Abstract
Cerebral near-infrared spectroscopy (NIRS) has long represented an exciting prospect for the noninvasive monitoring of cerebral tissue oxygenation and perfusion in the context of traumatic brain injury (TBI), although uncertainty still exists regarding the reliability of this technology specifically within this field. We have undertaken a review of the existing literature relating to the application of NIRS within TBI. We discuss current “state-of-the-art” NIRS monitoring, provide a brief background of the technology, and discuss the evidence regarding the ability of NIRS to substitute for established invasive monitoring in TBI.
Key words: : brain, injury, near-infrared, review, spectroscopy, trauma
Background
Approximately every 90 seconds an individual in the United Kingdom sustains a traumatic brain injury (TBI), with 10,000–20,000 people every year sustaining a serious brain injury requiring hospital admission and intensive therapy.1 A key facet in the management of the injured brain is close monitoring to guide intervention.
Cerebral near-infrared spectroscopy (NIRS) has long represented an exciting prospect for the noninvasive monitoring of cerebral tissue oxygenation and perfusion in the context of brain injury, and as a “work-in-progress,” has demonstrated great potential.2 Recent developments in both the NIRS technology and techniques used to derive cerebral NIRS measures are leading to more accurate and usable data to guide therapy, and thus NIRS is potentially closer to becoming a mainstream method of cerebral monitoring.
Cerebral NIRS (Fig. 1), as a noninvasive and easily applied method, has exciting potential to allow significantly earlier commencement of cerebral tissue monitoring with minimal interoperator variability in output. Conceivably, this could be applied at the earliest phase of brain injury resuscitation (e.g., from the point of first pre-hospital contact), as opposed to current invasive monitoring, which needs admission to a specialized intensive therapy unit. The purpose of this review is to describe the technology and to evaluate the current evidence surrounding its use in the context of adult TBI, and to consider the current limitations and criticisms surrounding NIRS in these circumstances.
FIG. 1.
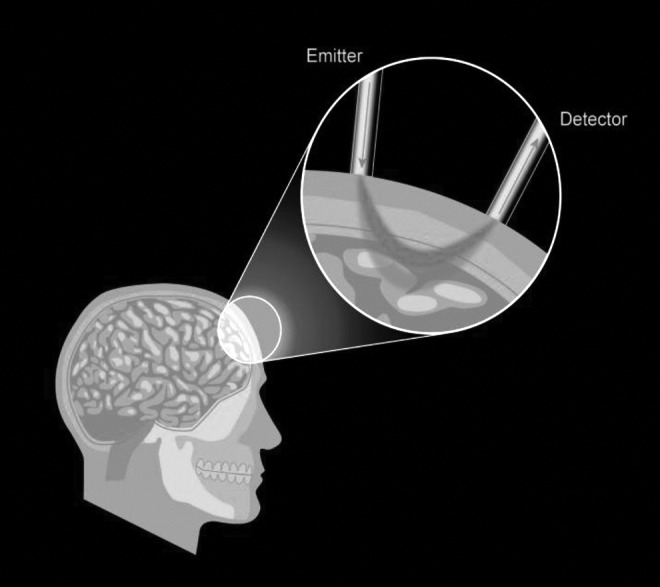
Near-infrared spectroscopy (NIRS) Illustrated: NIR light is applied to the surface, typically using fiber optics, and the transmitted/reflected signal is measured via a detector fiber. The path of light is diffuse, its spectrally varying attenuation providing information about bulk concentrations of “chromophores” in tissue.
Introduction to the Technology
Sir William Herschel (1738–1822) first described infrared radiation in 1800 while observing sunlight through a red colored filter; he passed the resultant filtrate through a prism and observed the surprising heat adjacent to the limits of the visible red light. This led to the deduction that invisible portions of the light/electromagnetic spectrum were responsible for the localized rise in temperature, and the concept of infrared light was established.
“Near-infrared” (NIR) is a term used to define light with wavelengths varying from ∼600–1000 nm, with “true” infrared light having wavelengths of up to 1 mm. This wavelength range describes a window within which biological tissues are relatively translucent because of the low molar absorptivity of the tissue's key “chromophore” constituents (Fig. 2). One of the fundamental principles of NIRS is that the absorption characteristics of each chromophore are unique; therefore, detector signals can be “unmixed” to quantify the relative amounts of each chromophore in a target tissue. As light wavelengths expand >1000 nm, absorption by water becomes too significant to allow effective transmission through tissues, and<600 nm, scattering and absorption lead to the same operational shortcomings.3
FIG. 2.
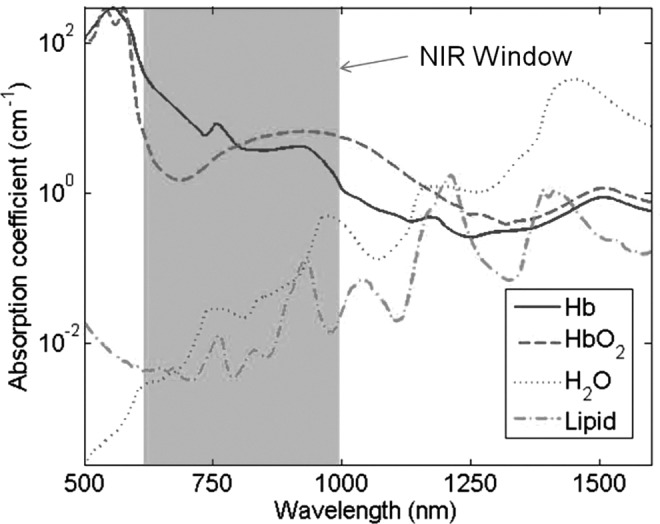
Absorption coefficients of oxy (HbO2) and deoxy (Hb) hemoglobin, water, and lipids showing the basis for the near-infrared (NIR) window.
Between 600 and 1000 nm, absorption is relatively low; therefore, scattering is the dominant tissue interaction process for NIR light. A detailed physical explanation of this is beyond the scope of this review; however, the scatter allows NIR light to be transmitted several centimeters into biological tissue, hence its potentially useful role in noninvasive medical imaging and spectroscopy. Although NIR light can be utilized for functional imaging, giving a spatially resolved map of chromophore concentrations, the majority of instruments currently in use are small- scale NIR spectroscopes that provide a global measurement of chromophore concentrations within the target tissue. Oxygenated and deoxygenated hemoglobin (Hb) are the most commonly targeted chromophores, and knowledge as to the signal strength of these substances delivers useful clinical information regarding blood supply and oxygen transport within the tissues of interest.
