Abstract
Aging is accompanied by endothelial dysfunction due to reduced bioavailability of nitric oxide (NO) and/or reduced endothelium-dependent hyperpolarizations (EDH). This study examines the hypothesis that hypertension aggravates the impairment of EDH-type relaxation due to aging. EDH-type relaxations were studied in superior mesenteric arteries isolated from Wistar Kyoto (WKY) and spontaneously hypertensive (SHR) rats of 12, 36, 60, and 72 weeks of age. EDH-type relaxations in WKY were reduced with aging, and this was associated with an impairment of the function of small-conductance calcium-activated potassium channels (SKCa) and sodium-potassium ATPase (Na-K ATPase). EDH-type relaxation in SHR was smaller than that in WKY arteries, and further reduction occurred with aging. Pharmacological experiments suggested a reduced involvement of SKCa and Na-K ATPase and activation of adenosine monophosphate-activated protein kinase and silent information regulator T1 (sirtuin-1; SIRT1) in mesenteric arteries of 12-week-old SHR. These pharmacological findings suggest that in superior mesenteric arteries of the rat, the reduction in EDH-type relaxation occurs with aging and that such a reduction is exacerbated in hypertension. The latter exacerbation appears to involve proteins associated with the process of cellular senescence and is related to impaired function of SKCa and Na-K ATPase, a phenomenon that is also observed in mesenteric arteries of older normotensive rats.
Keywords: Aging, endothelial dysfunction, endothelium-derived hyperpolarization, hypertension, small-conductance calcium-activated potassium channel, sodium–potassium ATPase
Introduction
The endothelium is a monolayer of cells that contributes to the regulation of vascular tone, inflammation, and angiogenesis (Félétou and Vanhoutte 2007). The modulation of vascular tone by endothelial cells is accomplished by vasodilator [mainly nitric oxide (NO) produced by endothelial NO synthase (eNOS) and endothelium-dependent hyperpolarization (EDH)] and vasoconstrictor [endothelin-1 and endothelium-derived contracting prostanoids] signals (Furchgott and Vanhoutte 1989; Vanhoutte et al., 2009). The healthy endothelium maintains a balance between the release of the contracting and relaxing factors in response to stimuli such as sheer stress and endogenous neurohumoral compounds. Abnormality of endothelial cell function accompanies diseases such as ischemia, hypertension, atherosclerosis, and diabetes, and is indeed one of the major predictors of cardiovascular events (Lüscher et al. 1993; Félétou and Vanhoutte 2007; Vanhoutte et al., 2009).
Aging has been linked to the development of endothelial senescence, a condition of permanent growth arrest, and the deterioration of endothelial function resulting in the alteration of the release of endothelium-derived vasoactive substances (Brandes et al. 2005; Csiszar et al. 2008; Erusalimsky 2009). Abnormalities in the morphology of endothelial cells, resembling those occurring with aging, have been demonstrated in experimental hypertension (Haudenschild et al. 1981). In hypertension, blood vessels are exposed to increased sheer stress and this chronic state causes progressive damage to the endothelial cell layer (Mantelli et al. 1995; Cardillo and Panza 1998). As such, it appears that chronic exposure to a high-arterial blood pressure induces and/or accelerates senescence of endothelial cells. Silent information regulator T1 (SIRT1) is an important regulator of the process of aging and cellular senescence (Bitterman et al. 2002; Ota et al. 2007; Donmez and Guarente 2010; Wang et al. 2011). It is a nicotinamide adenine dinucleotide-dependent deacetylase that hydrolyzes lysine residues in various proteins, including histones (North and Verdin 2004). Its activation results in inhibition of senescence (Wang et al. 2011). In the vasculature, SIRT1 activates eNOS, induces NO production and hence promotes endothelium-dependent relaxation (Mattagajasingh et al. 2007). One of the downstream cellular targets of SIRT1 in endothelial cells is adenosine monophosphate-activated protein kinase (AMPK; Zu et al. 2010; Wang et al. 2011), which, like SIRT1, promotes endothelium-dependent relaxations in the aorta and mesenteric arteries through phosphorylation of eNOS and hence increased production of NO (Fisslthaler and Fleming 2009; Ford et al. 2012; Dolinsky et al. 2013). In addition, genetic deletion of endothelial AMPK results in high-blood pressure and reduced EDH-mediated relaxations in mouse mesenteric arteries (Enkhjargal et al. 2014), suggesting a role of AMPK in the EDH signaling pathway.
Therefore, this study examines the hypothesis that hypertension is associated with a more advanced stage of endothelial dysfunction than natural aging, and that EDH-type relaxations are impaired at an earlier age in hypertensive animals compared to normotensive controls. Moreover, the possible involvements of SIRT1 and AMPK in the progressive impairment of endothelium-dependent relaxations were determined in aged and hypertensive rats. A better understanding of the events underlying the progression of endothelial dysfunction with aging and its acceleration by hypertension will facilitate the design of pharmacological interventions alleviating/preventing vascular complications in the older population.
Materials and Methods
Animals
Male normotensive Wistar-Kyoto (WKY) and spontaneously hypertensive (SHR) rats at 12, 36, 60, or 72-week old (purchased from the Chinese University of Hong Kong) were used. The animals were housed in a temperature-controlled room under a 12-h light/12-h dark cycle and fed with standard laboratory chow and water ad libitum. They were allowed to age under these conditions starting at the age of 12 weeks. The study was approved by the committee on the use of live animals in teaching and research of the University of Hong Kong.
Isolation of mesenteric artery
The rats were anesthetized with pentobarbital sodium (70 mg/ml/kg; Ganes Chemicals Inc., Pennsville, NJ). At the time of sacrifice, their arterial blood pressure was measured by means of a catheter placed in the carotid artery and attached to a pressure transducer [P23XL (Grass Technologies, Warwick, RI, USA)] connected to a recording physiography [MK IV (Narco Bio-Systems, Austin, TX, USA)]. The superior mesenteric artery was excised quickly, cleared of adhering tissue and kept in Krebs-Ringer bicarbonate buffer (control solution) of the following composition (in 10−3 M): NaCl (118), KCl (4.7), CaCl2 (2.5), MgSO4 (1.2), KH2PO4 (1.2), NaHCO3 (25), and glucose (11.1).
