Abstract
Nicotinic receptors are not only expressed by excitable tissues, but have been identified in various epithelia. One aim of this study was to investigate the expression of nicotinic receptors and their involvement in the regulation of ion transport across colonic epithelium. Ussing chamber experiments with putative nicotinic agonists and antagonists were performed at rat colon combined with reverse transcription polymerase chain reaction (RT-PCR) detection of nicotinic receptor subunits within the epithelium. Dimethylphenylpiperazinium (DMPP) and nicotine induced a tetrodotoxin-resistant anion secretion leading to an increase in short-circuit current (Isc) across colonic mucosa. The response was suppressed by the nicotinic receptor antagonist hexamethonium. RT-PCR experiments revealed the expression of α2, α4, α5, α6, α7, α10, and β4 nicotinic receptor subunits in colonic epithelium. Choline, the product of acetylcholine hydrolysis, is known for its affinity to several nicotinic receptor subtypes. As a strong acetylcholinesterase activity was found in colonic epithelium, the effect of choline on Isc was examined. Choline induced a concentration-dependent, tetrodotoxin-resistant chloride secretion which was, however, resistant against hexamethonium, but was inhibited by atropine. Experiments with inhibitors of muscarinic M1 and M3 receptors revealed that choline-evoked secretion was mainly due to a stimulation of epithelial M3 receptors. Although choline proved to be only a partial agonist, it concentration-dependently desensitized the response to acetylcholine, suggesting that it might act as a modulator of cholinergically induced anion secretion. Thus the cholinergic regulation of colonic ion transport – up to now solely explained by cholinergic submucosal neurons stimulating epithelial muscarinic receptors – is more complex than previously assumed.
Keywords: Choline, intestinal epithelium, ion transport, muscarinic receptors, nicotinic receptors.
Introduction
Acetylcholine, produced by enteric neurons (e.g., Harrington et al. 2010) as well as by the colonic epithelium (Klapproth et al. 1997; Bader et al. 2014) is a central regulator of many gastrointestinal functions. The actions of this transmitter are mediated either by muscarinic or nicotinic receptors. Muscarinic receptors are metabotropic, G-protein-coupled receptors with five subtypes (M1–M5). The muscarinic receptors M2 and M4 couple to the Giα subunit and thus inhibit the adenylate cyclase, whereas M1, M3, and M5 couple to Gq/11α subunits. The subsequent activation of phospholipase C leads to stimulation of protein kinase C and increased levels of the cytosolic Ca2+ concentration (Hirota and McKay 2006). Muscarinic receptors are widely expressed in the alimentary tract. The M1 subtype has been shown to localize on enteric neurons (Khan et al. 2013), on intestinal glands (Wessler and Kirkpatrick 2008) as well as on colonic epithelial cells (Haberberger et al. 2006; Wessler and Kirkpatrick 2008; Khan et al. 2013), whereas the M3 subtype is localized on the epithelium (Hirota and McKay 2006; Wessler and Kirkpatrick 2008).
In contrast, nicotinic receptors are homo- or heteropentamers enclosing an ion channel, that is, they function as ionotropic receptors. Until now, the following subunits have been identified in vertebrates: 10 α subunits (α1–α10), four β subunits (β1–β4), one γ subunit, one δ subunit, and one ε subunit. They were classified into neuronal-type and muscle-type nicotinic receptors (Schuller 2009). The neuronal nicotinic receptors are either homomers consisting of five identical α7, (α8), or α9 subunits or they are heteropentamers consisting of combinations of α2–α6 or α10 subunits with β2–β4 subunits (Schuller 2009). The muscle nicotinic receptors are comprised of two α1 subunits in combination with β1, γ, and ε in adult skeletal muscle (Kalamida et al. 2007).
However, the expression of nicotinic receptors is not restricted to excitable tissues such as nerves or skeletal muscle, they were also found in epithelia of, for example, placenta (Lips et al. 2005), trachea (Kummer et al. 2008), urinary bladder (Haberberger et al. 2002; Beckel 2005), and skin (for review see Wessler and Kirkpatrick 2008). There is evidence that epithelial nicotinic receptors are involved in tumorgenesis in the respiratory and the gastrointestinal tract (Schuller 2009; Improgo et al. 2013). Although there are hints for the expression of nicotinic receptors in colonic epithelium, there is no study about the distribution of nicotinic receptor subunits in native colonic epithelial cells. Furthermore, it remains unclear whether nicotinic receptors are involved in the regulation of colonic ion transport, one of the fundamental functions of this tissue. Therefore, in this study, we investigated the expression of nicotinic receptor subunits in isolated colonic crypts and the effect on ion secretion of presumed nicotinic agonists across rat distal colon.
Materials and Methods
Animals
Female and male Wistar rats with a body mass of 160–240 g were used. The animals were bred and housed at the Institute of Veterinary Physiology and Biochemistry of the Justus-Liebig-University at an ambient temperature of 22.5°C and air humidity of 50–55% on a 12:12 h light-dark cycle with free access to water and food until the time of the experiment. Animals were stunned by a blow on the head and killed by exsanguination (approved by Regierungspräsidium Giessen, Germany).
Solutions
If not indicated differently (e.g., in ion substitution experiments), all Ussing chamber experiments were carried out in a bathing solution containing (in mmol/L): 107 NaCl, 4.5 KCl, 25 NaHCO3, 1.8 Na2HPO4, 0.2 NaH2PO4, 1.25 CaCl2, 1 MgSO4, and 12.2 glucose. The solution was gassed with 5% (v/v) CO2 and 95% (v/v) O2 at 37°C and had a pH of 7.4 (adjusted by NaHCO3/HCl). For the Cl−-free buffer, NaCl and KCl were equimolarly substituted by Na gluconate (NaGluc) and K gluconate (KGluc), respectively. To obtain a Ca2+-free buffer, CaCl2 was omitted from the buffer without additional administration of a Ca2+-chelating agent.
For crypt isolation, a Ca2+- and Mg2+-free Hanks balanced salt solution containing 10 mmol/L ethylenediaminotetraacetic acid (EDTA) was used. The pH was adjusted to 7.4 by tris(hydroxymethyl)-aminomethane. The isolated crypts were stored in a high potassium Tyrode solution consisting of (in mmol/L): 100 K gluconate, 30 KCl, 20 NaCl, 1.25 CaCl2, 1 MgCl2, 10 HEPES, 12.2 glucose, 5 Na pyruvate, and 1 g/L bovine serum albumin; pH was 7.4 (adjusted by KOH). Tissue was fixed in 100 mmol/L phosphate buffer (pH 7.4) containing 40 g/L paraformaldehyde. For the histochemical staining of acetylcholinesterase activity, a citrate buffer (100 mmol/L, pH 5.0) was used containing (in mmol/L) 2.5 CuSO4, 5 K3[Fe(CN)6], and 1 acetylthiocholine chloride. For the rehydration of the colon sections, a 100 mmol/L sodiumhydrogen maleate buffer (pH 6.0) was used.
Tissue preparation
The distal colon was quickly removed and placed in ice-cold Ussing chamber bathing solution. The colon was mounted on a thin plastic rod. A circular incision was made near the distal end with a blunt scalpel. The serosa and muscularis propria were stripped off to obtain a mucosa–submucosa preparation. This preparation was either directly used for Ussing chamber experiments or for the preparation of the mucosa. For the latter, the mucosa–submucosa was opened along the mesenteric border and placed onto a glass plate. The proximal end of the tissue was clamped with a clip. The distal end of the colon was fixed with another slide. With a sharp glass slide the mucosa was carefully separated from the submucosal layer in order to obtain a mucosa preparation.
Ussing chamber experiments
Either mucosa preparations (for the experiments with nicotine) or mucosa–submucosa preparations (for the experiments with choline) were fixed in a modified Ussing chamber, bathed with a volume of 3.5 mL on each side of the mucosa. The tissue was incubated at 37°C and short-circuited by a computer-controlled voltage-clamp device (Ingenieur Büro für Mess- und Datentechnik Mussler, Aachen, Germany) with correction for solution resistance. Tissue conductance (Gt) was measured every minute by the voltage deviation induced by a current pulse (±50 μA, duration 200 msec) under open-circuit conditions. Short-circuit current (Isc) was continuously recorded. Isc is expressed as μEq/h per cm2, that is, the flux of a monovalent ion per time and area, with 1 μEq/h per cm2 = 26.9 μA/cm2. All drugs were administered to the serosal side of the tissue. The maximal increase in Isc evoked by an agonist is given as difference to the baseline just prior administration of the drug.
