Abstract
Background: Lymph is pumped through the collecting lymphatic vessels by both intrinsic and extrinsic forces, propelling it downstream back into circulation. The intrinsic lymph pump relies on the spontaneous contraction of lymphatic muscles to generate the force of pumping lymph (Plp). However, the association between leg edema and reduced leg Plp in the general population is unknown. Therefore, this study determined the association of leg Plp and edema complaints and quality of life in healthy participants.
Methods and Results: A total of 465 healthy volunteers (78 men and 387 women, age 30–85 years) filled out a questionnaire and medical history to rule out severe systemic diseases and local venous/lymphatic diseases. Quality of life was assessed using the Medical Outcome Study Short Form 36 (SF36). Leg Plp was measured using minimally invasive indocyanine green fluorescence lymphography and an occlusion cuff technique while sitting. All participants were divided into three groups according to the Plp values, as follows: Participants with Plp >40 mmHg in both legs, 20 mmHg–40 mmHg in either leg; and <20 mmHg in both legs were divided into the good (n=100), moderate (n=314), and poor (n=51) Plp groups, respectively. The survey revealed the poor leg Plp group was associated with more frequently complaints of leg edema, as well as lower quality of life than the moderate and good Plp groups.
Conclusion: Reduced leg Plp is significantly associated with leg edema complaints and lower quality of life in the general population.
Introduction
The lymphatic system plays an important role in maintaining the balance of body fluids, macromolecules, lipid absorption, and immunity. Lymphatic vessels are responsible for transporting lymph from the tissues back to the circulation. After uptake by initial lymphatic vessels, lymph is pumped through the collecting lymphatic vessels by both extrinsically and intrinsically generated forces; this combination of forces is essential for propelling lymph downstream back into the circulation. The extrinsic forces are generated by the contraction of skeletal muscles adjacent to the lymphatic vessels, as well as influences of cardiac and arterial pulsations, respiration, and central venous pressure fluctuations. On the other hand, the intrinsic lymph pump relies on the spontaneous contraction of lymphatic muscles to generate force.1,2 The combination of forces can transport lymph via lymphatic vessels against hydrostatic pressure in the legs.
Decreases in lymphatic pumping may reduce the lymph velocity and cause lymph stasis or lymphedema. Although the lymphatic intrinsic pumping function is considered important for maintaining the homeostasis of interstitial fluid and molecule balances in local tissue and may be critical for total body health,3,4 the effect of reduced lymphatic pumping pressure (Plp) in the legs on general health has not been studied. Many people complain of leg edema in daily life. Although some visit hospital outpatient clinics, most do not undergo a detailed examination, and are prescribed diuretic drugs or ordered to wear compression stockings without any evidence of lymph stasis. Although there are numerous methods for visualizing lymphatic circulation, including lymphoscintigraphy, MRI, and direct lymphography, it is difficult to screen lymphatic function in such patients in daily practice.
Until recently, the measurement of leg Plp in a large-scale human study was considered to be impossible because of the lack of a noninvasive measurement method. However, near-infrared fluorescence imaging using indocyanine green and an occlusion cuff technique is a new minimally invasive technique for measuring Plp in human legs.5,6 Using this technique, we identified Plp decreases in the early phase of secondary lymphedema compared to control subjects.5 We also identified age-related decreases in Plp by measuring leg Plp in healthy volunteers.7 In this study, we continued measuring Plp in healthy volunteers and investigated its association with the onset of leg edema and quality of life (QOL).
Materials and Methods
Ethical approval
This study was approved by the Ethics Committee of Hamamatsu University School of Medicine and was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Subjects
The participants were recruited at the University Hospital of Hamamatsu University School of Medicine. Potential volunteers were found by advertising the project locally and with poster boards in the most frequented areas of the university hospital. Advertisements were placed with the assistance of The Hamamatsu Chamber of Commerce and Industry. Individuals who were older than 30 years old were invited to participate in the study. After preliminary screening, subjects with no serious allergies or history of leg injury or surgery were included in the study. According to the industry's recommendations (Daiichi-Sankyo Pharmaceutical CO), those with iodine allergy were excluded, because ICG contains iodine.