Optical monitoring of living tissues in the NIR region of the spectrum (600– 1000 nm) was first demonstrated in 1977 by Jobsis et al.3 Here, observations were made in feline (brain) and canine (heart) models looking at chromophores such as oxygenated and deoxygenated Hb and certain essential cytochromes (a,a3) involved in oxidative metabolism. In 1985, Ferrari et al.4 published the first description of the application of cerebral monitoring using NIRS, and in 1993, the first commercially available NIRS device was marketed by Somanetics (INVOS® 3100).
Since this initial introduction to the clinical environment, the use of NIRS has become widespread. It has been applied in obtaining an average value for oxygen saturation across arteriolar, capillary, and venous compartments in numerous types of observed tissues. Similar technology that uses pulsed signals to resolve arterial/arteriolar components in the measurement of peripheral Hb oxygen saturation 5 (i.e., pulse oximetry) is almost universally used across all acute clinical disciplines in some form or another.
Physical Principles and Detection Modes
The light that is received and processed after its transmission through the brain tissue is the primary means by which the concentrations of the target chromophores can be deduced. Because of the highly scattering nature of NIR light in tissue (Fig. 1), light does not travel on a linear path. Therefore, variability in the detected signal cannot simply be attributed to changes in chromophore concentration. Consequently, some form of computational reconstruction is required.6
The Beer–Lambert law (see Eqn. [1]) is a form of logical modelling relating the absorption of light to the properties of the material through which the light is travelling.
 |
In this model, ɛ is used to represent the known molar absorptivity of the target chromophore (specific extinction coefficient), I represents the intensity of the pre (I) and post (I0) transmission light and, using these, the concentration of the chromophore, c, can be derived (i.e., oxygenated Hb [OHb]).
As we observe, this law assumes 100% transmission of light through the examined media and no scattering affect. To account for this, a modified Beer–Lambert law (Eqn. [2]) was developed by Delpy and colleagues7 in 1988, employing differential path length (L) to calculate multiple chromophore concentrations (C), using measured attenuation (A) at multiple wavelengths (λ).
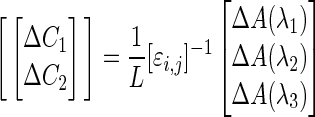 |
Extinction coefficients, ɛ, were provided in a matrix with a value for every chromophore (i) at a given wavelength (j). Despite this advance, the model is still limited by blanket assumptions (regarding homogeneity of the tissues) and a single adaption to consider path length, both of which hinder (but do not exclude) its usefulness in clinical devices for detecting the concentration of chromophore in tissues where scatter is significant. Most commercially available NIRS devices are still underpinned by this principle. Recent advances in modelling that seek to improve on the sensitivity of the technology are discussed later in this review.
Whereas modelling is important to the accuracy of NIRS systems, it is also influenced by the quality of the obtained detector measurements. There are three different detection modes used in NIRS: continuous wave (CW), frequency domain (FD), and time domain (TD) (Fig. 3).
FIG. 3.

Examples of input and output data for the three main types of instruments; (A) continuous wave, (B) frequency domain, (C) time domain.
CW
CW instruments are the most simplistic of the three types. They rely solely on measuring attenuation of the input light (Fig. 3A) and thus provide a fast and relatively inexpensive method of assessment; however, they cannot give absolute measurements. There is also no inherent information generated about the spatial origins of the obtained signal; for this multiple location measurements are required. The In Vivo Optical Spectroscopy (INVOS) System produced by Somanetics is an example of a system using CW resolution technology. This is by far the most popular system employed in clinical practice.
FD
In FD instruments, the source light is amplitude modulated at frequencies in the MHz range. The high-frequency modulation employed in FD systems allows measurement of both phase and intensity of generated signals (Fig. 3B). This allows for a more quantitative assessment of the optical properties of the tissues, as phase data can be used to calculate a more accurate path length for the medium.
TD
TD systems measure the time of flight of photons and give the best spatial resolution when it comes to locating the origin of a chromophore signal; however, they are the most expensive instruments, as picosecond NIR sources and gated detectors are required. The broadening of the output light yields information about the scattering properties of the medium, and the decay in amplitude infers information about the light absorption characteristics of the tissue (Fig. 3C).
Recent developments
The vast majority of clinical investigations utilizing cerebral NIRS in trauma employed CW technology with spatial/depth resolution (particular indices discussed later in this review). The theoretical benefits of more complex NIRS devices incorporating frequency modulation are yet to be properly investigated.
Recent developments in software modelling for light absorption and scatter through biological tissue have enabled far more accurate predictions of how the light emitted from these detectors travels through the tissues of the scalp, cranium, and brain.8 Modelling methods, such as those employed by the NIRFAST,9 use atlas-based anatomical tissue registration and finite element models to predict absorption and scatter through tissues (Fig. 4). These computational methods have been useful in validating the origins of the output data obtained from NIRS equipment,8 demonstrating whether the parameters observed by the clinician are indeed reflective of characteristics of deeper relevant tissue. Model based analysis can also be advantageous in deciding which commercially available device is most sensitive in observing the required target tissue depth.
FIG. 4.
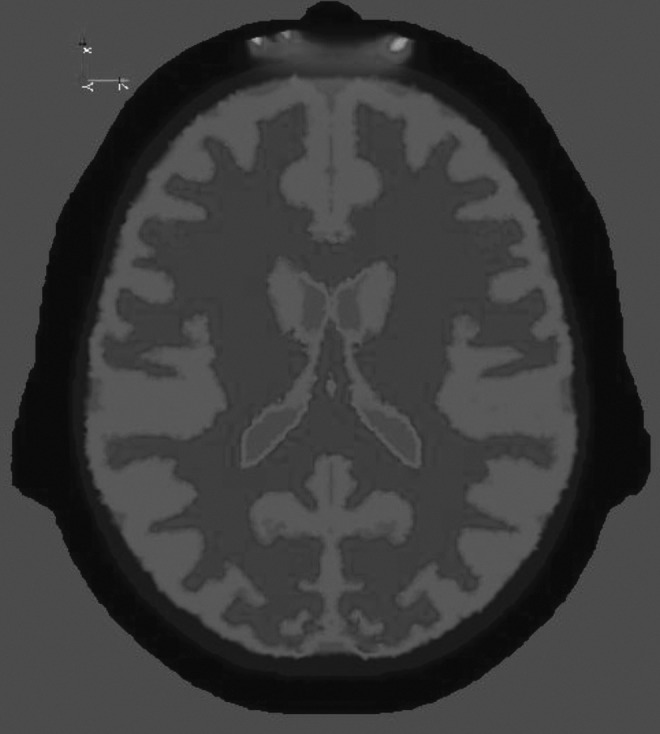
Example of computational models used to predict light path within a three-dimensional complex structure.
