Abstract
Small molecule inhibitors of α2β1 integrin, a major cellular collagen receptor, have been reported to inhibit platelet function, kidney injury, and angiogenesis. Since α2β1 integrin is abundantly expressed on various inflammation-associated cells, we tested whether recently developed α2β1 blocking sulfonamides have anti-inflammatory properties. Integrin α2β1 inhibitors were shown to reduce the signs of inflammation in arachidonic acid-induced ear edema, PAF stimulated air pouch, ovalbumin-induced skin hypersensitivity, adjuvant arthritis, and collagen-induced arthritis. Thus, these sulfonamides are potential drugs for acute and allergic inflammation, hypersensitivity, and arthritis. One sulfonamide with potent anti-inflammatory activity has previously been reported to be selective for activated integrins, but not to inhibit platelet function. Thus, the experiments also revealed fundamental differences in the action of nonactivated and activated α2β1 integrins in inflammation when compared to thrombosis.
Keywords: Cell adhesion, collagen, inflammation, integrin, sulfonamide
Introduction
The members of the integrin family mediate adhesion to extracellular matrix, cell–cell adhesion and the binding of platelets and inflammatory cells to plasma proteins. Platelet fibrinogen receptor, αIIbβ3 integrin, and leukocyte integrins α4β1/β7 and αLβ2 have been targets for intensive and successful drug development (Goodman and Picard 2012). The collagen receptors form a special subgroup among the integrin family (Heino 2007). These heterodimeric receptors share a common β1 subunit in complex with α1, α2, α10, or α11 subunit. The α subunits bind to collagens and other ligands via their αI (also called αA) domains (Emsley et al. 2000).
Integrin α2β1 is expressed on platelets, epithelial cells and large variety of mesenchymal cells, including endothelial cells (Zutter and Santoro 1990). Genetic mouse models have indicated that the deficiency of α2β1 leads to impaired platelet binding to collagen (Chen et al. 2002; Holtkötter et al. 2002), accelerated neoangiogenesis (Grenache et al. 2007; Zweers et al. 2007; Zhang et al. 2008) and defects in innate immunity (McCall-Culbreath et al. 2008). Integrin α2β1 has also been shown to regulate collagen synthesis in vitro (Ivaska et al. 1999) and in vivo (Borza et al. 2012). This collagen receptor was originally named as very late activation antigen 2 (VLA-2) because its expression started on T lymphocytes only several days after activation (Hemler et al. 1985). The fact that α2β1 is a dominating collagen receptor on neutrophils (Werr et al. 2000), mast cells (Edelson et al. 2004) and Th17 lymphocytes (Boisvert et al. 2010) may explain its role during infection and inflammation. New observations have also indicated that α2β1 is a marker for type 1 T regulatory cells, that have an essential role in promoting and maintaining tolerance (Gagliani et al. 2013). Furthermore, in transmigration from blood to bone marrow α2β1 is essential for CD4+ memory T-cell precursors (Hanazawa et al. 2013). In addition to various collagen subtypes, α2β1 can mediate cell adhesion to numerous other extracellular matrix proteins, such as tenascin, laminins, and proteoglycans (Heino, 2007). Moreover, integrin α2β1 can also recognize collectin family members (Zutter and Edelson 2007).
Integrin α2β1 has successfully been used as a target molecule in development of direct and allosteric inhibitors (Choi et al. 2007; Käpylä et al. 2007; Nissinen et al. 2010; Momic et al. 2015) as well as inhibitors of α2β1 synthesis (Mita et al. 2011). Inhibitors of α2β1 function and expression are generally considered as potential drugs for many human diseases. Animal models support this opinion, since specific monoclonal antibodies can inhibit cancer-related angiogenesis (Senger et al. 1997), contact hypersensitivity reaction (de Fougerolles et al. 2000), experimental colitis (Gillberg et al. 2013) and collagen-induced arthritis (El Azreq et al. 2013). A monoclonal antibody against α2β1 integrin (Vatelizumab; GBR 500) is in Phase II clinical trials for acute relapse in multiple sclerosis and inflammatory bowel disease (www.glenmarkpharma.com).
There are still several open questions related to the role of α2β1 integrin in inflammatory diseases and the usefulness of small molecule inhibitors as drug molecules. In animal models α2β1 antibodies have shown anti-inflammatory effects (Senger et al. 1997; de Fougerolles et al. 2000; El Azreq et al. 2013; Gillberg et al. 2013), but often the influence of the blocking antibodies has been moderate and only simultaneous blocking of both α1β1 and α2β1 has given more remarkable effects (Senger et al. 1997; de Fougerolles et al. 2000). An important unanswered question is, which α2β1 conformation should be targeted in drug development? The three major activation states of integrins are considered to represent (i) bent, αI domain closed conformation, (ii) extended, αI domain closed conformation, and (iii) extended, αI domain open conformation (Luo et al. 2007). Our results with blood platelets indicate that most effective inhibitors are the ones that actually recognize the nonactivated conformation (Nissinen et al. 2012).
We have previously described two sulfonamide-type molecules that both inhibit α2β1 function in vitro by about similar EC50 value (Nissinen et al. 2012). However, the two molecules have different binding mechanism to α2β1 integrin and they also have shown selectivity in the recognition of different α2β1 conformations (Nissinen et al. 2012). Here, we have tested three sulfonamides in different in vivo models of inflammation. Our results indicate that blocking of α2β1 function is an effective strategy to suppress inflammation. Furthermore, the ability to block the nonactivated α2β1 integrin is not critical in inflammation-associated cellular mechanism as it is on platelets. Thus, α2β1 integrin may function in a fundamentally different manner in inflammation when compared to thrombosis.
Materials and Methods
Ethics statement
The animal experiments were approved by the Committee on Animal Care and Use of the Free State of Saxony and carried out following the German Law on the Protection of Animals. Local ethical guidelines for experimental investigations in animals used in the rat adjuvant arthritis model were followed and the regional ethical committee of Szeged University, Hungary, approved the experimental protocol.
Drugs
BTT-3016, BTT-3033, and BTT-3034 were obtained from Pharmatory Oy (Oulu, Finland), dexamethasone and Diclofenac-Na were purchased from Fluka Chemie (Buchs, Switzerland) and Sigma (Budapest, Hungary), respectively. The α2-antibody (Hal/29) was obtained from BD Biosciences Pharmingen (San Diego, CA). No significant adverse effects for thromboxane A2 or cyclooxygenase COX-1 were detected for BTT-3016, BTT-3033, and BTT-3034 (MDS Pharma Services, Taipei, Taiwan).