Isolation of RNA from intact colonic crypts
The colon was rinsed with Hank’s balanced salt solution. The mucosa–submucosa preparation was fixed on a plastic holder with tissue adhesive and transferred for 6 min to Hank’s-EDTA solution. The tissue was vibrated for 30 sec in order to release crypts. This results in a pure preparation of viable crypts which have been intensively used for electrophysiological or imaging experiments (Schultheiss et al. 2002).
Crypts were collected in a high potassium Tyrode and centrifuged 3 min at 1000 g. Total RNA was isolated from the pellet using the NucleoSpin® RNA kit (Macherey-Nagel, Düren, Germany) according to the manufacturer’s protocol. Contaminating DNA was digested by rDNase (provided with the latter kit).
Reverse transcriptase polymerase chain reaction experiments
RNA was reverse transcribed with Tetro cDNA synthesis kit (Bioline, Luckenwalde, Germany) or with Superscript II cDNA synthesis kit (Life Technologies, Darmstadt, Germany). For subsequent reverse transcription polymerase chain reaction (RT-PCR) with the primer pairs (Eurofins Genomics, Ebersberg, Germany) listed in Table1, MangoMix™ mastermix (Bioline, Luckenwalde, Germany) was used with 2.5 mmol/L MgCl2. Due to higher sensitivity, a touchdown PCR was performed. For α1, α2, α3, α4, α5, α6, α9, α10, β2, and β3 cycling conditions were: after an initial denaturation for 10 min at 95°C, 10 cycles were run with of 95°C for 45 sec, annealing for 45 sec with an initial temperature of 67°C (decreasing by 1°C per cycle), and 72°C for 45 sec. This was followed by 30 cycles of 95°C for 45 sec, 57°C for 45 sec, 72°C for 45 min, with a final extension at 72°C for 7 min. For α7, β1, β4 cycling conditions were: after an initial denaturation for 10 min at 95°C, 10 cycles were run with of 95°C for 45 sec, annealing for 45 sec with an initial temperature of 71°C (decreasing by 1°C per cycle), and 72°C for 45 sec. This was followed by 30 cycles of 95°C for 45 sec, 61°C for 45 sec, 72°C for 45 min, with a final extension at 72°C for 7 min. For M1 and M3 receptors cycling conditions were as follows: after an initial denaturation for 3 min at 95°C, 10 cycles were run with of 95°C for 45 sec, annealing for 45 sec with an initial temperature of 65°C (decreasing by 1°C per cycle), and 72°C for 60 sec. This was followed by 30 cycles of 95°C for 45 sec, 55°C for 45 sec, 72°C for 60 sec, with a final extension at 72°C for 10 min. For an internal control of the PCR reaction, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used. PCR products were visualized after electrophoresis in a 2.5% (w/v) agarose gel (Peqlab, Erlangen, Germany) with GelRed™ (Biotium, Hayward, CA) or Roti®-GelStain (Roth, Karlsruhe, Germany).
Table 1.
Primers used for RT-PCR
| Sequence | Product length | Accession no. | Reference | |
|---|---|---|---|---|
| α1 | ||||
| Forward | AACTTCATGGAGAGCGGAGA | 285 | NM_024485 | Schirmer et al. (2011) |
| Reverse | CAGCTCCACAATGACGAGAA | |||
| α2 | ||||
| Forward | CTTCGGTGAAGGAAGACTGG | 154 | L 10077 | Lips et al. (2005) |
| Reverse | GGAGCCAACATGAAGGACAT | |||
| α3 | ||||
| Forward | GCCAACCTCACAAGAAGCTC | 208 | NM_052805 | Schirmer et al. (2011) |
| Reverse | CCAGGATGAAAACCCAGAGA | |||
| α4 | ||||
| Forward | GACTTTGCAGTCACCCACCT | 197 | M 15682 | Lips et al. (2005) |
| Reverse | CGGCTATGCATGCTCACTAA | |||
| α5 | ||||
| Forward | ACCCTACCAATTTGCAACCA | 146 | J 05231 | Lips et al. (2005) |
| Reverse | GACCCAAAGACCCATTCTGA | |||
| α6 | ||||
| Forward | TGGTGTTAAGGACCCCAAAA | 142 | L 08227 | Lips et al. (2005) |
| Reverse | GCTGCTGGCTTAACCTCTTG | |||
| α7 | ||||
| Forward | GGAGGCTGTACAAGGAGCTG | 446 | L 31619 | Lips et al. (2005) |
| Reverse | ACCCTCCATAGGACCAGGAC | |||
| α10 | ||||
| Forward | TCTGACCTCACAACCCACAA | 168 | AF 196344 | Lips et al. (2005) |
| Reverse | TCCTGTCTCAGCCTCCATGT | |||
| β1 | ||||
| Forward | TCCTAAGCGTGGTGGTCCTC | 151 | NM_012528 | Mikulski et al. (2010) |
| Reverse | TGTGGTTCGGGTAGTTGGTC | |||
| β2 | ||||
| Forward | AGCCTTCTTTGGCTGTGCTC | 116 | NM_019297 | Mikulski et al. (2010) |
| Reverse | GAGCCGTTAGTAGCTGGACGA | |||
| β3 | ||||
| Forward | CACTCTGCGCTTGAAAGGAA | 196 | NM_133597 | Mikulski et al. (2010) |
| Reverse | GCGGACCCATTTCTGGTAAC | |||
| β4 | ||||
| Forward | ATGAAGCGTCCCGGTCTTGAAGTC | 300 | NM_052806 | Liu et al. (2004) |
| Reverse | GGTCATCGCTCTCCAGATGCTGGG | |||
| M1 | ||||
| Forward | GCACAGGCACCCACCAAGCAG | 373 | M16406 | Wei et al. (1994) |
| Reverse | AGAGCAGCAGCAGGCGGAACG | |||
| M3 | ||||
| Forward | GTCTGGCTTGGGTCATCTCCT | 434 | M16409 | Wei et al. (1994) |
| Reverse | GCTGCTGCTGTGGTCTTGGTC | |||
Sequence, expected product length of the amplificate, and gene accession code (http://www.ncbi.nlm.nih.gov) of the primers used. RT-PCR, reverse transcription polymerase chain reaction.
Enzyme-histochemical detection of acetylcholinesterase activity
In order to locate acetylcholinesterase activity in rat colonic epithelium, we used the method of Karnovky and Roots (1964) with slight modifications (Tsuji and Larabi 1983). This method employs acetylthiocholine as a substrate for acetylcholinesterase. The cleavage product of the esterase activity, thiocholine, reduces ferricyanide to ferrocyanide and this in turn is precipitated by Cu2+ as copper ferrocyanide.
Distal colon was fixed with 40 g/L paraformaldehyde in phosphate buffer at 4°C overnight. After washing three times in phosphate buffer, the tissue was embedded in gelatin and cryofixed with N2-cooled isopentane. Sections (about 6 μm thick) were mounted on glass slides coated with gelatin containing chromium(III) potassium sulfate (0.5 g/L). After rehydration in maleate buffer, the sections were incubated with the incubation medium overnight at 4°C. The sections were rinsed in maleate buffer again (four times for 2 min) and subsequently coverslipped. As negative control, other sections were incubated in a medium without acetylthiocholine chloride.
Drugs
Bumetanide and forskolin were dissolved in ethanol (final maximal volume concentration of ethanol 2.5 mL/L). Calmidazolium, darifenacin, J104129 (Tocris, Bristol, UK), and trifluoperazine were dissolved in dimethylsulfoxide (DMSO; final maximal DMSO volume concentration 2.5 mL/L). Tetrodotoxin was dissolved in 2 × 102 mol/L citrate buffer. Acetylcholine chloride, acetylthiocholine chloride, atropine, α-bungarotoxin (Tocris), carbachol, choline, dihydro-β-erythroidine (Tocris), DMPP, hexamethonium, nicotine hydrogen tartrate, pirenzepine, strychnine, and telenzepine were dissolved in distilled water. If not indicated differently, drugs were purchased from Sigma, Taufkirchen, Germany.