From September 2009 to September 2013, a total of 465 healthy volunteers (78 men and 387 women, aged 30–85 years) participated in this study. A standard questionnaire including complaints of leg edema, body weight, and height (to calculate body mass index [BMI]), smoking status, and co-morbid diseases (e.g., hypertension, diabetes mellitus, and hyperlipidemia) was performed.
A leg edema complaint was defined as a feeling of edema or persisting visible sock line at least 5 days per week even before noon. Previous smokers were defined as people who smoked for more than 10 years during their life. Subjects with the following past histories were excluded from the study, such as varicose veins in the legs (C3,4,5: CEAP classification), medical history of lymphedema, deep vein thrombosis, radiation therapy to abdomen or legs, prescription of cancer agents, current pregnancy, or leg trauma.
Medical Outcome Study Short Form 36
The Medical Outcome Study Short Form 36 (SF36) is a health survey questionnaire comprising 36 items that was used to obtain information regarding participants' subjective health conditions to assess QOL.8 The SF36 is a well-validated instrument that provides estimates of the following eight health concepts: physical functioning (PF), role functioning-physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role functioning-emotional (RE), and mental health (MH). The responses to the 36 questions are transformed into these concepts, providing a scale from 0 (worst possible health) to 100 (best possible health).
Leg lymphatic pumping pressure measurement
Plp was measured in both legs of participants as described previously.5,7 Briefly, while the participant was seated, a custom-made transparent sphygmomanometer cuff (17 cm length) connected to a standard mercury sphygmomanometer was wrapped around the participant's lower leg just below the popliteal fossa. A larger cuff (18 cm length) was used if the subject had thick legs. Using a 27-gauge needle, 0.3 mL of indocyanine green (ICG: Diagnogreen 0.5%; Daiichi-Sankyo Pharmaceutical, Tokyo, Japan) was subcutaneously injected into the dorsum of each participant's foot.
The indocyanine green fluorescence signal was observed in real time using a near-infrared camera system (PDE™; Hamamatsu Photonics K.K. Hamamatsu, Japan). The light source was a light emitting diode (LED) that emitted light at the wavelength of 760 nm, and the detector was a charge-coupled device (CCD) camera with a filter used to filter out light with a wavelength <820 nm.9,10 When the indocyanine green was injected, the transparent cuff was inflated to 70 mmHg, then gradually deflated, lowering the pressure in 5 mmHg increments every 2 min, in which the time interval was determined according to the lymph movement time along the cuff. Plp was defined as the value of the cuff pressure when the indocyanine green fluorescence signal exceeded the upper border of the cuff. During the measurement, the patients were in a sitting position and kept their legs still to exclude extrinsic forces generated by the leg muscles.
Statistical analysis
Data are expressed as mean±SD. The χ2 test was used to analyze differences in numerical variables among two or three groups, one-way ANOVA was used to analyze parametric data among three groups, and Student's t-test was used to analyze parametric data between two groups. Variables exhibiting significant differences in the ANOVA were submitted to post-hoc pairwise comparisons of PF and GH among the groups by Tukey's test. The level of statistical significance was defined as p<0.05. SPSS for Windows (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
The Plp measurement and the SF36 were successfully performed in all participants. No side effects or complications occurred during leg Plp measurement with indocyanine green fluorescence lymphography. The average Plp decreased with age from participants in their thirties to eighties (p<0.01, r=−0.30) (Table 1). However, there was no significant difference between men and women. Approximately, 10% of participants complained of leg edema. The frequency of complaints of edema was similar among all age groups and both sexes.
Table 1.