Animals
Depending on the experimental model, different mice strains were used: NMRI mice, BALB/cAnNCrl (both from Charles River, Sulzfeld, Germany), and DBA/1JNCrlj (Janvier Labs, Le Genest St. Isle, France). They were housed in groups of five to six under standard conditions on a 12 h light/dark cycle (light on at 06:00 h) with ad libitum access to water and food (ssniff M/R 15, Spezialdiäten GmbH, Soest, Germany). Guinea pigs obtained from Charles River were housed in groups of four under standard conditions with ad libitum access to food and water (ssniff MS-H 4 mm, hey cobs 15 mm, Spezialdiäten GmbH, Germany). The Lewis rats used in the adjuvant arthritis were obtained from Charles River (Budapest, Hungary). They were housed in groups of five animals in plastic cages (Macrolon 2) in the same room under temperature and light-controlled conditions at the rodent facility. Rats were maintained on a standard pellet rodent diet (Bioplan Ltd, Isaszeg, Hungary) with tap water available ad libitum. The number of animals that were used in each experiment is given in the method description.
Drug administration
For oral administration BTT-3016 was suspended in Tween 80/0.5% hydroxyethylcellulose (2/98 v/v). BTT-3033 and BTT-3034 were dissolved in PEG 400/30% hydroxypropyl-β-cyclodextrin (HP-β-CDX) or 30% captisol (80/20 v/v). Dexamethasone was suspended in PEG 300/0.5% hydroxyethylcellulose (10/90 v/v). Diclofenac-Na was prepared in 0.25% of methylcellulose (MC) each day. All compounds were administered orally with an administration volume of 10 mL/kg, 5 mL/kg and 2 mL/kg in mice, rats and guinea pigs, respectively. Except, BTT-3016, which was administered using dosing volume of 10 mL/kg in rats. The monoclonal antibody Hal/29 was directly administered into air pouch. Pretreatment time was different depending on the different experimental settings.
PAF-stimulated air pouch model
To induce an air pouch, a volume of 5 mL of sterile filtered air was injected subcutaneously under the back skin of anesthetized female NMRI mice (22.5–31.3 g; Charles River) on day 1. The procedure was repeated on day 3 and day 6 (2.5 mL of air into the existing pouch). On day 7 inflammation was induced by application of 0.7 mL of a 10−6 mol/L/mL platelet-activating factor (PAF, Sigma Aldrich, Deisenhofen, Germany) in Phosphate buffered saline, pH 7.4 (PBS) into the pouch. About 6 h after PAF stimulation the animals were euthanized and the air pouches were lavaged with 1 mL ice-cold PBS. The number of leukocytes in the lavage was counted under microscope. Test or reference compounds or the corresponding vehicle were administered orally 24 h and 2 h prior to application of the inflammatory stimulants (group size: n = 8).
Arachidonic acid-induced ear edema model
Before the experiment the basal thickness of both ears of each female NMRI mouse (23.2–29.3 g; Charles River) was measured (group size: n = 6). The inflammatory response was induced by administration of 20 μL arachidonic acid (Sigma Aldrich) solution (164 mmol/L in acetone) on the left ears. The right ears were treated with the same volume of acetone and served as a negative control. 60 min after the arachidonic acid challenge ear thickness was measured again. Test or reference compound and the corresponding vehicle were given once daily 48 h, 24 h and 3 h before the challenge with arachidonic acid, respectively. Formulation used has no effect when compared the sham control (data not shown).
Mouse skin hypersensitivity model
Female BALB/cAnNCrl mice (16.5–22.2 g; Charles River) were sensitized subcutaneously twice with 0.4 mL of saline containing 100 μg ovalbumin (grade VI; Sigma Aldrich) and 3.3 mg Al(OH)3 (Sigma Aldrich) on day 1 and day 8. Starting on day 13, mice were treated with test or reference compound and the corresponding vehicle 48 h, 24 h, and 2 h before ovalbumin challenge (group size: n = 10). On day 15 basic thickness of both ears of each mouse was measured. After that the right ear was treated with 10 μL PBS, while the left one was treated with 10 μL of 1% ovalbumin solution, both intradermally. About 24 h and 48 h later ear thickness was measured again.
Guinea pig skin allergic inflammation model
At two consecutive days male HA guinea pigs (387.9–514.4 g; Charles River) were sensitized by intraperitoneal injections of 20 μg ovalbumin (grade VI; Sigma Aldrich) and 20 mg Al(OH)3 (Sigma Aldrich). Fourteen days later the guinea pigs were challenged with 50 μL of a 0.1% ovalbumin solution in four different locations under the back skin. The response was measured by determining the diameter of the allergic wheal that developed 60 min after the challenge. Based on the data nonresponders were excluded from further testing. The responders were allowed to rest for 7 days. Prior to second antigen challenge the guinea pigs were treated orally with the test or reference compound or the corresponding vehicle once daily for 3 consecutive days (group size: n = 6). Last treatment was given 2 h prior to second antigen challenge. Each location was then challenged by intradermal injection of 50 μl of 0.05% OVA solution. Sixty minutes after antigen challenge, the area of the allergic wheals was measured as described above.
Arthritis models
In adjuvant arthritis model male Lewis inbred rats (157–186 g, age 6–7 weeks; Charles River) were injected intradermally, 2 cm from the base of the tail with 0.5 mg of killed Mycobacterium butyricum (Difco, BD Biosciences, San Jose, CA) in 0.1 mL of liquid paraffin. The severity of the disease was determined prior to adjuvant injection and then on days 4, 7, 11, 14, 18, 21, 25, and 28 by scoring by two independent examiners. On days 0, 10, 21, and 28 the volume of both left and right hind paws was measured.
In collagen-induced arthritis model 150 μL of emulsion containing in 2:1 ratio bovine type II collagen solution (MD Biosciences, Zürich, Switzerland) and complete Freund’s adjuvant (Sigma Aldrich) was injected intradermally into the base of the tail of male DBA/1JNCrlj mice (20.6–27.3 g; Janvier Lab). The same injection was repeated at day 21. Test compounds, the reference compound or vehicle were administered orally once or twice daily from day 21 until day 44 (group size: n = 10). The severity of arthritis was evaluated every day for 26 days. Scoring from 0 to 4 was done using a published scale (Holmdahl et al., 2002). Additionally, the thickness of hind paw was measured three times a week.