Statistics
Results are given as mean ± standard error of the mean (SEM) with the number (n) of investigated tissues. For the comparison of two groups either Student’s t-test or Mann–Whitney-U-test was applied. An F-test decided which test method had to be used. Both paired and unpaired two-tailed Student’s t-tests were applied as appropriate. P < 0.05 was considered to be statistically significant.
Results
RT-PCR detection of nicotinic receptor subunits in rat colonic epithelium
To determine which of the nicotinic receptor subunits are expressed by rat colonic epithelium, RT-PCR was performed using mRNA from isolated crypts, that is, epithelial cells devoid of neuronal contamination, as starting material. Skeletal muscle was used as positive control for α1- and β1-subunit, for all the other subunits spinal cord served as positive control. The following subunits were consistently (in each of n ≥ 3 independent assays for each mRNA) detected in colonic epithelial cells: α2, α4, α5, α6, α7, α10, and β4. The mRNA for β2 was only inconsistently detected in two of five experiments (Fig.1). Not detectable in colonic epithelium, but in control tissues were the α1-, α3-, β1-, and β3-subunits. The α9 subunit was neither detectable in the positive control nor in the colonic epithelium, although different primers from the literature (Glowatzki et al. 1995; Hecker et al. 2009; Mikulski et al. 2010; Schirmer et al. 2011) were tested (see Discussion for possible reasons). Water controls without template did not show any amplificates (Fig.1).
Figure 1.
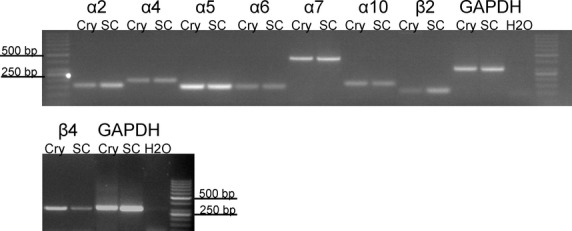
RT-PCR detection of mRNA expression of nicotinic acetylcholine receptor subunits in isolated rat colonic crypts. The agarose gel shows bands of cDNA fragments amplified using primers for α2 (154 bp), α4 (197 bp), α5 (146 bp), α6 (142 bp), α7 (446 bp), α10 (168 bp), β2 (116 bp), and β4 (300 bp). cDNA from spinal cord was used as positive, water instead of cDNA as negative control. The efficiency of RNA isolation and cDNA synthesis was verified by GAPDH-specific primers (303 bp). Each RT-PCR reaction was performed in at least three independent experiments. The mRNA for β2 was only inconsistently detected in two of five experiments, whereas the signals for the other nicotinic receptor subunits depicted here were found consistently. Cry, colonic crypts; SC, spinal cord; RT-PCR, reverse transcription polymerase chain reaction; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Functional evidence for nicotinic receptors in rat colonic epithelium
Ussing chamber experiments were performed with DMPP (10−4 mol/L), a nicotinic receptor agonist (Tapper and Lewand 1981), in order to investigate if there is functional evidence for the expression of nicotinic receptors in colonic epithelium. The experiments were carried out on mucosa preparations of rat distal colon (i.e., in the absence of the myenteric and the submucosal plexus) and in the continuous presence of the neurotoxin tetrodotoxin (10−6 mol/L) in order to prevent effects of DMPP (or nicotine) on enteric neurons of the mucosal plexus, which is still present in these preparations (Bridges et al. 1986). DMPP induced a transient, tetrodotoxin-resistant increase in Isc of 2.87 ± 1.00 μEq/h per cm2 (n = 6; Fig.2A), which was suppressed, when the tissue was pretreated with the nicotinic receptor antagonist hexamethonium (10−5 mol/L, Fig.2B).
Figure 2.
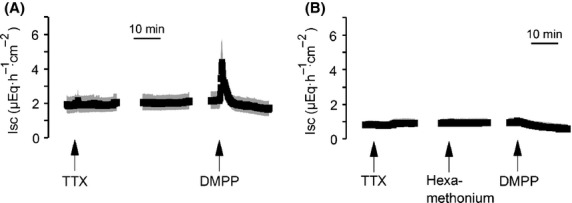
Stimulation of epithelial nicotinic receptors evokes an increase in Isc. Dimethylphenylpiperazinium (DMPP, 10−4 mol/L) induces an increase in Isc in the presence of tetrodotoxin (TTX; 10−6 mol/L) across mucosa preparations from rat distal colon (A), which was suppressed by preincubation with hexamethonium (10−5 mol/L; B). Values are given as means (black symbols) ± SEM (gray area), n = 6. Line interruptions are caused by omission of time intervals in order to synchronize the tracings of individual records to the administration of drugs.
The effect of DMPP was mimicked by nicotine (Fig.3). Nicotine evoked a concentration-dependent increase in Isc. For lower agonist concentrations (<10−4 mol/L), the serosal compartment was washed three times with the fivefold chamber volume before the next concentration was administered. Due to an apparent desensitization of the Isc response evoked by nicotine at higher concentrations, nicotine in concentrations ≥10−4 mol/L was administered only once to the same tissue. A maximal response was evoked at a concentration of 3 × 10−4 mol/L, which declined when the concentration was elevated to 10−3 mol/L probably reflecting desensitization as nicotinic receptors are well known to possess the ability to transform into the desensitized state after agonist binding without prior transition into the activated state (Giniatullin et al. 2005).
Figure 3.
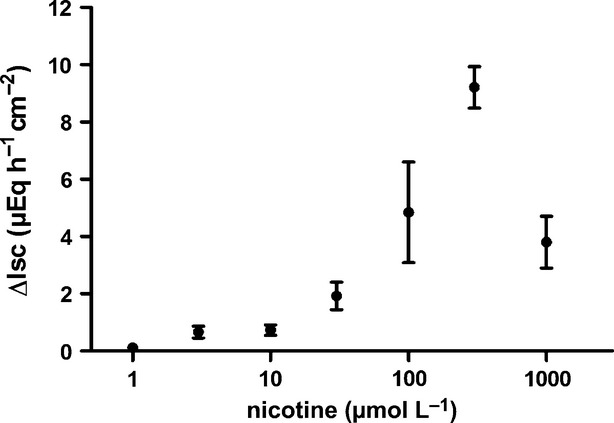
Concentration-dependent increase in Isc across mucosa preparations from rat distal colon induced by serosal administration of nicotine (in the presence of tetrodotoxin). Values are given as increase in Isc (ΔIsc) above baseline just prior administration of the respective nicotine concentration and are means (filled circles) ± SEM (vertical lines), n = 6–8.
Sensitivity of nicotinic receptor subunit-specific inhibitors on the nicotine-induced Isc
In order to further characterize the presumed epithelial nicotinic receptor subunits functionally, the interference of presumed subunit specific inhibitors with the secretory response induced by nicotinic receptor stimulation was tested. Inhibitors used were as follows: α-bungarotoxin, an inhibitor of α7, α8, and α9*-subunits, strychnine, which inhibits α7, α8, α9α10-subunits, dihydro-β-erythroidine, an inhibitor of, for example, α4β2 and α3β2 nicotinic receptors (for references to the inhibitors used, see Chavez-Noriega et al. 1997; Wonnacott and Barik 2007), and atropine, which (in concentrations higher than those needed for muscarinic receptor blockade) has been reported to inhibit, for example, α3β2, α3β4, α4β2, or α4β4 nicotinic receptors (Parker et al. 2003). The preincubation for 20 min with strychnine (10−5 mol/L) and atropine (2.5 × 10−5 mol/L) significantly inhibited the increase in Isc induced by nicotine (10−4 mol/L). In contrast, α-bungarotoxin (10−6 mol/L) or dihydro-β-erythroidine (10−5 mol/L) were ineffective (Fig.4). Even when the tissue was pretreated for 60 min with α-bungarotoxin (10−6 mol/L) to allow a better diffusion of this macromolecule to its presumed action sites, the nicotine-evoked increase in Isc was unaffected, as it amounted to 6.53 ± 1.39 μEq/h per cm2 (n = 6) in the absence and 7.27 ± 1.36 μEq/h per cm2 (n = 6) in the presence of this neurotoxin.
Figure 4.