Current Application
Interest in the utilization of NIRS monitoring in adult clinical practice is based on some of the inherent advantages that it has over other mainstream invasive methods of cerebral parameter monitoring, most notably its noninvasive nature, its ease of use, and the minimal interoperator variability in detection. Its versatility, in terms of potential for use in a variety of environments, is also of great interest, including the possibility/viability of NIRS use in pre-hospital care10 and initial patient resuscitation. Despite this, the implementation of NIRS techniques into mainstream clinical practice has lagged behind its widespread use in pre-clinical research.2,11 Many reasons have been speculated on for this dichotomy, including the problems of potential contamination of the signal by activity in superficial tissues.
Table 1.
Summary of Key Works
| Author/Year | Device | n | NIRS ability | Conclusion | Comment |
|---|---|---|---|---|---|
| Robertson et al.31 1997 | RunMan (US) | 300 | Hematoma development | Intracranial hematomas accurately detected at presentation. | Dissymmetry in optical density used as identifier. |
| Weerakkody et al.35 2012 | Hamamatsu NIRO 200 | 40 | Intracranial hypertension | Fluctuations in NIRS parameters predictive of the development of vasogenic waves in (raised) ICP during CSF infusion studies. | Correlation coefficient between Hb and HbO(2) as a marker of the slow vasogenic waves of ICP. Not in acute setting. Difficult to translate findings to traumatic brain injury setting. |
| Kampfl et al.37 | INVOS 3100A | 8 | Intracranial hypertention | Significant difference in NIRS parameters between individuals with intracranial hypertension (>25mmHg) and those without. | Very small investigation. Significant finding building on Weerakkody et al., although patients were preselected as having a raised ICP or not. |
| Leal-Noval et al.19 | INVOS 5100 | 22 | Brain tissue Oxygen tension | A robust relationship to significant changes in tissue oxygen tension, but not sufficiently sensitive to detect moderate or mild changes | Significant number of individuals excluded (46%).Concludes that NIRS cannot be used as a substitute for this modality. |
| Lewis et al.48 1996 | INVOS 3100 | 10 | Jugular bulb saturation | Poor correlation and agreement between modalities | 14 clinically significant episodes of jugular bulb desaturation missed by NIRS monitoring. |
NIRS, near-infrared spectroscopy; ICP, intracranial pressure; CSF, cerebrospinal fluid.
Currently, the most frequent use of cerebral NIRS outside of neonatology is in the monitoring of patients undergoing cardiac, great vessel, and carotid artery surgery.12,13 In these situations, changes in the initially measured baseline cerebral tissue Hb oxygen saturation (rSO2) are monitored, and the goal of supportive intervention is to maintain this value through surgery at a minimum of 75% of the baseline reading. Avoiding incidences of “desaturation” is believed to reduce complications relating to cerebral ischemia during these procedures and, although the evidence for this is not fully conclusive, data do indicate that NIRS- monitored patients with higher baseline rSO2 have a lower rate of mortality than those who are not monitored with this modality.14,15
The use of NIRS in the context of adult TBI is currently not widespread,2 although intuitively it is in these patients that cerebral NIRS would be most useful in terms of monitoring whether adequate brain tissue oxygenation and vascular hemostasis is maintained, not only in the neurological critical care environment but also in the resuscitation and post-resuscitation periods. However, the contamination of signal from extracranial tissue has been of concern for some time.16 Further, inherent difficulties in the implementation of NIRS techniques in the context of brain injury exist, which have inhibited the widespread introduction of NIRS techniques for these patients (such as scalp and facial injuries and the presence of hematomas). With current methods, the baseline reading obtained for rSO2 is highly variable, and rarely gives an accurate absolute value. Therefore, what is more useful in all clinical scenarios is to monitor the trend in terms of deviation from the baseline rSO2 levels. In the traumatically injured brain the variability in baseline saturation readings is even greater,2,15,16 because of loss of normal cerebral vascular autoregulation, and the subsequent changes from this baseline are more difficult to interpret. This, combined with the frequent presence of subdural, epidural, and extracranial hematomas that further confound saturation signals, has prevented NIRS-based observation in TBI from becoming widespread and routine. The presence of hematomas has such a profound effect on NIRS-derived rSO2 readings that this perturbation itself has been exploited to demonstrate the presence of intracranial haematomas.17,18 In addition, individuals who sustain a significant intracranial injury frequently have significant concurrent extracranial injuries. The systemic stress placed on an individual with such complex injuries has a profound effect on the quality of perfusion and oxygen consumption in the extracranial tissues (skin, scalp) and, therefore, the sophistication of differential algorithms employed by NIRS devices in such situations become all the more important. Therefore, currently with TBI, in which a patient requires cerebroprotective sedation and intensive monitoring, it is not accepted practice to use NIRS as a single modality for brain tissue monitoring.18–21
Spatially Resolved NIRS-Derived Output and Its Usefulness
Traditionally, the primary output of NIRS devices gives specific information on the saturation of Hb within the broadly observed target tissue. Recently, more eloquent algorithmic methods, based on multiple (spatially varying) detector measurements have produced specially resolved data that reflect concentrations of both OHb and deoxygenated Hb (HHb) specifically within the deeper/intracranial zone of observation. The following examples of spatially resolved parameters are not exhaustive, but serve as an illustration.