Plasma concentration
To investigate the pharmacokinetic profile of BTT-3033 and BTT-3034, male DBA/1 mice were orally administered with 10 mg/kg formulated in PEG400/captisol (80/20 v/v). Plasma concentrations of three mice per sampling point were determined by LC/MS/MS. Based on that, pharmacokinetic parameters were calculated from the composite profile using a noncompartmental pharmacokinetic model.
Statistical analysis
The data were analyzed by Student’s t-test (two groups) or by one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc comparisons tests (several groups). Two-way ANOVA with the post hoc Tukey’s test for individual comparison was used in the collagen-induced arthritis model. ANOVA followed by Newman-Keuls multiple comparison test was used in the adjuvant-induced arthritis model.
Results
Integrin α2β1 function blocking sulfonamides BTT-3016, BTT-3033 and BTT-3034 have anti-inflammatory effects in PAF-stimulated air pouch model
Sulfonamide BTT-3016 is an inhibitor of α2β1 integrin function (Nissinen et al. 2010). It is selective for α2β1 over other collagen receptor integrins, when α1+α2–α11+ Saos-wt are compared to their α2 overexpressing counterparts. BTT-3016 is an effective inhibitor of platelet binding to collagen in ex vivo assays and in vivo thrombosis models (Nissinen et al. 2010).
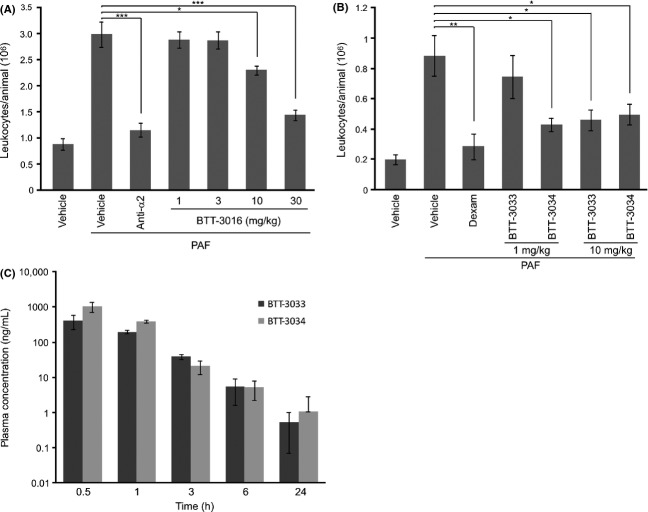
To analyze the effects of BTT-3016 on acute inflammation, we used a mouse skin air pouch model. This model measures, for example, the function of polymorphonuclear leukocytes, known to use α2β1 as their collagen receptor (Werr et al. 2000). BTT-3016 dose dependently suppressed the recruitment of leukocytes into the air pouch. With 100 mg/kg (oral administration) the effect size was in range of that of 0.1 mg/kg dexamethasone indicating a moderate anti-inflammatory effect (not shown). To further study the dose dependency of BTT-3016 effect we repeated the experiment. Low doses of BTT-3016 (1 and 3 mg/kg, p.o.) were not able to affect the number of leukocytes in inflamed air pouch of mice compared to untreated control group (Fig.1A). However, higher doses (10 and 30 mg/kg) dose dependently and in a statistically significant manner reduced the number of leukocytes after PAF treatment (Fig.1A). Importantly, specific monoclonal antibody against α2 integrin (Ha1/29) was only moderately more effective than BTT-3016. To conclude, our results indicate that orally administrated α2β1 blocking sulfonamides are anti-inflammatory agents in vivo.
Figure 1.
BTT-3016, BTT-3033, and BTT-3034 small molecular inhibitors of α2β1 integrin present anti-inflammatory effect in PAF-induced air pouch model. An air pouch was induced by injecting sterile air to the back of female NMRI mice. Platelet-activating factor (PAF, 0.7 mL of 10−6 mol/L solution/air pouch) was used to induce inflammation. (A) Indicated concentrations of BTT-3016 were administered p.o. and anti-α2 antibody (anti-α2, Ha1/29, 25 μg) directly into air pouch 24 h and 2 h before PAF induction. Number of mice in each group was 10. *P < 0.05; ***P < 0.001 (One-way ANOVA followed by Bonferroni’s post hoc comparisons tests). (B) BTT-3033 and BTT-3034 (both 1 mg/kg, n = 8; 10 mg/kg, n = 8), dexamethasone (Dexam, 0.1 mg/kg, n = 8), nonstimulated vehicle (n = 7) and PAF-stimulated vehicle (n = 8) were administrated p.o. 24 h and 2 h before PAF induction. *P < 0.05; **P < 0.01 (One-way ANOVA followed by Bonferroni’s post hoc comparisons tests). (A and B) The number of leukocytes was counted under microscope. The data are shown as mean ± SEM. (C) The pharmacokinetic profiles of BTT-3033 and BTT-3034 were studied in male DBA/1 mice by administrating single oral dose (10 mg/kg) of the compounds. Plasma concentrations of three animals per sampling time points are presented as mean ± SD. In 1 h time point the difference is statistically significant (P < 0.05; Student’s t-test).
BTT-3033 and BTT-3034 represent more potent α2β1 inhibiting sulfonamides (Nissinen et al. 2012). The two molecules have different binding mechanisms to α2I domain and we have reported that they have interesting differences in their action. BTT-3033 seems to be a better inhibitor of nonactivated α2β1 conformation than BTT-3034, whereas BTT-3034 seems to be more potent inhibitor of the activated integrin conformation, and only BTT-3033 can inhibit platelet binding to collagen under shear stress (Nissinen et al. 2012). The selectivity of BTT-3033 (8-fold) for α2β1 integrin when compared to α1β1 integrin is greater than that of BTT-3034 (twofold) (Nissinen et al. 2012).
To test the effects of BTT-3033 and BTT-3034 (1 or 10 mg/kg, per oral) on acute inflammation, we used mouse air pouch model (Fig.1B). BTT-3033 reduced the infiltration of leukocytes by about 50% at 10 mg/kg dose, while it had no significant effect at the dose of 1 mg/kg. BTT-3034 was considered to be more effective than BTT-3033, since it had similar effect at the smaller dose (1 mg/kg). Dexamethasone (0.1 mg/kg) was used as a reference substance and it reduced leukocyte infiltration about 70% (Fig.1B).