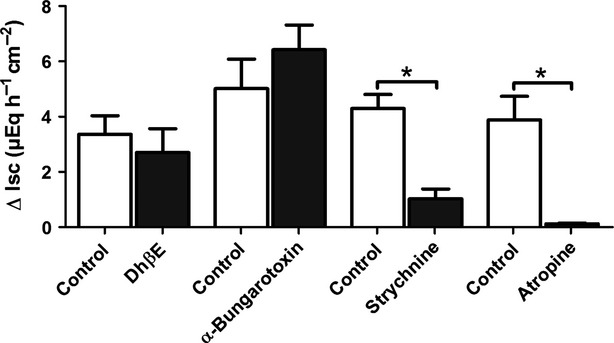
Effects of inhibitors of nicotinic acetylcholine receptor subunits on nicotine-evoked secretion. The Isc induced by nicotine (10−4 mol/L, applied in the presence of tetrodotoxin) across colonic mucosa preparations was tested either under control conditions (open bars) or in the presence of different inhibitors of nicotinic acetylcholine receptor subunits (solid bars). Inhibitors: dihydro-β-erythroidine (DhβE; 10−5 mol/L), α-bungarotoxin (10−6 mol/L), strychnine (10−5 mol/L), or (a high concentration of) atropine (2.5 × 10−5 mol/L). Values are given as increase in Isc (ΔIsc) above baseline just prior administration of nicotine and are means ± SEM; n = 5–6. *P < 0.05 vs. response evoked by nicotine in the respective control series.
Characterization of nicotinic receptor-induced anion secretion
In order to find out if the nicotine-induced Isc is due to Cl− secretion, anion substitution experiments were performed. In the presence of Cl−, nicotine (10−4 mol/L) evoked an increase in Isc of 5.58 ± 0.97 μEq/h per cm2 (n = 8, Fig.5). However, after substitution of Cl− on both sides of the tissue with the impermeable anion gluconate, the increase in Isc was significantly reduced and only amounted to 0.86 ± 0.33 μEq/h per cm2 (P < 0.05 vs. control, n = 8).
Figure 5.
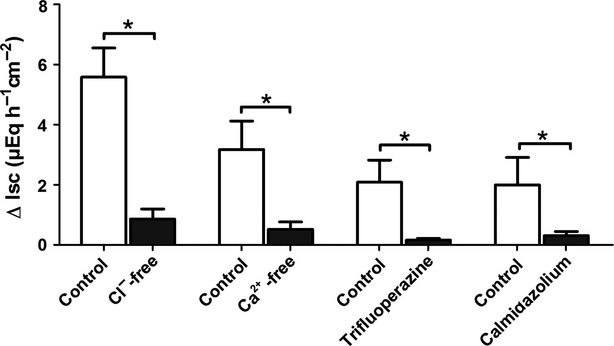
Nicotine induces a Ca2+-dependent Cl− secretion. The Isc induced by nicotine (10−4 mol/L, applied in the presence of tetrodotoxin) across mucosa preparations from rat distal colon was either tested under control conditions (open bars) or after ion substitution or in the presence of different inhibitors of the Ca2+ signaling pathway, respectively (solid bars). For the Cl−-free solution, Cl− was removed from the serosal and the mucosal compartment, for the Ca2+-free solution, Ca2+ was removed from the serosal compartment. Inhibitors: trifluoperazine (5 × 10−5 mol/L) and calmidazolium (5 × 10−7 mol/L). Values are given as increase in Isc (ΔIsc) above baseline just prior administration of nicotine and are means ± SEM; n = 6–10. *P < 0.05 vs. response evoked by nicotine in the respective control series.
Classically, nicotinic receptors function as ionotropic receptors mediating an influx of cations such as Na+ or Ca2+. As an increase in the cytosolic Ca2+ concentration is well known to induce epithelial Cl− secretion (e.g., Diener and Schultheiss 2002), we tested the dependence of the epithelial nicotine response on the presence of serosal Ca2+. In the nominal absence of serosal Ca2+, the tetrodotoxin-resistant Isc evoked by nicotine (10−4 mol/L) was reduced by more than 80% compared to the control group, where nicotine was administered in the presence of Ca2+ (n = 8, P < 0.05 vs. response in the presence of Ca2+, Fig.5). One of the central enzymes involved in Ca2+-signaling cascades is calmodulin. In order to test the involvement of this protein in the nicotine response, two chemically different calmodulin inhibitors were used, that is, trifluoperazine (e.g., Kleene 1994) and calmidazolium (Worell and Frizzell 1991). Both inhibitors significantly reduced the nicotine-evoked increase in Isc by more than 80% (P < 0.05 for both drugs, Fig.5).
Choline induces an increase in Isc in a concentration-dependent manner
Acetylcholinesterase has been detected in mucosal scrapings of the large intestine of different mammals (Sine et al. 1988), and the end product of the esterase reaction, choline, is known to exert affinity for different nicotinic receptors (Alkondon et al. 1997; Alkondon and Albuquerque 2006). In order to test the ability of the colonic epithelium to cleave acetylcholine and thus produce choline, which might potentially act at nicotinic receptors within the tissue, an enzyme-histochemical staining for acetylcholinesterase was performed. As can be seen in Figure6, the colonic epithelium expresses an acetylcholinesterase activity. The reaction product was observed all over the crypt axis, especially in the middle part of the crypts.
Figure 6.
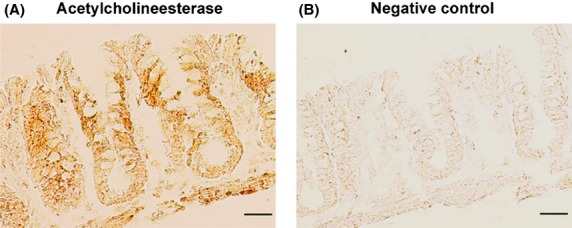
Histochemical detection of acetylcholinesterase activity in rat colonic epithelium. (A) Lower part: muscularis propria; upper part: surface area of the colonic epithelium. For the negative control (B), acetylthiocholine in the incubation medium was omitted. Typical results from three independent experiments; scale bars: 50 μm.
Thus we asked the question whether choline might stimulate nicotinic receptors in the colonic wall. As not only the epithelium (Fig.6) but also enteric nerves (Mestres et al. 1992) express high acetylcholinesterase activity, choline might be produced in close vicinity of enteric neurons, too. Therefore, for the Ussing chamber experiments with choline, a mucosa–submucosa preparation was used with an intact submucosal plexus in order not to overlook possible effects of choline at neuronal nicotinic receptors. Choline (10−3 mol/L to 10−2 mol/L) evoked a concentration-dependent increase in Isc (Fig.7). Because a strong desensitization was observed after repeated administration of the agonist to the same tissue (data not shown), choline was administered only once to the same tissue. Desensitization might also explain why the Isc response declined at the highest concentration tested in the concentration–response curve experiments (Fig.7).
Figure 7.
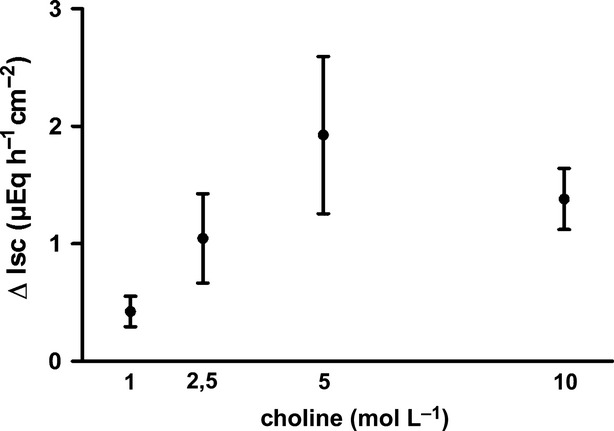
Concentration-dependent increase in Isc across mucosa–submucosa preparations from rat distal colon induced by serosal administration of choline (filled circles, black solid line), Because of a desensitization, each tissue was treated with only one concentration of choline. Values are given as increase in Isc (ΔIsc) above baseline just prior administration of the respective agonist concentration and are means (symbols) ± SEM (vertical lines), n = 6.