The tissue oxygenation index (TOI) and total Hb index (THI) generated by the NIRO Hamamatsu system (Hamamatsu Photonics KK, Hamamatsu, Japan) are derived NIRS parameters conceived to give a better reflection of the information derived from deeper target tissues. The TOI (expressed as a percentage index) is calculated using depth resolved total tissue Hb alongside the relative concentrations of OHb and HHb as identified by the relative NIRS tissue absorbance.10,22 It has been demonstrated to be a useful guide to the threshold of cerebral ischemia and a reflection of cerebral tissue oxygenation specifically in certain circumstances.22
Lam and coworkers23 demonstrated that a 13% decrease from baseline of the TOI was 100% sensitive and 93.2% specific in detecting clinically significant cerebral oxygen desaturation. This was established by the decrease in cerebral function (intraoperative cerebral function monitoring) during carotid artery clamping and bypass at endarterectomy using the Hamamatsu NIRO 300 NIRS monitor (space/distance resolved). Importantly, this investigation made significant progress in resolving component contributions from the external carotid artery (ECA) and internal carotid (ICA), minimizing the often confounding surface effect of skin and other extracranial tissue perfusions that have been described.20,22,24
The THI is an NIRS output parameter derived largely from a depth resolved combined tissue Hb signal strength20 and has been utilized primarily to provide a surrogate for total tissue Hb content within the observed tissue. Intuitively, this is not entirely accurate, as the exact total volume of tissue under observation through the absorption of the emitted light is not known (it is therefore expressed as an arbitrary unit), although educated estimates have been formulated through novel computational modelling processes.25 As an extrapolation of this work, the THI could be utilized as an indirect measure of blood flow and vasomotor activity in the observed tissue. In clinical observations involving patients in severe cardiovascular compromise, the THI (alongside other NIRS based parameters) has been demonstrated to be an effective reflection of tissue perfusion blood volume when correlated with established invasive parameters.26,27
Evidence for the Use of NIRS in TBI
As discussed earlier in this review, currently the most popular uses for cerebral NIRS in adult clinical practice are within the context of cardiac and vascular surgery, in which close monitoring of cerebral perfusion is required during periods of cardiopulmonary bypass. These situations most frequently involve normal cerebral anatomy and autoregulation. The fact that both of these are disrupted by TBI makes interpretation of the observed parameters in this situation that much more complicated. It is currently accepted that the intervention threshold for a drop in Hb saturation as observed by cerebral NIRS is between 13 and 17%13–15 to avoid damage to brain tissue, but in the context of TBI, the utility of this threshold is less clear.
Cerebral NIRS is a more established monitoring modality in pediatric and neonatal intensive care,2 because of favorable anatomical factors (e.g., decreased skull thickness), despite very little established research to demonstrate its clinical effectiveness in the treatment of TBI in this age group.28
The profound effect that the presence of hematomas or intracranial mass lesions can have on the cerebral NIRS parameters yielded within the context of TBI has led to this modality being examined as a screening tool for the presence of these lesions since the early 1990s.29–34 Primarily, these investigations examined the mean difference in optical density between each cerebral hemisphere (ΔOD) as a means of localizing pathology or predicting the presence of a localized hematoma or mass lesion. Within this area of study, work conducted by Robertson and coworkers31 examined parameters retrieved from>300 patients under observation after sustaining a TBI. The primary goal of this investigation was to establish if cerebral NIRS using ΔOD could predict the development of a significant secondary mass lesion. Fifty-nine patients in this cohort developed a secondary hematoma, with 93% (n=55) of these cases identified by a positive ΔOD. Other similar investigations support these levels of sensitivity at ∼ 90%,30,31 with Salonia and coworkers34 producing similarly sensitive and specific data in pediatric cases. NIRS, therefore, has exciting potential as a bedside screening tool for those individuals admitted after TBI to be observed for the development of secondary mass lesions, particularly if clinical examination is impractical because of sedation/general anesthesia, and if avoiding invasive intracranial monitoring is desired.
The use of NIRS in the detection of acute (primary) intracranial hematomas would be most useful in the out-of-hospital setting, where identification of an acute mass lesion would lead to earlier TBI-focused resuscitation techniques alongside expedited transportation to specialist neurosurgical centers. The role of NIRS for the initial detection of hematomas within the secondary or tertiary care setting would be considerably more limited, because of the availability of CT imaging. When axial imaging is available and indicated, NIRS currently does not offer an acceptable replacement or surrogate for these investigations on presentation of injury. However, as discussed for ongoing monitoring for the development of new mass lesions for which serial imaging is not desirable or practical, a potentially useful role exists for NIRS.
Currently, the majority of treatments implemented in the management of significant (moderate-severe) TBI consist of measures to normalize intracranial pressure (ICP) and maintaining an acceptable cerebral tissue perfusion pressure, with other invasive devices monitoring intracranial metabolic parameters such as cerebral tissue microdialysis and tissue oxygen tensor sensors. If cerebral NIRS is to provide a viable alternative to these established invasive monitoring technologies, then clearly there needs to be convincing evidence that NIRS can provide clinical data that are sufficiently accurate to do so. As mentioned, ICP is currently a cornerstone in the management of TBI.
In a small number of investigations, NIRS-based parameters have been demonstrated to have a reasonably robust temporal relationship with ICP15, 35–37 in both traumatic and nontraumatic causes of intracranial hypertension. However, data regarding the sensitivity of NIRS to detect or predict changes in ICP are sparse. Weerakkody and coworkers35,36 demonstrated in two studies that changes in NIRS parameters during cerebrospinal fluid (CSF) infusion very strongly correlated with vasogenic slow wave rises in ICP in a total of 59 post-TBI patients (p<0.001). A smaller investigation undertaken by Kampfl and coworkers37 investigated this specifically in the context of severe TBI, and demonstrated that individuals with an ICP of >25 mm Hg exhibited significantly different (reduced) NIRS parameters than those with an ICP <25 mm Hg, although this only included data on eight patients who were preselected based on their ICP. Unfortunately, this study focused primarily on the ability of NIRS to detect cerebral hypoxia within the context of a raised ICP (which was reported as satisfactory) and not on the ability of NIRS to determine if a change in ICP has occurred, and the nature of its temporal relationship. Budohoski and coworkers15 examined the response phasing of cerebral monitoring modalities to changes in arterial blood pressure (AP) and cerebral perfusion pressure (CPP) in 41 TBI patients, and demonstrated a robust relationship between significant changes in ICP (>5 mm Hg) and NIRS-based parameters (TOI+THI) in 121 pressure change “events” during ∼120 h of multimodal monitoring. A limitation of this study for supporting the concordance of ICP with NIRS was that only events in which there was a significant change in NIRS parameters that followed changes in ICP were considered for analysis. Therefore, within the hours of monitoring data, there were likely to have been numerous significant pressure-related events that evoked no demonstrable change in NIRS parameters. Consequently, it is difficult to determine a definitive sensitivity for changes in NIRS parameters reacting to variations in ICP.