Measurement of isometric tension
The arteries were cut into rings (approximately 2 mm in length) which were suspended in 5-ml organ chambers for the measurement of isometric tension. The changes in tension were detected by means of a strain gauge (MLT0201; AD Instruments, Sydney, Australia) connected to an Octal Bridge Amplifier (ML228; AD Instruments) and recorded with a data acquisition system (PowerLab@ 8/30; AD Instruments). The rings were allowed to equilibrate in oxygenated (95% O2/5% CO2) control solution at 37°C for 90 minutes under a resting tension of 1 g, which was the optimal basal tension for rat mesenteric arteries as determined in preliminary experiments (data not shown). They then were exposed to potassium chloride (6 × 10−2 M) to obtain a reference contraction. To confirm the presence of the endothelium, the rings were contracted with phenylephrine (10−6 M) first and then exposed to acetylcholine (10−6 M). In isolated arteries of the rat, in particular of the SHR, the endothelium-dependent relaxations to acetylcholine are blunted by the concomitant release of endothelium-derived vasoconstrictor prostanoids (Lüscher and Vanhoutte 1986; Takase et al. 1994; Lang et al. 1995; Gluais et al. 2005a,b; Wong et al. 2010; Li et al. 2011); to avoid this confounding factor all experiments were performed in the presence of the cyclooxygenase inhibitor indomethacin (10−5 M).
To study EDH-type responses, quiescent rings were incubated with Nω-nitro-L-arginine methyl ester (L-NAME, NO synthase inhibitor, 10−4 M) and indomethacin for 30 minutes (Tang et al. 2005; Yeung et al. 2013). Some preparations were treated with various pharmacological agents, including 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole [TRAM-34, intermediate-conductance calcium-activated potassium (IKCa) channel blocker, 5 × 10−7 M (Zou et al. 2010; Yeung et al. 2013)], 6,12,19,20,25,26-hexahydro-5,27:13,18:21,24-trietheno-11,7-metheno-7H-dibenzo[b,m][1,5,12,16]tetraazacyclotricosine-5,13-diium ditrifluoroacetate hydrate [UCL 1684, small-conductance calcium-activated potassium (SKCa) channel blocker, 5 × 10−7 M (Zou et al. 2010; Yeung et al. 2013)], ouabain [sodium-potassium ATPase (Na-K ATPase) inhibitor, 10−3 M (Doughty et al. 2000)], barium chloride [inwardly-rectifying potassium (KIR) channel blocker, 3 × 10−5 M (Doughty et al. 2000)], compound C [AMPK inhibitor, 10−5 M (Thors et al. 2008)] and 6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide [EX-527, selective silent information regulator T1 (SIRT1) inhibitor, 10−6 M (Orecchia et al. 2011)] for 40 minutes before evoking contractions with phenylephrine (EC80). Concentration-relaxation curves to the endothelium-dependent vasodilator acetylcholine (Furchgott and Zawadzki 1980) were obtained by cumulative additions (from 10−9 to 10−4 M) to the organ chamber. The concentrations of pharmacological inhibitors used were selected either from previous experience in the laboratory or from the literature.
Materials
Acetylcholine chloride, barium chloride, compound C, EX-527, indomethacin, L-NAME, ouabain, phenylephrine, TRAM-34, and UCL 1684 were purchased from Sigma (Saint Louis, MO, USA). All drugs were dissolved in distilled water except for indomethacin, TRAM-34 and UCL 1684 which were dissolved in dimethyl sulfoxide (DMSO). The final concentration of DMSO in the bath solution was equal to or less than 0.1%. All concentrations are expressed as final molar concentrations in the organ chambers.
Statistical analysis
Relaxations to acetylcholine are expressed as percent reversion of the sustained contraction induced by phenylephrine. Data are presented as means ± standard error of the mean (SEM). Statistical analysis was performed using the Prism version5 (GraphPad Software, San Diego, CA, USA): concentration-response curves of acetylcholine were compared with two-way analysis of variance followed by the Bonferroni post hoc test for multiple comparisons; whereas Student’s t-test was used for comparison between blood pressures of age-matched WKY and SHR. P values less than 0.05 were considered to indicate statistically significant differences.
Results
The WKY remained normotensive at all ages while the SHR became hypertensive from 12 weeks on (Fig.1). As they aged, there was no significant difference in weight between the WKY and the SHR (Table1).
Figure 1.
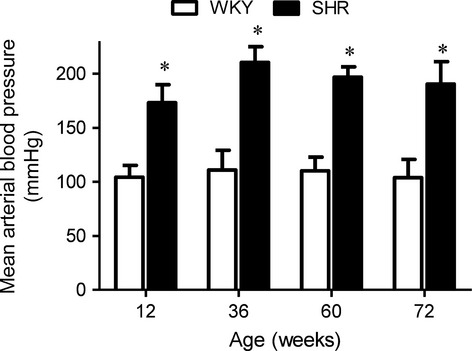
Mean arterial blood pressure of WKY and SHR at different ages. Values are means ± SEM of nine experiments. * P < 0.05 compared with corresponding WKY groups.
Table 1.
Body weight (g) in control and hypertensive rats of different ages.
| Age (weeks) | 12 | 36 | 60 | 72 |
|---|---|---|---|---|
| Control rats (WKY) | 310.0 ± 23.5 | 404.6 ± 20.5 | 460.8 ± 28.1 | 490.6 ± 17.3 |
| Hypertensive rats (SHR) | 301.1 ± 11.9 | 410.9 ± 19.3 | 449.1 ± 26.9 | 482.9 ± 16.1 |
Values are means ± SEM of nine animals.
