshida S, Maeda Y, Takahashi T, et al. Hydrogen sulfide as a novel nociceptive messenger. Pain. 2007;132:74–81. doi: 10.1016/j.pain.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Kodela R, Chattopadhyay M, Kashfi K. NOSH-aspirin: a novel nitric oxide-hydrogen sulfide-releasing hybrid: a new class of anti-inflammatory pharmaceuticals. ACS Med Chem Lett. 2012;3:257–262. doi: 10.1021/ml300002m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, McGeer E, Kodela R, Kashfi K, McGeer PL. NOSH-aspirin (NBS-1120), a novel nitric oxide and hydrogen sulfide releasing hybrid, attenuates neuroinflammation induced by microglial and astrocytic activation: a new candidate for treatment of neurodegenerative disorders. Glia. 2013;61:1724–1734. doi: 10.1002/glia.22553. [DOI] [PubMed] [Google Scholar]
- Li L, Bhatia M, Moore PK. Hydrogen sulphide–a novel mediator of inflammation? Curr Opin Pharmacol. 2006;6:125–129. doi: 10.1016/j.coph.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Li T, Zhao B, Wang C, Wang H, Liu Z, Li W, et al. Regulatory effects of hydrogen sulfide on IL-6, IL-8 and IL-10 levels in the plasma and pulmonary tissue of rats with acute lung injury. Exp Biol Med (Maywood) 2008;233:1081–1087. doi: 10.3181/0712-RM-354. [DOI] [PubMed] [Google Scholar]
- Lopes SC, da Silva AV, Arruda BR, Morais TC, Rios JB, Trevisan MT, et al. Peripheral antinociceptive action of mangiferin in mouse models of experimental pain: role of endogenous opioids, K(ATP)-channels and adenosine. Pharmacol Biochem Behav. 2013;110:19–26. doi: 10.1016/j.pbb.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Lorenzetti BB, Ferreira SH. Mode of analgesic action of dipyrone: direct antagonism of inflammatory hyperalgesia. Eur J Pharmacol. 1985;114:375–381. doi: 10.1016/0014-2999(85)90383-8. [DOI] [PubMed] [Google Scholar]
- Lorenzetti BB, Ferreira SH. Activation of the arginine-nitric oxide pathway in primary sensory neurons contributes to dipyrone-induced spinal and peripheral analgesia. Inflamm Res. 1996;45:308–311. doi: 10.1007/BF02280997. [DOI] [PubMed] [Google Scholar]
- Lukkarinen H, Laine J, Aho H, Asikainen E, Penttinen P, Kaapa P. Inhibition of COX-2 aggravates neutrophil migration and pneumocyte apoptosis in surfactant-depleted rat lungs. Pediatr Res. 2006;59:412–417. doi: 10.1203/01.pdr.0000200798.79840.3d. [DOI] [PubMed] [Google Scholar]
- Ma QP, Woolf CJ. Progressive tactile hypersensitivity: an inflammation-induced incremental increase in the excitability of the spinal cord. Pain. 1996;67:97–106. doi: 10.1016/0304-3959(96)03105-3. [DOI] [PubMed] [Google Scholar]
- Martinez V, Thakur S, Mogil JS, Tache Y, Mayer EA. Differential effects of chemical and mechanical colonic irritation on behavioral pain response to intraperitoneal acetic acid in mice. Pain. 1999;81:179–186. doi: 10.1016/s0304-3959(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Moncada S, Ferreira SH, Vane JR. Prostaglandins, aspirin-like drugs and the oedema of inflammation. Nature. 1973;246:217–219. doi: 10.1038/246217a0. [DOI] [PubMed] [Google Scholar]
- Moncada S, Ferreira SH, Vane JR. Inhibition of prostaglandin biosynthesis as the mechanism of analgesia of aspirin-like drugs in the dog knee joint. Eur J Pharmacol. 1975;31:250–260. doi: 10.1016/0014-2999(75)90047-3. [DOI] [PubMed] [Google Scholar]
- Moore PK, Bhatia M, Moochhala S. Hydrogen sulfide: from the smell of the past to the mediator of the future? Trends Pharmacol Sci. 2003;24:609–611. doi: 10.1016/j.tips.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Morucci F, Lopez P, Mino J, Ferraro G, Gorzalczany S. Antinociceptive activity of aqueous extract and isolated compounds of Lithrea molleoides. J Ethnopharmacol. 2012;142:401–406. doi: 10.1016/j.jep.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Nalamachu S, Pergolizzi JV, Raffa RB, Lakkireddy DR, Taylor R., Jr Drug-drug interaction between NSAIDS and low-dose aspirin: a focus on cardiovascular and GI toxicity. Expert Opin Drug Saf. 2014;13:903–917. doi: 10.1517/14740338.2014.924924. [DOI] [PubMed] [Google Scholar]