Importantly, the results indicate that BTT-3034 can inhibit inflammation despite the fact that in previously published experiments it had no inhibitory effect on platelet function under flow (Nissinen et al. 2012). Thus, α2β1 integrin seems to function by a different mechanism in inflammation than in thrombosis. The fact that BTT-3034 can also block α1β1 integrin function may partially explain the difference between BTT-3033 and BTT-3034, since α1 integrin antibodies can also potentiate the effects of α2 integrin antibodies (de Fougerolles et al. 2000). Furthermore, the pharmacokinetic profiles of two sulfonamides indicate that BTT-3034 plasma levels are higher during the first 1 h after oral administration (Fig.1C; P < 0.05; Student’s t-test, n = 3).
BTT-3033 and BTT-3034 have moderate anti-inflammatory effects in arachidonic acid-induced ear edema model
In the further experiments, the anti-inflammatory effects of BTT-3033 and BTT-3034 were tested using arachidonic acid-induced ear edema (Fig.2), a mouse model of acute inflammation (Young et al., 1984; Chang et al., 1986; Van der Mey et al., 2003). Sulfonamides were administrated orally 48 h, 24 h, and 3 h before ear swelling was induced by application of arachidonic acid. When ear swelling was measured 60 min later both BTT-3033 (10 mg/kg, P < 0.01) and BTT-3034 (2.5 mg/kg, P < 0.001; 10 mg/kg, P < 0.001) were clearly effective. However, they did not reach the potency of a reference compound (dexamethasone, 0.1 mg/kg). Thus, again inhibition of α2β1 integrin function was shown to have moderate anti-inflammatory effects and BTT-3034 was more effective than BTT-3033.
Figure 2.
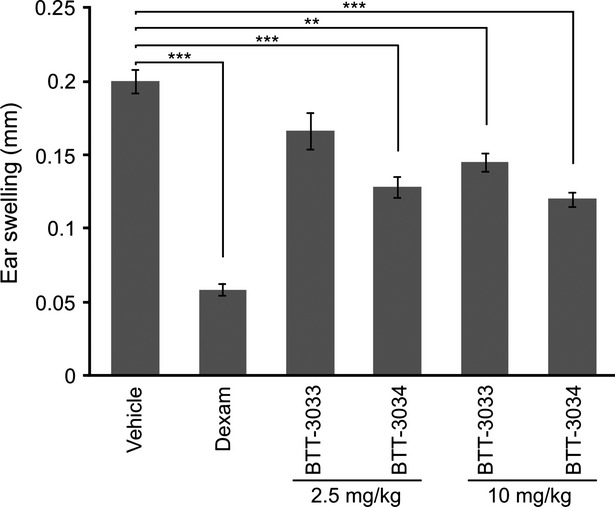
Both BTT-3033 and BTT-3034 demonstrate moderate anti-inflammatory effect in an acute inflammation model of mouse. The inflammatory response was induced with arachidonic acid (20 μL of 164 mmol/L solution in acetone) on left ears. The indicated concentrations of BTT-3033 and BTT-3034, dexamethasone (Dexam, 0.1 mg/kg, n = 6), and vehicle were administrated p.o. once daily 48 h, 24 h, and 3 h before arachidonic acid challenge. The number of mice in each group was 6. Ear thickness was measured 60 min after arachidonic acid challenge. The data are presented as mean ± SEM. **P < 0.01; ***P < 0.001 (One-way ANOVA followed by Bonferroni’s post hoc comparisons tests).
BTT-3034 inhibits inflammation in a mouse skin hypersensitivity model
To investigate the effects of the integrin inhibitor BTT-3034 on allergic inflammation, mice were sensitized with ovalbumin. This model measures delayed type hypersensitivity reaction. Ear thickness was determined 3 h, 24 h, and 48 h after the challenge. Dexamethasone significantly reduced inflammation (about 53% at 3 h, about 40% at 24 h, and about 47% at 48 h). BTT-3034 had also significant but slightly weaker inhibitory effect on inflammation at 48 h time point (about 35%) (Fig.3). BTT-3033 was not effective at 10 mg/kg but reduced ear thickness at 40 mg/kg at 24 h and 48 h time point (data not shown).
Figure 3.
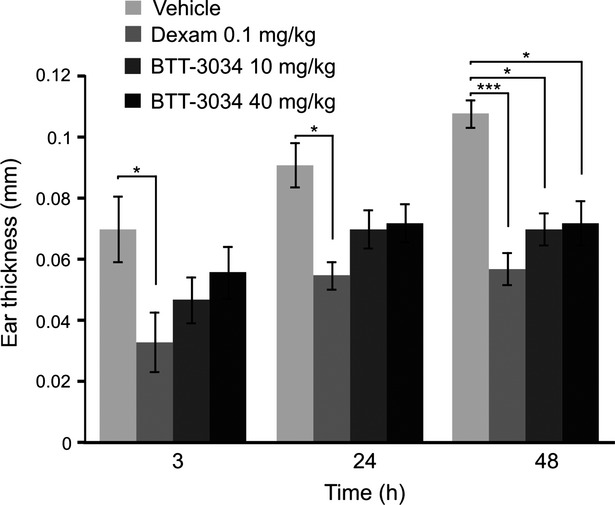
BTT-3034 inhibits inflammatory response in mouse skin hypersensitivity model. Subcutaneous sensitization with ovalbumin and Al(OH)3 (100 μg and 3.3 mg/400 μL, respectively) was done on day 1 and 8 for female BALB/cAnNCrl mice. On day 13 vehicle, dexamethasone (0.1 mg/kg) and the indicated concentrations of BTT-3034 were administrated p.o. 48 h, 24 h, and 2 h before ovalbumin challenge. There were 10 mice in each group. Ear thickness was measured 3 h, 24 h, and 48 h after ovalbumin challenge. *P < 0.05; ***P < 0.001 (One-way ANOVA followed by Bonferroni’s post hoc comparisons tests). The data are shown as mean ± SEM.