Collectively, such findings demonstrate that NIRS has the potential to be used in selected cases as a surrogate for invasive ICP measurement, although there are currently factors preventing this strategy from being implemented. First, the relationship between NIRS and ICP in terms of retrospective temporal/waveform analysis has been coupled in these reported investigations, but the extent to which NIRS can be relied upon to detect changes in ICP (in the absence of an invasive probe) has not been established. Further, the precise behavior of this relationship is not understood well enough to enable prospective predictions of how to interpret changes in NIRS parameters as changes in ICP (e.g., would a larger change in NIRS parameters reflect a more significant rise in ICP?). Much more work is required before a specific protocol can be developed to allow changes in NIRS activity to be directly “converted” into a change of ICP. As mentioned, NIRS parameters have not reliably demonstrated an ability to reflect true absolute values, and this is true in terms of any established relationship with ICP, although frequently in cases of moderate or mild TBI for which effective clinical monitoring is not possible, the clinician will require only knowledge of a substantial change in values. Therefore, using NIRS as a noninvasive alternative to ICP measurement could be feasible; however, more research is required to better establish the nature of the relationship.
Within the last decade, a number of alternative intracranial/cerebral tissue monitoring modalities have been developed with the view of directing therapy more precisely to maintain the most favorable environment for neurological tissue and the most consistent intracranial homeostasis. Cerebral tissue microdialysis and brain tissue oxygen tension (PbrO2) are perhaps the two most noteworthy developments within this field. As the focus of treatment in TBI moves toward these parameters and becomes less heavily reliant on ICP alone, it becomes increasingly important for cerebral NIRS to demonstrate its equivalence and effectiveness against these modalities.
PbrO2 is an invasive modality measuring the partial pressure of oxygen in extracellular tissues of the brain. It is, therefore, broadly a measure of the balance of consumption and the availability of oxygen for aerobic respiration. A normal physiological level of oxygen partial pressure in the brain is ∼25–30 mm Hg.38 This modality has been established as a promising and safe monitoring modality in TBI, and PbrO2-guided therapy has been linked to improved patient outcome.38,39 In terms of establishing the relationship between cerebral NIRS and PbrO2, in 2010, Leal-Noval and coworkers19 published a prospective observational investigation of 22 patients with severe TBI, specifically looking at this relationship. Each patient was observed over a 16 h period with a total of almost 42,000 paired NIRS/PbrO2 data points. The study concluded that NIRS was reliably sensitive in detecting relatively severe cerebral hypoxia (PbrO2<12 mm Hg), but in situations in which tissue hypoxia was less severe, the sensitivity of NIRS versus PbrO2 decreased significantly. Another limitation of this investigation that must be considered was that 45% of the originally recruited cohort was excluded for TBI-specific factors (e.g., scalp hematoma, surgery, or a poor NIRS signal). These authors recommended that the NIRS technology as it stood was not sufficiently sensitive to be used independently of PbrO2. Budohoski and coworkers15 juxtaposed NIRS and PbrO2 in 41 TBI patients, and noted a concordance between modalities of ∼77%, although principally, the aim of this investigation was to establish the temporal relationship among multiple modalities of brain tissue oxidative physiology. In this study, no comment was made on the clinical significance of these events and any differing agreement between the modalities with tissue hypoxia of differing magnitude. A largely negative outcome was reported in a more recent investigation by Rosenthal and coworkers40 that evaluated the effectiveness of NIRS technology when combined with ultrasound pulsing against invasive multimodal monitoring in 18 patients with severe TBI. They concluded that the parameters recovered by NIRS did not correlate to PbrO2 consistently (using the Licox® system).
These three aforementioned studies15,19,40 utilized the Licox (Clarke cell based) system for PbrO2 monitoring, without frequency domain or depth-resolved NIRS data acquisition. To date, no study has compared these modalities using either the alternative systems of measuring PbrO2 or any of the data manipulation techniques discussed, including better depth-resolved outputs such as the TOI and THI. Future investigations incorporating these technical modifications may produce different and more conclusive results. Nonetheless, currently available cerebral NIRS devices and application of the technology have not yet demonstrated sufficient accuracy for NIRS to be used as an independent surrogate for PbrO2 in TBI.
Jugular bulb venous oxygen saturation has been a mainstream and popular method of measuring cerebral oxygen saturation and oxygen consumption for more than two decades, and it has been demonstrated as a valuable tool in predicting outcome after severe TBI.41–44 The relationship and correlation of cerebral NIRS to this modality (inside and outside the context of TBI) has been reported, with positive results in multiple small observational investigations in children.45–47 As such, evidence for how changes in cerebral NIRS parameters reflect and predict changes in jugular bulb oximetry is sparse, particularly within the context of TBI. A small early prospective observational study conducted by Tateishi and coworkers47 indicated a positive correlation in the majority of their subjects, although only 10 cases were considered, and of these only 4 were cases of TBI. A subsequent investigation conducted by Lewis and coworkers48 (10 TBI patients), which incorporated a greater number of paired measurements and recorded clinically significant episodes of jugular bulb desaturation, reported very poor correlation between the modalities. This study concluded that cerebral NIRS was not at all useful in predicting changes in jugular bulb saturation. Both of these investigations were very limited in terms of number of patients, and the NIRS technology available at the time (1995–1996) did not make use of more recent advances in depth-resolved parameters. Therefore, new studies that make use of significant recent advances in NIRS technology and analysis are needed to provide evidence regarding the ability of cerebral NIRS to predict changes in jugular bulb venous saturation.
At this time, there are very few clinical studies assessing the relationship between cerebral microdialysis and NIRS parameters. Murine models provide encouraging initial data regarding the ability of NIRS to predict changes in lactate-to-pyruvate ratios in cerebral tissue,49 although subsequent clinical work within the context of TBI reported results to the contrary. Specifically, an investigation by Tachtsidis and coworkers50 involving eight TBI patients compared broadband NIRS parameters targeting cytochrome-oxidase with tissue ratios of lactate-to-pyruvate in cerebral tissue harvested via microdialysis. The reaction of these parameters to changes in blood CO2 and O2 was observed; however, the authors found no correlation or agreement between these two modalities of cerebral metabolism measurement.
Unfortunately, as with many other monitoring modalities within the field of brain injury medicine, there is very limited evidence to support the ability of NIRS to predict outcome after injury. Although frequently referred to in limited clinical investigations,17–19,29–37 no substantial prospective investigation provides useful information regarding how NIRS parameters can be used to predict the eventual impact of a particular injury. This in particular provides an opportunity for very meaningful investigations to be undertaken in the future.