Sensitivity of the choline response to tetrodotoxin and cholinergic antagonists
In order to investigate a possible contribution of secretomotor enteric neurons to the increase in Isc induced by choline, the tissue was pre-incubated with tetrodotoxin (10−6 mol/L). However, in the presence of this neurotoxin, choline (7.5 × 10−3 mol/L) induced an increase of 0.36 ± 0.06 μEq/h per cm2 (n = 6), which was not different from the increase in Isc evoked under control conditions, which amounted to 0.38 ± 0.10 μEq/h per cm2 (n = 6). Consequently, choline does not act via stimulation of neuronal cholinergic receptors.
The inhibition of nicotinic receptors with hexamethonium (10−4 mol/L) did not affect the choline-induced Isc (Fig.8). However, atropine, used in a low concentration (10−6 mol/L) that can be expected to selectively inhibit muscarinic (and not nicotinic) receptors, reduced the choline response significantly (P < 0.05 vs. control response in the absence of any inhibitor; n = 6; Fig.8).
Figure 8.
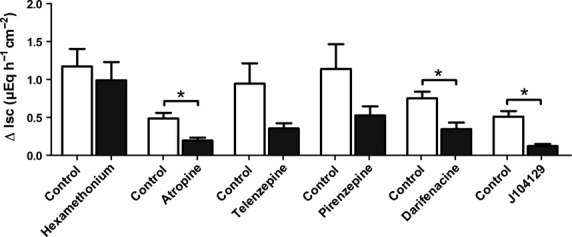
Effects of acetylcholine receptor antagonists on Isc induced by choline across colonic mucosa–submucosa preparations. The response to choline (7.5 × 10−3 mol/L except the initial series with hexamethonium, where a concentration of 5 × 10−3 mol/L was used) was either tested under control conditions (open bars) or in the presence of different inhibitors of acetylcholine receptors (solid bars). Inhibitors: hexamethonium (10−4 mol/L), atropine (10−6 mol/L), telenzepine (10−7 mol/L), pirenezepine (10−6 mol/L), darifenacine (10−6 mol/L), and J104129 (5 × 10−6 mol/L). Values are given as increase in Isc (ΔIsc) above baseline just prior administration of choline and are means ± SEM; n = 6–8. *P < 0.05 vs. response evoked by choline in the respective control series.
In murine colonic epithelium, the two isoforms M1 and M3 of the muscarinic receptors have been described (Haberberger et al. 2006). In order to find out, whether M1 receptors were involved, the effect of choline was tested in the absence or presence of pirenzepine or telenzepine, two M1 muscarinic receptor antagonists (Hammer and Giachetti 1980; Galvan et al. 1989). Telenzepine (10−7 mol/L) tended to reduce the choline-induced Isc as did pirenzepine (10−6 mol/L, Fig.8). However, these effects did not reach statistical significance. A significant inhibition, however, was observed, when the tissue was pretreated with darifenacin or J104129 (Fig.8), which inhibit M3 muscarinic receptors (Mitsuya et al. 1999; Zinner 2007). It can therefore be concluded that the choline-induced secretion is due to an activation of muscarinic receptors, particularly of the M3 subtype. Expression of both M1 and M3 muscarinic receptors in isolated rat colonic crypts was confirmed by RT-PCR experiments (Fig.9).
Figure 9.

RT-PCR detection of mRNA expression of muscarinic M1 and M3 acetylcholine receptor subunits in isolated rat colonic crypts. The agarose gel shows bands of cDNA fragments amplified using specific primers for M1 (373 bp) and M3 receptors (434 bp). cDNA from spinal cord was used as positive control. The efficiency of RNA isolation and cDNA synthesis was verified by using GAPDH-specific primers (303 bp). Representative results from three independent experiments. Cry, colonic crypts; SC, spinal cord; RT-PCR, reverse transcription polymerase chain reaction; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
In rat brain, the low-affinity stimulation of α7 nicotinic receptors by choline is thought to play mainly a modulatory role, as low concentrations of choline lead to a desensitization of the corresponding receptors (Alkondon et al. 1997). In order to investigate, whether a similar desensitization of cholinergic receptors might occur at colonic epithelium, tissues were desensitized to choline by three consecutive administrations of this agonist (10−3 mol/L). Indeed, the Isc evoked by a subsequent administration of acetylcholine (10−3 mol/L) was significantly attenuated compared to an untreated control (Fig.10). A pretreatment with choline reduced not only the amplitude of the Isc response, but also shortened the duration of the response (quantified as increase in Isc above baseline 10 min after administration of acetylcholine) significantly (P < 0.05). The desensitization of the acetylcholine response by choline was concentration-dependent (Table2).
Figure 10.
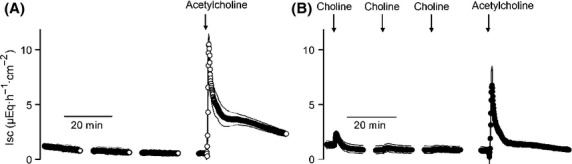
Choline desensitizes the tissue against acetylcholine. Acetylcholine (10−3 mol/L) induces a long-lasting increase in Isc across mucosa–submucosa preparations under control conditions (A), which is desensitized by three prior administrations of choline (10−3 mol/L; B). Values are given as means (symbols) ± SEM (lines), n = 6–7. Line interruptions are caused by omission of time intervals in order to synchronize the tracings of individual records to the administration of drugs. For statistics, see Table2.
Table 2.
Choline desensitizes the tissue against acetylcholine
| Conditions | Acetylcholine-induced increase in Isc | n | |
|---|---|---|---|
| Peak (ΔIsc; μEq/h per cm2) | Plateau (ΔIsc; μEq/h per cm2) | ||
| No choline | 10.96 ± 0.97 | 3.13 ± 0.81 | 6 |
| 10−3 mol/L choline | 6.68 ± 1.56* | 0.58 ± 0.42* | 6 |
| No choline | 9.88 ± 1.68 | 2.40 ± 0.97 | 6 |
| 5 × 10−4 mol/L choline | 7.50 ± 1.39 | 2.34 ± 1.31 | 7 |
| No choline | 8.88 ± 1.73 | 1.47 ± 0.92 | 6 |
| 10−4 mol/L choline | 10.34 ± 1.42 | 2.52 ± 0.80 | 6 |
Increase in Isc evoked by acetylcholine (10−3 mol/L) across mucosa–submucosa preparations from rat distal colon under control conditions (“no choline”) or after pretreatment with three different concentrations of choline (administered three times in intervals of 15 min). Both the maximal increase in Isc (peak) as well as the long-lasting rise in Isc (measured 10 min after administration of acetylcholine) are given. Values are given as increase in Isc above baseline just prior to administration of acetylcholine (ΔIsc) and are means ± SEM.
P < 0.05 vs. response evoked by acetylcholine in the respective control series.
Ionic nature of the Isc response to choline
Replacing Cl− in the buffer solution by the impermeant anion gluconate reduced the Isc evoked by choline (7.5 × 10−3 mol/L) by 75% (n = 7–8, P < 0.05, Table3). A similar inhibition was observed in the presence of bumetanide (10−4 mol/L), an inhibitor of the Na+-K+-2Cl− cotransporter responsible for the basolateral uptake of Cl− during Cl− secretion (n = 7–8, P < 0.05, Table3). Thus the choline-induced Isc is mediated by Cl− secretion.
Table 3.
Choline-induced Isc is carried by Cl− secretion
| Conditions | Choline-induced increase in Isc (ΔIsc; μEq/h per cm2) | n |
|---|---|---|
| With chloride | 1.25 ± 0.30 | 7 |
| Without chloride | 0.30 ± 0.19* | 8 |
| Without bumetanide | 0.64 ± 0.18 | 7 |
| With bumetanide | 0.15 ± 0.03* | 8 |
Chloride dependency of the Isc response evoked by choline (7.5 × 10−3 mol/L) across mucosa–submucosa preparations from rat distal colon. For each experimental series, the response to choline was tested in the presence of bumetanide (10−4 mol/L) or after chloride substitution (at the mucosal and serosal side), respectively, and compared to the response under control conditions. Values are given as increase in Isc above baseline just prior to administration of choline (ΔIsc) and are means ± SEM.
P < 0.05 vs. response evoked by choline in the respective control series.