Endothelium-dependent NO-mediated relaxations
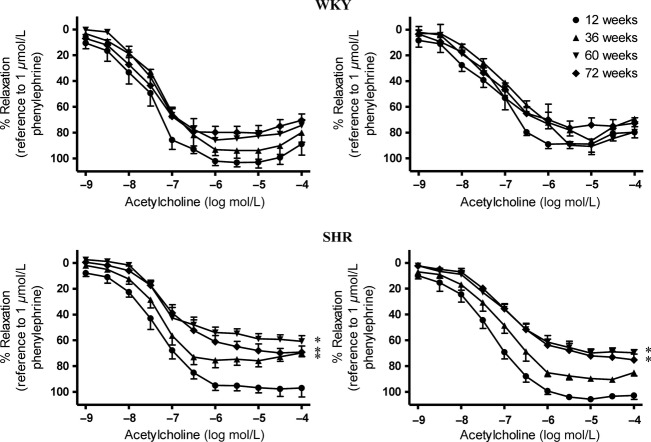
In mesenteric arteries isolated from WKY, acetylcholine-induced relaxations in the presence of indomethacin (10−5 M) alone (Fig.2, Upper Left) or in that of the combination of TRAM-34 [IKCa channel blocker, 5 × 10−7 M (Zou et al. 2010)] plus UCL 1684 [SKCa channel blocker, 5 × 10−7 M (Zou et al. 2010)] were similar among the different age groups (Fig.2, Upper Right). However, in SHR arteries, acetylcholine-induced relaxations in the presence of indomethacin decreased with aging (Fig.2, Lower Left). In the presence of indomethacin, TRAM-34 and UCL 1684, full relaxation in response to acetylcholine was observed in arteries of 12-week-old SHR, and this relaxation was reduced in 60- and 72-weeks-old SHR (Fig.2, Lower Right).
Figure 2.
Acetylcholine-induced relaxations of superior mesenteric arteries of (Upper) WKY and (Lower) SHR at various ages in the presence of (Left) indomethacin (cyclooxygenase inhibitor; 10−5 mol/L) or (Right) indomethacin (10−5 mol/L) alone, TRAM-34 (IKCa inhibitor; 5 × 10−7 mol/L) and UCL 1684 (SKCa inhibitor; 5 × 10−7 mol/L). Values are means ± SEM of six to eight experiments. *P < 0.05 compared with corresponding 12-week-old groups.
EDH-type relaxations
In the presence of indomethacin (10−5 M) and L-NAME (10−4 M), relaxations to acetylcholine in mesenteric arteries of WKY were impaired significantly in an age-dependent manner (Fig.3, Upper). Acetylcholine-induced relaxations in the presence of L-NAME and indomethacin were impaired in mesenteric arteries of 36-week-old SHR and abolished in those of 72-week-old SHR (Fig.3, Lower).
Figure 3.
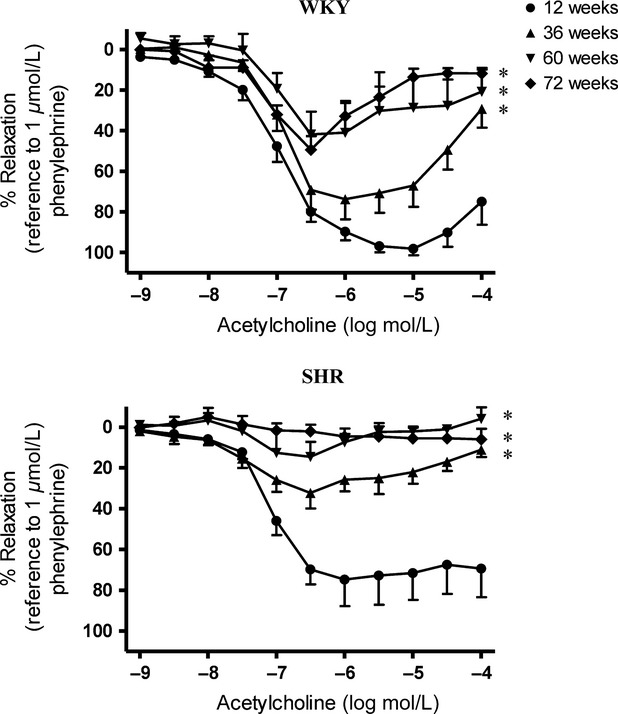
Acetylcholine-induced relaxations of superior mesenteric arteries of (Upper) WKY and (Lower) SHR at various ages in the presence of indomethacin (cyclooxygenase inhibitor; 10−5 mol/L) and L-NAME (nitric oxide synthase inhibitor; 10−4 mol/L). Values are means ± SEM of six to eight experiments. *P < 0.05 compared with corresponding 12-week-old groups.
Changes in IKCa- and SKCa-mediated relaxations in aging and hypertension
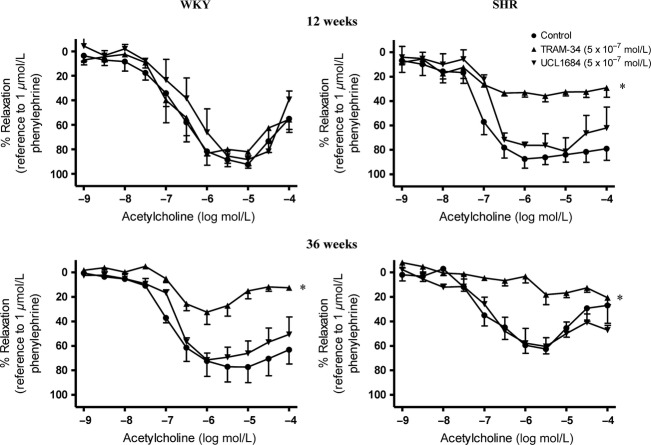
In mesenteric arteries of 12-week-old WKY, TRAM-34 (5 × 10−7 M) or UCL 1684 (5 × 10−7 M) alone did not affect the non-NO-non-prostaglandin-mediated relaxations (Fig.4, Upper Left). In 12-week-old SHR, the part of EDH-type relaxation in the presence of TRAM-34 (mediated by SKCa) was decreased significantly while that in the presence of UCL 1684 (mediated by IKCa) was not altered significantly (Fig.4, Upper Right).
Figure 4.