Age, Sex, Leg Lymphatic Pumping Pressure, and Edema Complaints
| Age (years) (mean±SD) | N (Male/Female) | Plp (mmHg) (mean±SD) | Edema complaints (Male/Female (total %)) |
|---|---|---|---|
| 30–39 (35.0±2.7) | 93 (24/69) | 26.9±16.2 | 0/9 (9.7%) |
| 40–49 (43.9±3.1) | 85 (15/70) | 24.7±15.5 | 0/10 (11.8%) |
| 50–59 (54.6±2.9) | 131 (9/122) | 22.3±15.1 | 2/12 (10.7%) |
| 60–69 (63.4±2.6) | 108 (18/90) | 20.6±14.6 | 3/6 (8.3%) |
| 70– (73.8±4.2) | 48 (12/36) | 19.5±14.4 | 1/4 (10.4%) |
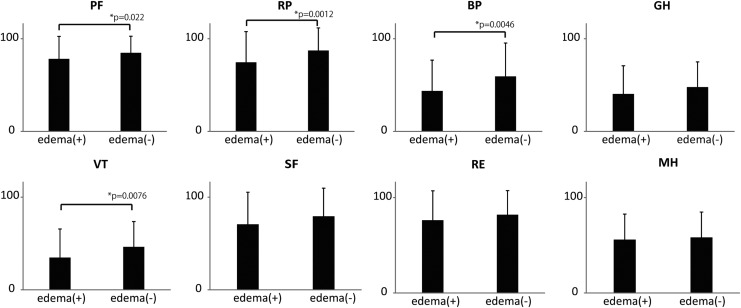
There were no significant differences between the edema (+) and edema (−) groups with respect to BMI, smoking status, or co-morbidities (e.g., hypertension, diabetes mellitus, and hyperlipidemia) (Table 2). The mean leg Plp in the edema (+) and edema (−) groups was 20.4±12.7 mmHg and 23.3±15.6 mmHg, respectively (p=0.23). The results of the SF36 showed participants complaining of edema in the lower legs had significantly lower PF, RP, BP, and VT scores than participants not complaining of edema in the lower legs (p=0.022, 0.0012, 0.0046, and 0.0076, respectively) (Fig. 1).
Table 2.
Participant Characteristics in the Edema (+) and Edema (−) Groups
| Edema (+)n=47 | Edema (−)n=418 | p-value | |
|---|---|---|---|
| Age (years; mean±SD) | 52.5±12.3 | 52.8±12.7 | 0.90 |
| Sex (male/female) | 6/41 | 72/346 | 0.44 |
| BMI (kg/m2; mean±SD) | 22.1±3.6 | 21.5±3.7 | 0.34 |
| Smoking | |||
| Previous | 2 (4.3%) | 42 (10.0%) | 0.20 |
| Current | 3 (6.4%) | 26 (6.2%) | 0.97 |
| Co-morbidities | |||
| Hypertension (%) | 5 (10.6%) | 66 (15.7%) | 0.35 |
| Diabetes mellitus (%) | 2 (4.3%) | 15 (3.6%) | 0.82 |
| Hyperlipidemia (%) | 10 (21.3%) | 78 (18.7%) | 0.66 |
| Plp (mmHg) | 20.4±12.7 | 23.3±15.6 | 0.23 |
FIG. 1.
SF-36 scores of participants with and without edema complaints BP, bodily pain; GH, general health; MH, mental health; PH, physical functioning; RE, role functioning-emotional; RP, role functioning-physical; SF, social functioning; VT, vitality.
According to the age-related values of Plp in a previous study7 and this study, the average Plp in the 20s is around 40 mmHg, while the average Plp over 70s is <20 mmHg. Therefore, participants with Plp >40 mmHg in both legs, 20–40 mmHg in either leg, and <20 mmHg in both legs were divided into good (n=100, 21.5%), moderate (n=314, 67.5%), and poor (n=51, 11.0%) Plp groups, respectively. The mean Plp of both legs in the good, moderate, and poor Plp groups was 44.0±7.4, 19.3±10.8, and 4.2±5.2 mmHg, respectively (Table 3).
Table 3.