Future Directions for NIRS
The recent developments in computational modelling of NIRS (as discussed earlier in this review) and the integration of NIRS-based parameters into subject- specific imaging currently represents an exciting and useful expansion of optical monitoring in brain pathology.51 Using contemporaneous patient images (e.g., CT, MR) and integrating the information that imaging provides (e.g., specific information regarding tissue thicknesses and the location of neurological structures) a far more sensitive and relevant interpretation can be made of the parameters recovered by NIRS. This technology exists, and initial pre-clinical modelling is promising.8,9,51
In order to determine the future role of cerebral NIRS in the management of TBI, work needs to be undertaken to establish a greater understanding of the relationship between NIRS-based monitoring and the other established modalities. Through these investigations, the significance of a change in NIRS parameters and its implications can be better interpreted and understood. To achieve this, a large prospective observational investigation comparing cerebral NIRS with established invasive monitoring methods is needed, which incorporates validated outcome measures at key time points in the patient journey. Should data from such a study be available, the justification of interventional investigations utilizing NIRS technology can be justified. Table 2 summarizes the currently viable roles for NIRS- based monitoring within the context of TBI based on the evidence presented.
Table 2.
Summary of Current Useful Application of NIRS
| + | − |
|---|---|
| Sensitive detection of mass lesions and midline shift | Cannot be used as a substitute for initial CT imaging |
| Detect the development of cerebral edema | No strong evidence to confidently couple NIRS parameters to any other invasive monitoring modality (ICP, tissue oxygen tension, jugular bulb saturation), and NIRS cannot be used in place of these where they are indicated |
| Sensitive in detecting a change in ICP (relationship of this change is uncertain) |
NIRS, near-infrared spectroscopy; ICP, intracranial pressure.
Recent technological advances have been made in the delivery of NIR light and its detection in biological tissues, rather than interoperating the conventional NIRS parameters in a more precise manner (combining it with other modalities). Of note are diffuse NIR correlation spectroscopy (DCS) and ultrasound pulsed conventional NIRS (UP-NIRS). DCS captures the very subtle fluctuations in light scatter that are observed during NIR detection through tissue (largely caused by the movement of the target chromophores within tissue). Its application provides derived values for tissue perfusion and blood flow,52 as opposed to total Hb and the saturation of that Hb with conventional NIRS. This technique has been largely pioneered at the University of Pennsylvania in Philadelphia.53 The advantages of this technique are clear, as information regarding the supply/quantity of blood actually reaching the tissue and the saturation of the transported Hb with oxygen gives far more specific feedback as to the effects of any intervention or therapy. Further, it also supplies the clinician with a more detailed idea as to how ICP and cerebral perfusion are affecting oxygen delivery at the capillary level. No current clinical evidence has been published using this method, largely because of the lack of commercial availability of this equipment and ongoing refinements in the technique. Nevertheless, DCS poses an exciting prospect in the field of noninvasive monitoring in TBI.
A similar adaptation of NIR technology to estimate values of flow, perfusion, and blood movement using ultrasound tagging of NIR parameters has also been developed, and demonstrates encouraging initial results in healthy volunteers.54 The advantages of this method of parameter recovery are very similar to those highlighted by DCS. Another positive facet of this particular development in NIR monitoring is that a commercially available stand-alone device is currently available (C-Flow™ – Ornim Medical, MA). Peer review publications regarding clinical implementation and effectiveness using this device are not yet available, although given the availability of the technology this progression in NIR imaging is another exciting avenue for development in the field of TBI.
Conclusion
On balance, NIRS technology currently has the potential to provide a useful noninvasive adjunct to mainstream monitoring in neurological trauma. The application for which the most evidential support exists (in its current technical state) is for the detection of intracranial (space occupying) hematomas. At present, there is not sufficient evidence to support its use as a surrogate or replacement for any invasive monitoring modality, as efforts to test the modality's ability to detect significant changes in ICP, brain tissue oxygen tension, or jugular bulb saturation have not yielded sufficiently consistent results.
However, the technology is improving rapidly, with introductions of novel methods of data acquisition and analysis, and its mergence with other imaging modalities. These exciting developments have the potential to enhance the utility of NIRS as a noninvasive neurological monitoring tool that can provide information that is as useful to the clinician as are currently available invasive modalities.
Acknowledgments
This article presents independent research funded by the National Institute for Health Research (NIHR) Surgical Reconstruction and Microbiology Research Centre (partnership between University Hospitals Birmingham NHS Foundation Trust, the University of Birmingham, and the Royal Centre for Defence Medicine). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hodgkinson S., Pollit V., Sharpin C., and Lecky F. (2014). Early management of head injury: summary of updated NICE guidance. BMJ (Clin. Res. Ed.) 348, g104. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh A., Elwell C., and Smith M. (2012). Review article: cerebral near-infrared spectroscopy in adults: a work in progress. Anesth. Analg. 115, 1373–1383 [DOI] [PubMed] [Google Scholar]
- 3.Jobsis F.F. (1977). Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198, 1264–1267 [DOI] [PubMed] [Google Scholar]