Effect of inhibition of intermediate- and small-conductance calcium-activated K+ channels on EDH-type relaxation in (Upper) 12- and (Lower) 36-week-old (Left) WKY and (Right) SHR superior mesenteric arteries. Arteries were incubated with TRAM-34 (IKCa inhibitor; 5 × 10−7 mol/L) or UCL 1684 (SKCa inhibitor; 5 × 10−7 mol/L) for 40 min in the presence of indomethacin (cyclooxygenase inhibitor; 10−5 mol/L) and L-NAME (nitric oxide synthase inhibitor; 10−4 mol/L). Values are means ± SEM of eight experiments. *P < 0.05 compared to the control.
In mesenteric arteries of 36-week-old WKY and SHR, UCL 1684 alone did not alter acetylcholine-induced relaxations in the presence of indomethacin and L-NAME (Fig.4, Lower Left and Lower Right). In the presence of TRAM-34, EDH-type relaxations were reduced significantly in preparations of 36-week-old WKY compared with those in arteries of 12-week-old animals (Fig.4).
Role of Na-K ATPase and KIR in EDH-type relaxations
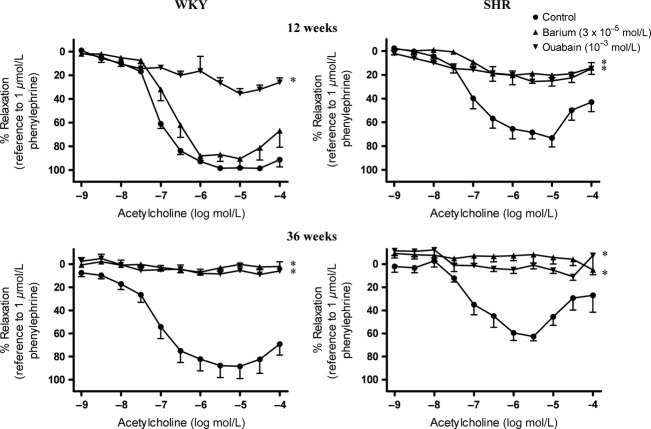
Ouabain [Na-K ATPase inhibitor, 10−3 M (Doughty et al. 2000)], but not barium chloride [KIR channel blocker, 3 × 10−5 M (Doughty et al. 2000)], abolished the acetylcholine-induced relaxation in the presence of indomethacin and L-NAME in arteries of 12-week-old WKY (Fig.5, Upper Left).
Figure 5.
Effect of inhibition of Na-K ATPase and inwardly rectifying K+ channels on EDH-type relaxation in (Upper) 12- and (Lower) 36-week-old (Left) WKY and (Right) SHR superior mesenteric arteries. Arteries were incubated with ouabain (Na-K ATPase inhibitor; 10−3 mol/L) and/or barium (KIR inhibitor; 3 × 10−5 mol/L) for 40 min in the presence of indomethacin (cyclooxygenase inhibitor; 10−5 mol/L) and L-NAME (nitric oxide synthase inhibitor; 10−4 mol/L). Values are means ± SEM of eight experiments. *P < 0.05 compared to the control.
Ouabain alone also abolished EDH-type relaxations in arteries of 12-week-old SHR (Fig.5, Upper Right). While barium chloride alone had no effect on this relaxation in arteries from 12-week-old WKY, it abolished it in preparations from SHR of the same age (Fig.5, Upper Left and Upper Right).
In 36-week-old WKY mesenteric arteries, acetylcholine-induced relaxations were inhibited by both ouabain and barium chloride (Fig.5, Lower Left), although in younger age group (12 weeks), only ouabain, but not barium chloride, inhibited the response (Fig.5, Upper Left). Inhibitions of the EDH-type relaxations by either ouabain or barium chloride were also observed in arteries of both 12–week- and 36-week-old SHR (Fig.5, Upper and Lower Right).
Role of SIRT-1 and AMPK in EDH-type relaxations
In the presence of indomethacin and L-NAME, EX-527 [SIRT1 inhibitor, 10−6 M (Orecchia et al. 2011)] or compound C [AMPK inhibitor, 10−5 M (Thors et al. 2008)] significantly inhibited acetylcholine-induced relaxation in mesenteric arteries of 12-week-old SHR but not in those of age-matched WKY (Figs.6, 7).
Figure 6.
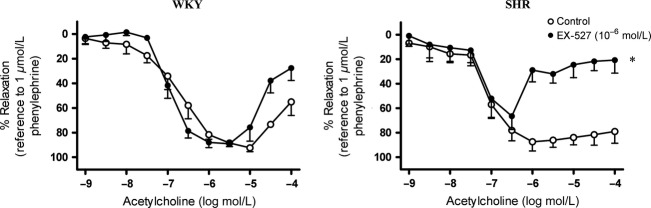
Effect of inhibition of SIRT1 on EDH-type relaxation in 12-week-old (Left) WKY and (Right) SHR superior mesenteric arteries. Arteries were incubated with EX-527 (SIRT1 inhibitor; 10−6 mol/L) for 40 min in the presence of indomethacin (cyclooxygenase inhibitor; 10−5 mol/L) and L-NAME (nitric oxide synthase inhibitor; 10−4 mol/L). Values are means ± SEM of six experiments. *P < 0.05 compared to the control.
Figure 7.
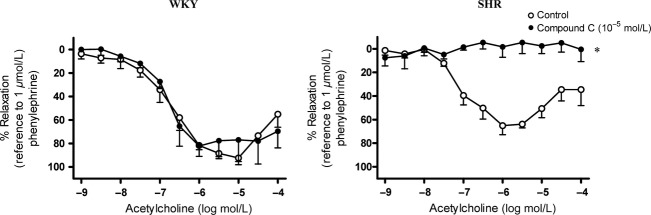
Effect of the inhibition of adenosine monophospate-actived protein kinase (AMPK) on EDH-type relaxation in 12-week-old (Left) WKY and (Right) SHR superior mesenteric arteries. Arteries were incubated with compound C (AMPK inhibitor; 10−5 mol/L) for 40 min in the presence of indomethacin (cyclooxygenase inhibitor; 10−5 mol/L) and L-NAME (nitric oxide synthase inhibitor; 10−4 mol/L). Values are means ± SEM of six experiments. *P < 0.05 compared to the control.