Discussion
This study shows that nicotinic receptors are expressed in colonic epithelial cells and that a stimulation of these receptors induces an anion secretion across colonic epithelium. The nicotinic agonist DMPP induces a transient increase in Isc across mucosa preparations, that is, preparations devoid of the submucosal and the myenteric plexus. In order to prevent effects of DMPP on enteric neurons of the mucosal plexus, the experiments were carried out in the continuous presence of the neurotoxin tetrodotoxin, suppressing the propagation of action potentials. The response to DMPP was inhibited by a preincubation with hexamethonium (Fig.2) supporting the assumption that there are functional nicotinic receptors on the colonic epithelium. Also another agonist at nicotinic receptors, nicotine itself, induced a concentration-dependent increase in Isc (Fig.3), which was carried by a Ca2+-dependent Cl− secretion (Fig.5). Classically, nicotinic receptors function as ionotropic receptors mediating an influx of cations such as Na+ or Ca2+. An increase in the cytosolic Ca2+ concentration is well known to induce epithelial Cl− secretion (e.g., Diener et al. 1989, Diener and Schultheiss 2002).
The transient time course of secretion induced by nicotinic agonists is in agreement with the effect of other Ca2+-dependent secretagogues. This is not only observed for endogenous agonists such as acetylcholine (Fig.10), which are quickly degraded enzymatically (in this case by the acetylcholine esterase expressed by the epithelium, Fig.6), as also the response to esterase-resistant acetylcholine derivatives (like carbachol) is only transient (Strabel and Diener 1995). As the increase in the cytosolic Ca2+ concentration evoked by Ca2+-dependent secretagogues lasts much longer than the secretory response of the epithelium (Diener et al. 1991), an active downregulation of transporters involved in anion secretion is generally assumed, which might involve release of fatty acids by activation of Ca2+-dependent phospholipase A2, which then inhibits ion channels (Devor and Frizzell 1998), a transactivation of receptors for epithelial growth factor (Keely and Barrett 2003), or internalization of the Na+-K+-Cl− cotransporter (NKCC1) thus reducing basolateral Cl− uptake (Calvo Del Castillo et al. 2005).
The RT-PCR experiments showed the consistent expression of various nicotinic receptor subunits, namely α2, α4, α5, α6, α7, α10, and β4, whereas the β2 subunit transcripts were found only in a part (40%) of the samples (Fig.1). For the α9 subunit, no amplification product was detected neither in the control tissue nor in the colonic epithelium. Mikulski et al. (2010) also described problems with the detection of the α9 subunit in rat alveolar macrophages. They were able to detect the α9 subunit only when using the Superscript II (Life Technologies, Darmstadt, Germany) reverse transcriptase system (for discussion of possible reasons, see Mikulski et al. 2010). However, even with this method to prepare cDNA from isolated crypts (and other tissues such as skin, urothelium or spinal cord), we were unable to detect a transcript (data not shown), which might indicate a low stability of the corresponding mRNA. A proof of these mRNA expression patterns on the protein level was not possible due to the lack of suitable antibodies (e.g., Moser et al. 2007). However, in accordance with our observations at native colonic epithelium, transcripts of various nicotinic receptor subunits (i.e., α4, α5, α7, and β1) have been detected by RT-PCR in the human colonic epithelial tumor cell line HT29 and shown to be involved in cytokine release (Summers et al. 2003) and cell proliferation (Wong et al. 2007).
Theoretically, the expression of the nicotinic receptor subunits found (Fig.1) would enable the formation of several nicotinic receptor subtypes such as homopentameric α7 or heteromers of α2β4, α4β4, and α5α7β4. For investigating the functional significance of the nicotinic receptor subunits expressed, we performed experiments with selective antagonists of different nicotinic receptor subunits. The preincubation of the tissue with dihydro-β-erythroidine, a potent inhibitor of, for example, α4β2 and α3β2 nicotinic receptors (Chavez-Noriega et al. 1997; Wonnacott and Barik 2007), as well as a preincubation with α-bungarotoxin, an inhibitor of α7, α8, and α9* receptors, did not affect the increase in Isc evoked by nicotine (Fig.4). In contrast, the nicotine response was significantly inhibited after a preincubation with the α7, α8, α9/α10 receptor antagonist strychnine or atropine (Fig.4). Atropine, although being the classical antagonist of muscarinic receptors, is known since long to inhibit – at higher concentrations – nicotinic receptors, too (Ambache 1955), an effect, which can also be seen on the level of acetylcholine- or nicotine-evoked inward currents at Xenopus oocytes heterologously expressing human nicotinic receptors (Zwart and Vijverberg 1997). Atropine has been reported to inhibit, for example, α3β2, α3β4, α4β2, or α4β4 receptors (Parker et al. 2003). This might – at first glance – allow different combinations of functional strychnine-sensitive (e.g., α10*) or atropine-sensitive (e.g., α4β4) epithelial nicotinic receptors when combining the inhibitor experiments (Fig.4) with the expression data (Fig.1). However, the observed inhibitor profile differs from classical observations obtained with nicotinic receptor subunit combinations from excitable tissues in two aspects. Usually, strychnine (blocker of α7, α8, α9α10-subunits) and α-bungarotoxin (blocker of α7, α8, and α9*-subunits) have a similar pharmacological profile, but at rat colonic epithelium only strychnine was an effective inhibitor. Furthermore, after heterologous expression of the corresponding human subunits in Xenopus oocytes, the atropine-sensitive heteromer α4β4 is highly sensitive to dihydro-β-erythroidine, which also proved to be without effect at rat colonic epithelium (Fig.4). Whether species differences or variations in the subunit combinations of nicotinic receptors between excitable and nonexcitable cells are responsible for this discrepancy, finally remains open. Furthermore, strychnine might have other effects such as, for example, the inhibition of glycine receptors, where strychnine acts as a classical antagonist (e.g., Grenningloh et al. 1987). Nevertheless, the observation that hexamathonium abolishes the tetrodotoxin-resistant increase Isc evoked by nicotinic agonists (Fig.2) together with the expression data on mRNA level (Fig.1) clearly shows a functional role of epithelial nicotinic receptors, although their subunit stoichiometry remains undefined.
A further unexpected result of this study was the observation that choline has the ability to act as an agonist of epithelial muscarinic receptors and thereby induces a Cl− secretion across the colonic epithelium (Fig.7, Table3). Choline is a cleavage product of the hydrolysis of acetylcholine by acetylcholinesterases. Mucosal intestinal scrapings from various mammals are known to express high cholinesterase activity (Sine et al. 1988). This study confirmed the expression of cholinesterase in rat colonic epithelium by histochemical staining demonstrating high cholinesterase activity all over the crypt axis (Fig.6). Since the histochemical assay was performed without addition of iso-tetraisopropylpyrophosphoramide (OMPA) (due to its high toxicity), a specific inhibitor of the butyrylcholinesterase (Koelle et al. 1973), it is not possible to differentiate if the reaction product is due to an acetylcholinesterase or butyrylcholinesterase activity.
Choline is well known for its affinity to some nicotinic receptors. For example, choline has been described as full agonist of α7 nicotinic receptors in rat neurons and as partial agonist of nicotinic receptors containing α3β4* subunits (Alkondon et al. 1997). In contrast, on nicotinic α4β4* receptors choline acts as an antagonist (Alkondon and Albuquerque 2006). Thus, we had expected that – if choline might be able to induce changes in electrogenic ion transport – the response to choline would be mediated by a stimulation of the nicotinic receptors found in the functional (Figs.4) and RT-PCR (Fig.1) experiments. However, although choline induced a tetrodotoxin-resistant Cl− secretion across rat colonic epithelium, this effect was unaffected by hexamethonium (Fig.8). In contrast, the choline-induced Isc was inhibited by the nonselective muscarinic antagonist atropine (Fig.8). Because M1 as well as M3 muscarinic receptors have been described to be expressed in murine colonic epithelium (Haberberger et al. 2006), the effect of choline was tested in the presence of specific antagonists of M1 or M3 acetylcholine receptors. Inhibition of M3 muscarinic receptors with darifenacin or J104129 significantly inhibited the increase in Isc evoked by choline (Fig.8). Thus choline induces an activation of epithelial muscarinic receptors in rat distal colon resulting in a Cl− secretion. Although choline is well known for its affinity to nicotinic receptors (see above), in the literature at least in one tissue muscarinic effects of choline have been described, that is, the stimulation of muscarinic receptors on atrial myocytes from canine and guinea pig heart (Shi et al. 1999).