- 4.Ferrari M., Giannini I., Sideri G., and Zanette E. (1985). Continuous non invasive monitoring of human brain by near infrared spectroscopy. Adv. Exp. Med. Biol. 191, 873–882 [DOI] [PubMed] [Google Scholar]
- 5.Boushel R., Langberg H., Olesen J., Gonzales–Alonzo J., Bulow J., and Kjaer M. (2001). Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand. J. Med. Sci. Sports 11, 213–222 [DOI] [PubMed] [Google Scholar]
- 6.Hongo K., Kobayashi S., Okudera H., Hokama M., and Nakagawa F. (1995). Noninvasive cerebral optical spectroscopy: depth-resolved measurements of cerebral haemodynamics using indocyanine green. Neurol. Res. 17, 89–93 [DOI] [PubMed] [Google Scholar]
- 7.Delpy D.T., Cope M., van der Zee P., Arridge S., Wray S., and Wyatt J. (1988). Estimation of optical pathlength through tissue from direct time of flight measurement. Phys. Med. Biol. 33, 1433–1442 [DOI] [PubMed] [Google Scholar]
- 8.Tian F., and Liu H. (2014). Depth-compensated diffuse optical tomography enhanced by general linear model analysis and an anatomical atlas of human head. NeuroImage 85 Pt 1, 166–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dehghani H., Eames M.E., Yalavarthy P.K., Davis S.C., Srinivasan S., Carpenter C.M., Pogue B.W., and Paulsen K.D. (2008). Near infrared optical tomography using NIRFAST: algorithm for numerical model and image reconstruction. Commun. Numer. Methods Eng. 25, 711–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weatherall A., Skowno J., Lansdown A., Lupton T., and Garner A. (2012). Feasibility of cerebral near-infrared spectroscopy monitoring in the pre-hospital environment. Acta anaesthesiologica Scandinavica 56, 172–177 [DOI] [PubMed] [Google Scholar]
- 11.Wolf M., Ferrari M., and Quaresima V. (2007). Progress of near-infrared spectroscopy and topography for brain and muscle clinical applications. J. Biomed. Opt. 12, 062104. [DOI] [PubMed] [Google Scholar]
- 12.Fedorow C., and Grocott H.P. (2010). Cerebral monitoring to optimize outcomes after cardiac surgery. Curr. Opin. Anaesthesiol. 23, 89–94 [DOI] [PubMed] [Google Scholar]
- 13.Vohra H.A., Modi A., and Ohri S.K. (2009). Does use of intra-operative cerebral regional oxygen saturation monitoring during cardiac surgery lead to improved clinical outcomes? Interact. Cardiovasc. Thorac. Surg. 9, 318–322 [DOI] [PubMed] [Google Scholar]
- 14.Murkin J.M., Adams S.J., Novick R.J., Quantz M., Bainbridge D., Iglesias I., Cleland A., Schaefer B., Irwin B., and Fox S. (2007). Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth. Analg. 104, 51–58 [DOI] [PubMed] [Google Scholar]
- 15.Budohoski K.P., Zweifel C., Kasprowicz M., Sorrentino E., Diedler J., Brady K.M., Smielewski P., Menon D.K., Pickard J.D., Kirkpatrick P.J., and Czosnyka M. (2012). What comes first? The dynamics of cerebral oxygenation and blood flow in response to changes in arterial pressure and intracranial pressure after head injury. Br. J. Anaesth. 108, 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Germon T.J., Kane N.M., Manara A.R., and Nelson R.J. (1994). Near-infrared spectroscopy in adults: effects of extracranial ischaemia and intracranial hypoxia on estimation of cerebral oxygenation. Br. J. Anaesth. 73, 503–506 [DOI] [PubMed] [Google Scholar]
- 17.Kessel B., Jeroukhimov I., Ashkenazi I., Khashan T., Oren M., Haspel J., Medvedev M., Nesterenko V., Halevy A., and Alfici R. (2007). Early detection of life-threatening intracranial haemorrhage using a portable near-infrared spectroscopy device. Injury 38, 1065–1068 [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q., Ma H., Nioka S., and Chance B. (2000). Study of near infrared technology for intracranial hematoma detection. J. Biomed. Opt. 5, 206–213 [DOI] [PubMed] [Google Scholar]
- 19.Leal–Noval S.R., Cayuela A., Arellano–Orden V., Marin–Caballos A., Padilla V., Ferrandiz–Millon C., Corcia Y., Garcia–Alfaro C., Amaya–Villar R., and Murillo–Cabezas F. (2010). Invasive and noninvasive assessment of cerebral oxygenation in patients with severe traumatic brain injury. Intensive Care Med. 36, 1309–1317 [DOI] [PubMed] [Google Scholar]
- 20.Matcher S.J., and Cooper C.E. (1994). Absolute quantification of deoxyhaemoglobin concentration in tissue near infrared spectroscopy. Phys. Med. Biol. 39, 1295–1312 [DOI] [PubMed] [Google Scholar]
- 21.Al-Rawi P.G., Smielewski P., and Kirkpatrick P.J. (2001). Evaluation of a near-infrared spectrometer (NIRO 300) for the detection of intracranial oxygenation changes in the adult head. Stroke 32, 2492–2500 [DOI] [PubMed] [Google Scholar]
- 22.Al-Rawi P.G., and Kirkpatrick P.J. (2006). Tissue oxygen index: thresholds for cerebral ischemia using near-infrared spectroscopy. Stroke 37, 2720–2725 [DOI] [PubMed] [Google Scholar]
- 23.Lam J.M., Smielewski P., al-Rawi P., Griffiths P., Pickard J.D., and Kirkpatrick P.J. (1997). Internal and external carotid contributions to near-infrared spectroscopy during carotid endarterectomy. Stroke 28, 906–911 [DOI] [PubMed] [Google Scholar]
- 24.Myers D., McGraw M., George M., Mulier K., and Beilman G. (2009). Tissue hemoglobin index: a non-invasive optical measure of total tissue hemoglobin. Crit. Care 13, Suppl. 5, S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franceschini M.A., Gratton E., Hueber D., and Fantini S. (1999). Near-Infrared absorbtion and scattering spectra of tissues in vivo. Proc. SPIE 3597, 526–531 [Google Scholar]
- 26.Mulier K.E., Skarda D.E., Taylor J.H., Myers D.E., McGraw M.K., Gallea B.L., and Beilman G.J. (2008). Near-infrared spectroscopy in patients with severe sepsis: correlation with invasive hemodynamic measurements. Surg. Infect. 9, 515–519 [DOI] [PubMed] [Google Scholar]
- 27.Skarda D.E., Mulier K.E., Myers D.E., Taylor J.H., and Beilman G.J. (2007). Dynamic near-infrared spectroscopy measurements in patients with severe sepsis. Shock 27, 348–353 [DOI] [PubMed] [Google Scholar]
- 28.Dieters E.I., Hidding S.H., Kalisvaart M., and Mik E.G. (2011). Near infrared spectrospocty: an asset to the diagnosis and treatment of traumatic brain injury. Erasmus J. Med. 1, 23–26 [Google Scholar]
- 29.Gopinath S.P., Robertson C.S., Grossman R.G., and Chance B. (1993). Near-infrared spectroscopic localization of intracranial hematomas. J. Neurosurg. 79, 43–47 [DOI] [PubMed] [Google Scholar]