Discussion
This study determined the effect of aging, with or without concurrent hypertension on endothelium-dependent relaxations in the rat mesenteric artery. In normotensive rats, aging did not reduce acetylcholine-induced, endothelium-dependent relaxations under control conditions. In arteries of young [12-week old] hypertensive rats, endothelium-dependent relaxations were still comparable to those observed in preparations of normotensive animals of the same age. However, as hypertensive rats aged, their overall endothelium-dependent relaxations decreased, suggesting that aging, when accompanied by hypertension, is associated with severe endothelial dysfunction in the mesenteric artery. This severe endothelial dysfunction could be attributed mainly to the impairment of EDH-type relaxations, although reduction in responses to endothelium-derived NO was also observed.
Endothelial dysfunction is manifested in part as an impairment of NO- and EDH-mediated relaxations (Félétou and Vanhoutte 2006; Vanhoutte et al., 2009). NO is synthesized by nitric oxide synthase in the endothelium (Palmer et al. 1987; Rees et al. 1990). Under the present experimental conditions, NO-mediated relaxations are the part of acetylcholine-induced decreases in tension that persist in the presence of indomethacin (to prevent the production of vasoactive prostanoids), TRAM-34 and UCL 1684 (IKCa and SKCa channel blockers, respectively, to prevent EDH-mediated responses) and are sensitive to the inhibitory effect of nitric oxide synthase inhibitor (e.g. L-NAME). Whereas the mechanism underlying EDH varies with species and blood vessel type (Chataigneau et al. 1998; Chaytor et al. 1998; Edwards et al. 1998; Fisslthaler et al. 1999; Morikawa et al. 2003; Shimokawa and Morikawa 2005), relaxations that are independent of either NO or prostanoids and require the activation of IKCa and SKCa are characteristic of EDH-mediated responses in rat arteries (Corriu et al. 1996; Chataigneau et al. 1998; Gluais et al. 2005a,b; Félétou and Vanhoutte 2006; Yeung et al. 2013). Therefore, the residual relaxations to acetylcholine observed here in mesenteric arteries incubated with indomethacin and L-NAME can be attributed to EDH, as they were abolished by the combination of IKCa and SKCa blockers, TRAM-34 and UCL 1684 (Fig. S1). In this study, a greater impairment of EDH-type than of NO-mediated relaxation with aging is observed in the WKY. Similar results were obtained in SHR mesenteric arteries, in which EDH-type relaxation is abolished at 72 weeks of age while NO still contributes significantly to acetylcholine-induced relaxation. These findings suggest that aging alone has minimal effect on NO-mediated relaxations, unlike on EDH-type responses. The data also suggest that endothelial dysfunction is not always associated with a reduced activity of NO, but may be initiated mainly by a reduced contribution of EDH-type responses.
Hypertension alone also does not affect NO-mediated relaxation of the rat mesenteric artery. However, NO-mediated relaxation decreases in preparations of aged [60- and 72-week old] SHR, but not in WKY. Thus, impairment of NO-mediated responses only occurs when aging is associated with hypertension. By contrast, the signaling pathway involved in EDH-type relaxation is dysfunctional at a younger age in the SHR, and hence at an earlier stage of hypertension than that for NO-mediated relaxation. Further increases in the age of SHR exacerbate this impairment until SHR mesenteric arteries no longer exhibit EDH-type relaxations at the age of 72 weeks. These findings are in agreement with an earlier report demonstrating that, while NO-mediated relaxation was only slightly impaired, EDH-mediated responses were completely lost in renal arteries of old (22 months) SHR (Büssemaker et al. 2003).
The present data suggest that aging and hypertension per se do not significantly affect endothelium-dependent NO-mediated relaxations in rat mesenteric arteries, but that impairment of NO-mediated responses only occurs when aging is associated with hypertension. On the other hand, EDH-type relaxations in mesenteric arteries decrease with either aging or hypertension, and these impairments are additive. At the early stage, hypertension is mostly asymptomatic; nevertheless, the exposure of endothelial cells to a chronically elevated arterial blood pressure likely predisposes to endothelial dysfunction thereby increasing the risk for vascular complications with aging. Therefore, treatment should be initiated as early as possible to prevent the progression of endothelial damage. As impairment occurs selectively in the signaling pathways leading to EDH-mediated responses, activation of other vasodilator mechanisms, for example, those involving NO-mediated response, would be more effective in reducing the impact of mechanical stress exerted on endothelial cells due to the high arterial blood pressure.
Activation of potassium channels is the key feature of the “classical” EDH pathway, as combination of IKCa and SKCa inhibitors can abolish EDH-mediated relaxation (Edwards et al. 1998; Gluais et al. 2005a). However, inhibition of either one of these potassium channels alone does not affect EDH-type responses in superior mesenteric arteries of the young (12-week old) WKY, as reported in the small (third order branch) mesenteric artery (Waldron and Garland 1994). The present experiments demonstrate that both aging and hypertension per se result in a significant impairment of SKCa-mediated relaxation in superior mesenteric arteries. On the other hand, IKCa-mediated relaxations in this artery are less susceptible to the effect of aging and/or hypertension, suggesting a predominant role of IKCa in the EDH pathway (Giachini et al. 2009). Reduced SKCa-mediated relaxations in aging [young versus old WKY] or hypertension [young WKY versus young SHR] alone may be due to reduced protein expression or reduced activity of SKCa. An earlier report demonstrating a significant reduction of SKCa expression in aged hypertensive animals (Weston et al. 2010) suggests that the contribution of SKCa to relaxation may indeed be impaired due to down-regulation.