The physiological relevance for direct stimulation of muscarinic epithelial receptors by choline, the degradation product of acetylcholine, is probably low. Millimolar concentrations of choline are required to induce an increase in Isc and even at the highest concentration used (10−2 mol/L) only a small secretory response was evoked by this agonist (Fig.7), suggesting that choline might act only as partial agonist. Furthermore, considering, for example, the physiological plasma concentration of choline, which amounts to about 10 μmol/L in humans (Zeisel 2000), it is quite improbable that millimolar concentrations are reached in vivo, although the cleavage of acetylcholine by esterases might transiently result in higher local concentrations of choline. However, for the action of choline at α7 nicotinic receptors in rat brain, a modulatory role of low concentrations of choline has been proposed, as these concentrations lead to a desensitization of the corresponding receptors (Alkondon et al. 1997). A similar desensitization by choline of the acetylcholine-induced Isc was observed in the colonic epithelium (Fig.10), suggesting that in vivo the degradation product of acetylcholine might well be able to modify cholinergic signaling to the epithelium.
Taken together the present experiments reveal a more complex cholinergic regulation of epithelial ion transport in colonic mucosa than previously thought. Acetylcholine, produced both by enteric neurons (Harrington et al. 2010) as well as by the colonic epithelium (Klapproth et al. 1997; Bader et al. 2014) modulates anion secretion both by epithelial muscarinic and by epithelial nicotinic receptors and also its degradation product, choline, is able to modify cholinergic transmission.
Acknowledgments
The diligent technical contributions of B. Brück, E. Haas, B. Schmidt, and A. Stockinger are gratefully acknowledged. This study was supported by the LOEWE research focus “Non-neuronal cholinergic systems”.
Glossary
- DMPP
dimethylphenylpiperazinium
- DMSO
dimethylsulfoxide
- EDTA
ethylenediaminotetraacetic acid
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- Gt
tissue conductance
- Isc
short-circuit current
- RT-PCR
reverse transcription polymerase chain reaction
- TTX
tetrodotoxin
Author Contributions
S. B. performed experiments. S. B. and M. D. conception and design of research, analysis of data, drafting, and editing of the manuscript.
Disclosure
None declared.
References
- Alkondon M, Albuquerque EX. Subtype-specific inhibition of nicotinic acetylcholine receptors by choline: a regulatory pathway. J Pharmacol Exp Ther. 2006;318:268–275. doi: 10.1124/jpet.106.103135. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci. 1997;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Ambache N. The use and limitations of atropine for pharmacological studies on autonomic effectors. Pharmacol Rev. 1955;7:467–494. [PubMed] [Google Scholar]
- Bader S, Klein J, Diener M. Choline acetyltransferase and organic cation transporters are responsible for synthesis and propionate-induced release of acetylcholine in colon epithelium. Eur J Pharmacol. 2014;733:23–33. doi: 10.1016/j.ejphar.2014.03.036. [DOI] [PubMed] [Google Scholar]
- Beckel JM. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am J Physiol Renal Physiol. 2005;290:F103–F110. doi: 10.1152/ajprenal.00098.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RJ, Rack M, Rummel W, Schreiner J. Mucosal plexus and electrolyte transport across the rat colonic mucosa. J Physiol. 1986;376:531–542. doi: 10.1113/jphysiol.1986.sp016168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo Del Castillo I, Fedor-Chaiken M, Song JC, Starlinger V, Yoo J, Matlin KS, et al. Dynamic regulation of Na+-K+-2Cl− cotransporter surface expression by PKC-epsilon in Cl−-secretory epithelia. Am J Physiol Cell Physiol. 2005;289:C1332–C1343. doi: 10.1152/ajpcell.00580.2004. [DOI] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors hα2β2, hα2β4, hα3β2, hα3β4, hα4β2, hα4β4 and hα7 expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;280:346–356. [PubMed] [Google Scholar]
- Devor DC, Frizzell RA. Modulation of K+ channels by arachidonic acid in T84 cells. I. Inhibition of the Ca2+-dependent K+ channel. Am J Physiol. 1998;274:C138–C148. doi: 10.1152/ajpcell.1998.274.1.C138. [DOI] [PubMed] [Google Scholar]
- Diener M, Schultheiss G. Ca2+-signaling in intestinal epithelial cells. Curr Top Pharmacol. 2002;6:163–169. [Google Scholar]
- Diener M, Knobloch SF, Bridges RJ, Keilmann T, Rummel W. Cholinergic-mediated secretion in the rat colon: neuronal and epithelial muscarinic responses. Eur J Pharmacol. 1989;168:219–229. doi: 10.1016/0014-2999(89)90568-2. [DOI] [PubMed] [Google Scholar]
- Diener M, Eglème C, Rummel W. Phospholipase C-induced anion secretion and its interaction with carbachol in the rat colonic mucosa. Eur J Pharmacol. 1991;200:267–276. doi: 10.1016/0014-2999(91)90581-a. [DOI] [PubMed] [Google Scholar]
- Galvan M, Boer R, Schudt C. Interaction of telenzepine with muscarinic receptors in mammalian sympathetic ganglia. Eur J Pharmacol. 1989;167:1–10. doi: 10.1016/0014-2999(89)90741-3. [DOI] [PubMed] [Google Scholar]
- Giniatullin R, Nistri A, Yakel JL. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci. 2005;28:371–378. doi: 10.1016/j.tins.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Wild K, Brandle U, Fakler G, Fakler B, Zenner HP, et al. Cell-specific expression of the alpha9 n-ACh receptor subunit in auditory hair cells revealed by single-cell RT-PCR. Proc R Soc Lond B. 1995;262:141–147. doi: 10.1098/rspb.1995.0188. [DOI] [PubMed] [Google Scholar]
- Grenningloh G, Rienitz A, Schmitt B, Methfessel C, Zensen M, Beyreuther K, et al. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987;328:215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Haberberger RV, Pfeil U, Lips KS, Kummer W. Expression of the high-affinity choline transporter, CHT1, in the neuronal and non-neuronal cholinergic system of human and rat skin. J Invest Dermatol. 2002;119:943–948. doi: 10.1046/j.1523-1747.2002.00182.x. [DOI] [PubMed] [Google Scholar]
- Haberberger R, Schultheiss G, Diener M. Epithelial muscarinic M1 receptors contribute to carbachol-induced ion secretion in mouse colon. Eur J Pharmacol. 2006;530:229–233. doi: 10.1016/j.ejphar.2005.11.055. [DOI] [PubMed] [Google Scholar]
- Hammer R, Giachetti A. Muscarinic receptor subtypes M1 and M2. Biochemical and functional classification. Life Sci. 1980;31:2991–2998. doi: 10.1016/0024-3205(82)90066-2. [DOI] [PubMed] [Google Scholar]
- Harrington AM, Hutson JM, Southwell BR. Cholinergic neurotransmission and muscarinic receptors in the enteric nervous system. Prog Histochem Cytochem. 2010;44:173–202. doi: 10.1016/j.proghi.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Hecker A, Mikulski Z, Lips KS, Pfeil U, Zakrzewicz A, Wilker S, et al. Pivotal advance: Up-regulation of acetylcholine synthesis and paracrine cholinergic signaling in intravascular transplant leukocytes during rejection of rat renal allografts. J Leukoc Biol. 2009;86:13–22. doi: 10.1189/jlb.1107722. [DOI] [PubMed] [Google Scholar]