Participant Characteristics According to Plp
| Good Plp group | Moderate Plp group | Poor Plp group | ||
|---|---|---|---|---|
| N=100 (21.5%) | N=314 (67.5%) | N=51 (11.0%) | p-value | |
| Age | 51.2±12.8 | 52.9±12.3 | 55.0±13.8 | 0.20 |
| Sex Male/Female | 16 / 84 | 55 / 259 | 7 / 44 | 0.78 |
| Body Mass Index (kg/m2; mean±SD) | 21.6±3.0 | 21.5±3.7 | 22.3±4.3 | 0.39 |
| Previous smoking | 7 (7.0%) | 33 (10.5%) | 4 (7.8%) | 0.53 |
| Current smoking | 4 (4.0%) | 20 (6.4%) | 5 (9.8%) | 0.37 |
| Hypertension (%) | 12 (12.0%) | 51 (16.2%) | 8 (15.7%) | 0.59 |
| Diabetes mellitus (%) | 4 (4.0%) | 10 (3.2%) | 3 (5.9%) | 0.62 |
| Hyperlipidemia (%) | 19 (19.0%) | 58 (18.5%) | 11 (21.6%) | 0.87 |
| Edema complaints (%) | 4 (4.0%) | 35 (11.1%) | 8 (15.7%)* | 0.044 |
| Plp (mmHg) | 44.0±7.4 | 19.3±10.8 | 4.2±5.2* | <0.001 |
Statistically significant
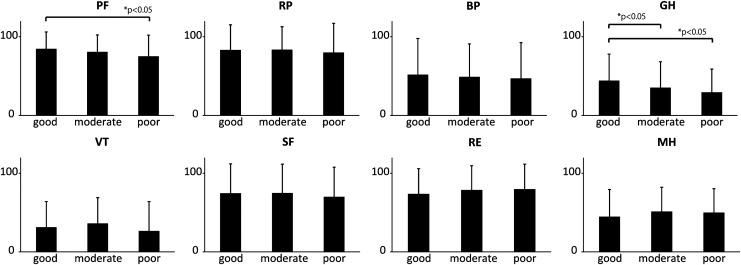
There were no significant differences among the three groups with respect to sex, smoking status, BMI, or co-morbidities (Table 3). In the good, moderate, and poor Plp groups, 4%, 11.1%, and 15.7% of subjects complained of leg edema in daily life (p=0.044) The SF36 revealed that the poor Plp group had a significantly lower PF score than the good Plp group. The poor Plp group also had a significantly lower GH score than both the moderate and good Plp groups (Fig. 2).
FIG. 2.
SF-36 scores according to Plp. Good Plp group: Participants with >40 mmHg in both legs. Moderate Plp group: Participants with Plp 20–40 mmHg in either leg. Poor Plp group: Participants with Plp <20 mmHg in both legs.
Discussion
In this large-scale study of healthy volunteers, indocyanine green fluorescence lymphography and an occlusion cuff technique were used to analyze the associations of leg Plp with leg edema and QOL as assessed by the SF36, which is the most widely used generic health profile.11,12 Among a total of 465 participants aged 30–85 years, leg Plp decreased with age, as we reported previously.7
The standard questionnaire including presence or absence of leg edema complaints, BMI, smoking status, past medical history, and co-morbidities revealed that almost 10% of the participants complained of daily leg edema, even before noon. The participants with leg edema complaints had significantly lower SF36 scores than those without leg edema complaints, suggesting that leg edema is associated with lower QOL. However, there were no significant differences in Plp between the 2 groups; this suggests Plp is not necessarily a decisive factor provoking edema complaints.
Other variables, including, BMI, smoking status, co-morbid diseases (hypertension, diabetes, and hyperlipidemia) also did not differ significantly between those with and without edema complaints. Furthermore, the participants were divided into three groups according to leg Plp. The analysis revealed the poor leg Plp group was not only significantly associated with more frequent complaints of leg edema, but also significantly associated with lower QOL than the moderate and good Plp groups. These results show an association between poor leg Plp and leg edema complaints.
Leg edema generally occurs because of systemic diseases such as liver cirrhosis, congestive heart failure, scleroderma, renal failure, hypoproteinemia; the prescription of calcium channel blockers, prednisone, and anti-inflammatory drugs;13 or localized problems such as leg infection, trauma, chronic venous insufficiency, and lymphatic dysfunction. Subjects with the above-mentioned systemic diseases were excluded from the present study such that participants were as healthy as possible. However, the recruitment method used, which focused on our university hospital area, might not have gathered a sample representative of the general population.
The present participants might have been more interested in their general health conditions, particularly leg health conditions, than the general population, which might be the limitation of this study. Indeed, the sample included several nurses working at the hospital, relatives of patients admitted to the hospital, and patients who visited the outpatient clinic for treatment for co-morbid diseases (i.e., hypertension, lipemia) who were otherwise healthy. Therefore, the prevalence of leg edema complaints in the present study might be greater than that of the general populations. However, the prevalence of leg edema in the general population is largely unknown. Moffatt et al. reported the prevalence of chronic leg edema is 1.33/1000 people in southwest London and estimated more than 100,000 patients suffered from chronic leg edema in the United Kingdom.14 They concluded that leg lymphedema attributable to reasons besides cancer treatment is much more prevalent than generally thought. The present results corroborate their conclusion.