Activation of Na-K ATPase and opening of KIR channels are two of the major mechanisms responsible for the EDH of vascular smooth muscle cells (Edwards et al. 1998). They act as the downstream signaling pathways of the IKCa and SKCa, respectively, to facilitate hyperpolarization of the vascular smooth muscle and hence their relaxation (Dora and Garland 2001; Edwards et al. 2010; Weston et al. 2010). Indeed, SKCa and IKCa generate EDH through two distinct pathways: SKCa triggers myocyte hyperpolarization via activation of KIR in caveolae, whereas IKCa activates the downstream Na-K ATPase in myoendothelial projections (Weston et al. 2010). The finding that ouabain, but not barium chloride, abolished the EDH-type relaxations in arteries of 12-week-old WKY suggests that, in young WKY, Na-K ATPase plays a dominant role in EDH-type response. By contrast, in arteries of aging and hypertensive animals, EDH-type relaxation is abolished not only by inhibition of Na-K ATPase alone, but also by inhibition of KIR alone. Thus, KIR may serve as a compensatory mechanism for EDH-type relaxation in mesenteric arteries of aging and hypertensive rats.
In this study, inhibition of SIRT1 did not affect the EDH-type relaxation in the mesenteric arteries of normotensive rats; however, it reduced that in SHR preparations. These data suggest that SIRT1 is activated and contributes to EDH-type relaxations only under the latter pathological condition. Activation of SIRT1 is regulated by AMPK, and SIRT1 also activates AMPK (Zu et al. 2010; Wang et al. 2011). As with inhibition of SIRT1, inhibition of AMPK also reduced EDH-type relaxations in SHR mesenteric arteries, without affecting such responses in WKY preparations. The involvement of SIRT1 and AMPK in EDH-type relaxations in mesenteric arteries of hypertensive, but not normotensive, rats suggests the development of accelerated senescence in hypertensive blood vessels. The present findings then indicate that at the early stage of hypertension, AMPK restores EDH-mediated relaxations. These findings are in line with the reports that the protein expression of AMPK increases in mesenteric arteries of SHR at 20 to 24 weeks of age (Ford et al. 2012) and that mice deficient in endothelial AMPK have high-blood pressure and exhibit reduced EDH-like responses in their mesenteric arteries (Enkhjargal et al. 2014). Activation of AMPK leads to up-regulation of the anti-oxidative enzyme, heme oxygenase-1 (Li et al. 2012, 2013), which in hypertension may counteract the increased production of reactive oxygen species that contributes to the impairment of EDH-mediated relaxation (Li et al. 2013; Rashid et al. 2014). A caveat is that compound C, at the concentration used in the present experiments, only preferentially inhibits AMPK, but also competes for the ATP binding sites of other enzymes (Bain et al. 2007). Therefore, the interpretation based on the use of this pharmacological inhibitor warrants further investigation.
In conclusion, the present data suggest that EDH-type response in the rat mesenteric artery undergoes an age-dependent impairment, which is also observed in hypertensive rats but is exacerbated when aging is associated with hypertension. The impairment due to aging or hypertension can be attributed to reduced involvement of SKCa (Fig.8), suggesting that high arterial blood pressure may cause similar damage to endothelial function as aging. The remaining EDH-type relaxation in young hypertensive animal appears to depend on the activation of SIRT1 and AMPK, which may serve to combat the impairment of endothelium-dependent relaxation in hypertension.
Figure 8.
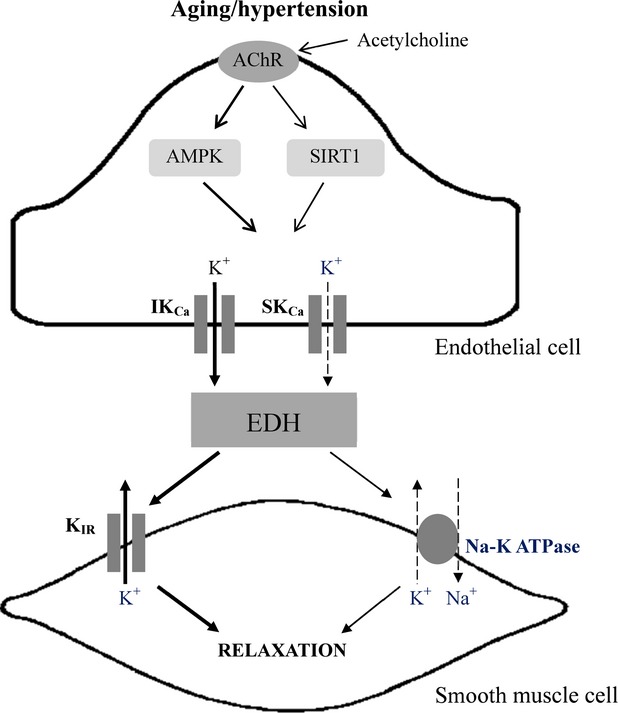
The mechanism underlying the impairment of endothelium-dependent hyperpolarization (EDH)-type relaxations in aging or hypertensive rats. Aging or hypertension causes the dysfunction of small-conductance calcium-activated potassium chanels (SKCa) and sodium-potassium ATPase (Na-K ATPase), thus impairing EDH-mediated responses. In young hypertensive animals, the impairment is compensated by the facilitation of the activity of intermediate-conductance calcium-activated potassium chanels (IKCa), and the activation of adenosine monophosphate-activated protein kinase (AMPK) and silent information regulator T1 (sirtuin-1; SIRT1).
Acknowledgments
This study was supported by Research Grant Council General Research Fund (HKU769907M; HKU769808), Hong Kong SAR.
Author Contribution
Kong, Man, Gao, Vanhoutte, and Leung participated in research design and wrote or contributed to the writing of the manuscripts; Kong conducted the experiments; Kong and Leung performed data analysis
Disclosures
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Acetylcholine-induced relaxations of superior mesenteric arteries of (Left) Wistar Kyoto (WKY) and (Right) spontaneously hypertensive rats (SHR) at various ages in the presence of indomethacin (cyclooxygenase inhibitor; 10−5 mol/L) with and without L-NAME (nitric oxide synthase inhibitor; 10−4 mol/L), TRAM-34 (inhibitor of intermediate-conductance calciumactivated potassium channel; 5 × 10−7 mol/L) plus UCL 1684 (inhibitor of small-conductance calcium-activated potassium channel; 5 × 10−7 mol/L), and their combination. Values are means ± SEM of five to eight experiments.