BTT-3034 inhibits inflammation in guinea pig skin allergic inflammation model
BTT-3034 was further tested using a guinea pig allergic skin inflammation model. For this experiment, guinea pigs were sensitized to ovalbumin in a way (i.p.) to promote allergic immune responses. BTT-3034 inhibited the ovalbumin-induced increase in the area of allergic wheals when administrated in doses 0.75 mg/kg, 2.5 mg/kg or 10 mg/kg (Fig.4). Thus, BTT-3034 inhibited both hypersensitivity and allergic inflammation.
Figure 4.
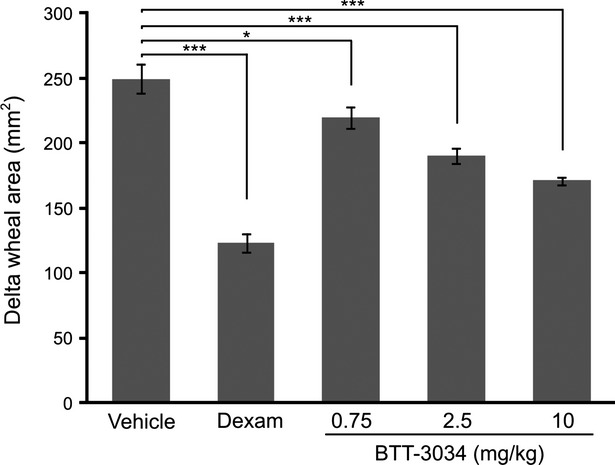
BTT-3034 inhibits inflammatory response in guinea pig skin allergic inflammation model. Male HA guinea pigs were sensitized intraperitoneally with ovalbumin and Al(OH)3 (20 μg and 20 mg, respectively). In the final experiment after 3 weeks the guinea pigs were challenged with 0.1% ovalbumin solution on the back skin. Vehicle (n = 6), dexamethasone (Dexam, 0.1 mg/kg, n = 5), and indicated concentrations of BTT-3034 (n = 6 in both groups) were administrated orally once a day for 3 days before the ovalbumin treatment. The response was measured 60 min after the challenge by measuring the diameter of allergic wheals. The data are presented as change in the area of allergic wheals. *P < 0.05; ***P < 0.001 (One-way ANOVA followed by Bonferroni’s post hoc comparisons tests). The data are shown as mean ± SEM.
Inhibitors of α2β1 integrin have anti-inflammatory effects in arthritis models
To test, whether α2β1 integrin inhibitors can inhibit the signs of chronic inflammation we tested the potency of intraperitoneally administered BTT-3016 (5 mg/kg, once a day) in a rat model of adjuvant-induced arthritis. Orally administered diclofenac (2 mg/kg, twice a day) was used as a positive control. BTT-3016 treatment reduced substantially and statistically significantly the score reflecting the clinical severity of the arthritis (Fig.5A). The effect was comparable with that caused by the reference compound diclofenac (Fig.5A). BTT-3016 also significantly decreased the volume of angle edema, but the effect was smaller than that of diclofenac (Fig.5B). Thus, in principle small molecule inhibitors of α2β1 integrin are effective anti-inflammatory compounds in chronic inflammation.
Figure 5.
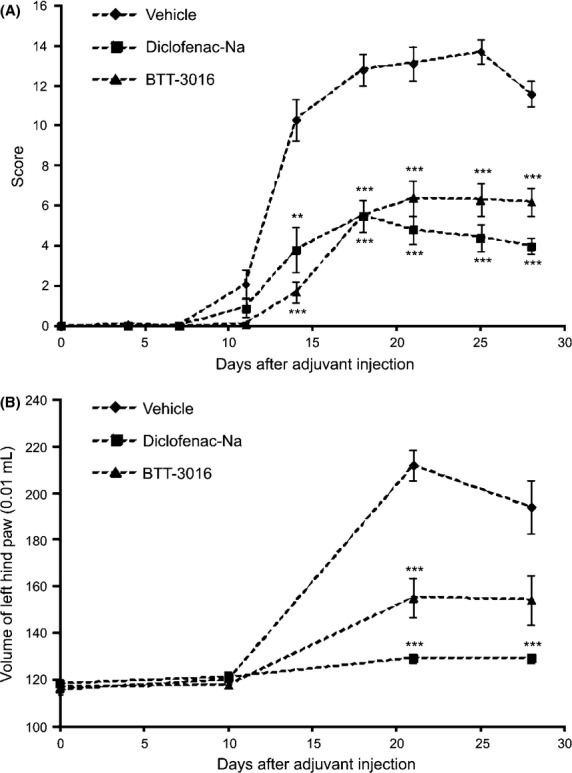
Inhibition of α2β1 integrin has anti-inflammatory effect in adjuvant-induced arthritis model. The male Lewis inbred rats were injected with 0.5 mg of killed Mycobacterium butyrium. Vehicle (n = 10), Diclofenac-Na (2 mg/kg, n = 10) and BTT-3016 (5 mg/kg, n = 10) were administrated i.p. once a day from day 1 to 27 after the challenge. The severity of the disease was determined by (A) scoring and by (B) measuring the volume of left hind paw at time points indicated. (A, B) The data are represented as mean ± SEM. **P < 0.01; ***P < 0.001 (ANOVA followed by Newman-Keuls multiple comparison test).
In another series of experiments arthritis in DBA/1J mice was induced by two immunizations with bovine collagen type II in the interval of 3 weeks. This disease model is influenced by the activity of α2β1-positive Th17 cell. Furthermore, the antibodies against α2β1 have been reported to decrease the severity of arthritis (El Azreq et al. 2013). Dexamethasone significantly reduced paw edema, thickness, and redness. BTT-3033 (10 mg/kg) had no statistically significant effects. BTT-3034 (10 mg/kg) significantly suppressed paw thickness (Fig.6A) but not paw redness (Fig.6B) or paw edema (Fig.6C). The results with BTT-3034 confirm the data from BTT-3016 experiments. Weaker potency of BTT-3034 is most probably due to its relative short half-life in plasma.
Figure 6.