At present, the diagnosis of lymphatic dysfunction (i.e., intrinsic pump failure) can only be made by dynamic lymphoscintigraphy by indirectly measuring radioactive tracer movement.15,16 However, this method is expensive, time-consuming, and involves radiation exposure, which is unsuitable for screening lymphatic function in healthy volunteers.17 The novel method of measuring Plp using indocyanine green lymphography with an occlusion cuff technique enables the minimally invasive screening of lymphatic function. Using this method, the present study demonstrated poor Plp might account for leg edema in a substantial proportion of healthy participants.
On the contrary, the results also revealed that several participants complained of leg edema even though their Plp was normal. Therefore, routine measurement of Plp in patients with leg edema complaints may aid the differential diagnosis of chronic edema due to intrinsic lymphatic pumping failure. Moreover, near-infrared fluorescence technique including the indocyanine green fluorescence lymphography can only detect superficial lymph signals no more than 2 cm depth, so that measurement of Plp in the superficial collecting vessels may overlook dysfunction of the deeper lymphatic systems.
In this study, most participants complaining of leg edema reported bilateral leg symptoms, suggesting the edema might not be due to unilateral venous/lymph drainage impairment but rather filtration edema. Although neither duplex scanning for venous insufficiency nor lymphoscintigraphy for diagnosing lymphedema was performed to rule out the possibility of local disorder of venous/lymph drainage in the present study, careful medical history review and leg observations were performed to exclude unilateral venous/lymph drainage impairment.
Besides systemic diseases such as cardiac failure or nephrotic syndrome, filtration edema could also be caused by long periods of standing/sitting postures, including working with such conditions. During sustained standing/sitting postures, increased transcapillary filtration and reduced reabsorption of tissue fluid due to increased hydrostatic pressure in the blood vessels of the leg lead to increased extravascular fluid volume of the lower leg. The increased capillary filtration can be compensated by lymph pumping. If the burden of capillary filtration overwhelms the lymph pumping capacity, filtration edema can occur.18 In the poor leg Plp group, decreased leg Plp might not compensate for the increased capillary filtration causing filtration edema.
The results of the SF36 revealed participants with leg edema complaints had significantly lower scores in various categories than those without complaints, namely PF, RP, BP, and VT. Although little is known about the QOL of the general population complaining of occasional leg edema, several studies that investigated QOL in patients with leg lymphedema reported a clear deficit in the sub-scores of the SF36 in these patients.14,19,20 In lymphedema patients, leg edema causes difficulties in functioning at home and/or work, altered body image, low self-esteem, and loss of interest in social activities. In the present study, the poor Plp group had significantly lower QOL scores than the moderate and good Plp groups. Although the reduction in QOL is not substantial as that reported in lymphedema patients,14 poor leg Plp itself might be associated with lower QOL in the general population.
In conclusion, this study investigated the association of leg Plp measured by indocyanine green lymphography with an occlusion cuff technique with the onset of leg edema and QOL in healthy volunteers. The results indicate reduced leg Plp is significantly associated with leg edema complaints and lower QOL.
Acknowledgments
The authors thank Tsuyako Ida, Makiko Kato, and Yuichi Ito of the Angiology Laboratory of Hamamatsu University School of Medicine for their assistance and support in this study.
Author Disclosure Statement
Hamamatsu University School of Medicine holds a patent for “Lymphatic pressure-measuring system and method for controlling same” (PCT/JP2010/051706). Naoki Unno is an inventor in the patent.
Funding: This study was supported by a Grant-in-Aid for Scientific Research (C) (22591400) (to NU) and (B)(26293310) (to NU) from the Japan Society for the Promotion of Science.