- Hirota CL, McKay DM. Cholinergic regulation of epithelial ion transport in the mammalian intestine. Br J Pharmacol. 2006;149:463–479. doi: 10.1038/sj.bjp.0706889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Improgo MR, Soll LG, Tapper AR, Gardner PD. Nicotinic acetylcholine receptors mediate lung cancer growth. Front Physiol. 2013;4:251. doi: 10.3389/fphys.2013.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamida D, Poulas K, Avramopoulou V, Fostieri E, Lagoumintzis G, Lazaridis K, et al. Muscle and neuronal nicotinic acetylcholine receptors. FEBS J. 2007;274:3799–3845. doi: 10.1111/j.1742-4658.2007.05935.x. [DOI] [PubMed] [Google Scholar]
- Karnovky MJ, Roots L. A “direct-coloring” thiocholine method for cholineesterases. J Histochem Cytochem. 1964;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Keely SJ, Barrett KE. p38 mitogen-activated protein kinase inhibits calcium-dependent chloride secretion in T84 colonic epithelial cells. Am J Physiol Cell Physiol. 2003;284:C339–C348. doi: 10.1152/ajpcell.00144.2002. [DOI] [PubMed] [Google Scholar]
- Khan RI, Anisuzzaman AS, Semba S, Ma Y, Uwada J, Hayashi H, et al. M1 is a major subtype of muscarinic acetylcholine receptors on mouse colonic epithelial cells. J Gastroenterol. 2013;48:885–896. doi: 10.1007/s00535-012-0718-5. [DOI] [PubMed] [Google Scholar]
- Klapproth H, Reinheimer T, Metzen J, Munch M, Bittinger F, Kirkpatrick CJ, et al. Non-neuronal acetylcholine, a signalling molecule synthezised by surface cells of rat and man. Naunyn-Schmiedeberg’s Arch Pharmacol. 1997;355:515–523. doi: 10.1007/pl00004977. [DOI] [PubMed] [Google Scholar]
- Kleene SJ. Inhibition of olfactory cyclic nucleotide-activated current by calmodulin antagonists. Br J Pharmacol. 1994;111:469–472. doi: 10.1111/j.1476-5381.1994.tb14760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle GB, Davis R, Diliberto EJ, Koelle WA. Selective, near-total, irreversible inactivation of peripheral pseudocholin-esterase and acetylcholinesterase in cats in vivo. Biochem Pharmacol. 1973;23:175–188. doi: 10.1016/0006-2952(74)90408-0. [DOI] [PubMed] [Google Scholar]
- Kummer W, Lips KS, Pfeil U. The epithelial cholinergic system of the airways. Histochem Cell Biol. 2008;130:219–234. doi: 10.1007/s00418-008-0455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips KS, Brüggmann D, Pfeil U, Vollerthun R, Grando SA, Kummer W. Nicotinic acetylcholine receptors in rat and human placenta. Placenta. 2005;26:735–746. doi: 10.1016/j.placenta.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Liu R, Mizuta M, Matsukura S. The expression and functional role of nicotinic acetylcholine receptors in rat adipocytes. J Pharmacol Exp Ther. 2004;310:52–58. doi: 10.1124/jpet.103.065037. [DOI] [PubMed] [Google Scholar]
- Mestres M, Diener M, Rummel W. Histo- and immunocytochemical characterization of the neurons of the mucosal plexus in the rat colon. Acta Anat. 1992;143:268–274. doi: 10.1159/000147261. [DOI] [PubMed] [Google Scholar]
- Mikulski Z, Hartmann P, Jositsch G, Zaslona Z, Lips KS, Pfeil U, et al. Nicotinic receptors on rat alveolar macrophages dampen ATP-induced increase in cytosolic calcium concentration. Respir Res. 2010;11:133. doi: 10.1186/1465-9921-11-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuya M, Mase T, Tsuchiya Y, Kawakami K, Hattori H, Kobayashi K, et al. J-104129, a novel muscarinic M3 receptor antagonist with high selectivity for M3 over M2 receptors. Bioorg Med Chem. 1999;7:2555–2567. doi: 10.1016/s0968-0896(99)00177-7. [DOI] [PubMed] [Google Scholar]
- Moser N, Mechawar N, Jones I, Gochberg-Sarver A, Orr-Urtreger A, Plomann M, et al. Evaluating the suitability of nicotinic acetylcholine receptor antibodies for standard immunodetection procedures. J Neurochem. 2007;102:479–492. doi: 10.1111/j.1471-4159.2007.04498.x. [DOI] [PubMed] [Google Scholar]
- Parker JC, Sarkar D, Quick MW, Lester RAJ. Interactions of atropine with heterologously expressed and native α3 subunit-containing nicotinic acetylcholine receptors. Br J Pharmacol. 2003;138:801–810. doi: 10.1038/sj.bjp.0705124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer SU, Eckhardt I, Lau H, Klein J, DeGraaf YC, Lips KS, et al. The cholinergic system in rat testis is of non-neuronal origin. Reproduction. 2011;14:157–166. doi: 10.1530/REP-10-0302. [DOI] [PubMed] [Google Scholar]
- Schuller HM. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat Rev Cancer. 2009;9:195–205. doi: 10.1038/nrc2590. [DOI] [PubMed] [Google Scholar]
- Schultheiss G, Kocks LS, Diener M. Methods for the study of ionic currents and Ca2+-signals in isolated colonic crypts. Biol Proced Online. 2002;3:70–78. doi: 10.1251/bpo25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Wang H, Lu Y, Yang B, Wang Z. Choline modulates cardiac membrane repolarization by activating an M3 muscarinic receptor and its coupled K+ channel. J Membr Biol. 1999;169:55–64. doi: 10.1007/pl00005901. [DOI] [PubMed] [Google Scholar]
- Sine JP, Ferrand R, Colas B. Acetylcholinesterase and butyrylcholinesterase in the gut mucosal cells of various mammal species: distribution along the intestine and molecular forms. Comp Biochem Physiol C. 1988;91:597–602. doi: 10.1016/0742-8413(88)90084-9. [DOI] [PubMed] [Google Scholar]
- Strabel D, Diener M. Evidence against direct activation of chloride secretion by carbachol in the rat distal colon. Eur J Pharmacol. 1995;274:181–191. doi: 10.1016/0014-2999(94)00728-p. [DOI] [PubMed] [Google Scholar]
- Summers AE, Whelan CJ, Parsons ME. Nicotinic acetylcholine receptor subunits and receptor activity in the epithelial cell line HT29. Life Sci. 2003;72:2091–2094. doi: 10.1016/s0024-3205(03)00089-4. [DOI] [PubMed] [Google Scholar]
- Tapper EJ, Lewand DL. Actions of a nicotinic agonist, DMPP, on intestinal ion transport in vitro. Life Sci. 1981;28:155–162. doi: 10.1016/0024-3205(81)90547-6. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Larabi Y. A modification of thiocholine-ferricyanide method of Karnovsky and Roots for localization of acetylcholinesterase activity without interference by Koelle’s copper thiocholine iodide precipitate. Histochemistry. 1983;78:317–323. doi: 10.1007/BF00496619. [DOI] [PubMed] [Google Scholar]
- Wei J, Walton EA, Milici A, Buccafusco JJ. m1–m5 Muscarinic receptor distribution in rat CNS by RT-PCR and HPLC. J Neurochem. 1994;63:815–851. doi: 10.1046/j.1471-4159.1994.63030815.x. [DOI] [PubMed] [Google Scholar]
- Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154:1558–1571. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HPS, Yu L, Lam EKY, Tai EKK, Wu WKK, Cho CH. Nicotine promotes cell proliferation via α7-nicotinic acetylcholine receptor and catecholamine-synthesizing enzymes-mediated pathway in human colon adenocarcinoma HT-29 cells. Toxicol Appl Pharmacol. 2007;221:261–267. doi: 10.1016/j.taap.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Barik J. Nicotinic ACh receptors. Tocris Rev. 2007;28:1–20. [Google Scholar]
- Worell RT, Frizzell RA. CaMKII mediates stimulation of chloride conductance by calcium in T84 cells. Am J Physiol. 1991;260:C877–C882. doi: 10.1152/ajpcell.1991.260.4.C877. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. Choline: An essential nutrient for humans. Nutrition. 2000;16:669–671. doi: 10.1016/s0899-9007(00)00349-x. [DOI] [PubMed] [Google Scholar]
- Zinner N. Darifenacin: a muscarinic M3-selective receptor antagonist for the treatment of overactive bladder. Exp Opin Pharmacother. 2007;8:511–523. doi: 10.1517/14656566.8.4.511. [DOI] [PubMed] [Google Scholar]
- Zwart R, Vijverberg HPM. Potentiation and inhibition of neuronal nicotinic receptors by atropine: Competitive and noncompetitive effects. Mol Pharmacol. 1997;52:886–895. doi: 10.1124/mol.52.5.886. [DOI] [PubMed] [Google Scholar]