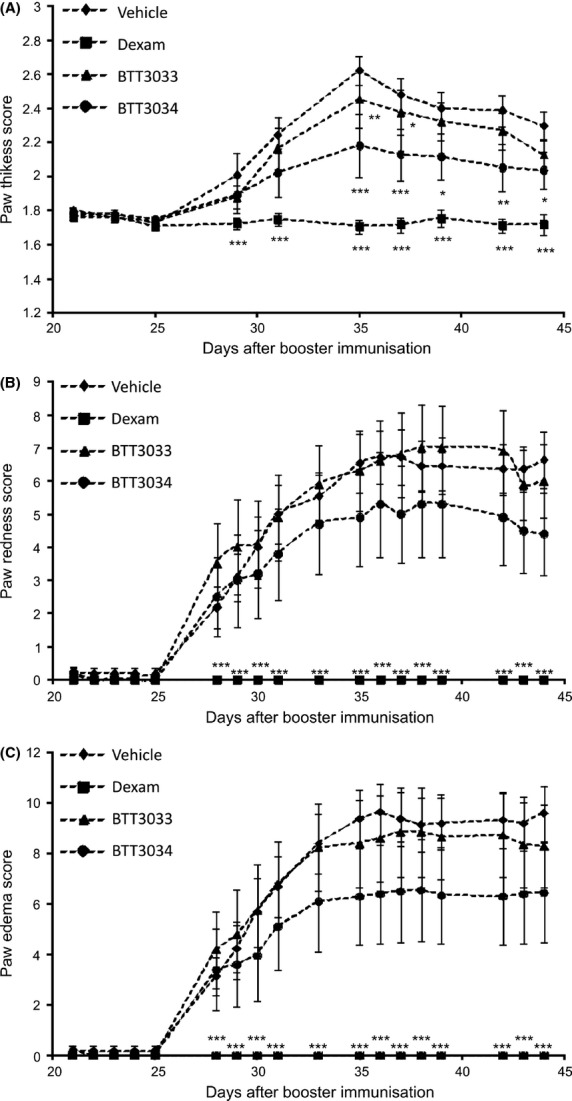
α2β1 integrin inhibitor BTT-3034 presents anti-inflammatory effect in collagen-induced arthritis model. Male DBA/1JNCrlj mice were challenged with bovine type II collagen and Freud′s adjuvant. Vehicle (n = 11), BTT-3033 (10 mg/kg, n = 10) and BTT-3034 (10 mg/kg, n = 10) were administrated orally twice a day and dexamethasone (Dexam, 0.1 mg/kg, n = 10) once a day from day 1 to 26 after the challenge. The severity of arthritis was evaluated with (A) paw thickness score, (B) paw redness score, and (C) paw edema score at time points indicated. (A–C) The data are shown as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 (Two-Way ANOVA with the post hoc Tukey’s test).
Discussion
Small molecule inhibitors of α2β1 integrin have been used in in vivo animal models of human diseases and shown to inhibit thrombosis (Miller et al. 2009; Nissinen et al. 2010), cancer-related angiogenesis (San Antonio et al. 2009), and kidney injury (Borza et al. 2012). Here, we report that sulfonamide-type inhibitors are also potent anti-inflammatory agents.
Small molecule inhibitors are also valuable tools in studies on integrin biology. Firstly, the use of small size, nonprotein ligands gives new insight when compared to function blocking antibodies. Even more so, because in many cases the in vivo results obtained with specific antibodies are in conflict with integrin knockout phenotypes. For example α2β1-binding proteins and antibodies against α2β1 prevent angiogenesis (Senger et al. 1997; Woodall et al. 2008), but α2 deficiency leads to accelerated angiogenesis (Grenache et al. 2007; Zweers et al. 2007; Zhang et al. 2008). Secondly, small molecules can be selective to different integrin conformations and therefore be used to study the biology of different activation stages of integrins (Nissinen et al. 2012). New insight into the integrin function is also essential for drug development.
Skin air pouch model has been reported to give conflicting information about the role of α2β1 integrin depending, whether the experiments are based on the use of specific antibodies against α2β1 integrin (de Fougerolles et al. 2000) or genetic deficiency of this collagen receptor (Zweers et al. 2006). Here, we have shown that different sulfonamide-type inhibitors of α2β1 are effective anti-inflammatory agents. Thus, our data indicate that the previous observations received with specific antibodies are based on the inhibition of integrin function and not any antibody related secondary effect, for example, induction of integrin clustering. We also noticed that, in the air pouch model BTT-3034 was more potent inhibitor than BTT-3033. This result is opposite to what we have previously detected in experiments measuring platelet function (Nissinen et al. 2012). We have proposed that under shear stress nonactivated platelet α2β1 integrin, presumably in bent conformation, can mediate binding to collagen and that BTT-3033 inhibits this interaction. Here, our observations based on several models of acute and chronic inflammation, indicate that in inflammation α2β1 behaves in a fundamentally different manner. Thus, we propose that in the inflammation regulating cells α2β1 must be preactivated to be functional.
In adjuvant arthritis the potency of sulfonamide BTT-3016 was comparable to that of diclofenac, stressing the important role of α2β1 in joint inflammation. This is in accordance with previous observations based on the use of function blocking antibodies (de Fougerolles et al. 2000; El Azreq et al. 2013). Similarly, genetic deficiency of α2 integrin suppresses inflammation and cartilage destruction (Peters et al. 2012). The abundant expression of α2β1 on various leukocytes, for example, neutrophils, T lymphocytes and mast cells (Werr et al. 2000; Edelson et al. 2004; Boisvert et al. 2010; Gagliani et al. 2013), and the large number of its putative ligands (Heino et al., 2007) makes it difficult to speculate, what is the most critical step that requires the presence of α2β1 in distinct animal models. BTT-3034 also inhibited hypersensitivity and allergic inflammation. Integrin α2β1 has been reported to augment T-cell receptor-dependent production of interferon-γ in T cells and therefore suggested to control Th1 response (Boisvert et al. 2007). Furthermore, α2β1 is required for IL-6 secretion by mast cells (McCall-Culbreath et al. 2011).
As a summary, we have shown using sulfonamide-type α2β1 integrin inhibitors, that the collagen receptor plays an essential role in in vivo animal models of acute inflammation, allergy, hypersensitivity, and arthritis. Furthermore, our data propose a fundamental difference in α2β1 function when inflammation is compared to thrombosis (Nissinen et al. 2012). We suggest that in inflammation, unlike on platelets, α2β1 integrins are preactivated before ligand binding.
Acknowledgments
Auni Juhankoski (Biotie Therapies Corp) is acknowledged for determination of analytical and bioanalytical samples used in these in vitro and in vivo experiments.