References
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 2002;227:45009–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Büssemaker E, Popp R, Fisslthaler B, Larson CM, Fleming I, Busse R, et al. Aged spontaneously hypertensive rats exhibit a selective loss of EDHF-mediated relaxation in the renal artery. Hypertension. 2003;42:562–568. doi: 10.1161/01.HYP.0000088852.28814.E2. [DOI] [PubMed] [Google Scholar]
- Cardillo C, Panza JA. Impaired endothelial regulation of vascular tone in patients with systemic arterial hypertension. Vasc Med. 1998;3:138–144. doi: 10.1177/1358836X9800300208. [DOI] [PubMed] [Google Scholar]
- Chataigneau T, Félétou M, Duhault J, Vanhoutte PM. Epoxyeicosatrienoic acids, potassium channel blockers and endothelium-dependent hyperpolarization in the guinea-pig carotid artery. Br J Pharmacol. 1998;123:574–580. doi: 10.1038/sj.bjp.0701629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor AT, Evans WH, Griffith TM. Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries. J Physiol. 1998;508:561–573. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriu C, Félétou M, Canet E, Vanhoutte PM. Endothelium-derived factors and hyperpolarization of the carotid artery of the guinea-pig. Br J Pharmacol. 1996;119:959–964. doi: 10.1111/j.1476-5381.1996.tb15765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J Appl Physiol. 2008;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinsky VW, Chakrabarti S, Pereira TJ, Oka T, Levasseur J, Beker D, et al. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim Biophys Acta. 2013;1832:1723–1733. doi: 10.1016/j.bbadis.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Donmez G, Guarente L. Aging and disease: connections to sirtuins. Aging Cell. 2010;9:285–290. doi: 10.1111/j.1474-9726.2010.00548.x. [DOI] [PubMed] [Google Scholar]
- Dora KA, Garland CJ. Properties of smooth muscle hyperpolarization and relaxation to K+ in the rat isolated mesenteric artery. Am J Physiol Heart Circ Physiol. 2001;280:H2424–H2429. doi: 10.1152/ajpheart.2001.280.6.H2424. [DOI] [PubMed] [Google Scholar]
- Doughty JM, Boyle JP, Langton PD. Potassium does not mimic EDHF in rat mesenteric arteries. Br J Pharmacol. 2000;130:1174–1182. doi: 10.1038/sj.bjp.0703412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- Edwards G, Félétou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflügers Arch. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- Enkhjargal B, Godo S, Sawada A, Suvd N, Saito H, Noda K, et al. Endothelial AMP-activated protein kinase regulates blood pressure and coronary flow responses through hyperpolarization mechanism in mice. Arterioscler Thromb Vasc Biol. 2014;34:1505–1513. doi: 10.1161/ATVBAHA.114.303735. [DOI] [PubMed] [Google Scholar]
- Erusalimsky JD. Vascular endothelial senescence: from mechanisms to pathophysiology. J Appl Physiol. 2009;106:326–332. doi: 10.1152/japplphysiol.91353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. Endothelium-dependent hyperolarizations: past beliefs and present facts. Annals of Med. 2007;39:495–516. doi: 10.1080/07853890701491000. [DOI] [PubMed] [Google Scholar]
- Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, et al. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- Fisslthaler B, Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res. 2009;105:114–127. doi: 10.1161/CIRCRESAHA.109.201590. [DOI] [PubMed] [Google Scholar]
- Ford RJ, Teschke SR, Reid B, Durham KK, Kroetsch JT, Rush JWE. AMP-activated protein kinase activator AICAR acutely lowers blood pressure and relaxes isolated resistance arteries of hypertensive rats. J Hypertens. 2012;30:725–733. doi: 10.1097/HJH.0b013e32835050ca. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3:2007–2018. [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Giachini FR, Carneiro FS, Lima VV, Carneiro ZN, Dorrance A, Webb RC, et al. Upregulation of intermediate calcium-activated potassium channels counterbalance the impaired endothelium-dependent vasodilation in stroke-prone spontaneously hypertensive rats. Transl Res. 2009;154:183–193. doi: 10.1016/j.trsl.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluais P, Edwards G, Weston AH, Falck JR, Vanhoutte PM, Félétou M. Role of SK(Ca) and IK(Ca) in endothelium-dependent hyperpolarizations of the guinea-pig isolated carotid artery. Br J Pharmacol. 2005a;144:477–485. doi: 10.1038/sj.bjp.0706003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluais P, Lonchampt M, Morrow JD, Vanhoutte PM, Feletou M. Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: the Janus face of prostacyclin. Br J Pharmacol. 2005b;146:834–845. doi: 10.1038/sj.bjp.0706390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudenschild CC, Prescott MF, Chobanian AV. Aortic endothelial and subendothelial cells in experimental hypertension and aging. Hypertension. 1981;3(Suppl. I):I148–I153. doi: 10.1161/01.hyp.3.3_pt_2.i148. [DOI] [PubMed] [Google Scholar]
- Lang MG, Noll G, Lüscher TF. Effect of aging and hypertension on contractility of resistance arteries: modulation by endothelial factors. Am J Physiol. 1995;269:H837–H844. doi: 10.1152/ajpheart.1995.269.3.H837. [DOI] [PubMed] [Google Scholar]
- Li FY, Lam KS, Tse HF, Chen C, Wang Y, Vanhoutte PM, et al. Endothelium-selective activation of AMP-activated protein kinase prevents diabetes mellitus-induced impairment in vascular function and reendothelialization via induction of heme oxygenase-1 in mice. Circulation. 2012;126:1267–1277. doi: 10.1161/CIRCULATIONAHA.112.108159. [DOI] [PubMed] [Google Scholar]
- Li Z, Wang Y, Man RYK, Vanhoutte PM. Upregulation of heme oxygenase-1 potentiates EDH-type relaxations in the mesenteric artery of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2013;305:H1474–H1483. doi: 10.1152/ajpheart.00962.2012. [DOI] [PubMed] [Google Scholar]