- 30.Robertson C.S., Gopinath S.P., and Chance B. (1995). A new application for near-infrared spectroscopy: detection of delayed intracranial hematomas after head injury. J. Neurotrauma 12, 591–600 [DOI] [PubMed] [Google Scholar]
- 31.Robertson C.S., Gopinath S., and Chance B. (1997). Use of near infrared spectroscopy to identify traumatic intracranial hemotomas. J. Biomed. Optics 2, 31–41 [DOI] [PubMed] [Google Scholar]
- 32.Francis S.V., Ravindran G., Visvanathan K., and Ganapathy K. (2005). Screening for unilateral intracranial abnormalities using near infrared spectroscopy: a preliminary report. J. Clin. Neurosci. 12, 291–295 [DOI] [PubMed] [Google Scholar]
- 33.Kahraman S., Kayali H., Atabey C., Acar F., and Gocmen S. (2006). The accuracy of near-infrared spectroscopy in detection of subdural and epidural hematomas. J. Trauma 61, 1480–1483 [DOI] [PubMed] [Google Scholar]
- 34.Salonia R., Bell M.J., Kochanek P.M., and Berger R.P. (2012). The utility of near infrared spectroscopy in detecting intracranial hemorrhage in children. J. Neurotrauma 29, 1047–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weerakkody R.A., Czosnyka M., Zweifel C., Castellani G., Smielewski P., Brady K., Pickard J.D., and Czosnyka Z. (2012). Near infrared spectroscopy as possible non-invasive monitor of slow vasogenic ICP waves. Acta Neurochir. Suppl. 114, 181–185 [DOI] [PubMed] [Google Scholar]
- 36.Weerakkody R.A., Czosnyka M., Zweifel C., Castellani G., Smielewski P., Keong N., Haubrich C., Pickard J., and Czosnyka Z. (2010). Slow vasogenic fluctuations of intracranial pressure and cerebral near infrared spectroscopy—an observational study. Acta Neurochir. (Wien) 152, 1763–1769 [DOI] [PubMed] [Google Scholar]
- 37.Kampfl A., Pfausler B., Denchev D., Jaring H.P. and Schmutzhard E. (1997). Near infrared spectroscopy (NIRS) in patients with severe brain injury and elevated intracranial pressure. A pilot study. Acta Neurochir. Suppl. 70, 112–114 [DOI] [PubMed] [Google Scholar]
- 38.Narotam P.K., Morrison J.F., and Nathoo N. (2009). Brain tissue oxygen monitoring in traumatic brain injury and major trauma: outcome analysis of a brain tissue oxygen-directed therapy. J. Neurosurg. 111, 672–682 [DOI] [PubMed] [Google Scholar]
- 39.De Georgia M.A. (2014). Brain Tissue Oxygen Monitoring in Neurocritical Care. J. Intensive Care Med. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Rosenthal G., Furmanov A., Itshayek E., Shoshan Y., and Singh V. (2014). Assessment of a noninvasive cerebral oxygenation monitor in patients with severe traumatic brain injury. J. Neurosurg. 120, 901–907 [DOI] [PubMed] [Google Scholar]
- 41.Gopinath S.P., Robertson C.S., Contant C.F., Hayes C., Feldman Z., Narayan R.K., and Grossman R.G. (1994). Jugular venous desaturation and outcome after head injury. J. Neurol. Neurosurg. Psychiatry 57, 717–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cormio M., Valadka A.B., and Robertson C.S. (1999). Elevated jugular venous oxygen saturation after severe head injury. J. Neurosurg. 90, 9–15 [DOI] [PubMed] [Google Scholar]
- 43.Lewis S.B., Myburgh J.A., and Reilly P.L. (1995). Detection of cerebral venous desaturation by continuous jugular bulb oximetry following acute neurotrauma. Anaesth. Intensive Care 23, 307–314 [DOI] [PubMed] [Google Scholar]
- 44.Daubeney P.E., Pilkington S.N., Janke E., Charlton G.A., Smith D.C., and Webber S.A. (1996). Cerebral oxygenation measured by near-infrared spectroscopy: comparison with jugular bulb oximetry. Ann. Thorac. Surg. 61, 930–934 [DOI] [PubMed] [Google Scholar]
- 45.Abdul–Khaliq H., Troitzsch D., Berger F., and Lange P.E. (2000). Regional transcranial oximetry with near infrared spectroscopy (NIRS) in comparison with measuring oxygen saturation in the jugular bulb in infants and children for monitoring cerebral oxygenation [in German]. Biomed. Tech. 45, 328–332 [DOI] [PubMed] [Google Scholar]
- 46.Lynch J.M., Buckley E.M., Schwab P.J., Busch D.R., Hanna B.D., Putt M.E., Licht D.J., and Yodh A.G. (2014). Noninvasive optical quantification of cerebral venous oxygen saturation in humans. Acad. Radiol. 21, 162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tateishi A., Maekawa T., Soejima Y., Sadamitsu D., Yamamoto M., Matsushita M., and Nakashima K. (1995). Qualitative comparison of carbon dioxide-induced change in cerebral near-infrared spectroscopy versus jugular venous oxygen saturation in adults with acute brain disease. Crit. Care Med. 23, 1734–1738 [DOI] [PubMed] [Google Scholar]
- 48.Lewis S.B., Myburgh J.A., Thornton E.L., and Reilly P.L. (1996). Cerebral oxygenation monitoring by near-infrared spectroscopy is not clinically useful in patients with severe closed-head injury: a comparison with jugular venous bulb oximetry. Crit. Care Med. 24, 1334–1338 [DOI] [PubMed] [Google Scholar]
- 49.Kuo J.R., Lin B.S., Cheng C.L., and Chio C.C. (2014). Hypoxic-state estimation of brain cells by using wireless near-infrared spectroscopy. IEEE J. Biomed. Health Inform. 18, 167–173 [DOI] [PubMed] [Google Scholar]
- 50.Tachtsidis I., Tisdall M.M., Pritchard C., Leung T.S., Ghosh A., Elwell C.E., and Smith M. (2011). Analysis of the changes in the oxidation of brain tissue cytochrome-c-oxidase in traumatic brain injury patients during hypercapnoea: a broadband NIRS study. Adv. Exp. Med. Biol. 701, 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhan Y., Eggebrecht A.T., Culver J.P., and Dehghani H. (2012). Image quality analysis of high-density diffuse optical tomography incorporating a subject-specific head model. Front. Neuroenergetics 4, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diop M., Verdecchia K., Lee T.Y., and St. Lawrence K. (2011). Calibration of diffuse correlation spectroscopy with a time-resolved near-infrared technique to yield absolute cerebral blood flow measurements. Biomed. Opt. Express 2, 2068–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Durduran T., and Yodh A.G. (2014). Diffuse correlation spectroscopy for non-invasive, micro-vascular cerebral blood flow measurement. NeuroImage 85 Pt. 1, 51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schytz H.W., Guo S., Jensen L.T., Kamar M., Nini A., Gress D.R., and Ashina M. (2012). A new technology for detecting cerebral blood flow: a comparative study of ultrasound tagged NIRS and 133Xe-SPECT. Neurocrit. Care 17, 139–145 [DOI] [PubMed] [Google Scholar]