Glossary
- ANOVA
analysis of variance
- HP-β-CDX
hydroxypropyl-β-cyclodextrin
- MC
methylcellulose
- PAF
platelet-activating factor
Author Contributions
Liisa Nissinen, Anne Marjamäki, Jarmo Käpylä, and Jyrki Heino defined the experimental design of this study and wrote the final version of this manuscript.
Marika Ojala, Barbara Langen, and Rita Dost designed, managed, and analyzed the experimental in vivo animal models and development of formulations used in drug administration under supervision of Anne Marjamäki. They also contributed in description of the experimental procedures of this study and writing of this manuscript.
Marjo Pihlavisto and Anne Marjamäki were responsible for design, synthesis, and chemical characterization of small molecule inhibitors.
Liisa Nissinen and Jarmo Käpylä were responsible for pharmacological in vitro characterization of compounds selected for in vivo experiments.
Conflicts of Interest
Marjo Pihlavisto is a current employee of Biotie Therapies Corp. Liisa Nissinen, Marika Ojala, and Anne Marjamäki are former employees of Biotie Therapies Corp. Jyrki Heino is a former consultant of Biotie Therapies Corp. Barbara Langen and Rita Dost are former employees of Biotie Therapies GmbH and current employees of BioCrea GmbH (Radebeul, Germany).
References
- Boisvert M, Gendron S, Chetoui N, Aoudjit F. α2β1 integrin signaling augments T cell receptor-dependent production of interferon-gamma in human T cells. Mol Immunol. 2007;44:3732–3740. doi: 10.1016/j.molimm.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Boisvert M, Chetoui N, Gendron S, Aoudjit F. α2β1 integrin is the major collagen-binding integrin expressed on human Th17 cells. Eur J Immunol. 2010;40:2710–2719. doi: 10.1002/eji.201040307. [DOI] [PubMed] [Google Scholar]
- Borza CM, Su Y, Chen X, Yu L, Mont S, Chetyrkin S, et al. Inhibition of integrin α2β1 ameliorates glomerular injury. J Am Soc Nephrol. 2012;23:1027–1038. doi: 10.1681/ASN.2011040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Carlson RP, O’Neill-Davis L, Lamb B, Sharma RN, Lewis AJ. Correlation between mouse skin inflammation induced by arachidonic acid and eicosanoid synthesis. 1986;10:205–214. doi: 10.1007/BF00916116. [DOI] [PubMed] [Google Scholar]
- Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM. The α2 integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol. 2002;161:337–344. doi: 10.1016/s0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Vilaire G, Marcinkiewicz C, Winkler JD, Bennett JS, DeGrado WF. Small molecule inhibitors of integrin α2β1. J Med Chem. 2007;50:5457–5462. doi: 10.1021/jm070252b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson BT, Li Z, Pappan LK, Zutter MM. Mast cell-mediated inflammatory responses require the α2β1 integrin. Blood. 2004;103:2214–2220. doi: 10.1182/blood-2003-08-2978. [DOI] [PubMed] [Google Scholar]
- El Azreq M-A, Boisvert M, Cesaro A, Pagé N, Loubaki L, Alleys I, et al. α2β1 integrin regulates Th17 cell activity and its neutralization decreases the severity of collagen-induced arthritis. J Immunol. 2013;191:5941–5950. doi: 10.4049/jimmunol.1301940. [DOI] [PubMed] [Google Scholar]
- Emsley J, Knight CG, Farndale RW, Barnes MJ, Liddington RC. Structural basis of collagen recognition by integrin α2β1. Cell. 2000;101:47–56. doi: 10.1016/S0092-8674(00)80622-4. [DOI] [PubMed] [Google Scholar]
- de Fougerolles AR, Sprague AG, Nickerson-Nutter CL, Chi-Rosso G, Rennert PD, Gardner H, et al. Regulation of inflammation by collagen-binding integrins α1β1 and α2β1 in models of hypersensitivity and arthritis. J Clin Invest. 2000;105:721–729. doi: 10.1172/JCI7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. 2013;19:739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- Gillberg L, Berg S, de Verdier PJ, Lindbom L, Werr J, Hellström PM. Effective treatment of mouse experimental colitis by α2 integrin antibody: comparison with α4 antibody and conventional therapy. Acta Physiol (Oxf) 2013;207:326–336. doi: 10.1111/apha.12017. [DOI] [PubMed] [Google Scholar]
- Goodman SL, Picard M. Integrins as therapeutic targets. Trends Pharmacol Sci. 2012;33:405–412. doi: 10.1016/j.tips.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Grenache DG, Zhang Z, Wells LE, Santoro SA, Davidson JM, Zutter MM. Wound healing in the α2β1 integrin-deficient mouse: altered keratinocyte biology and dysregulated matrix metalloproteinase expression. J Invest Dermatol. 2007;127:455–466. doi: 10.1038/sj.jid.5700611. [DOI] [PubMed] [Google Scholar]
- Hanazawa A, Hayashizaki K, Shinoda K, Yagita H, Okumura K, Löhning M, et al. CD49b-dependent establishment of T helper cell memory. Immunol Cell Biol. 2013;91:524–531. doi: 10.1038/icb.2013.36. [DOI] [PubMed] [Google Scholar]
- Heino J. The collagen family members as cell adhesion proteins. BioEssays. 2007;29:1001–1010. doi: 10.1002/bies.20636. [DOI] [PubMed] [Google Scholar]
- Hemler ME, Jacobson JG, Strominger JL. Biochemical characterization of VLA-1 and VLA-2. Cell surface heterodimers on activated T cells. J Biol Chem. 1985;260:15246–15252. [PubMed] [Google Scholar]
- Holmdahl R, Bockermann R, Bäcklund J, Yamada H. The molecular pathogenesis of collagen-induced arthritis in mice–a model for rheumatoid arthritis. Ageing Res Rev. 2002;1:135–147. doi: 10.1016/s0047-6374(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Holtkötter O, Nieswandt B, Smyth N, Müller W, Hafner M, Schulte V, et al. Integrin α2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J Biol Chem. 2002;277:10789–10794. doi: 10.1074/jbc.M112307200. [DOI] [PubMed] [Google Scholar]