- Li Z, Wang Y, Vanhoutte PM. Upregulation of heme oxygenase 1 by hemin impairs endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. Hypertension. 2011;58:926–934. doi: 10.1161/HYPERTENSIONAHA.111.173807. [DOI] [PubMed] [Google Scholar]
- Lüscher TF, Tanner FC, Tschudi MR, Noll G. Endothelial dysfunction in coronary artery disease. Annu Rev Med. 1993;44:395–418. doi: 10.1146/annurev.me.44.020193.002143. [DOI] [PubMed] [Google Scholar]
- Lüscher TF, Vanhoutte PM. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension. 1986;8:344–348. doi: 10.1161/01.hyp.8.4.344. [DOI] [PubMed] [Google Scholar]
- Mantelli L, Amerini S, Ledda F. Roles of nitric oxide and endothelium-derived hyperpolarizing factor in vasorelaxant effect of acetylcholine as influenced by aging and hypertension. J Cardiovasc Pharmacol. 1995;25:595–602. doi: 10.1097/00005344-199504000-00013. [DOI] [PubMed] [Google Scholar]
- Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa K, Shimokawa H, Matoba T, Kubota H, Akaike T, Talukder MA, et al. Pivotal role of Cu, Zn-superoxide dismutase in endothelium-dependent hyperpolarization. J Clin Invest. 2003;112:1871–1189. doi: 10.1172/JCI19351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North BJ, Verdin E. Sirtuins: Sir2-related NAD-dependent protein deacetylases. Genome Biol. 2004;5:224. doi: 10.1186/gb-2004-5-5-224. doi: 10.1186/gb-2004-5-5-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orecchia A, Scarponi C, Di Felice F, Cesarini E, Avitabile S, Mai A, et al. Sirtinol treatment reduces inflammation in human dermal microvascular endothelial cells. PLoS ONE. 2011;6:e24307. doi: 10.1371/journal.pone.0024307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota H, Akishita M, Eto M, Iijima K, Kaneki M, Ouchi Y. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol. 2007;43:571–579. doi: 10.1016/j.yjmcc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ferrigo AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–525. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Rashid SK, Khodja NI, Auger C, Alhosin M, Boehm N, Oswald-Mammosser M, et al. Probiotics (VSL#3) prevent endothelial dysfunction in rats with portal hypertension: role of the angiotensin system. PLoS ONE. 2014;9:e97458. doi: 10.1371/journal.pone.0097458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees DD, Palmer RMJ, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa H, Morikawa K. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J Mol Cell Cardiol. 2005;39:725–732. doi: 10.1016/j.yjmcc.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Takase H, Dohi Y, Kojima M, Sato K. Changes in the endothelial cyclooxygenase pathway in resistance arteries of spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1994;23:326–330. [PubMed] [Google Scholar]
- Tang EHC, Félétou M, Huang Y, Man RYK, Vanhoutte PM. Acetylcholine and sodium nitroprusside cause long-term inhibition of EDCF-mediated contractions. Am J Physiol Heart Circ Physiol. 2005;289:2434–2340. doi: 10.1152/ajpheart.00568.2005. [DOI] [PubMed] [Google Scholar]
- Thors B, Halldórsson H, Jónsdóttir G, Thorgeirsson G. Mechanism of thrombin mediated eNOS phosphorylation in endothelial cells is dependent on ATP levels after stimulation. Biochim Biophys Acta. 2008;1783:1893–1902. doi: 10.1016/j.bbamcr.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Shimokawa H, Tang EHC, Félétou M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- Waldron GJ, Garland CJ. Contribution of both nitric oxide and a change in membrane potential to acetylcholine-induced relaxation in the rat small mesenteric artery. Br J Pharmacol. 1994;112:831–836. doi: 10.1111/j.1476-5381.1994.tb13154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liang Y, Vanhoutte PM. SIRT1 and AMPK in regulating mammalian senescence: a critical review and a working model. FEBS Lett. 2011;585:986–994. doi: 10.1016/j.febslet.2010.11.047. [DOI] [PubMed] [Google Scholar]
- Weston AH, Porter EL, Harno E, Edwards G. Impairment of endothelial SKCa channels and of downstream hyperpolarizing pathways in mesenteric arteries from spontaneously hypertensive rats. Br J Pharmacol. 2010;160:836–843. doi: 10.1111/j.1476-5381.2010.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MS, Delansorne R, Man RY, Svenningsen P, Vanhoutte PM. Chronic treatment with vitamin D lowers arterial blood pressure and reduces endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2010;299:H1226–H1234. doi: 10.1152/ajpheart.00288.2010. [DOI] [PubMed] [Google Scholar]
- Yeung YYT, Lee SS, Vanhoutte PM, Leung SWS. Prolonged exposure to lopinavir impairs endothelium-dependent hyperpolarization-mediated relaxation in rat mesenteric arteries. J Cardiovasc Pharmacol. 2013;62:397–404. doi: 10.1097/FJC.0b013e31829fdd01. [DOI] [PubMed] [Google Scholar]
- Zou Q, Leung SW, Vanhoutte PM. Activation of nicotinic receptors can contribute to endothelium-dependent relaxations to acetylcholine in the rat aorta. J Pharmacol Exp Ther. 2010;341:756–763. doi: 10.1124/jpet.112.192229. [DOI] [PubMed] [Google Scholar]
- Zu Y, Liu L, Lee MYK, Xu C, Liang Y, Man RYK, et al. SIRT1 promotes proliferation and prevents senescence through targeting LKB1 in primary porcine aortic endothelial cells. Circ Res. 2010;106:1384–1393. doi: 10.1161/CIRCRESAHA.109.215483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Acetylcholine-induced relaxations of superior mesenteric arteries of (Left) Wistar Kyoto (WKY) and (Right) spontaneously hypertensive rats (SHR) at various ages in the presence of indomethacin (cyclooxygenase inhibitor; 10−5 mol/L) with and without L-NAME (nitric oxide synthase inhibitor; 10−4 mol/L), TRAM-34 (inhibitor of intermediate-conductance calciumactivated potassium channel; 5 × 10−7 mol/L) plus UCL 1684 (inhibitor of small-conductance calcium-activated potassium channel; 5 × 10−7 mol/L), and their combination. Values are means ± SEM of five to eight experiments.