References
- 1.Gashev AA. Lymphatic vessels: Pressure- and flow- dependent regulatory reactions. Ann NY Acad Sci 2008;1131:100–109 [DOI] [PubMed] [Google Scholar]
- 2.Zawieja DC. Contractile physiology of lymphatics. Lymphat Res Biol 2009;7:87–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodge LM. Osteopathic lymphatic pump techniques to enhance immunity and treat pneumonia. Int J Osteopath Med 2012;15:13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schander A, Padro D, King HH, Downey HF, Hodge LM. Lymphatic pump treatment repeatedly enhances the lymphatic and immune systems. Lymphat Res Biol 2013;11:219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unno N, Nishiyama M, Suzuki M, Tanaka H, Yamamoto N, Sagara D, Mano Y, Konno H. A novel method of measuring human lymphatic pumping using indocyanine green fluorescence lymphography. J Vasc Surg 2010;52:946–952 [DOI] [PubMed] [Google Scholar]
- 6.Okitsu T, Tsuji T, Fujii T, Mihara M, Hara H, Kisu I, Aoki D, Miyata C, Otaka Y, Liu M. Natural history of lymph pumping pressure after pelvic lymphadenectomy. Lymphology 2012;45:165–176 [PubMed] [Google Scholar]
- 7.Unno N, Tanaka H, Suzuki M, Yamamoto N, Mano Y, Sano M, Saito T, Konno H. Influence of age and gender on human lymphatic pumping pressure in the leg. Lymphology 2011;44:113–120 [PubMed] [Google Scholar]
- 8.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–483 [PubMed] [Google Scholar]
- 9.Unno N, Inuzuka K, Suzuki M, Yamamoto N, Sagara D, Nishiyama M, Konno H. Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema. J Vasc Surg 2007;45:1016–1021 [DOI] [PubMed] [Google Scholar]
- 10.Unno N, Nishiyama M, Suzuki M, Yamamoto N, Inuzuka K, Sagara D, Tanaka H, Konno H. Quantitative lymph imaging for assessment of lymph function using indocyanine green fluorescence lymphography. Eur J Vasc Endovasc Surg 2008;36:230–236 [DOI] [PubMed] [Google Scholar]
- 11.Stewart AL, Hays RD, Ware JE., Jr The MOS short-form general health survey. Reliability and validity in a patient population. Med Care 1988;26:724–735 [DOI] [PubMed] [Google Scholar]
- 12.Jenkinson C, Wright L, Coulter A. The SF 36 health survey questionnaire. …If used within its limits. BMJ 1993;307:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ely JW, Osheroff JA, Chambliss ML, Ebell MH. Approach to leg edema of unclear etiology. J Am Board Family Med 2006;19:148–160 [DOI] [PubMed] [Google Scholar]
- 14.Moffatt CJ, Franks PJ, Doherty DC, Williams AF, Badger C, Jeffs E, Bosanquet N, Mortimer PS. Lymphoedema: An underestimated health problem. QJM 2003;96:731–738 [DOI] [PubMed] [Google Scholar]
- 15.Szuba A, Shin WS, Strauss HW, Rockson S. The third circulation: Radionuclide lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med 2003;44:43–57 [PubMed] [Google Scholar]
- 16.Modi S, Stanton AWB, Svensson WE, Peters AM, Mortimer PS, Levick JR. Human lymphatic pumping measured in healthy and lymphoedematous arms by lymphatic congestion lymphoscintigraphy. J Physiol 2007;583:271–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiwari A, Myint F, Hamilton G. Management of lower limb lymphoedema in the United Kingdom. Eur J Vasc Endovasc Surg 2006;31:311–315 [DOI] [PubMed] [Google Scholar]
- 18.Mortimer PS, Levick JR. Chronic peripheral oedema: The critical role of the lymphatic system. Clin Med 2004;4:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franks PJ, Moffatt CJ, Doherty DC, Williams AF, Jeffs E, Mortimer PS. Assessment of health-related quality of life in patients with lymphedema of the lower limb. Wound Repair Regen 2006;14:110–118 [DOI] [PubMed] [Google Scholar]
- 20.Kim SJ, Park YD. Effects of complex decongestive physiotherapy on the oedema and the quality of life of lower unilateral lymphoedema following treatment for gynecological cancer. Eur J Cancer Care (Engl) 2008;17:463–468 [DOI] [PubMed] [Google Scholar]