- Ivaska J, Reunanen H, Westermarck J, Koivisto L, Kähäri VM, Heino J. Integrin α2β1 mediates isoform-specific activation of p38 and upregulation of collagen gene transcription by a mechanism involving the α2 cytoplasmic tail. J Cell Biol. 1999;147:401–416. doi: 10.1083/jcb.147.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käpylä J, Pentikäinen OT, Nyrönen T, Nissinen L, Lassander S, Jokinen J, et al. Small molecule designed to target metal binding site in the α2I domain inhibits integrin function. J Med Chem. 2007;50:2742–2746. doi: 10.1021/jm070063t. [DOI] [PubMed] [Google Scholar]
- Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall-Culbreath KD, Li Z, Zutter MM. Crosstalk between the α2β1 integrin and c-met/HGF-R regulates innate immunity. Blood. 2008;111:3562–3570. doi: 10.1182/blood-2007-08-107664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall-Culbreath KD, Li Z, Zhang Z, Lu LX, Orear L, Zutter MM. Selective, a2ß1 integrin-dependent secretion of IL-6 by connective tissue mast cells. J Innate Immun. 2011;3:459–470. doi: 10.1159/000324832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Basra S, Kulp DW, Billings PC, Choi S, Beavers MP, et al. Small-molecule inhibitors of integrin α2β1 that prevent pathological thrombus formation via an allosteric mechanism. Proc Natl Acad Sci USA. 2009;106:719–724. doi: 10.1073/pnas.0811622106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita M, Kelly KR, Mita A, Ricart AD, Romero O, Tolcher A, et al. Phase I study of E7820, an oral inhibitor of integrin α2 expression with antiangiogenic properties, in patients with advanced malignancies. Clin Cancer Res. 2011;17:193–200. doi: 10.1158/1078-0432.CCR-10-0010. [DOI] [PubMed] [Google Scholar]
- Momic T, Katzhendler J, Shai E, Noy E, Senderowitz H, Eble JA, et al. Vipegitide: a folded peptidomimetic partial antagonist of α2β1 integrin with antiplatelet aggregation activity. Drug Des Devel Ther. 2015;9:291–304. doi: 10.2147/DDDT.S72844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissinen L, Pentikäinen OT, Jouppila A, Käpylä J, Ojala M, Nieminen J, et al. A small-molecule inhibitor of integrin α2β1 introduces a new strategy for antithrombotic therapy. Thromb Haemost. 2010;103:387–397. doi: 10.1160/TH09-06-0358. [DOI] [PubMed] [Google Scholar]
- Nissinen L, Koivunen J, Käpylä J, Salmela M, Nieminen J, Jokinen J, et al. Novel α2β1 integrin inhibitors reveal that integrin binding to collagen under shear stress conditions does not require receptor preactivation. J Biol Chem. 2012;287:44694–44702. doi: 10.1074/jbc.M111.309450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters MA, Wendholt D, Strietholt S, Frank S, Pundt N, Korb-Pap A, et al. The loss of α2β1 integrin suppresses joint inflammation and cartilage destruction in mouse models of rheumatoid arthritis. Arthritis Rheum. 2012;64:1359–1368. doi: 10.1002/art.33487. [DOI] [PubMed] [Google Scholar]
- San Antonio JD, Zoeller JJ, Habursky K, Turner K, Pimtong W, Burrows M, et al. A key role for the integrin α2β1 in experimental and developmental angiogenesis. Am J Pathol. 2009;175:1338–1347. doi: 10.2353/ajpath.2009.090234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger DR, Claffey KP, Benes JE, Perruzzi CA, Sergiou AP, Detmar M. Angiogenesis promoted by vascular endothelial growth factor: regulation through α1β1 and α2β1 integrins. Proc Natl Acad Sci USA. 1997;94:13612–13617. doi: 10.1073/pnas.94.25.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werr J, Johansson J, Eriksson EE, Hedqvist P, Ruoslahti E, Lindbom L. Integrin α2β1 (VLA-2) is a principal receptor used by neutrophils for locomotion in extravascular tissue. Blood. 2000;95:1804–1809. [PubMed] [Google Scholar]
- Woodall BP, Nyström A, Iozzo RA, Eble JA, Niland S, Krieg T, et al. Integrin α2β1 is the required receptor for endorepellin angiostatic activity. J Biol Chem. 2008;283:2335–2343. doi: 10.1074/jbc.M708364200. [DOI] [PubMed] [Google Scholar]
- Van der Mey M, Bommelé KM, Boss H, Hatzelmann A, Van Slingerland M, Sterk GJ. Synthesis and structure-activity relationships of cis-tetrahydrophthalazinone/pyridazinone hybrids: a novel series of potent dual PDE3/PDE4 inhibitory agents. J Med Chem. 2003;46:2008–2016. doi: 10.1021/jm030776l. , et al. ( [DOI] [PubMed] [Google Scholar]
- Young JM, Spires DA, Bedord CJ, Wagner B, Ballaron SJ, De Young LM. The mouse ear inflammatory response to topical arachidonic acid. J Invest Dermatol. 1984;82:367–371. doi: 10.1111/1523-1747.ep12260709. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Ramirez NE, Yankeelov TE, Li Z, Ford LE, Qi Y, et al. α2β1 integrin expression in the tumor microenvironment enhances tumor angiogenesis in a tumor cell-specific manner. Blood. 2008;111:1980–1988. doi: 10.1182/blood-2007-06-094680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zutter MM, Edelson BT. The α2β1 integrin: a novel collectin/C1q receptor. Immunobiology. 2007;212:343–353. doi: 10.1016/j.imbio.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Zutter MM, Santoro SA. Widespread histologic distribution of the α2β1 integrin cell-surface collagen receptor. Am J Pathol. 1990;137:113–120. [PMC free article] [PubMed] [Google Scholar]
- Zweers MC, Siewe L, Wickenhauser C, Krieg T, Roers A, Eckes B. Integrin α2β1 deficiency does not affect contact hypersensitivity. Arch Dermatol Res. 2006;298:201–205. doi: 10.1007/s00403-006-0688-7. [DOI] [PubMed] [Google Scholar]
- Zweers MC, Davidson JM, Pozzi A, Hallinger R, Janz K, Quondamatteo F, et al. Integrin α2β1 is required for regulation of murine wound angiogenesis but is dispensable for reepithelialization. J Invest Dermatol. 2007;127:467–478. doi: 10.1038/sj.jid.5700546. [DOI] [PubMed] [Google Scholar]