- Nia KV, Kodela R, Chattopadhyay M, Kashfi K. The dual nitric oxide and hydrogen sulfide-releasing nonsteroidal anti-inflammatory drugs, NOSH-aspirin, NOSH- naproxen, and NOSH-sulindac are safe to the stomach and have strong anti-inflammatory, analgesic, antipyretic, anti-platelet, and anti-cancer properties. Gastroenterology. 2013;144:S-596. [Google Scholar]
- Patricio JP, Barbosa JP, Ramos RM, Antunes NF, de Melo PC. Relative cardiovascular and gastrointestinal safety of non-selective non-steroidal anti-inflammatory drugs versus cyclo-oxygenase-2 inhibitors: implications for clinical practice. Clin Drug Investig. 2013;33:167–183. doi: 10.1007/s40261-013-0052-6. [DOI] [PubMed] [Google Scholar]
- Phillipson M, Henriksnas J, Holstad M, Sandler S, Holm L. Inducible nitric oxide synthase is involved in acid-induced gastric hyperemia in rats and mice. Am J Physiol Gastrointest Liver Physiol. 2003;285:G154–G162. doi: 10.1152/ajpgi.00432.2002. [DOI] [PubMed] [Google Scholar]
- Qandil AM. Prodrugs of nonsteroidal anti-inflammatory drugs (NSAIDs), more than meets the eye: a critical review. Int J Mol Sci. 2012;13:17244–17274. doi: 10.3390/ijms131217244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues AR, Duarte ID. The peripheral antinociceptive effect induced by morphine is associated with ATP-sensitive K(+) channels. Br J Pharmacol. 2000;129:110–114. doi: 10.1038/sj.bjp.0703038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosland JH, Hunskaar S, Hole K. Diazepam attenuates morphine antinociception test-dependently in mice. Pharmacol Toxicol. 1990;66:382–386. doi: 10.1111/j.1600-0773.1990.tb00766.x. [DOI] [PubMed] [Google Scholar]
- Rossoni G, Manfredi B, Tazzari V, Sparatore A, Trivulzio S, Del Soldato P, et al. Activity of a new hydrogen sulfide-releasing aspirin (ACS14) on pathological cardiovascular alterations induced by glutathione depletion in rats. Eur J Pharmacol. 2010;648:139–145. doi: 10.1016/j.ejphar.2010.08.039. [DOI] [PubMed] [Google Scholar]
- Sachs D, Cunha FQ, Ferreira SH. Peripheral analgesic blockade of hypernociception: activation of arginine/NO/cGMP/protein kinase G/ATP-sensitive K+ channel pathway. Proc Natl Acad Sci USA. 2004;101:3680–3685. doi: 10.1073/pnas.0308382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer TJ, Kivitz AJ, Lipetz RS, Sanders N, Hee A. Comparison of the COX-inhibiting nitric oxide donator AZD3582 and rofecoxib in treating the signs and symptoms of Osteoarthritis of the knee. Arthritis Rheum. 2005;53:827–837. doi: 10.1002/art.21586. [DOI] [PubMed] [Google Scholar]
- Soares AC, Duarte ID. Dibutyryl-cyclic GMP induces peripheral antinociception via activation of ATP-sensitive K(+) channels in the rat PGE2-induced hyperalgesic paw. Br J Pharmacol. 2001;134:127–131. doi: 10.1038/sj.bjp.0704224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares AC, Leite R, Tatsuo MA, Duarte ID. Activation of ATP-sensitive K(+) channels: mechanism of peripheral antinociceptive action of the nitric oxide donor, sodium nitroprusside. Eur J Pharmacol. 2000;400:67–71. doi: 10.1016/s0014-2999(00)00355-1. [DOI] [PubMed] [Google Scholar]
- Staurengo-Ferrari L, Mizokami SS, Silva JJ, da Silva FO, Sousa EH, da Franca LG, et al. The ruthenium NO donor, [Ru(bpy)2(NO)SO3](PF6), inhibits inflammatory pain: involvement of TRPV1 and cGMP/PKG/ATP-sensitive potassium channel signaling pathway. Pharmacol Biochem Behav. 2013;105:157–165. doi: 10.1016/j.pbb.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Verri WA, Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther. 2006;112:116–138. doi: 10.1016/j.pharmthera.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Miller MJ. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000;119:512–520. doi: 10.1053/gast.2000.9304. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Ignarro LJ, Fiorucci S. Potential cardioprotective actions of no-releasing aspirin. Nat Rev Drug Discov. 2002;1:375–382. doi: 10.1038/nrd794. [DOI] [PubMed] [Google Scholar]
- Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- Zarpelon AC, Souza GR, Cunha TM, Schivo IR, Marchesi M, Casagrande R, et al. The nitroxyl donor, Angeli’s salt, inhibits inflammatory hyperalgesia in rats. Neuropharmacology. 2013;71:1–9. doi: 10.1016/j.neuropharm.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


